Rapamycin Perfluorocarbon Nanoparticle Mitigates Cisplatin-Induced Acute Kidney Injury
Abstract
1. Introduction
2. Results
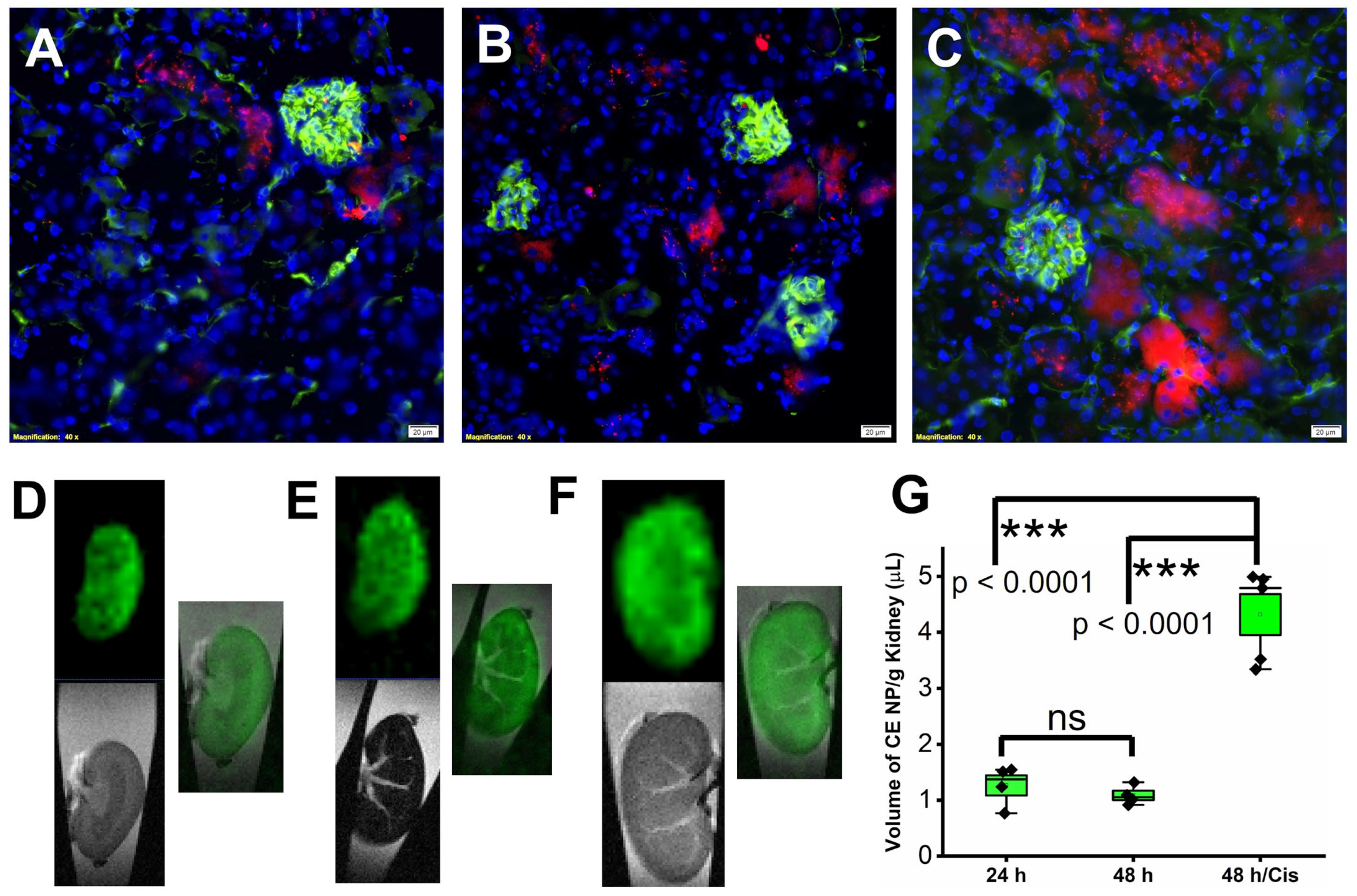
2.1. Accumulation of Rapamycin PFC NP in Kidney after Systemic Administration
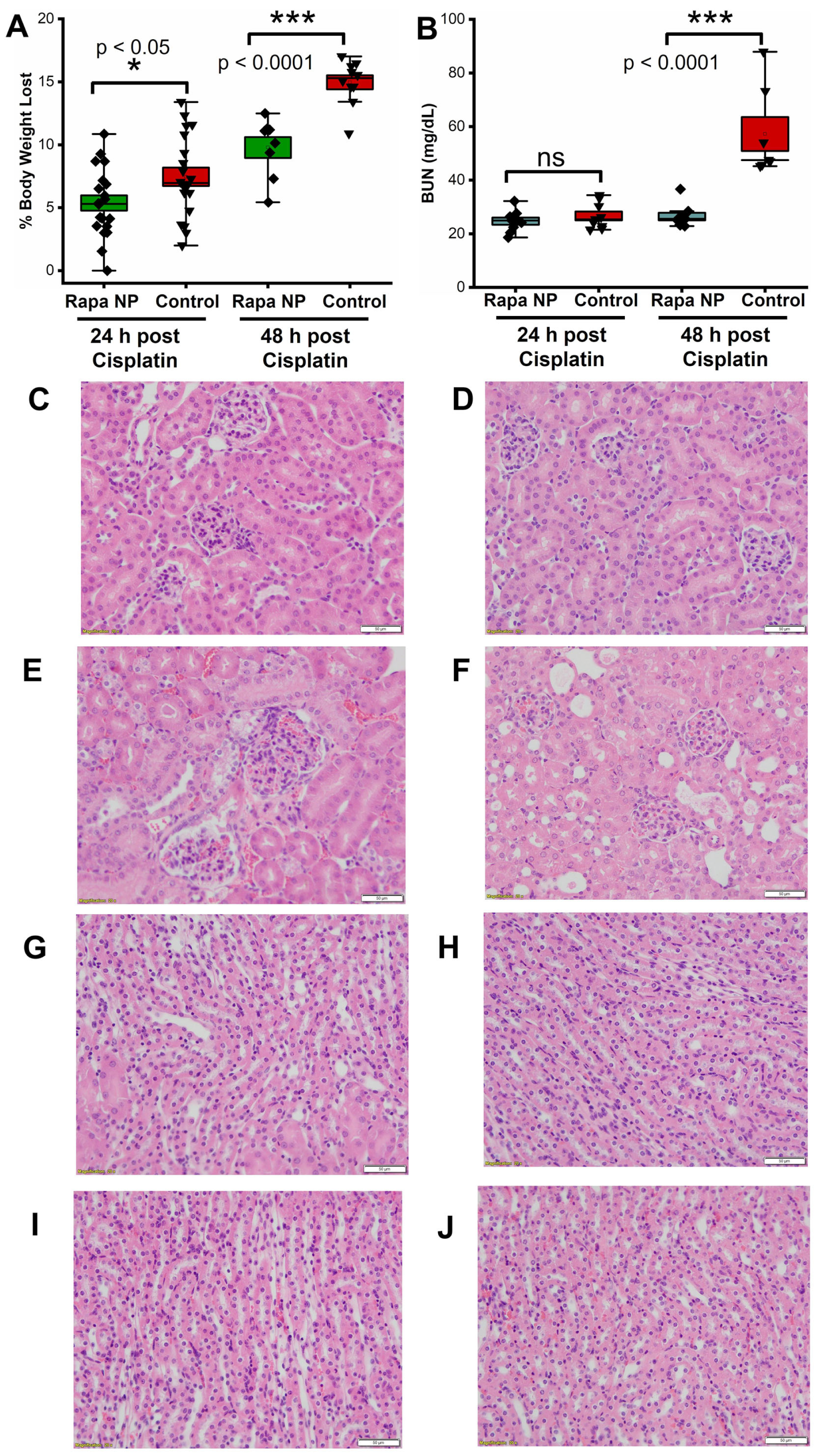
2.2. Rapamycin PFC NP Treatment Mitigated Renal Dysfunction Induced by Cisplatin
2.3. Rapamycin PFC NP Enhanced Autophagy in Kidney
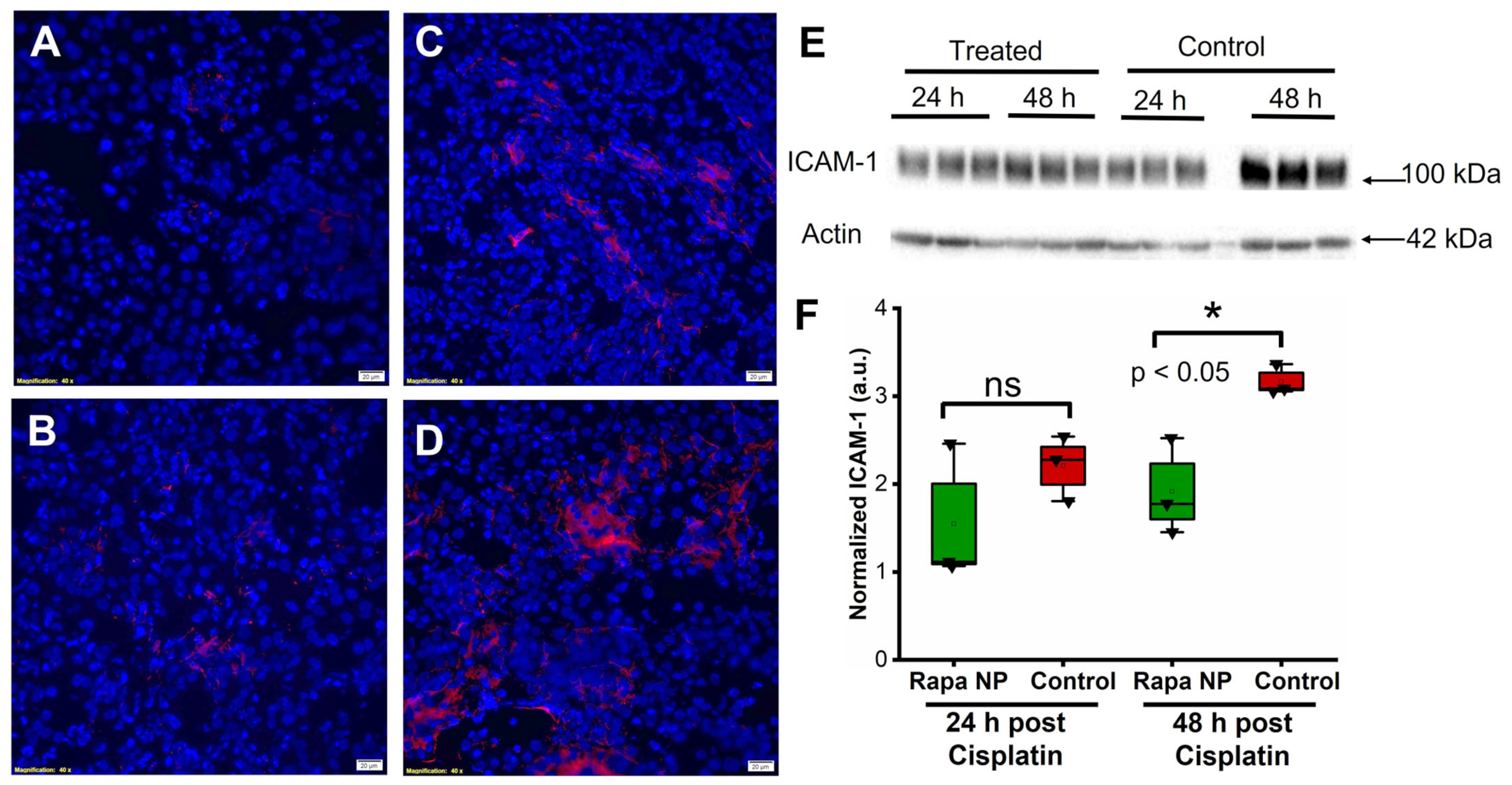
2.4. Rapamycin PFC NP Treatment Suppressed Renal Endothelial Inflammation
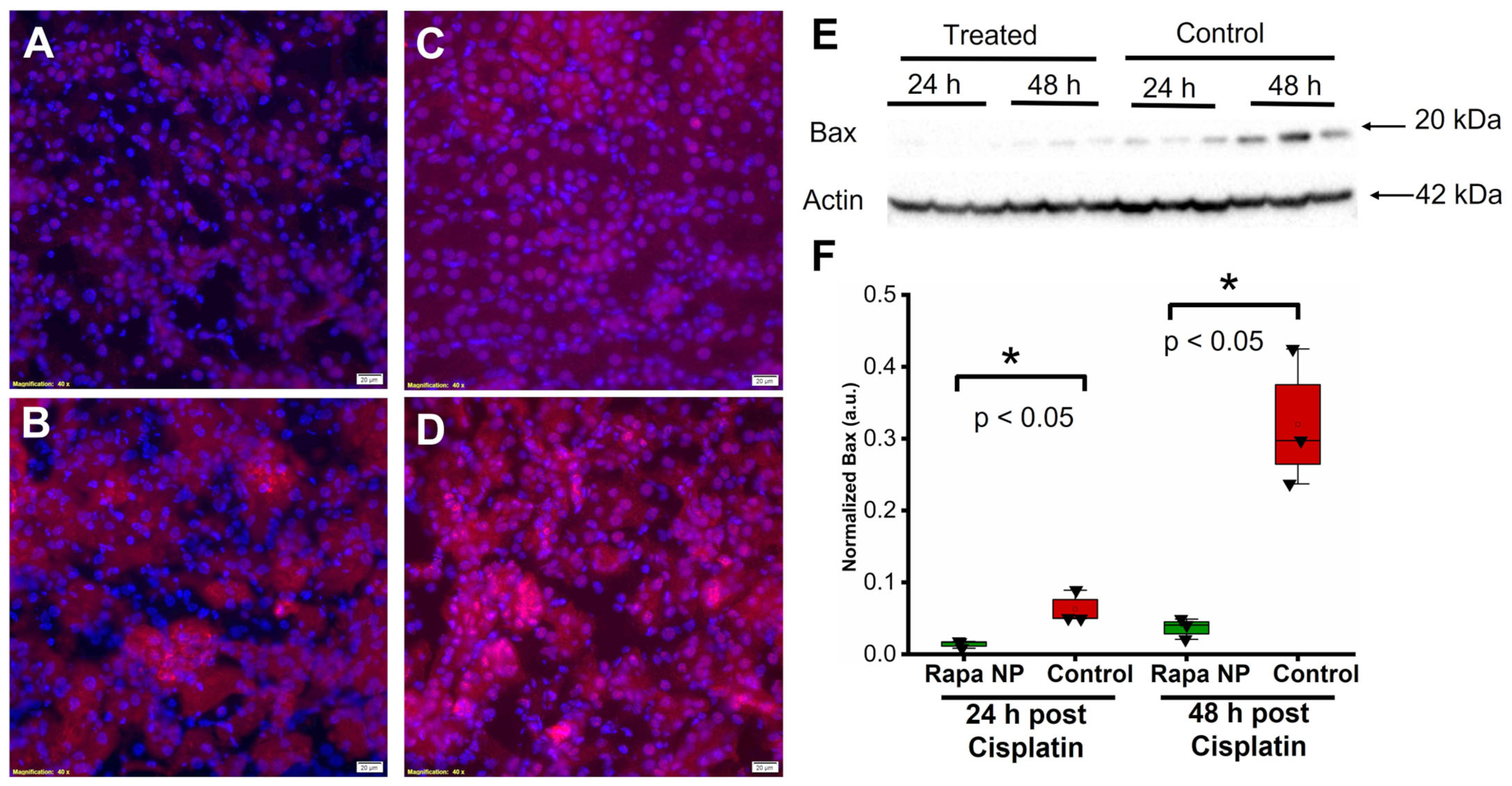
2.5. Rapamycin PFC NP Treatment Reduced Cell Death
3. Discussion
4. Materials and Methods
4.1. Nanoparticle Formulation
4.2. Nanoparticle Distribution and Zeta Potential Measurement
4.3. Acute Kidney Injury Model
4.4. Tissue Collection and Preservation
4.5. 19F magnetic Resonance Spectroscopy and Magnetic Resonance Imaging on 11.7 T
4.6. Serum Preparation
4.7. Blood Urea Nitrogen (BUN) Test
4.8. Histology
4.9. Protein Extraction
4.10. Western Blot
4.11. TUNEL Staining
4.12. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NCI. The "Accidental" Cure—Platinum-based Treatment for Cancer: The Discovery of Cisplatin. 2014. Available online: https://www.cancer.gov/research/progress/discovery/cisplatin (accessed on 1 February 2020).
- Lippman, A.J.; Helson, C.; Helson, L.; Krakoff, I.H. Clinical trials of cis-diamminedichloroplatinum (NSC-119875). Cancer Chemother. Rep. 1973, 57, 191–200. [Google Scholar]
- Manohar, S.; Leung, N. Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Malyszko, J.; Kozlowska, K.; Kozlowski, L.; Malyszko, J. Nephrotoxicity of anticancer treatment. Nephrol. Dial. Transpl. 2017, 32, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, H.; Furuya, R.; Yasuda, H.; Yamamoto, T.; Hishida, A.; Kitagawa, M. Anti-cancer agent-induced nephrotoxicity. Anticancer. Agents Med. Chem. 2014, 14, 921–927. [Google Scholar] [CrossRef]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.C.; Wu, C.H.; Huang, T.M.; Wang, C.Y.; Lai, C.F.; Shiao, C.C.; Chang, C.H.; Lin, S.L.; Chen, Y.Y.; Chen, Y.M.; et al. Long-term risk of coronary events after AKI. J. Am. Soc. Nephrol. 2014, 25, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Bucaloiu, I.D.; Kirchner, H.L.; Norfolk, E.R.; Hartle, J.E., 2nd; Perkins, R.M. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012, 81, 477–485. [Google Scholar] [CrossRef]
- Kaushal, G.P.; Shah, S.V. Autophagy in acute kidney injury. Kidney Int. 2016, 89, 779–791. [Google Scholar] [CrossRef]
- Kaushal, G.P.; Kaushal, V.; Herzog, C.; Yang, C. Autophagy delays apoptosis in renal tubular epithelial cells in cisplatin cytotoxicity. Autophagy 2008, 4, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Takabatake, Y.; Takahashi, A.; Isaka, Y. Chloroquine in cancer therapy: A double-edged sword of autophagy. Cancer Res. 2013, 73, 3–7. [Google Scholar] [CrossRef]
- He, L.; Livingston, M.J.; Dong, Z. Autophagy in acute kidney injury and repair. Nephron Clin. Pract. 2014, 127, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Chen, W.; Zou, Y.; Huang, H.; Pan, B.; Jin, S.; Huang, R.; Nie, S.; Kong, G. AMP-activated protein kinase regulates autophagic protection against cisplatin-induced tissue injury in the kidney. Genet. Mol. Res. 2015, 14, 12006–12015. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Shu, S.; Cai, J.; Tang, C.; Dong, Z. AMPK/mTOR Signaling in Autophagy Regulation During Cisplatin-Induced Acute Kidney Injury. Front. Physiol. 2020, 11, 619730. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Kimura, T.; Takabatake, Y.; Namba, T.; Kaimori, J.; Kitamura, H.; Matsui, I.; Niimura, F.; Matsusaka, T.; Fujita, N.; et al. Autophagy guards against cisplatin-induced acute kidney injury. Am. J. Pathol. 2012, 180, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wei, Q.; Dong, G.; Komatsu, M.; Su, Y.; Dong, Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012, 82, 1271–1283. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z.V.; Hill, J.A.; Lin, F. New autophagy reporter mice reveal dynamics of proximal tubular autophagy. J. Am. Soc. Nephrol. 2014, 25, 305–315. [Google Scholar] [CrossRef]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. BioMed Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef]
- Zuk, A.; Bonventre, J.V. Acute Kidney Injury. Annu. Rev. Med. 2016, 67, 293–307. [Google Scholar] [CrossRef]
- Shigemitsu, K.; Tsujishita, Y.; Hara, K.; Nanahoshi, M.; Avruch, J.; Yonezawa, K. Regulation of translational effectors by amino acid and mammalian target of rapamycin signaling pathways. Possible involvement of autophagy in cultured hepatoma cells. J. Biol. Chem. 1999, 274, 1058–1065. [Google Scholar] [CrossRef]
- Peterson, T.R.; Laplante, M.; Thoreen, C.C.; Sancak, Y.; Kang, S.A.; Kuehl, W.M.; Gray, N.S.; Sabatini, D.M. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 2009, 137, 873–886. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Ali, S.M.; Kim, D.H.; Guertin, D.A.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004, 14, 1296–1302. [Google Scholar] [CrossRef]
- Frias, M.A.; Thoreen, C.C.; Jaffe, J.D.; Schroder, W.; Sculley, T.; Carr, S.A.; Sabatini, D.M. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr. Biol. 2006, 16, 1865–1870. [Google Scholar] [CrossRef]
- Jacinto, E.; Facchinetti, V.; Liu, D.; Soto, N.; Wei, S.; Jung, S.Y.; Huang, Q.; Qin, J.; Su, B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 2006, 127, 125–137. [Google Scholar] [CrossRef]
- Yang, Q.; Inoki, K.; Ikenoue, T.; Guan, K.L. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006, 20, 2820–2832. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Doherty, J.; Akk, A.; Springer, L.E.; Fan, P.; Spasojevic, I.; Halade, G.V.; Yang, H.; Pham, C.T.N.; Wickline, S.A.; et al. Safety Profile of Rapamycin Perfluorocarbon Nanoparticles for Preventing Cisplatin-Induced Kidney Injury. Nanomaterials 2022, 12, 336. [Google Scholar] [CrossRef]
- Mayer, J.; Donnelly, T.M. Clinical Veterinary Advisor: Birds and Exotic Pets; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Gan, Y.; Lu, F.; Qin, Y. Radiotherapy plus cetuximab or cisplatin in head and neck squamous cell carcinoma: An updated systematic review and meta-analysis of randomized controlled trials. Eur. Arch. Otorhinolaryngol. 2023, 280, 11–22. [Google Scholar] [CrossRef]
- Bookman, M.A. Optimal primary therapy of ovarian cancer. Ann. Oncol. 2016, 27 (Suppl. 1), i58–i62. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.A.; Petty, W.J.; Urbanic, J.; Bernstein, E.D.; Ahmed, T. Cure of Oligometastatic Classic Biphasic Pulmonary Blastoma Using Aggressive Tri-modality Treatment: Case Series and Review of the Literature. Cureus 2018, 10, e3586. [Google Scholar] [CrossRef] [PubMed]
- Kris, M.G.; Gaspar, L.E.; Chaft, J.E.; Kennedy, E.B.; Azzoli, C.G.; Ellis, P.M.; Lin, S.H.; Pass, H.; Seth, R.; Shepherd, F.A.; et al. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA Completely Resected Non-Small-Cell Lung Cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 2960–2974. [Google Scholar] [CrossRef]
- McWhinney, S.R.; Goldberg, R.M.; McLeod, H.L. Platinum neurotoxicity pharmacogenetics. Mol. Cancer Ther. 2009, 8, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Mukherjea, D.; Rybak, L.P. Pharmacogenomics of cisplatin-induced ototoxicity. Pharmacogenomics 2011, 12, 1039–1050. [Google Scholar] [CrossRef]
- Hardaker, W.T., Jr.; Stone, R.A.; McCoy, R. Platinum nephrotoxicity. Cancer 1974, 34, 1030–1032. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.M.; Young, D.M.; Fauvie, K.A.; Wolpert, M.K.; Davis, R.; Guarino, A.M. Comparative nephrotoxicity of platinum cancer chemotherapeutic agents. Cancer Treat. Rep. 1976, 60, 1675–1678. [Google Scholar]
- Gonzalez-Vitale, J.C.; Hayes, D.M.; Cvitkovic, E.; Sternberg, S.S. The renal pathology in clinical trials of cis-platinum (II) diamminedichloride. Cancer 1977, 39, 1362–1371. [Google Scholar] [CrossRef]
- Kodama, A.; Watanabe, H.; Tanaka, R.; Kondo, M.; Chuang, V.T.G.; Wu, Q.; Endo, M.; Ishima, Y.; Fukagawa, M.; Otagiri, M.; et al. Albumin fusion renders thioredoxin an effective anti-oxidative and anti-inflammatory agent for preventing cisplatin-induced nephrotoxicity. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, M.K.; Burkhardt, G.; Kohler, H. Insights into potential cellular mechanisms of cisplatin nephrotoxicity and their clinical application. Nephrol. Dial. Transplant. 1997, 12, 2478–2480. [Google Scholar] [CrossRef]
- Pabla, N.; Murphy, R.F.; Liu, K.; Dong, Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am. J. Physiol.-Renal Physiol. 2009, 296, F505–F511. [Google Scholar] [CrossRef]
- Ciarimboli, G.; Ludwig, T.; Lang, D.; Pavenstädt, H.; Koepsell, H.; Piechota, H.-J.; Haier, J.; Jaehde, U.; Zisowsky, J.; Schlatter, E. Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am. J. Pathol. 2005, 167, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Bibee, K.P.; Cheng, Y.; Ching, J.K.; Marsh, J.N.; Li, A.J.; Keeling, R.M.; Connolly, A.M.; Golumbek, P.T.; Myerson, J.W.; Hu, G.; et al. Rapamycin nanoparticles target defective autophagy in muscular dystrophy to enhance both strength and cardiac function. FASEB J. 2014, 28, 2047–2061. [Google Scholar] [CrossRef]
- Moore, J.K.; Chen, J.; Pan, H.; Gaut, J.P.; Jain, S.; Wickline, S.A. Quantification of vascular damage in acute kidney injury with fluorine magnetic resonance imaging and spectroscopy. Magn. Reson. Med. 2018, 79, 3144–3153. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chen, J.; Yang, X.; Senpan, A.; Allen, J.S.; Yanaba, N.; Caruthers, S.D.; Lanza, G.M.; Hammerman, M.R.; Wickline, S.A. Assessing intrarenal nonperfusion and vascular leakage in acute kidney injury with multinuclear 1H/19F MRI and perfluorocarbon nanoparticles. Magn. Reson. Med. 2014, 71, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Soultati, A.; Mountzios, G.; Avgerinou, C.; Papaxoinis, G.; Pectasides, D.; Dimopoulos, M.-A.; Papadimitriou, C. Endothelial vascular toxicity from chemotherapeutic agents: Preclinical evidence and clinical implications. Cancer Treat. Rev. 2012, 38, 473–483. [Google Scholar] [CrossRef]
- Licciardello, J.T.W.; Moake, J.L.; Rudy, C.K.; Karp, D.D.; Hong, W.K. Elevated plasma von Willebrand factor levels and arterial occlusive complications associated with cisplatin-based chemotherapy. Oncology 1985, 42, 296–300. [Google Scholar] [CrossRef]
- Lechner, D.; Kollars, M.; Gleiss, A.; Kyrle, P.A.; Weltermann, A. Chemotherapy-induced thrombin generation via procoagulant endothelial microparticles is independent of tissue factor activity. J. Thromb. Haemost. JTH 2007, 5, 2445–2452. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Z.; Guo, X.-Y.; Wang, Z.; Xing, W.-G. Influence of Vein Injury in Different Methods of Chemotherapy in Mice. Clin. Oncol. Cancer Res. 2011, 8, 100–105. [Google Scholar] [CrossRef]
- Cameron, A.C.; McMahon, K.; Hall, M.; Neves, K.B.; Rios, F.J.; Montezano, A.C.; Welsh, P.; Waterston, A.; White, J.; Mark, P.B.; et al. Comprehensive Characterization of the Vascular Effects of Cisplatin-Based Chemotherapy in Patients with Testicular Cancer. J. Am. Coll. Cardiol. CardioOncol. 2020, 2, 443–455. [Google Scholar] [CrossRef]
- Meijer, C.; Nuver, J.; De Haas, E.C.; Van Zweeden, M.; Gietema, J.A. Vascular damage in testicular cancer patients: A study on endothelial activation by bleomycin and cisplatin in vitro. Oncol. Rep. 2010, 23, 247–253. [Google Scholar] [CrossRef]
- Takizawa, K.; Kamijo, R.; Ito, D.; Hatori, M.; Sumitani, K.; Nagumo, M. Synergistic induction of ICAM-1 expression by cisplatin and 5-fluorouracil in a cancer cell line via a NF-κB independent pathway. Br. J. Cancer 1999, 80, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhai, G.; Gu, X.; Sun, K. ATF3 and PRAP1 play important roles in cisplatin-induced damages in microvascular endothelial cells. Gene 2018, 672, 93–105. [Google Scholar] [CrossRef]
- Akcay, A.; Nguyen, Q.; Edelstein, C.L. Mediators of inflammation in acute kidney injury. Mediat. Inflamm. 2009, 2009, 137072. [Google Scholar] [CrossRef]
- Chargi, N.; Molenaar-Kuijsten, L.; Huiskamp, L.F.; Devriese, L.A.; de Bree, R.; Huitema, A.D. The association of cisplatin pharmacokinetics and skeletal muscle mass in patients with head and neck cancer: The prospective PLATISMA study. Eur. J. Cancer 2021, 160, 92–99. [Google Scholar] [CrossRef]
- Singh, G.K.; Patil, V.M.; Noronha, V.; Joshi, A.; Menon, N.; Lashkar, S.G.; Mathrudev, V.; Satam, K.N.; Prabhash, K. Weight loss and its impact on outcome in head and cancer patients during chemo-radiation. Oral Oncol. 2021, 122, 105522. [Google Scholar] [CrossRef]
- Hess, L.; Barakat, R.; Tian, C.; Ozols, R.; Alberts, D. Weight change during chemotherapy as a potential prognostic factor for stage III epithelial ovarian carcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2007, 107, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Topkan, E.; Parlak, C.; Selek, U. Impact of weight change during the course of concurrent chemoradiation therapy on outcomes in stage IIIB non-small cell lung cancer patients: Retrospective analysis of 425 patients. Int. J. Radiat. Oncol. 2013, 87, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; MacLeod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Awadalla, A.; Hussein, A.M.; El-Far, Y.M.; El-Senduny, F.F.; Barakat, N.; Hamam, E.T.; Abdeen, H.M.; El-Sherbiny, M.; Serria, M.S.; Sarhan, A.A.; et al. Rapamycin Improves Adipose-Derived Mesenchymal Stem Cells (ADMSCs) Renoprotective Effect against Cisplatin-Induced Acute Nephrotoxicity in Rats by Inhibiting the mTOR/AKT Signaling Pathway. Biomedicines 2022, 10, 1295. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.J.; Hou, J.G.; Ma, Z.N.; Wang, Z.; Ren, S.; Wang, Y.P.; Liu, W.C.; Chen, C.; Li, W. Ginsenoside Rb3 provides protective effects against cisplatin-induced nephrotoxicity via regulation of AMPK-/mTOR-mediated autophagy and inhibition of apoptosis in vitro and in vivo. Cell Prolif. 2019, 52, e12627. [Google Scholar] [CrossRef]
- Bao, H.; Zhang, Q.; Liu, X.; Song, Y.; Li, X.; Wang, Z.; Li, C.; Peng, A.; Gong, R. Lithium targeting of AMPK protects against cisplatin-induced acute kidney injury by enhancing autophagy in renal proximal tubular epithelial cells. FASEB J. 2019, 33, 14370–14381. [Google Scholar] [CrossRef]
- Andrianova, N.V.; Zorova, L.D.; Babenko, V.A.; Pevzner, I.B.; Popkov, V.A.; Silachev, D.N.; Plotnikov, E.Y.; Zorov, D.B. Rapamycin Is Not Protective against Ischemic and Cisplatin-Induced Kidney Injury. Biochemistry 2019, 84, 1502–1512. [Google Scholar] [CrossRef]
- Roberts, J.J.; Friedlos, F. Quantitative estimation of cisplatin-induced DNA interstrand cross-links and their repair in mammalian cells: Relationship to toxicity. Pharmacol. Ther. 1987, 34, 215–246. [Google Scholar] [CrossRef] [PubMed]
- Chu, G. Cellular responses to cisplatin. The roles of DNA-binding proteins and DNA repair. J. Biol. Chem. 1994, 269, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Dong, G.; Franklin, J.; Dong, Z. The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int. 2007, 72, 53–62. [Google Scholar] [CrossRef]
- Karch, J.; Molkentin, J.D. Regulated necrotic cell death: The passive aggressive side of Bax and Bak. Circ. Res. 2015, 116, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Cohn, C.S.; Cushing, M.M. Oxygen therapeutics: Perfluorocarbons and blood substitute safety. Crit. Care Clin. 2009, 25, 399–414. [Google Scholar] [CrossRef]
- Spahn, D.R.; van Brempt, R.; Theilmeier, G.; Reibold, J.P.; Welte, M.; Heinzerling, H.; Birck, K.M.; Keipert, P.E.; Messmer, K.; Heinzerling, H.; et al. Perflubron emulsion delays blood transfusions in orthopedic surgery. European Perflubron Emulsion Study Group. Anesthesiology 1999, 91, 1195–1208. [Google Scholar] [CrossRef]
- Kerins, D.M. Role of the perfluorocarbon Fluosol-DA in coronary angioplasty. Am. J. Med. Sci. 1994, 307, 218–221. [Google Scholar] [CrossRef]
- Desai, U.R.; Peyman, G.A.; Harper, C.A., 3rd. Perfluorocarbon liquid in traumatic vitreous hemorrhage and retinal detachment. Ophthalmic Surg. 1993, 24, 537–541. [Google Scholar] [CrossRef]
- Chen, J.; Pan, H.; Lanza, G.M.; Wickline, S.A. Perfluorocarbon nanoparticles for physiological and molecular imaging and therapy. Adv. Chronic Kidney Dis. 2013, 20, 466–478. [Google Scholar] [CrossRef]
- Kaneda, M.M.; Caruthers, S.; Lanza, G.M.; Wickline, S.A. Perfluorocarbon nanoemulsions for quantitative molecular imaging and targeted therapeutics. Ann. Biomed. Eng. 2009, 37, 1922–1933. [Google Scholar] [CrossRef]
- Palekar, R.U.; Jallouk, A.P.; Lanza, G.M.; Pan, H.; Wickline, S.A. Molecular imaging of atherosclerosis with nanoparticle-based fluorinated MRI contrast agents. Nanomedicine 2015, 10, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Morawski, A.M.; Lanza, G.A.; Wickline, S.A. Targeted contrast agents for magnetic resonance imaging and ultrasound. Curr. Opin. Biotechnol. 2005, 16, 89–92. [Google Scholar] [CrossRef]
- Hu, L.; Pan, H.; Wickline, S.A. Fluorine (19F) MRI to Measure Renal Oxygen Tension and Blood Volume: Experimental Protocol. Methods Mol. Biol. 2021, 2216, 509–518. [Google Scholar] [PubMed]
- Neubauer, A.M.; Sim, H.; Winter, P.M.; Caruthers, S.D.; Williams, T.A.; Robertson, J.D.; Sept, D.; Lanza, G.M.; Wickline, S.A. Nanoparticle pharmacokinetic profiling in vivo using magnetic resonance imaging. Magn. Reson. Med. 2008, 60, 1353–1361. [Google Scholar] [CrossRef]
- Winter, P.M.; Neubauer, A.M.; Caruthers, S.D.; Harris, T.D.; Robertson, J.D.; Williams, T.A.; Schmieder, A.H.; Hu, G.; Allen, J.S.; Lacy, E.K.; et al. Endothelial αvβ3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol 2006, 26, 2103–2109. [Google Scholar] [CrossRef]
- Waters, E.A.; Chen, J.; Yang, X.; Zhang, H.; Neumann, R.; Santeford, A.; Arbeit, J.; Lanza, G.M.; Wickline, S.A. Detection of targeted perfluorocarbon nanoparticle binding using 19F diffusion weighted MR spectroscopy. Magn. Reson. Med. 2008, 60, 1232–1236. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Quirk, J.D.; Hu, Y.; Yan, H.; Gaut, J.P.; Pham, C.T.N.; Wickline, S.A.; Pan, H. Rapamycin Perfluorocarbon Nanoparticle Mitigates Cisplatin-Induced Acute Kidney Injury. Int. J. Mol. Sci. 2023, 24, 6086. https://doi.org/10.3390/ijms24076086
Zhou Q, Quirk JD, Hu Y, Yan H, Gaut JP, Pham CTN, Wickline SA, Pan H. Rapamycin Perfluorocarbon Nanoparticle Mitigates Cisplatin-Induced Acute Kidney Injury. International Journal of Molecular Sciences. 2023; 24(7):6086. https://doi.org/10.3390/ijms24076086
Chicago/Turabian StyleZhou, Qingyu, James D. Quirk, Ying Hu, Huimin Yan, Joseph P. Gaut, Christine T. N. Pham, Samuel A. Wickline, and Hua Pan. 2023. "Rapamycin Perfluorocarbon Nanoparticle Mitigates Cisplatin-Induced Acute Kidney Injury" International Journal of Molecular Sciences 24, no. 7: 6086. https://doi.org/10.3390/ijms24076086
APA StyleZhou, Q., Quirk, J. D., Hu, Y., Yan, H., Gaut, J. P., Pham, C. T. N., Wickline, S. A., & Pan, H. (2023). Rapamycin Perfluorocarbon Nanoparticle Mitigates Cisplatin-Induced Acute Kidney Injury. International Journal of Molecular Sciences, 24(7), 6086. https://doi.org/10.3390/ijms24076086






