Regulation of Aqueous Humor Secretion by Melatonin in Porcine Ciliary Epithelium
Abstract
1. Introduction
2. Results
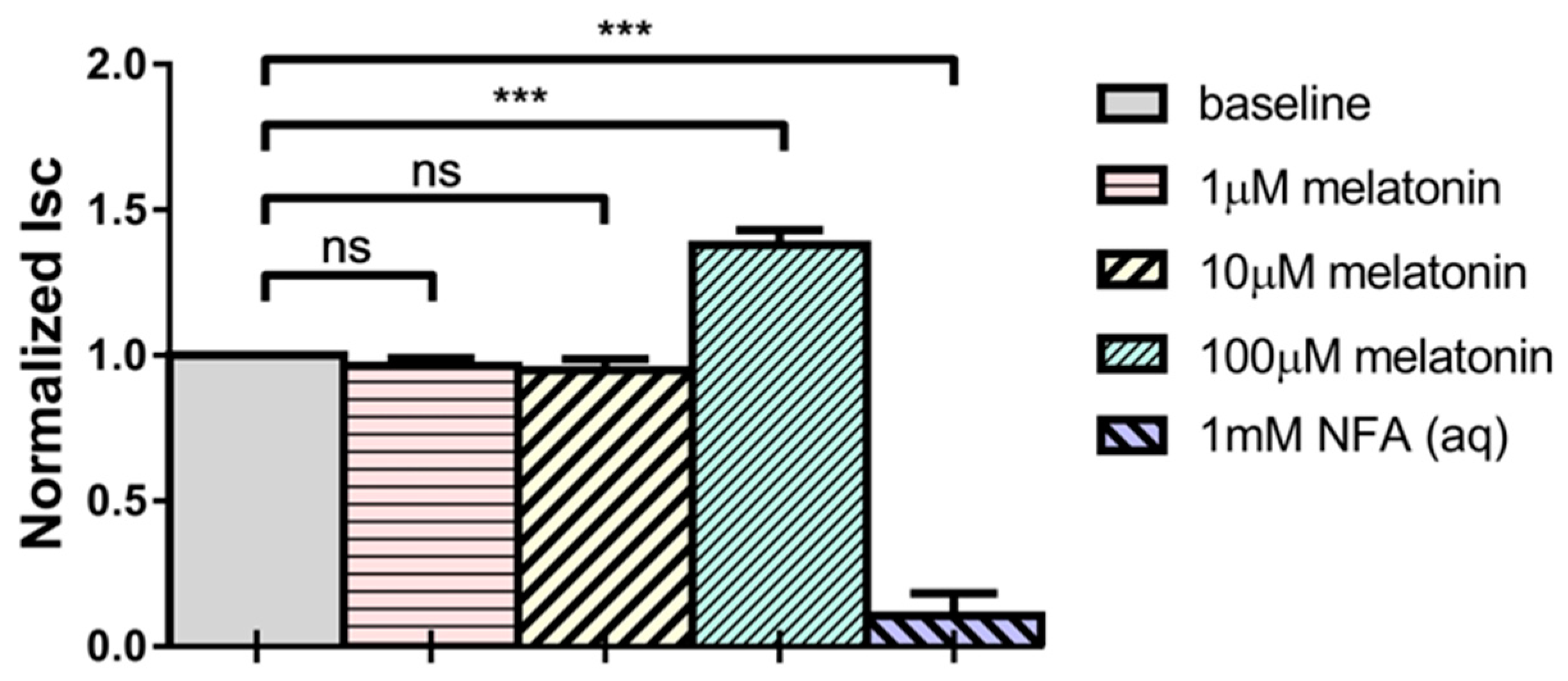
2.1. Melatonin Increases Short-Circuit Current (Isc) across Porcine Ciliary Epithelium
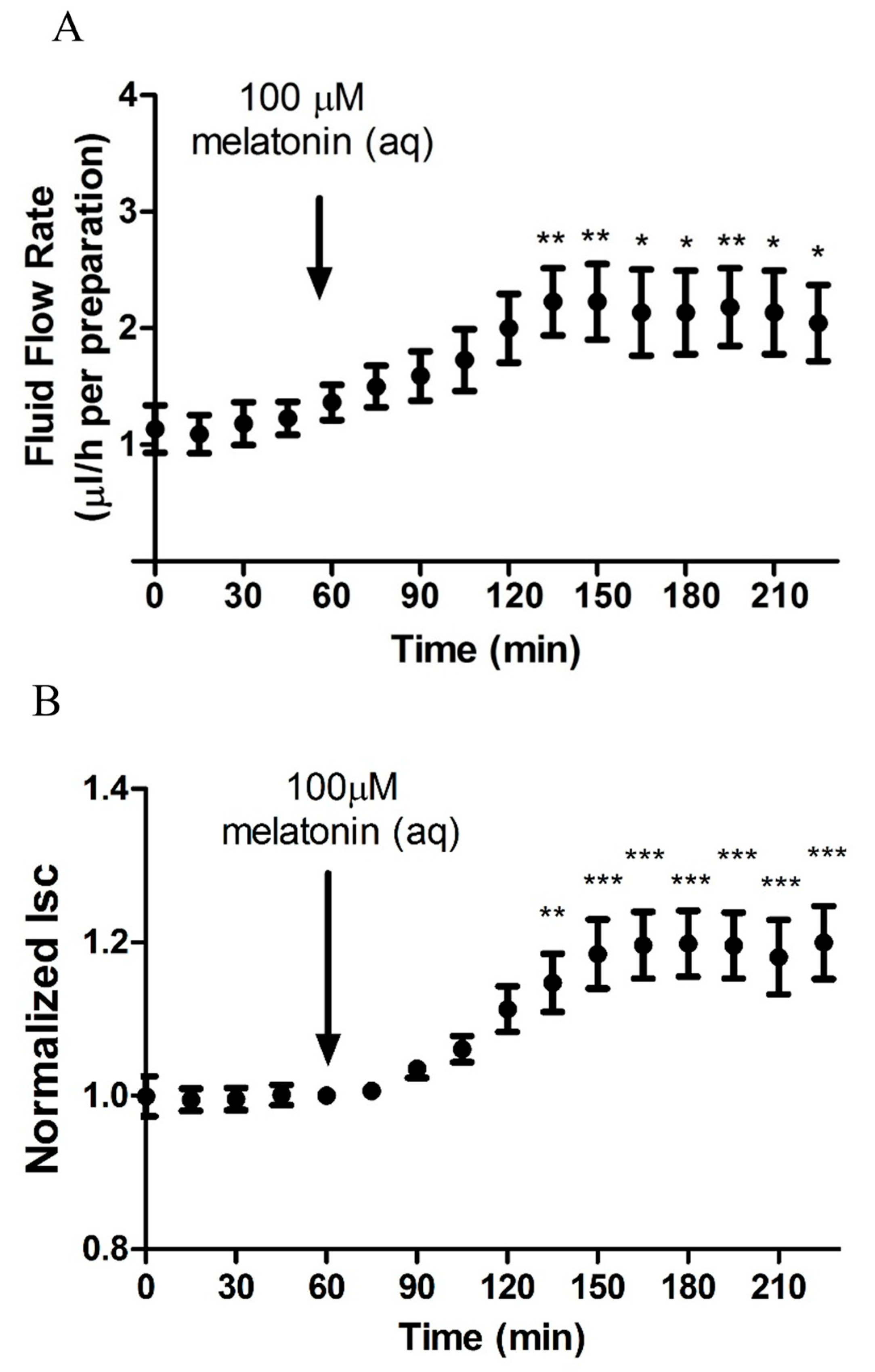
2.2. Melatonin Stimulates Transepithelial Fluid Flow and Electrical Measurements
2.3. Melatonin Enhances Gap Junction Permeability between PE and NPE Cells
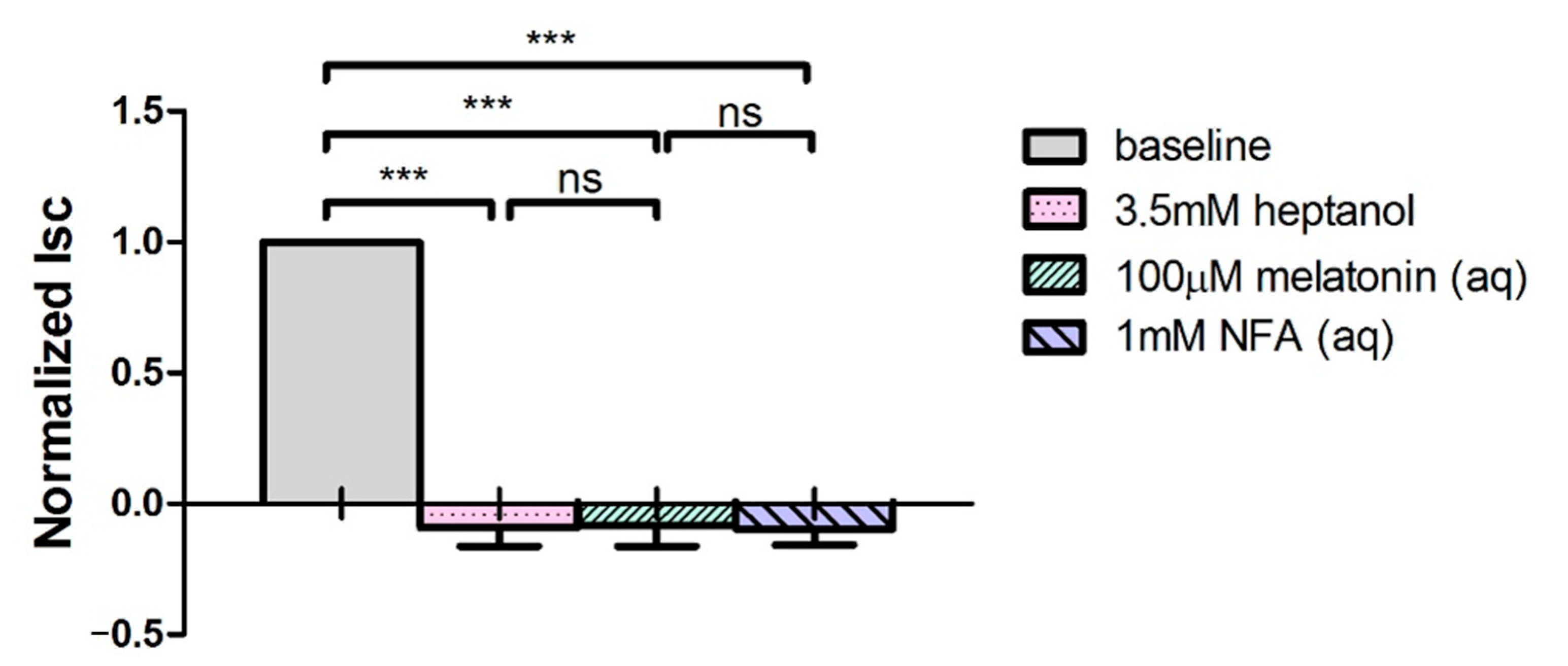
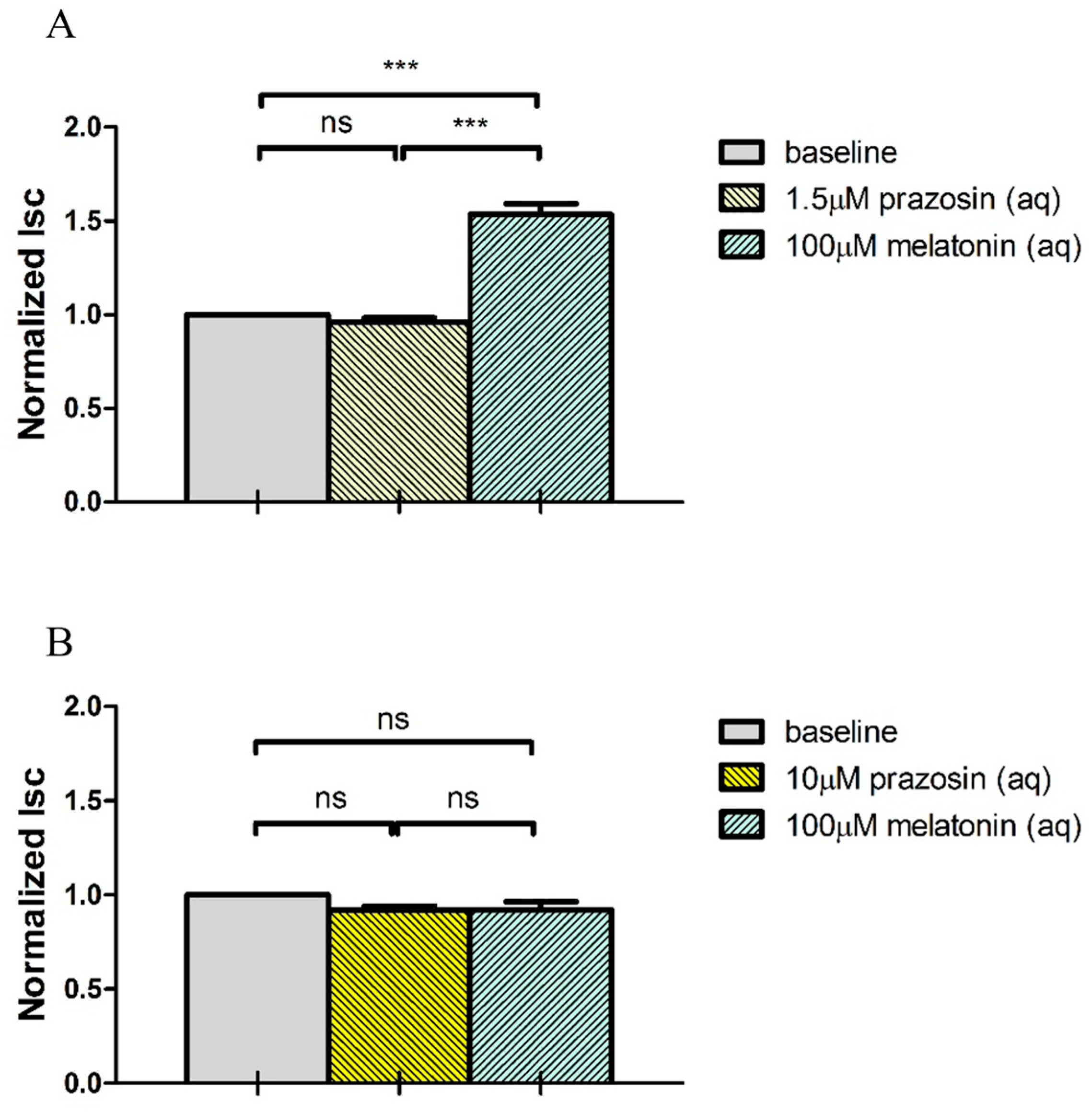
2.4. Melatonin Stimulates Cl− Secretion via MT3 Receptor
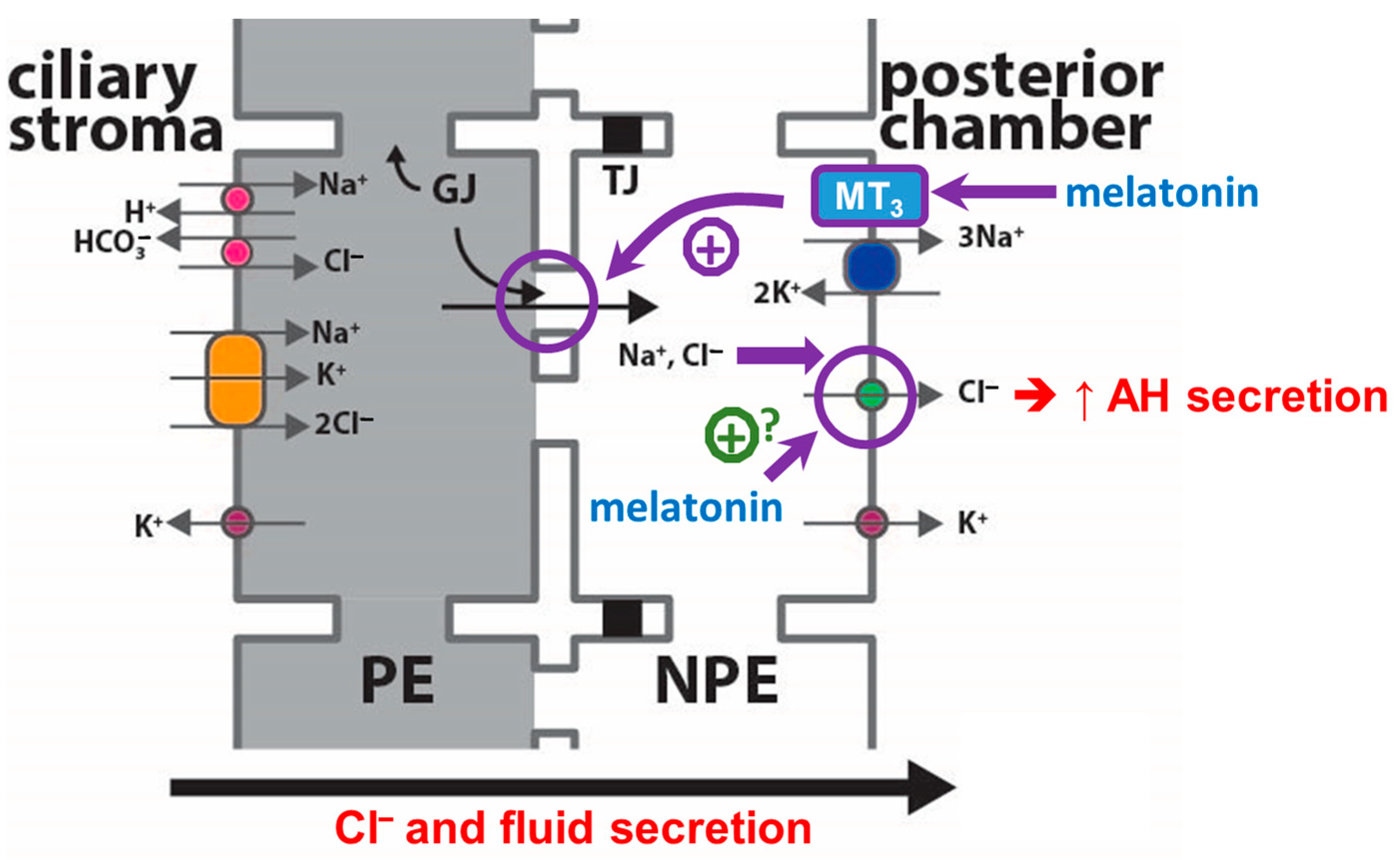
3. Discussion
4. Materials and Methods
4.1. Transepithelial Electrical Measurements with Modified Ussing Chamber
4.2. Simultaneous Electrical and Fluid Flow Measurements with Custom-Built Chamber
4.3. Measurement of Gap Junction Permeability with Lucifer Yellow (LY) Dye Transfer
4.4. Reverse Transcription-Real Time Polymerase Chain Reaction (RT-qPCR)
4.5. Solutions and Pharmacological Agents
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- To, C.H.; Kong, C.W.; Chan, C.Y.; Shahidullah, M.; Do, C.W. The mechanism of aqueous humour formation. Clin. Exp. Optom. 2002, 85, 335–349. [Google Scholar] [PubMed]
- To, C.H.; Mok, K.H.; Do, C.W.; Lee, K.L.; Millodot, M. Chloride and sodium transport across bovine ciliary body/epithelium (CBE). Curr. Eye Res. 1998, 17, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Delamere, N.A.; Shahidullah, M. Ion transport regulation by TRPV4 and TRPV1 in lens and ciliary epithelium. Front. Physiol. 2021, 12, 834916. [Google Scholar] [CrossRef] [PubMed]
- Shahidullah, M.; Delamere, N.A. Connexins form functional hemichannels in porcine ciliary epithelium. Exp. Eye Res. 2014, 118, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Li, S.K.L.; Shan, S.W.; Li, H.L.; Cheng, A.K.W.; Pan, F.; Yip, S.P.; Civan, M.M.; To, C.H.; Do, C.W. Characterization and regulation of gap junctions in porcine ciliary epithelium. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3461–3468. [Google Scholar] [CrossRef] [PubMed]
- Do, C.W.; Civan, M.M. Basis of chloride transport in ciliary epithelium. J. Membr. Biol. 2004, 200, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Do, C.W.; Lu, W.; Mitchell, C.H.; Civan, M.M. Inhibition of swelling-activated Cl− currents by functional anti-ClC-3 antibody in native bovine non-pigmented ciliary epithelial cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 948–955. [Google Scholar] [CrossRef]
- Alkozi, H.A.; Navarro, G.; Franco, R.; Pintor, J. Melatonin and the control of intraocular pressure. Prog. Retin. Eye Res. 2020, 75, 100798. [Google Scholar] [CrossRef]
- Alarma-Estrany, P.; Pintor, J. Melatonin receptors in the eye: Location, second messengers and role in ocular physiology. Pharmacol. Ther. 2007, 113, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Wiechmann, A.F.; Summers, J.A. Circadian rhythms in the eye: The physiological significance of melatonin receptors in ocular tissues. Prog. Retin. Eye Res. 2008, 27, 137–160. [Google Scholar] [CrossRef]
- Pevet, P. The internal time-giver role of melatonin. A key for our health. Rev. Neurol. 2014, 170, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Claustrat, B. Melatonin: An Introduction to Its Physiological and Pharmacological Effects in Humans; Springer: New Delhi, India, 2014. [Google Scholar]
- Von Gall, C.; Stehle, J.H.; Weaver, D.R. Mammalian melatonin receptors: Molecular biology and signal transduction. Cell Tissue Res. 2002, 309, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Nosjean, O.; Ferro, M.; Coge, F.; Beauverger, P.; Henlin, J.M.; Lefoulon, F.; Fauchere, J.L.; Delagrange, P.; Canet, E.; Boutin, J.A. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J. Biol. Chem. 2000, 275, 31311–31317. [Google Scholar] [CrossRef]
- Osborne, N.N.; Chidlow, G. The presence of functional melatonin receptors in the iris-ciliary processes of the rabbit eye. Exp. Eye Res. 1994, 59, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.E.; Wiechmann, A.F.; Hu, D.N. Melatonin receptors in human uveal melanocytes and melanoma cells. J. Pineal Res. 2000, 28, 165–171. [Google Scholar] [CrossRef]
- Wiechmann, A.F.; Wirsig-Wiechmann, C.R. Melatonin receptor mRNA and protein expression in Xenopus laevis nonpigmented ciliary epithelial cells. Exp. Eye Res. 2001, 73, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Lundmark, P.O.; Pandi-Perumal, S.R.; Srinivasan, V.; Cardinali, D.P.; Rosenstein, R.E. Melatonin in the eye: Implications for glaucoma. Exp. Eye Res. 2007, 84, 1021–1030. [Google Scholar] [CrossRef]
- Ismail, S.A.; Mowafi, H.A. Melatonin provides anxiolysis, enhances analgesia, decreases intraocular pressure, and promotes better operating conditions during cataract surgery under topical anesthesia. Anesth. Analg. 2009, 108, 1146–1151. [Google Scholar] [CrossRef]
- Pescosolido, N.; Gatto, V.; Stefanucci, A.; Rusciano, D. Oral treatment with the melatonin agonist agomelatine lowers the intraocular pressure of glaucoma patients. Ophthalmic. Physiol. Opt. 2015, 35, 201–205. [Google Scholar] [CrossRef]
- Gubin, D.; Neroev, V.; Malishevskaya, T.; Cornelissen, G.; Astakhov, S.Y.; Kolomeichuk, S.; Yuzhakova, N.; Kabitskaya, Y.; Weinert, D. Melatonin mitigates disrupted circadian rhythms, lowers intraocular pressure, and improves retinal ganglion cells function in glaucoma. J. Pineal Res. 2021, 70, e12730. [Google Scholar] [CrossRef]
- Pintor, J.; Martin, L.; Pelaez, T.; Hoyle, C.H.; Peral, A. Involvement of melatonin MT3 receptors in the regulation of intraocular pressure in rabbits. Eur. J. Pharmacol. 2001, 416, 251–254. [Google Scholar] [CrossRef]
- Pintor, J.; Pelaez, T.; Hoyle, C.H.; Peral, A. Ocular hypotensive effects of melatonin receptor agonists in the rabbit: Further evidence for an MT3 receptor. Br. J. Pharmacol. 2003, 138, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Aguila, A.; Fonseca, B.; Perez de Lara, M.J.; Pintor, J. Effect of melatonin and 5-Methoxycarbonylamino-N-acetyltryptamine on the intraocular pressure of normal and glaucomatous Mice. J. Pharmacol. Exp. Ther. 2016, 357, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Serle, J.B.; Wang, R.F.; Peterson, W.M.; Plourde, R.; Yerxa, B.R. Effect of 5-MCA-NAT, a putative melatonin MT3 receptor agonist, on intraocular pressure in glaucomatous monkey eyes. J. Glaucoma. 2004, 13, 385–388. [Google Scholar] [CrossRef]
- Kiuchi, Y.; Mockovak, M.E.; Gregory, D.S. Melatonin does not increase IOP significantly in rabbits. Curr. Eye Res. 1993, 12, 181–190. [Google Scholar] [CrossRef]
- Rohde, B.H.; McLaughlin, M.A.; Chiou, L.Y. Existence and role of endogenous ocular melatonin. J. Ocul. Pharmacol. 1985, 1, 235–243. [Google Scholar] [CrossRef]
- Alkozi, H.; Sanchez-Naves, J.; de Lara, M.J.; Carracedo, G.; Fonseca, B.; Martinez-Aguila, A.; Pintor, J. Elevated intraocular pressure increases melatonin levels in the aqueous humour. Acta Ophthalmol. 2017, 95, e185–e189. [Google Scholar] [CrossRef]
- Cheng, A.K.; Civan, M.M.; To, C.H.; Do, C.W. cAMP stimulates transepithelial short-circuit current and fluid transport across porcine ciliary epithelium. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6784–6794. [Google Scholar] [CrossRef]
- Blackman, C.F.; Andrews, P.W.; Ubeda, A.; Wang, X.; House, D.E.; Trillo, M.A.; Pimentel, M.E. Physiological levels of melatonin enhance gap junction communication in primary cultures of mouse hepatocytes. Cell Biol. Toxicol. 2001, 17, 1–9. [Google Scholar] [CrossRef]
- Cos, S.; Fernandez, R. Melatonin effects on intercellular junctional communication in MCF-7 human breast cancer cells. J. Pineal Res. 2000, 29, 166–171. [Google Scholar] [CrossRef]
- Liu, J.H.; Dacus, A.C. Endogenous hormonal changes and circadian elevation of intraocular pressure. Investig. Ophthalmol. Vis. Sci. 1991, 32, 496–500. [Google Scholar]
- Chiquet, C.; Claustrat, B.; Thuret, G.; Brun, J.; Cooper, H.M.; Denis, P. Melatonin concentrations in aqueous humor of glaucoma patients. Am. J. Ophthalmol. 2006, 142, 325–327. [Google Scholar] [CrossRef]
- Aydin, E.; Sahin, S. Increased melatonin levels in aqueous humor of patients with proliferative retinopathy in type 2 diabetes mellitus. Int. J. Ophthalmol. 2016, 9, 721–724. [Google Scholar] [PubMed]
- Martinez-Aguila, A.; Fonseca, B.; Bergua, A.; Pintor, J. Melatonin analogue agomelatine reduces rabbit’s intraocular pressure in normotensive and hypertensive conditions. Eur. J. Pharmacol. 2013, 701, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jeong, A.R.; Chin, H.S.; Kim, N.R. Melatonin levels in patients with primary open-angle glaucoma with high or low intraocular pressure. J. Glaucoma. 2019, 28, 154–160. [Google Scholar] [CrossRef]
- Alcantara-Contreras, S.; Baba, K.; Tosini, G. Removal of melatonin receptor type 1 increases intraocular pressure and retinal ganglion cells death in the mouse. Neurosci. Lett. 2011, 494, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Do, C.W.; To, C.H. Chloride secretion by bovine ciliary epithelium: A model of aqueous humor formation. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1853–1860. [Google Scholar]
- Huete-Toral, F.; Crooke, A.; Martinez-Aguila, A.; Pintor, J. Melatonin receptors trigger cAMP production and inhibit chloride movements in nonpigmented ciliary epithelial cells. J. Pharmacol. Exp. Ther. 2015, 352, 119–128. [Google Scholar] [CrossRef]
- Do, C.W.; Kong, C.W.; To, C.H. cAMP inhibits transepithelial chloride secretion across bovine ciliary body/epithelium. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3638–3643. [Google Scholar] [CrossRef]
- Kojima, T.; Mochizuki, C.; Mitaka, T.; Mochizuki, Y. Effects of melatonin on proliferation, oxidative stress and Cx32 gap junction protein expression in primary cultures of adult rat hepatocytes. Cell Struct. Funct. 1997, 22, 347–356. [Google Scholar] [CrossRef]
- Martinez, A.D.; Saez, J.C. Regulation of astrocyte gap junctions by hypoxia-reoxygenation. Brain Res. Rev. 2000, 32, 250–258. [Google Scholar] [CrossRef]
- Ubeda, A.; Trillo, M.A.; House, D.E.; Blackman, C.F. Melatonin enhances junctional transfer in normal C3H/10T1/2 cells. Cancer Lett. 1995, 91, 241–245. [Google Scholar] [CrossRef]
- Do, C.W.; Civan, M.M. Species variation in biology and physiology of the ciliary epithelium: Similarities and differences. Exp. Eye Res. 2009, 88, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Candia, O.A.; To, C.H.; Gerometta, R.M.; Zamudio, A.C. Spontaneous fluid transport across isolated rabbit and bovine ciliary body preparations. Investig. Ophthalmol. Vis. Sci. 2005, 46, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Gerometta, R.M.; Malgor, L.A.; Vilalta, E.; Leiva, J.; Candia, O.A. Cl− concentrations of bovine, porcine and ovine aqueous humor are higher than in plasma. Exp. Eye Res. 2005, 80, 307–312. [Google Scholar] [CrossRef]
- McLaughlin, C.W.; Peart, D.; Purves, R.D.; Carre, D.A.; Macknight, A.D.; Civan, M.M. Effects of HCO3− on cell composition of rabbit ciliary epithelium: A new model for aqueous humor secretion. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1631–1641. [Google Scholar]
- Crook, R.B.; Takahashi, K.; Mead, A.; Dunn, J.J.; Sears, M.L. The role of NaKCl cotransport in blood-to-aqueous chloride fluxes across rabbit ciliary epithelium. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2574–2583. [Google Scholar]
- To, C.H.; Do, C.W.; Zamudio, A.C.; Candia, O.A. Model of ionic transport for bovine ciliary epithelium: Effects of acetazolamide and HCO3−. Am. J. Physiol. Cell Physiol. 2001, 280, C1521–C1530. [Google Scholar] [CrossRef] [PubMed]
- Beauchemin, M.L. The fine structure of the pig’s retina. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 1974, 190, 27–45. [Google Scholar] [CrossRef]
- Kong, C.W.; Li, K.K.; To, C.H. Chloride secretion by porcine ciliary epithelium: New insight into species similarities and differences in aqueous humor formation. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5428–5436. [Google Scholar] [CrossRef]
- Shahidullah, M.; Yap, M.; To, C.H. Cyclic GMP, sodium nitroprusside and sodium azide reduce aqueous humour formation in the isolated arterially perfused pig eye. Br. J. Pharmacol. 2005, 145, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Simoens, P.; De Schaepdrijver, L.; Lauwers, H. Morphologic and clinical study of the retinal circulation in the miniature pig. A: Morphology of the retinal microvasculature. Exp. Eye Res. 1992, 54, 965–973. [Google Scholar] [CrossRef]
- Chan, H.C.; Lui, K.M.; Wong, W.S.; Poon, A.M. Effect of melatonin on chloride secretion by human colonic T84 cells. Life Sci. 1998, 62, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Martin, X.D.; Malina, H.Z.; Brennan, M.C.; Hendrickson, P.H.; Lichter, P.R. The ciliary body—The third organ found to synthesize indoleamines in humans. Eur. J. Ophthalmol. 1992, 2, 67–72. [Google Scholar] [CrossRef]
- Dortch-Carnes, J.; Tosini, G. Melatonin receptor agonist-induced reduction of SNP-released nitric oxide and cGMP production in isolated human non-pigmented ciliary epithelial cells. Exp. Eye Res. 2013, 107, 1–10. [Google Scholar] [CrossRef]
- Zhelev, Z.; Ivanova, D.; Bakalova, R.; Aoki, I.; Higashi, T. Synergistic cytotoxicity of melatonin and new-generation anticancer drugs against leukemia lymphocytes but not normal lymphocytes. Anticancer Res. 2017, 37, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Ekmekcioglu, C. Melatonin receptors in humans: Biological role and clinical relevance. Biomed. Pharmacother. 2006, 60, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Angus, J.A.; Wright, C.E. Novel alpha1-adrenoceptor antagonism by the fluroquinolone antibiotic trovafloxacin. Eur. J. Pharmacol. 2016, 791, 179–184. [Google Scholar] [CrossRef]
- Jerez, S.; Sierra, L.; Scacchi, F.; Peral de Bruno, M. Hypercholesterolemia modifies angiotensin II desensitisation and cross talk between alpha (1)-adrenoceptor and angiotensin AT (1) receptor in rabbit aorta. Eur. J. Pharmacol. 2010, 635, 149–155. [Google Scholar] [CrossRef]
- Peitzman, E.R.; Zaidman, N.A.; Maniak, P.J.; O’Grady, S.M. Carvedilol binding to beta2-adrenergic receptors inhibits CFTR-dependent anion secretion in airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L50–L58. [Google Scholar] [CrossRef] [PubMed]
- Pelis, R.M.; Shahidullah, M.; Ghosh, S.; Coca-Prados, M.; Wright, S.H.; Delamere, N.A. Localization of multidrug resistance-associated protein 2 in the nonpigmented ciliary epithelium of the eye. J. Pharmacol. Exp. Ther. 2009, 329, 479–485. [Google Scholar] [CrossRef]
- Karlsson, A.M.; Lerner, M.R.; Unett, D.; Lundstrom, I.; Svensson, S.P. Melatonin-induced organelle movement in melanophores is coupled to tyrosine phosphorylation of a high molecular weight protein. Cell Signal 2000, 12, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Godson, C.; Reppert, S.M. The Mel1a melatonin receptor is coupled to parallel signal transduction pathways. Endocrinology 1997, 138, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Pacheco, A.; Araiza-Saldana, C.I.; Granados-Soto, V.; Mixcoatl-Zecuatl, T. Possible participation of the nitric oxide-cyclic GMP-protein kinase G-K+ channels pathway in the peripheral antinociception of melatonin. Eur. J. Pharmacol. 2008, 596, 70–76. [Google Scholar] [CrossRef]
- Choi, S.I.; Joo, S.S.; Yoo, Y.M. Melatonin prevents nitric oxide-induced apoptosis by increasing the interaction between 14-3-3β and p-Bad in SK-N-MC cells. J. Pineal Res. 2008, 44, 95–100. [Google Scholar]
- Fukunaga, K.; Horikawa, K.; Shibata, S.; Takeuchi, Y.; Miyamoto, E. Ca2+/calmodulin-dependent protein kinase II-dependent long-term potentiation in the rat suprachiasmatic nucleus and its inhibition by melatonin. J. Neurosci. Res. 2002, 70, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, F.; Canonico, B.; Betti, M.; Arcangeletti, M.; Pilolli, F.; Piroddi, M.; Canesi, L.; Papa, S.; Galli, F. Melatonin signaling and cell protection function. FASEB J. 2010, 24, 3603–3624. [Google Scholar] [CrossRef] [PubMed]
- Do, C.W.; Civan, M.M. Swelling-activated chloride channels in aqueous humour formation: On the one side and the other. Acta Physiol. 2006, 187, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Rich, A.; Farrugia, G.; Rae, J.L. Effects of melatonin on ionic currents in cultured ocular tissues. Am. J. Physiol. 1999, 276, C923–C929. [Google Scholar] [CrossRef]
- Kahn, M.G.; Giblin, F.J.; Epstein, D.L. Glutathione in calf trabecular meshwork and its relation to aqueous humor outflow facility. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1283–1287. [Google Scholar]
- Zhou, L.; Li, Y.; Yue, B.Y. Oxidative stress affects cytoskeletal structure and cell-matrix interactions in cells from an ocular tissue: The trabecular meshwork. J. Cell Physiol. 1999, 180, 182–189. [Google Scholar] [CrossRef]
- Li, H.L.; Shan, S.W.; Stamer, W.D.; Li, K.K.; Chan, H.H.; Civan, M.M.; To, C.H.; Lam, T.C.; Do, C.W. Mechanistic effects of baicalein on aqueous humor drainage and intraocular pressure. Int. J. Mol. Sci. 2022, 23, 7372. [Google Scholar] [CrossRef] [PubMed]
- Do, C.W.; Peterson-Yantorno, K.; Civan, M.M. Swelling-activated Cl− channels support Cl− secretion by bovine ciliary epithelium. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2576–2582. [Google Scholar] [CrossRef]
- Ostrin, L.A. Ocular and systemic melatonin and the influence of light exposure. Clin. Exp. Optom. 2019, 102, 99–108. [Google Scholar] [CrossRef]
- Candia, O.A.; To, C.H.; Law, C.S. Fluid transport across the isolated porcine ciliary epithelium. Investig. Ophthalmol. Vis. Sci. 2007, 48, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Do, C.W.; Valiunas, V.; Leung, C.T.; Cheng, A.K.; Clark, A.F.; Wax, M.B.; Chatterton, J.E.; Civan, M.M. Regulation of gap junction coupling in bovine ciliary epithelium. Am. J. Physiol. Cell Physiol. 2010, 298, C798–C806. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.-L.; Shan, S.-W.; Lin, F.-Y.; Ling, C.-Y.; Wong, N.-W.; Li, H.-L.; Han, W.; To, C.-H.; Do, C.-W. Regulation of Aqueous Humor Secretion by Melatonin in Porcine Ciliary Epithelium. Int. J. Mol. Sci. 2023, 24, 5789. https://doi.org/10.3390/ijms24065789
Li K-L, Shan S-W, Lin F-Y, Ling C-Y, Wong N-W, Li H-L, Han W, To C-H, Do C-W. Regulation of Aqueous Humor Secretion by Melatonin in Porcine Ciliary Epithelium. International Journal of Molecular Sciences. 2023; 24(6):5789. https://doi.org/10.3390/ijms24065789
Chicago/Turabian StyleLi, Ka-Lok, Sze-Wan Shan, Fang-Yu Lin, Choi-Ying Ling, Nga-Wai Wong, Hoi-Lam Li, Wei Han, Chi-Ho To, and Chi-Wai Do. 2023. "Regulation of Aqueous Humor Secretion by Melatonin in Porcine Ciliary Epithelium" International Journal of Molecular Sciences 24, no. 6: 5789. https://doi.org/10.3390/ijms24065789
APA StyleLi, K.-L., Shan, S.-W., Lin, F.-Y., Ling, C.-Y., Wong, N.-W., Li, H.-L., Han, W., To, C.-H., & Do, C.-W. (2023). Regulation of Aqueous Humor Secretion by Melatonin in Porcine Ciliary Epithelium. International Journal of Molecular Sciences, 24(6), 5789. https://doi.org/10.3390/ijms24065789





