Long-Lasting Therapeutic Response following Treatment with Pembrolizumab in Patients with Non-Small Cell Lung Cancer: A Real-World Experience
Abstract
1. Introduction
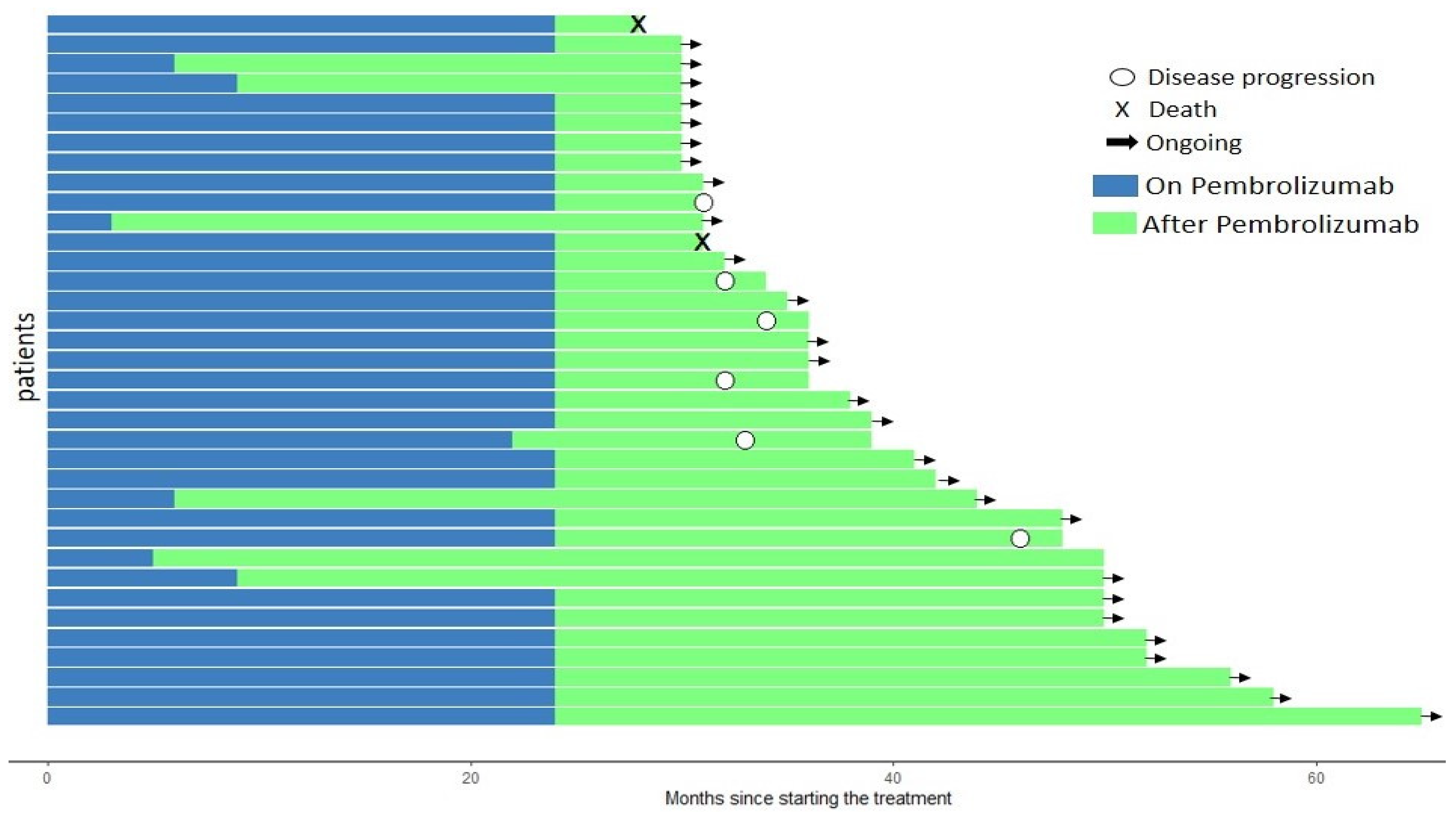
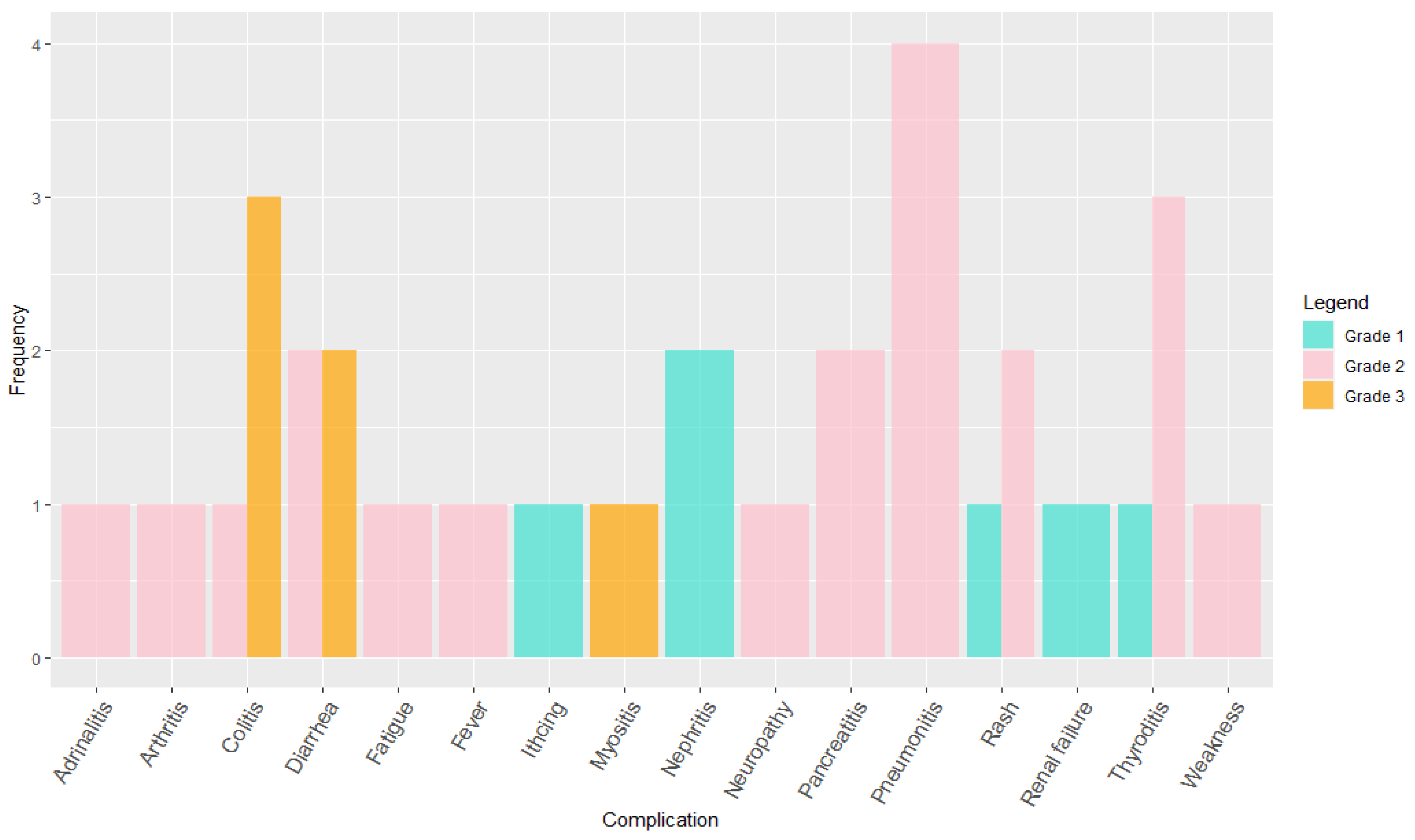
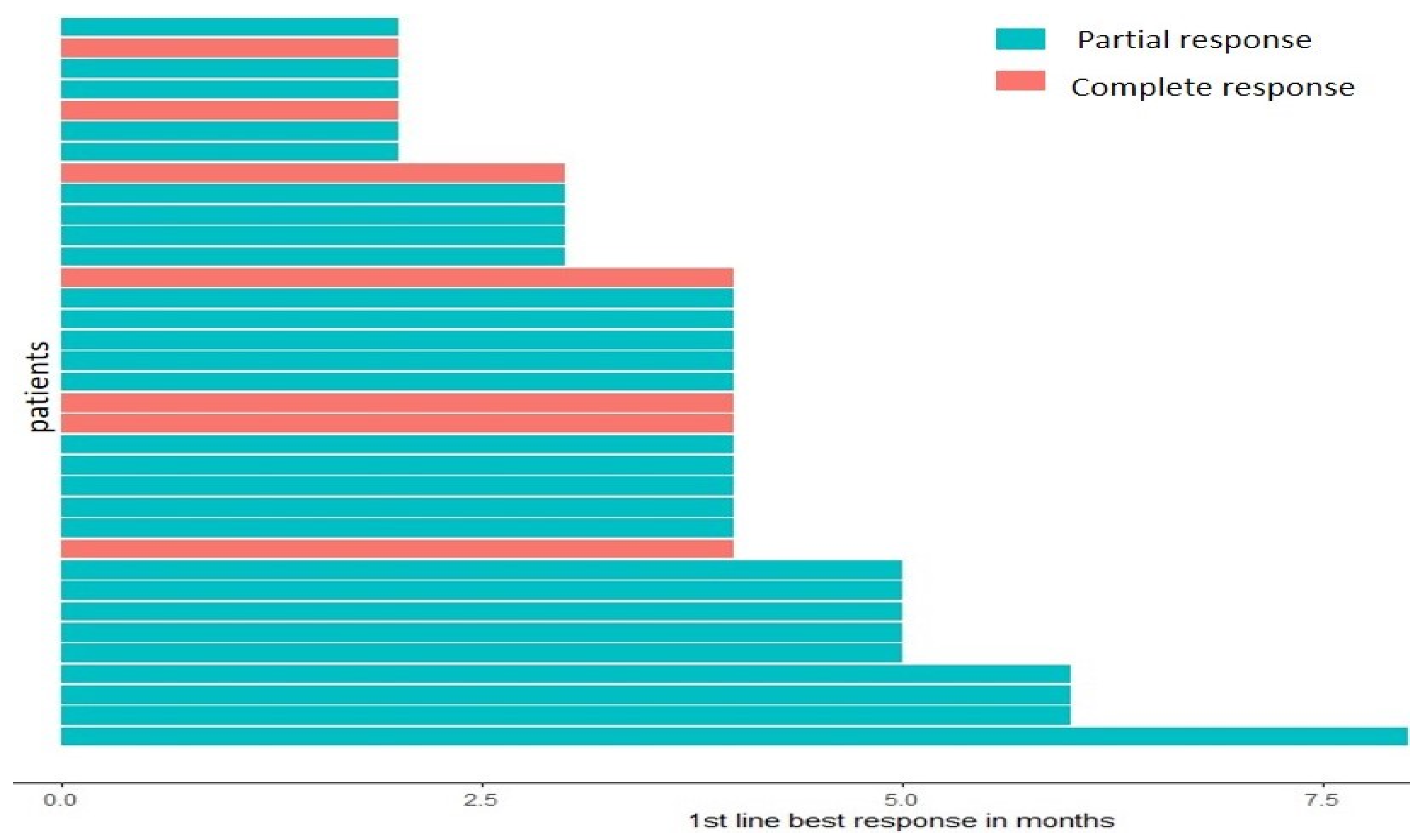
2. Results
3. Discussion
4. Material and Methods
4.1. Patient Selection
4.2. The Inclusion Criteria for the Study
4.3. Treatment Received
4.3.1. Adenocarcinoma, Giant Cell, and Adenocarcinoma with Neuroendocrine Features
4.3.2. Squamous Cell Carcinoma Adenosquamous Patients
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shalata, W.; Yakobson, A.; Weissmann, S.; Oscar, E.; Iraqi, M.; Kian, W.; Peled, N.; Agbarya, A. Crizotinib in MET Exon 14-Mutated or MET-Amplified in Advanced Disease Non-Small Cell Lung Cancer: A Retrospective, Single Institution Experience. Oncology 2022, 100, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Rafei, H.; El-Bahesh, E.; Finianos, A.; Nassereddine, S.; Tabbara, I. Immune-based Therapies for Non-small Cell Lung Cancer. Anticancer Res. 2017, 37, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Szeto, C.H.; Shalata, W.; Yakobson, A.; Agbarya, A. Neoadjuvant and Adjuvant Immunotherapy in Early-Stage Non-Small-Cell Lung Cancer, Past, Present, and Future. J. Clin. Med. 2021, 10, 5614. [Google Scholar] [CrossRef] [PubMed]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.; Morales, J.; Presley, C. Checkpoint Inhibitors for Non-Small Cell Lung Cancer Among Older Adults. Curr. Oncol. Rep. 2017, 19, 62. [Google Scholar] [CrossRef]
- Shalata, W.; Jacob, B.M.; Agbarya, A. Adjuvant Treatment with Tyrosine Kinase Inhibitors in Epidermal Growth Factor Receptor Mutated Non-Small-Cell Lung Carcinoma Patients, Past, Present and Future. Cancers 2021, 13, 4119. [Google Scholar] [CrossRef]
- Shalata, W.; Iraqi, M.; Bhattacharya, B.; Fuchs, V.; Roisman, L.C.; Cohen, A.Y.; Massalha, I.; Yakobson, A.; Prasad, M.; Elkabets, M.; et al. Rapid Response to the Combination of Lenvatinib and Pembrolizumab in Patients with Advanced Carcinomas (Lung Adenocarcinoma and Malignant Pleural Mesothelioma). Cancers 2021, 13, 3630. [Google Scholar] [CrossRef]
- Zhang, C.; Leighl, N.B.; Wu, Y.L.; Zhong, W.Z. Emerging therapies for non-small cell lung cancer. J. Hematol. Oncol. 2019, 12, 45. [Google Scholar] [CrossRef]
- Gerber, D.E.; Schiller, J.H. Maintenance chemotherapy for advanced non-small-cell lung cancer: New life for an old idea. J. Clin. Oncol. 2013, 31, 1009–1020. [Google Scholar] [CrossRef]
- Shalata, W.; Abu-Salman, A.; Steckbeck, R.; Mathew Jacob, B.; Massalha, I.; Yakobson, A. Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: A Systematic Review. Cancers 2021, 13, 5218. [Google Scholar] [CrossRef]
- Shalata, W.; Weissmann, S.; Itzhaki Gabay, S.; Sheva, K.; Abu Saleh, O.; Jama, A.A.; Yakobson, A.; Rouvinov, K. A Retrospective, Single-Institution Experience of Bullous Pemphigoid as an Adverse Effect of Immune Checkpoint Inhibitors. Cancers 2022, 14, 5451. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef]
- Gadgeel, S.; Rodríguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Dómine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F.; et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 1505–1517. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Vicente, D.; Tafreshi, A.; Robinson, A.; Soto Parra, H.; Mazières, J.; Hermes, B.; Cicin, I.; Medgyasszay, B.; Rodríguez-Cid, J.; et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J. Thorac. Oncol. 2020, 15, 1657–1669. [Google Scholar] [CrossRef]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef]
- Patel, S.A.; Weiss, J. Advances in the Treatment of Non-Small Cell Lung Cancer: Immunotherapy. Clin. Chest Med. 2020, 41, 237–247. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Powell, S.F.; Rodríguez-Abreu, D.; Langer, C.J.; Tafreshi, A.; Paz-Ares, L.; Kopp, H.-G.; Rodríguez-Cid, J.; Kowalski, D.M.; Cheng, Y.; Kurata, T.; et al. Outcomes With Pembrolizumab Plus Platinum-Based Chemotherapy for Patients With NSCLC and Stable Brain Metastases: Pooled Analysis of KEYNOTE-021, -189, and -407. J. Thorac. Oncol. 2021, 16, 1883–1892. [Google Scholar] [CrossRef] [PubMed]
- Shalata, W.; Peled, N.; Gabizon, I.; Abu Saleh, O.; Kian, W.; Yakobson, A. Associated Myocarditis: A Predictive Factor for Response? Case Rep. Oncol. 2020, 13, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Komiya, K.; Nakamura, T.; Abe, T.; Ogusu, S.; Nakashima, C.; Takahashi, K.; Kimura, S.; Sueoka-Aragane, N. Discontinuation due to immune-related adverse events is a possible predictive factor for immune checkpoint inhibitors in patients with non-small cell lung cancer. Thorac. Cancer 2019, 10, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- Sugano, T.; Seike, M.; Saito, Y.; Kashiwada, T.; Terasaki, Y.; Takano, N.; Hisakane, K.; Takahashi, S.; Tanaka, T.; Takeuchi, S.; et al. Immune checkpoint inhibitor-associated interstitial lung diseases correlate with better prognosis in patients with advanced non-small-cell lung cancer. Thorac. Cancer 2020, 11, 1052–1060. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer, Version 2.2021: 15 December 2020. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 1 February 2023).
- Evelson, L.; Geger, E.; Kozlova, I. Applying methods of twin comparing quantitative and binary samples in biomedical information systems for decision making. Vestn. ASTU Ser. Manag. Comput. Sci. Inform. 2022, 2, 87–96. [Google Scholar] [CrossRef]

| Age, median (range) | 65.1 (46–83) |
| male | 65.5 (46–85) |
| female | 64.9 (53–71) |
| Sex | |
| male | 23 (63.9%) |
| Female | 13 (36.1%) |
| Smoking status at diagnosis | |
| Current | 15 (41.6%) |
| Former | 15 (41.6%) |
| Never | 5 (16.8%) |
| ECOG status | |
| 0 | 34 (94.4%) |
| 1 | 2 (5.6%) |
| Mode of biopsy | |
| Tissue | 38 (100%) |
| Mode of Biopsy | |
|---|---|
| Tissue | 38 (100%) |
| Histology | |
| Adenocarcinoma | 22 (60%) |
| Squamous | 11 (31.6%) |
| Other * | 3 (8.3%) |
| Lymph nodes—extrathoracic at diagnosis | 26 (72.2%) |
| Contralateral lung affected | 25 (69.4%) |
| Bone metastasis at diagnosis | 14 (33.3%) |
| Adrenal metastasis at diagnosis | 6 (16.6%) |
| Brain metastasis at diagnosis | 4 (11.2%) |
| Liver metastasis at diagnosis | 1 (2.8%) |
| Pleural effusion at diagnosis | 1 (2.8%) |
| Pericardial effusion at diagnosis | 1 (2.8%) |
| Spleen metastasis at diagnosis | 0 (0%) |
| Peritoneal metastasis at diagnosis | 0 (0%) |
| PDL1 Expression (%) | >75% | >50% | 1–49% | <1% | Not Performed |
|---|---|---|---|---|---|
| Adenocarcinoma | 4 | 14 | 2 | 1 | 1 |
| Adenosquamous | 0 | 0 | 1 | 0 | 0 |
| Adenocarcinoma with neuroendocrine features | 0 | 0 | 1 | 0 | 0 |
| Giant cell carcinoma | 0 | 1 | 0 | 0 | 0 |
| Squamous cell carcinoma | 3 | 3 | 2 | 3 | 0 |
| Total (%) | 7 (19.4%) | 18 (50%) | 6 (16.7%) | 4 (11.1%) | 1 (2.8%) |
| kras (G12C) | 3 in adenocarcinoma |
| kras (G12D) | 1 in adenocarcinoma |
| braf (V600G) | 1 in adenocarcinoma |
| braf (G466E) | 1 in adenocarcinoma |
| FGFR3 (Phe 384 Leu) | 1 in adenocarcinoma |
| braf (G469V) | 1 in adenosquamous |
| kras (G13D) | 1 in squamous cell carcinoma |
| FGFR2 (1144T>G) | 1 in squamous cell carcinoma |
| PTEN (209+5G>A) | 1 in squamous cell carcinoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalata, W.; Zolnoorian, J.; Migliozzi, G.; Jama, A.A.; Dudnik, Y.; Cohen, A.Y.; Meirovitz, A.; Yakobson, A. Long-Lasting Therapeutic Response following Treatment with Pembrolizumab in Patients with Non-Small Cell Lung Cancer: A Real-World Experience. Int. J. Mol. Sci. 2023, 24, 5938. https://doi.org/10.3390/ijms24065938
Shalata W, Zolnoorian J, Migliozzi G, Jama AA, Dudnik Y, Cohen AY, Meirovitz A, Yakobson A. Long-Lasting Therapeutic Response following Treatment with Pembrolizumab in Patients with Non-Small Cell Lung Cancer: A Real-World Experience. International Journal of Molecular Sciences. 2023; 24(6):5938. https://doi.org/10.3390/ijms24065938
Chicago/Turabian StyleShalata, Walid, Jeremy Zolnoorian, Gabrielle Migliozzi, Ashraf Abu Jama, Yulia Dudnik, Ahron Yehonatan Cohen, Amichay Meirovitz, and Alexander Yakobson. 2023. "Long-Lasting Therapeutic Response following Treatment with Pembrolizumab in Patients with Non-Small Cell Lung Cancer: A Real-World Experience" International Journal of Molecular Sciences 24, no. 6: 5938. https://doi.org/10.3390/ijms24065938
APA StyleShalata, W., Zolnoorian, J., Migliozzi, G., Jama, A. A., Dudnik, Y., Cohen, A. Y., Meirovitz, A., & Yakobson, A. (2023). Long-Lasting Therapeutic Response following Treatment with Pembrolizumab in Patients with Non-Small Cell Lung Cancer: A Real-World Experience. International Journal of Molecular Sciences, 24(6), 5938. https://doi.org/10.3390/ijms24065938









