Comparative Efficacy of Intra-Articular Injection, Physical Therapy, and Combined Treatments on Pain, Function, and Sarcopenia Indices in Knee Osteoarthritis: A Network Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Results
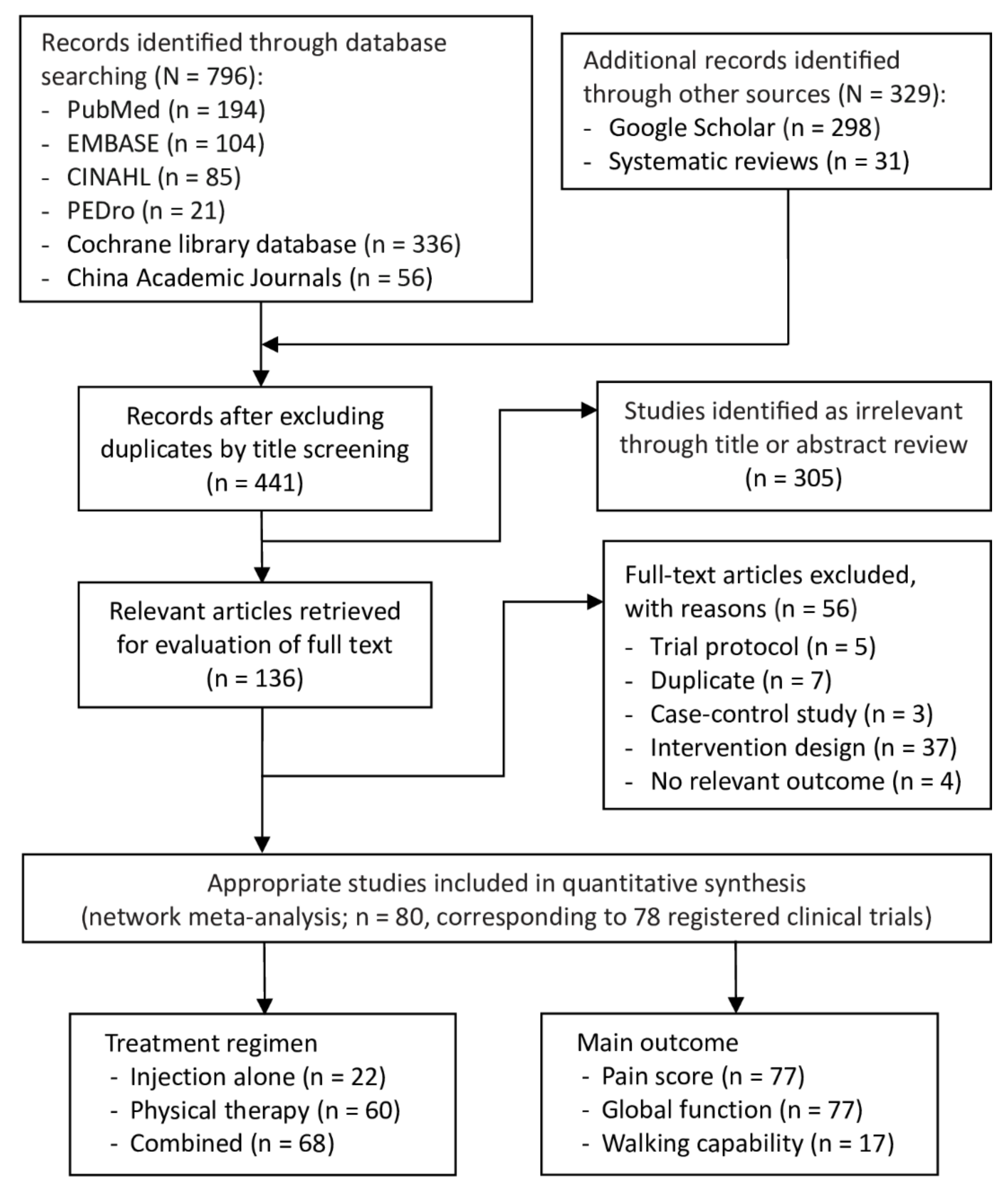
2.1. Selection of Studies
2.2. Characteristics of Analyzed Patients
2.3. Injection Treatment and Physical Therapy Characteristics
2.4. Quality and Risk of Bias in Analyzed Studies
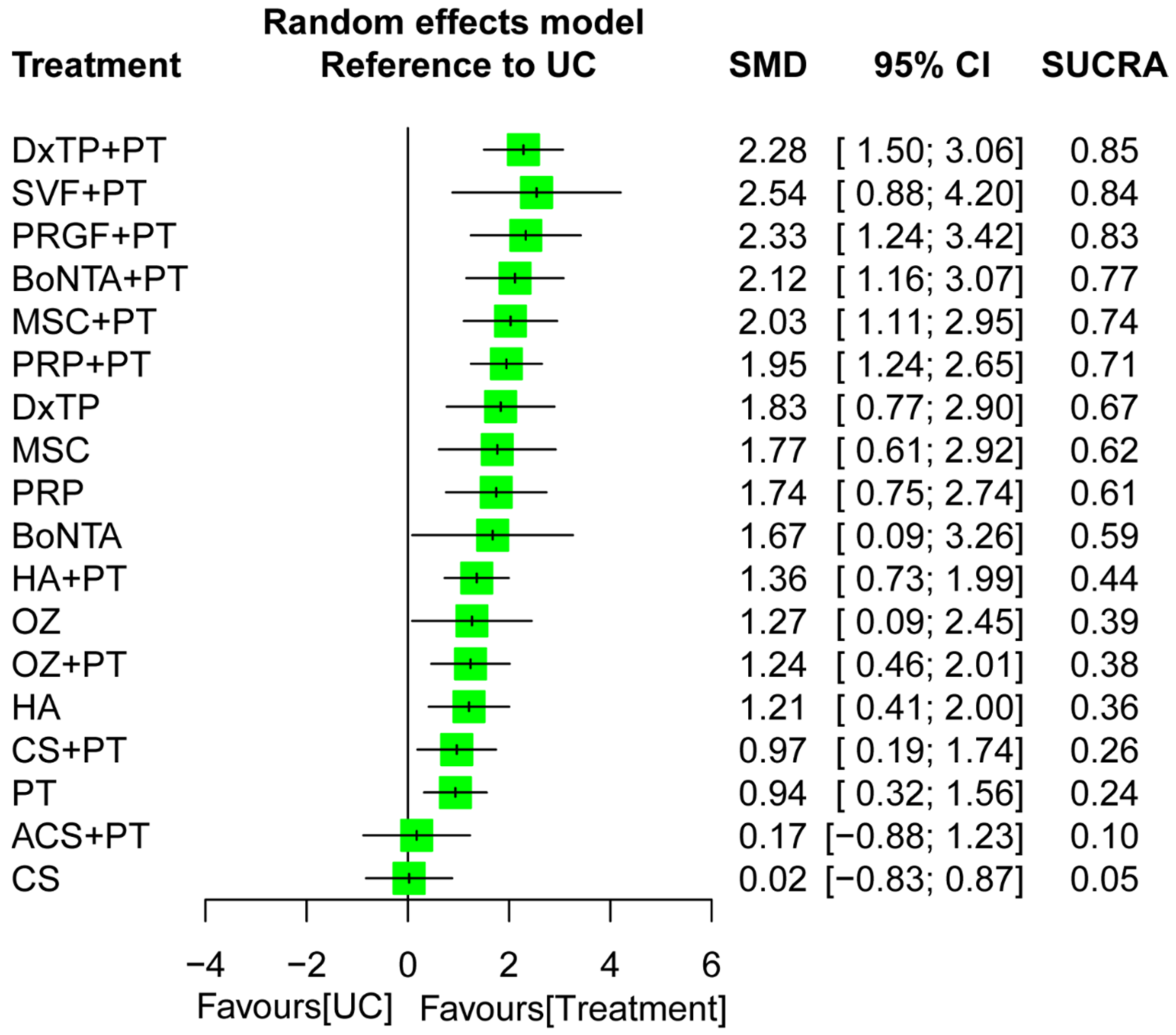
2.5. Effectiveness of Treatment for Pain Reduction
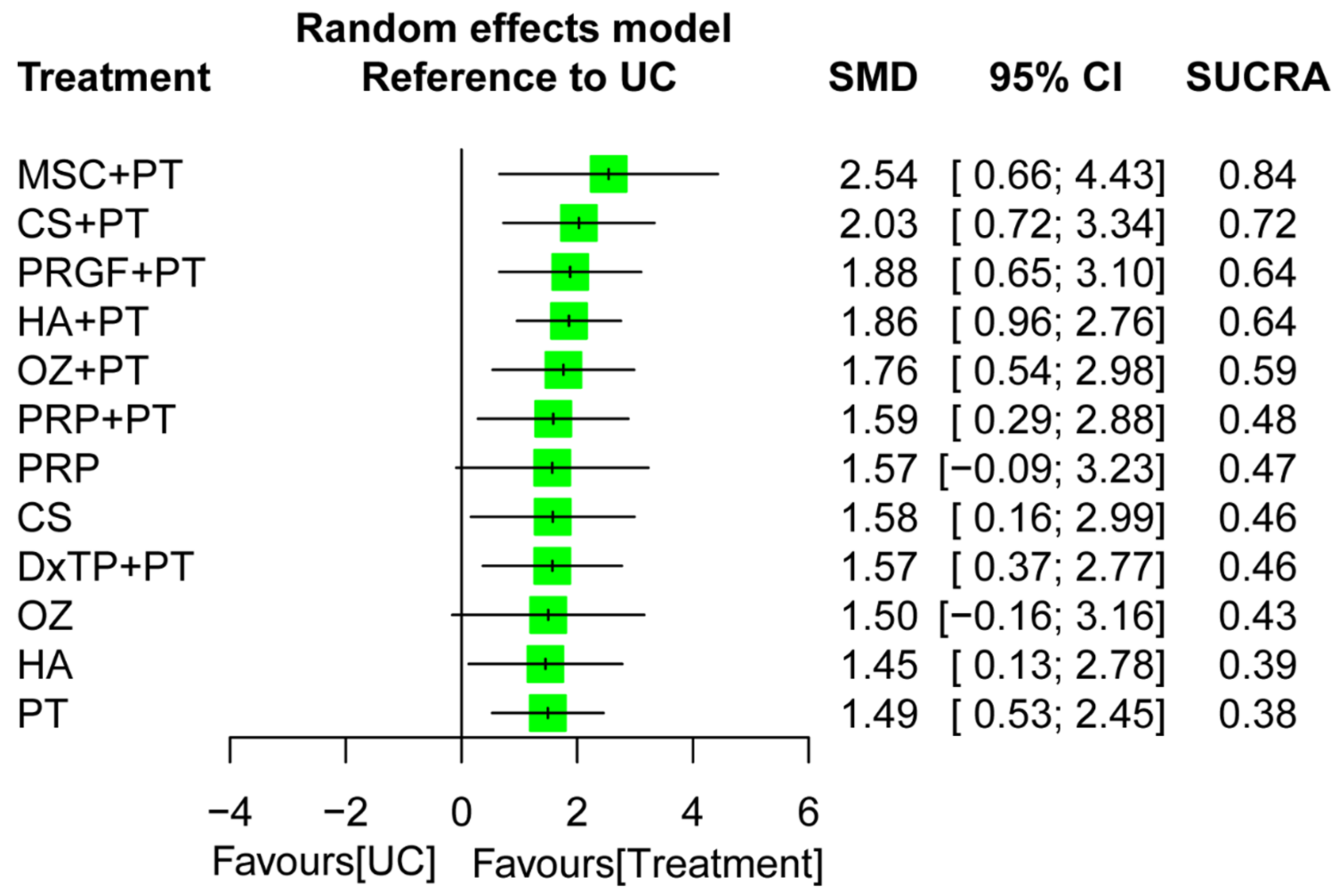
2.6. Effectiveness of Treatment for Global Function
2.7. Effectiveness of Treatment for Walking Capability
2.8. NMR Results for Potential Moderators of Treatment Effects
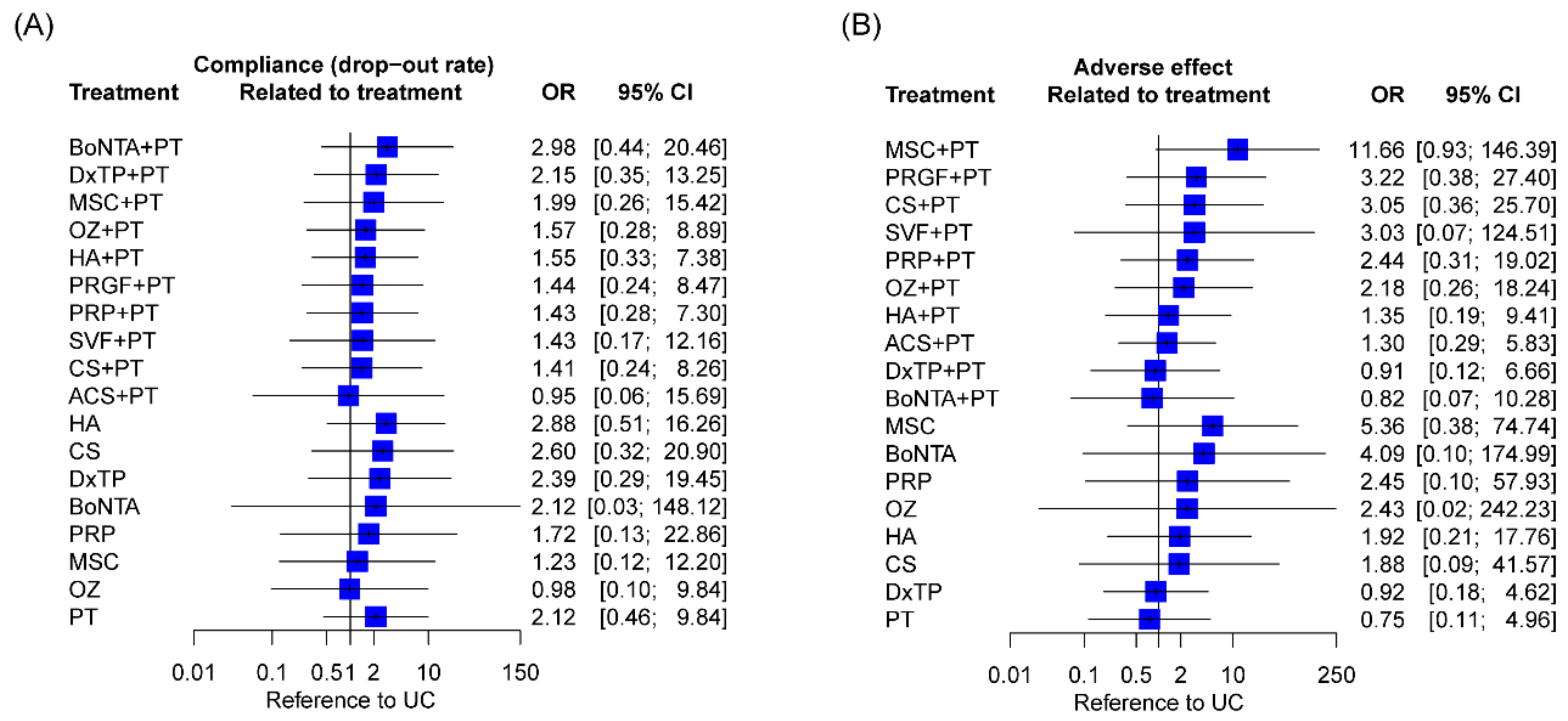
2.9. Compliance and Adverse Effects
2.10. Publication Bias
3. Discussion
3.1. Summary of Main Findings
3.2. Comparisons of this NMA with Previous Studies
3.3. Explorations and Possible Mechanisms of Treatment Effects
3.4. Moderator of Relative Efficiency among Treatment Regimens
3.5. Certainty of the Evidence of Treatment Options for each Main Outcome
3.6. The Needs of Multimodal Approach for Management of KOA
3.7. Strengths and Limitations
4. Materials and Methods
4.1. Study Design and Protocol Registration
4.2. Search Strategy and Study Selection
4.3. Study Selection Criteria
4.4. Outcome Measures
4.5. Data Collection and Extraction
4.6. Assessment of Bias Risk and Methodological Quality of Analyzed Studies
4.7. Data Synthesis and Analysis
4.8. Certainty of Evidence
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Culvenor, A.G.; Ruhdorfer, A.; Juhl, C.; Eckstein, F.; Øiestad, B.E. Knee Extensor Strength and Risk of Structural, Symptomatic, and Functional Decline in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2017, 69, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Omori, G.; Koga, Y.; Tanaka, M.; Nawata, A.; Watanabe, H.; Narumi, K.; Endoh, K. Quadriceps muscle strength and its relationship to radiographic knee osteoarthritis in Japanese elderly. J. Orthop. Sci. 2013, 18, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Villanueva, D.; Gómez-Cabello, A.; Marín-Puyalto, J.; Moreno, L.A.; Vicente-Rodríguez, G.; Casajús, J.A. Frailty and Physical Fitness in Elderly People: A Systematic Review and Meta-analysis. Sports Med. 2021, 51, 143–160. [Google Scholar] [CrossRef]
- Veronese, N.; Maggi, S.; Trevisan, C.; Noale, M.; De Rui, M.; Bolzetta, F.; Zambon, S.; Musacchio, E.; Sartori, L.; Perissinotto, E.; et al. Pain Increases the Risk of Developing Frailty in Older Adults with Osteoarthritis. Pain Med. 2017, 18, 414–427. [Google Scholar] [CrossRef]
- Ondresik, M.; Azevedo Maia, F.R.; da Silva Morais, A.; Gertrudes, A.C.; Dias Bacelar, A.H.; Correia, C.; Goncalves, C.; Radhouani, H.; Amandi Sousa, R.; Oliveira, J.M.; et al. Management of knee osteoarthritis. Current status and future trends. Biotechnol. Bioeng. 2017, 114, 717–739. [Google Scholar] [CrossRef]
- Brophy, R.H.; Fillingham, Y.A. AAOS Clinical Practice Guideline Summary: Management of Osteoarthritis of the Knee (Nonarthroplasty), Third Edition. J. Am. Acad. Orthop. Surg. 2022, 30, e721–e729. [Google Scholar] [CrossRef]
- Chu, C.R.; Rodeo, S.; Bhutani, N.; Goodrich, L.R.; Huard, J.; Irrgang, J.; LaPrade, R.F.; Lattermann, C.; Lu, Y.; Mandelbaum, B.; et al. Optimizing Clinical Use of Biologics in Orthopaedic Surgery: Consensus Recommendations From the 2018 AAOS/NIH U-13 Conference. J. Am. Acad. Orthop. Surg. 2019, 27, e50–e63. [Google Scholar] [CrossRef]
- Zhang, Z.; Schon, L. The Current Status of Clinical Trials on Biologics for Cartilage Repair and Osteoarthritis Treatment: An Analysis of ClinicalTrials.gov Data. Cartilage 2022, 13, 19476035221093065. [Google Scholar] [CrossRef]
- McLarnon, M.; Heron, N. Intra-articular platelet-rich plasma injections versus intra-articular corticosteroid injections for symptomatic management of knee osteoarthritis: Systematic review and meta-analysis. BMC Musculoskelet. Disord. 2021, 22, 550. [Google Scholar] [CrossRef]
- Anil, U.; Markus, D.H.; Hurley, E.T.; Manjunath, A.K.; Alaia, M.J.; Campbell, K.A.; Jazrawi, L.M.; Strauss, E.J. The efficacy of intra-articular injections in the treatment of knee osteoarthritis: A network meta-analysis of randomized controlled trials. Knee 2021, 32, 173–182. [Google Scholar] [CrossRef]
- Anitua, E.; Sánchez, M.; Aguirre, J.J.; Prado, R.; Padilla, S.; Orive, G. Efficacy and safety of plasma rich in growth factors intra-articular infiltrations in the treatment of knee osteoarthritis. Arthroscopy 2014, 30, 1006–1017. [Google Scholar] [CrossRef]
- Han, S.B.; Seo, I.W.; Shin, Y.S. Intra-Articular Injections of Hyaluronic Acid or Steroids Associated With Better Outcomes Than Platelet-Rich Plasma, Adipose Mesenchymal Stromal Cells, or Placebo in Knee Osteoarthritis: A Network Meta-analysis. Arthroscopy 2021, 37, 292–306. [Google Scholar] [CrossRef]
- Sax, O.C.; Chen, Z.; Mont, M.A.; Delanois, R.E. The Efficacy of Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis Symptoms and Structural Changes: A Systematic Review and Meta-Analysis. J. Arthroplast. 2022, 37, 2282–2290. [Google Scholar] [CrossRef]
- Shen, L.; Yuan, T.; Chen, S.; Xie, X.; Zhang, C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2017, 12, 16. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; You, D.; Zhao, S.; Zhu, Z.; Xu, M. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: A meta-analysis. Medicine 2020, 99, e19388. [Google Scholar] [CrossRef]
- Cortez, V.S.; Moraes, W.A.; Taba, J.V.; Condi, A.; Suzuki, M.O.; Nascimento, F.S.D.; Pipek, L.Z.; Mattos, V.C.; Torsani, M.B.; Meyer, A.; et al. Comparing dextrose prolotherapy with other substances in knee osteoarthritis pain relief: A systematic review. Clinics 2022, 77, 100037. [Google Scholar] [CrossRef]
- Hong, M.; Cheng, C.; Sun, X.; Yan, Y.; Zhang, Q.; Wang, W.; Guo, W. Efficacy and Safety of Intra-Articular Platelet-Rich Plasma in Osteoarthritis Knee: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2021, 2021, 2191926. [Google Scholar] [CrossRef]
- Javadi Hedayatabad, J.; Kachooei, A.R.; Taher Chaharjouy, N.; Vaziri, N.; Mehrad-Majd, H.; Emadzadeh, M.; Abolghasemian, M.; Ebrahimzadeh, M.H. The Effect of Ozone (O3) versus Hyaluronic Acid on Pain and Function in Patients with Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Arch. Bone Jt. Surg. 2020, 8, 343–354. [Google Scholar] [CrossRef]
- Li, Q.; Qi, X.; Zhang, Z. Intra-articular oxygen-ozone versus hyaluronic acid in knee osteoarthritis: A meta-analysis of randomized controlled trials. Int. J. Surg. 2018, 58, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Monticone, M.; Frizziero, A.; Rovere, G.; Vittadini, F.; Uliano, D.; Bruna, S.L.A.; Gatto, R.; Nava, C.; Leggero, V.; Masiero, S. Hyaluronic acid intra-articular injection and exercise therapy: Effects on pain and disability in subjects affected by lower limb joints osteoarthritis. A systematic review by the Italian Society of Physical and Rehabilitation Medicine (SIMFER). Eur. J. Phys. Rehabil. Med. 2016, 52, 389–399. [Google Scholar] [PubMed]
- Chen, Y.W.; Lin, Y.N.; Chen, H.C.; Liou, T.H.; Liao, C.D.; Huang, S.W. Effectiveness, Compliance, and Safety of Dextrose Prolotherapy for Knee Osteoarthritis: A Meta-Analysis and Metaregression of Randomized Controlled Trials. Clin. Rehabil. 2022, 36, 740–752. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Pan, J.K.; Yang, W.Y.; Han, Y.H.; Zeng, L.F.; Liang, G.H.; Liu, J. Intra-Articular Injections of Platelet-Rich Plasma, Adipose Mesenchymal Stem Cells, and Bone Marrow Mesenchymal Stem Cells Associated With Better Outcomes Than Hyaluronic Acid and Saline in Knee Osteoarthritis: A Systematic Review and Network Meta-analysis. Arthroscopy 2021, 37, 2298–2314.e2210. [Google Scholar] [CrossRef]
- Aletto, C.; Francesco, O.; Maffulli, N. Knee intra-articular administration of stromal vascular fraction obtained from adipose tissue: A systematic review. J. Clin. Orthop. Trauma 2022, 25, 101773. [Google Scholar] [CrossRef]
- Bolia, I.K.; Bougioukli, S.; Hill, W.J.; Trasolini, N.A.; Petrigliano, F.A.; Lieberman, J.R.; Weber, A.E. Clinical Efficacy of Bone Marrow Aspirate Concentrate Versus Stromal Vascular Fraction Injection in Patients With Knee Osteoarthritis: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2022, 50, 1451–1461. [Google Scholar] [CrossRef]
- Acosta-Olivo, C.; Esponda-Colmenares, F.; Vilchez-Cavazos, F.; Lara-Arias, J.; Mendoza-Lemus, O.; Ramos-Morales, T. Platelet rich plasma versus oral paracetamol for the treatment of early knee osteoarthritis. Preliminary study. Cir Cir 2014, 82, 163–169. [Google Scholar]
- Akan, Ö.; Sarıkaya, N.Ö.; Hikmet, K. Efficacy of platelet-rich plasma administration in patients with severe knee osteoarthritis: Can platelet-rich plasma administration delay arthroplasty in this patient population? Int. J. Clin. Exp. Med. 2018, 11, 9473–9483. [Google Scholar]
- Altman, R.D.; Rosen, J.E.; Bloch, D.A.; Hatoum, H.T.; Korner, P. A double-blind, randomized, saline-controlled study of the efficacy and safety of EUFLEXXA for treatment of painful osteoarthritis of the knee, with an open-label safety extension (the FLEXX trial). Semin. Arthritis Rheum. 2009, 39, 1–9. [Google Scholar] [CrossRef]
- Angoorani, H.; Mazaherinezhad, A.; Marjomaki, O.; Younespour, S. Treatment of knee osteoarthritis with platelet-rich plasma in comparison with transcutaneous electrical nerve stimulation plus exercise: A randomized clinical trial. Med. J. Islam Repub. Iran 2015, 29, 223. [Google Scholar]
- Anz, A.W.; Hubbard, R.; Rendos, N.K.; Everts, P.A.; Andrews, J.R.; Hackel, J.G. Bone Marrow Aspirate Concentrate Is Equivalent to Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis at 1 Year: A Prospective, Randomized Trial. Orthop. J. Sports Med. 2020, 8, 2325967119900958. [Google Scholar] [CrossRef]
- Atamaz, F.; Kirazli, Y.; Akkoc, Y. A comparison of two different intra-articular hyaluronan drugs and physical therapy in the management of knee osteoarthritis. Rheumatol. Int. 2006, 26, 873–878. [Google Scholar] [CrossRef]
- Auerbach, B.; Melzer, C. Cross-linked hyaluronic acid in the treatment of osteoarthritis of the knee--results of a prospective randomized trial. Zentralbl. Chir. 2002, 127, 895–899. [Google Scholar] [CrossRef]
- Babaei-Ghazani, A.; Najarzade, S.; Madani, P.; Azar, M.; Tirandazi, B. A comparison of ultrasound guided corticosteroid injection versus ozone injection in grade 3 knee osteoarthritis. Tehran Univ. Med. J. 2019, 77, 373–381. [Google Scholar]
- Babaei-Ghazani, A.; Najarzadeh, S.; Mansoori, K.; Forogh, B.; Madani, S.P.; Ebadi, S.; Fadavi, H.R.; Eftekharsadat, B. The effects of ultrasound-guided corticosteroid injection compared to oxygen–ozone (O2–O3) injection in patients with knee osteoarthritis: A randomized controlled trial. Clin. Rheumatol. 2018, 37, 2517–2527. [Google Scholar] [CrossRef]
- Bao, X.; Tan, J.W.; Flyzik, M.; Ma, X.C.; Liu, H.; Liu, H.Y. Effect of therapeutic exercise on knee osteoarthritis after intra-articular injection of botulinum toxin type A, hyaluronate or saline: A randomized controlled trial. J. Rehabil. Med. 2018, 50, 534–541. [Google Scholar] [CrossRef]
- Baranova, I.V. The use of the functional state of the joints for the estimation of the effectiveness of the application of oxygen/ozone therapy for the rehabilitative treatment of the patients suffering from knee arthritis. Vopr. Kurortol. Fizioter. Lech. Fiz. Kult. 2018, 95, 42–48. [Google Scholar] [CrossRef]
- Başar, B.; Başar, G.; Büyükkuşçu, M.Ö.; Başar, H. Comparison of physical therapy and arthroscopic partial meniscectomy treatments in degenerative meniscus tears and the effect of combined hyaluronic acid injection with these treatments: A randomized clinical trial. J. Back Musculoskelet. Rehabil. 2021, 34, 767–774. [Google Scholar] [CrossRef]
- Baygutalp, F.; Celik, M.; Oztürk, M.U.; Yayık, A.M.; Ahıskalıoglu, A. Comparison of the Efficacy of Dextrose Prolotherapy and Ozone in Patients with Knee Osteoarthritis: A Randomized Cross-Sectional Study. Appl. Sci. 2021, 11, 9991. [Google Scholar] [CrossRef]
- Bayramoğlu, M.; Karataş, M.; Çetin, N.; Akman, N.; Sözay, S.; Dilek, A. Comparison of two different viscosupplements in knee osteoarthritis—A pilot study. Clin. Rheumatol. 2003, 22, 118–122. [Google Scholar] [CrossRef]
- Centeno, C.; Sheinkop, M.; Dodson, E.; Stemper, I.; Williams, C.; Hyzy, M.; Ichim, T.; Freeman, M. A specific protocol of autologous bone marrow concentrate and platelet products versus exercise therapy for symptomatic knee osteoarthritis: A randomized controlled trial with 2 year follow-up. J. Transl. Med. 2018, 16, 355. [Google Scholar] [CrossRef]
- Chen, W.L.; Hsu, W.C.; Lin, Y.J.; Hsieh, L.F. Comparison of intra-articular hyaluronic acid injections with transcutaneous electric nerve stimulation for the management of knee osteoarthritis: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2013, 94, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Cole, B.J.; Karas, V.; Hussey, K.; Pilz, K.; Fortier, L.A. Hyaluronic Acid Versus Platelet-Rich Plasma: A Prospective, Double-Blind Randomized Controlled Trial Comparing Clinical Outcomes and Effects on Intra-articular Biology for the Treatment of Knee Osteoarthritis. Am. J. Sports Med. 2017, 45, 339–346. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Stagno, D.; Minetto, M.A.; Cisari, C.; Baricich, A.; Invernizzi, M. Long-term effects of intra-articular oxygen-ozone therapy versus hyaluronic acid in older people affected by knee osteoarthritis: A randomized single-blind extension study. J. Back Musculoskelet. Rehabil. 2020, 33, 347–354. [Google Scholar] [CrossRef] [PubMed]
- DeCaria, J.E.; Montero-Odasso, M.; Wolfe, D.; Chesworth, B.M.; Petrella, R.J. The effect of intra-articular hyaluronic acid treatment on gait velocity in older knee osteoarthritis patients: A randomized, controlled study. Arch. Gerontol. Geriatr. 2012, 55, 310–315. [Google Scholar] [CrossRef]
- Delgado-Enciso, I.; Paz-Garcia, J.; Valtierra-Alvarez, J.; Preciado-Ramirez, J.; Almeida-Trinidad, R.; Guzman-Esquivel, J.; Mendoza-Hernandez, M.A.; Garcia-Vega, A.; Soriano-Hernandez, A.D.; Cortes-Bazan, J.L.; et al. A phase I-II controlled randomized trial using a promising novel cell-free formulation for articular cartilage regeneration as treatment of severe osteoarthritis of the knee. Eur. J. Med. Res. 2018, 23, 52. [Google Scholar] [CrossRef]
- Delgado-Enciso, I.; Valtierra-Alvarez, J.; Paz-Garcia, J.; Preciado-Ramirez, J.; Soriano-Hernandez, A.D.; Mendoza-Hernandez, M.A.; Guzman-Esquivel, J.; Cabrera-Licona, A.; Delgado-Enciso, J.; Cortes-Bazan, J.L.; et al. Patient-reported health outcomes for severe knee osteoarthritis after conservative treatment with an intra-articular cell-free formulation for articular cartilage regeneration combined with usual medical care vs. Usual medical care alone: A randomized controlled trial. Exp. Ther. Med. 2019, 17, 3351–3360. [Google Scholar] [CrossRef]
- Deyle, G.D.; Allen, C.S.; Allison, S.C.; Gill, N.W.; Hando, B.R.; Petersen, E.J.; Dusenberry, D.I.; Rhon, D.I. Physical Therapy versus Glucocorticoid Injection for Osteoarthritis of the Knee. N. Engl. J. Med. 2020, 382, 1420–1429. [Google Scholar] [CrossRef]
- Di Sante, L.; Paoloni, M.; Dimaggio, M.; Colella, L.; Cerino, A.; Bernetti, A.; Murgia, M.; Santilli, V. Ultrasound-guided aspiration and corticosteroid injection compared to horizontal therapy for treatment of knee osteoarthritis complicated with Baker’s cyst: A randomized, controlled trial. Eur. J. Phys. Rehabil. Med. 2012, 48, 561–567. [Google Scholar]
- Dumais, R.; Benoit, C.; Dumais, A.; Babin, L.; Bordage, R.; de Arcos, C.; Allard, J.; Bélanger, M. Effect of regenerative injection therapy on function and pain in patients with knee osteoarthritis: A randomized crossover study. Pain Med. 2012, 13, 990–999. [Google Scholar] [CrossRef]
- Elerian, A.E.; Ewidea, T.A. Effect of shock wave therapy versus corticosteroid injection in management of knee osteoarthritis. Int. J. Physiother. 2016, 3, 246–251. [Google Scholar] [CrossRef]
- Elgendy, M.H.; Elsamahy, S.A.; Mostafa, M.S.E.M.; Hamza, M.S.K. Efficacy of shockwave therapy versus intra-articular platelet-rich plasma injection in management of knee osteoarthritis: A randomized controlled trial. Int. J. Pharm. Res. 2020, 12, 4283–4289. [Google Scholar] [CrossRef]
- Elik, H.; Doğu, B.; Yılmaz, F.; Begoğlu, F.A.; Kuran, B. The efficiency of platelet-rich plasma treatment in patients with knee osteoarthritis. J. Back Musculoskelet. Rehabil. 2020, 33, 127–138. [Google Scholar] [CrossRef]
- Filardo, G.; Di Matteo, B.; Di Martino, A.; Merli, M.L.; Cenacchi, A.; Fornasari, P.; Marcacci, M.; Kon, E. Platelet-Rich Plasma Intra-articular Knee Injections Show No Superiority Versus Viscosupplementation: A Randomized Controlled Trial. Am. J. Sports Med. 2015, 43, 1575–1582. [Google Scholar] [CrossRef]
- Forogh, B.; Mianehsaz, E.; Shoaee, S.; Ahadi, T.; Raissi, G.R.; Sajadi, S. Effect of single injection of platelet-rich plasma in comparison with corticosteroid on knee osteoarthritis: A double-blind randomized clinical trial. J. Sports Med. Phys. Fitness 2016, 56, 901–908. [Google Scholar]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.; Boyd, R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regen. Med. 2019, 14, 213–230. [Google Scholar] [CrossRef]
- Gaballa, N.M.; Mohammed, Y.A.; Kamel, L.M.; Mahgoub, H.M. Therapeutic efficacy of intra-articular injection of platelet–rich plasma and ozone therapy in patients with primary knee osteoarthritis. Egypt. Rheumatol. 2019, 41, 183–187. [Google Scholar] [CrossRef]
- García-Triana, S.A.; Toro-Sashida, M.F.; Larios-González, X.V.; Fuentes-Orozco, C.; Mares-País, R.; Barbosa-Camacho, F.J.; Guzmán-Ramírez, B.G.; Pintor-Belmontes, K.J.; Rodríguez-Navarro, D.; Brancaccio-Pérez, I.V.; et al. The Benefit of Perineural Injection Treatment with Dextrose for Treatment of Chondromalacia Patella in Participants Receiving Home Physical Therapy: A Pilot Randomized Clinical Trial. J. Altern. Complement. Med. 2021, 27, 38–44. [Google Scholar] [CrossRef]
- Garza, J.R.; Campbell, R.E.; Tjoumakaris, F.P.; Freedman, K.B.; Miller, L.S.; Santa Maria, D.; Tucker, B.S. Clinical Efficacy of Intra-articular Mesenchymal Stromal Cells for the Treatment of Knee Osteoarthritis: A Double-Blinded Prospective Randomized Controlled Clinical Trial. Am. J. Sports Med. 2020, 48, 588–598. [Google Scholar] [CrossRef]
- Ghai, B.; Gupta, V.; Jain, A.; Goel, N.; Chouhan, D.; Batra, Y.K. Effectiveness of platelet rich plasma in pain management of osteoarthritis knee: Double blind, randomized comparative study. Braz. J. Anesthesiol. 2019, 69, 439–447. [Google Scholar] [CrossRef]
- Hawkins, K.; Ghazi, F. The addition of a supervised exercise class to a home exercise programme in the treatment of patients with knee osteoarthritis following corticosteroid injection: A pilot study. Int. Musculoskelet. Med. 2012, 34, 159–165. [Google Scholar] [CrossRef]
- Henriksen, M.; Christensen, R.; Klokker, L.; Bartholdy, C.; Bandak, E.; Ellegaard, K.; Boesen, M.P.; Riis, R.G.; Bartels, E.M.; Bliddal, H. Evaluation of the benefit of corticosteroid injection before exercise therapy in patients with osteoarthritis of the knee: A randomized clinical trial. JAMA Intern. Med. 2015, 175, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Hermans, J.; Bierma-Zeinstra, S.M.A.; Bos, P.K.; Niesten, D.D.; Verhaar, J.A.N.; Reijman, M. The effectiveness of high molecular weight hyaluronic acid for knee osteoarthritis in patients in the working age: A randomised controlled trial. BMC Musculoskelet. Disord. 2019, 20, 196. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.H.; Yang, R.C.; Lee, C.L.; Chen, T.W.; Wang, M.C. Preliminary results of integrated therapy for patients with knee osteoarthritis. Arthritis Rheum. 2005, 53, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Ip, D.; Fu, N.Y. Can combined use of low-level lasers and hyaluronic acid injections prolong the longevity of degenerative knee joints? Clin. Interv. Aging 2015, 10, 1255–1258. [Google Scholar] [CrossRef]
- İşik, R.; Karapolat, H.; Bayram, K.B.; Uşan, H.; Tanıgör, G.; Atamaz Çalış, F. Effects of Short Wave Diathermy Added on Dextrose Prolotherapy Injections in Osteoarthritis of the Knee. J. Altern. Complement. Med. 2020, 26, 316–322. [Google Scholar] [CrossRef]
- Jhan, S.W.; Wang, C.J.; Wu, K.T.; Siu, K.K.; Ko, J.Y.; Huang, W.C.; Chou, W.Y.; Cheng, J.H. Comparison of Extracorporeal Shockwave Therapy with Non-Steroid Anti-Inflammatory Drugs and Intra-Articular Hyaluronic Acid Injection for Early Osteoarthritis of the Knees. Biomedicines 2022, 10, 202. [Google Scholar] [CrossRef]
- Karatosun, V.; Unver, B.; Gocen, Z.; Sen, A.; Gunal, I. Intra-articular hyaluranic acid compared with progressive knee exercises in osteoarthritis of the knee: A prospective randomized trial with long-term follow-up. Rheumatol. Int. 2006, 26, 277–284. [Google Scholar] [CrossRef]
- Kaszyński, J.; Bąkowski, P.; Kiedrowski, B.; Stołowski, Ł.; Wasilewska-Burczyk, A.; Grzywacz, K.; Piontek, T. Intra-Articular Injections of Autologous Adipose Tissue or Platelet-Rich Plasma Comparably Improve Clinical and Functional Outcomes in Patients with Knee Osteoarthritis. Biomedicines 2022, 10, 684. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kurosawa, H.; Ikeda, H.; Takazawa, Y.; Ishijima, M.; Kubota, M.; Kajihara, H.; Maruyama, Y.; Kim, S.G.; Kanazawa, H.; et al. Therapeutic home exercise versus intraarticular hyaluronate injection for osteoarthritis of the knee: 6-month prospective randomized open-labeled trial. J. Orthop. Sci. 2009, 14, 182–191. [Google Scholar] [CrossRef]
- Khalifeh Soltani, S.; Forogh, B.; Ahmadbeigi, N.; Hadizadeh Kharazi, H.; Fallahzadeh, K.; Kashani, L.; Karami, M.; Kheyrollah, Y.; Vasei, M. Safety and efficacy of allogenic placental mesenchymal stem cells for treating knee osteoarthritis: A pilot study. Cytotherapy 2019, 21, 54–63. [Google Scholar] [CrossRef]
- Kon, E.; Engebretsen, L.; Verdonk, P.; Nehrer, S.; Filardo, G. Clinical Outcomes of Knee Osteoarthritis Treated With an Autologous Protein Solution Injection: A 1-Year Pilot Double-Blinded Randomized Controlled Trial. Am. J. Sports Med. 2018, 46, 171–180. [Google Scholar] [CrossRef]
- Kon, E.; Engebretsen, L.; Verdonk, P.; Nehrer, S.; Filardo, G. Autologous Protein Solution Injections for the Treatment of Knee Osteoarthritis: 3-Year Results. Am. J. Sports Med. 2020, 48, 2703–2710. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, B.Y.; Shin, W.Y.; An, M.J.; Jung, K.I.; Yoon, S.R. Effect of Extracorporeal Shockwave Therapy Versus Intra-articular Injections of Hyaluronic Acid for the Treatment of Knee Osteoarthritis. Ann. Rehabil. Med. 2017, 41, 828–835. [Google Scholar] [CrossRef]
- Liu, Z.C.; Song, J.; Zhang, Q.L. Extracorporeal shock wave therapy versus intra-articular injection of sodium hyaluronate for knee osteoarthritis. Chin. J. Tissue Eng. Res. 2019, 23, 2297–2302. [Google Scholar] [CrossRef]
- Lucangeli, A.; Gugelmetto, M.; Primon, D. Physical therapy and intraarticular hyaluronic acid in the treatment of osteoarthritis. Riv. Ital. Biol. Med. 2001, 21, 5–10. [Google Scholar]
- McAlindon, T.E.; Schmidt, U.; Bugarin, D.; Abrams, S.; Geib, T.; DeGryse, R.E.; Kim, K.; Schnitzer, T.J. Efficacy and safety of single-dose onabotulinumtoxinA in the treatment of symptoms of osteoarthritis of the knee: Results of a placebo-controlled, double-blind study. Osteoarthr. Cartil. 2018, 26, 1291–1299. [Google Scholar] [CrossRef]
- Nishida, Y.; Kano, K.; Nobuoka, Y.; Seo, T. Sustained-release diclofenac conjugated to hyaluronate (diclofenac etalhyaluronate) for knee osteoarthritis: A randomized phase 2 study. Rheumatology 2021, 60, 1435–1444. [Google Scholar] [CrossRef]
- Paker, N.; Tekdos, D.; Kesiktas, N.; Soy, D. Comparison of the therapeutic efficacy of TENS versus intra-articular hyaluronic acid injection in patients with knee osteoarthritis: A prospective randomized study. Adv. Ther. 2006, 23, 342–353. [Google Scholar] [CrossRef]
- Paolucci, T.; Agostini, F.; Bernetti, A.; Paoloni, M.; Mangone, M.; Santilli, V.; Pezzi, L.; Bellomo, R.G.; Saggini, R. Integration of focal vibration and intra-articular oxygen-ozone therapy in rehabilitation of painful knee osteoarthritis. J. Int. Med. Res. 2021, 49, 300060520986705. [Google Scholar] [CrossRef]
- Parfitt, N.; Parfitt, D. The effects of exercise following a corticosteroid injection for knee osteoarthritis: A pilot study. J. Orthop. Med. 2006, 28, 80–84. [Google Scholar] [CrossRef]
- Paterson, K.L.; Nicholls, M.; Bennell, K.L.; Bates, D. Intra-articular injection of photo-activated platelet-rich plasma in patients with knee osteoarthritis: A double-blind, randomized controlled pilot study. BMC Musculoskelet. Disord. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Petrella, R.J.; DiSilvestro, M.D.; Hildebrand, C. Effects of hyaluronate sodium on pain and physical functioning in osteoarthritis of the knee: A randomized, double-blind, placebo-controlled clinical trial. Arch. Intern. Med. 2002, 162, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A.; Mohsin, S.N.; Siddiqui, U.N.; Naz, S.; Danish, S. Effectiveness of platelet rich plasma for the management of knee osteoarthritis: A randomized placebo controlled trial. Pak. J. Med. Health Sci. 2021, 15, 1553–1556. [Google Scholar] [CrossRef]
- Rabago, D.; Patterson, J.J.; Mundt, M.; Kijowski, R.; Grettie, J.; Segal, N.A.; Zgierska, A. Dextrose prolotherapy for knee osteoarthritis: A randomized controlled trial. Ann. Fam. Med. 2013, 11, 229–237. [Google Scholar] [CrossRef]
- Raeissadat, S.A.; Gharooee Ahangar, A.; Rayegani, S.M.; Minator Sajjadi, M.; Ebrahimpour, A.; Yavari, P. Platelet-Rich Plasma-Derived Growth Factor vs Hyaluronic Acid Injection in the Individuals with Knee Osteoarthritis: A One Year Randomized Clinical Trial. J. Pain Res. 2020, 13, 1699–1711. [Google Scholar] [CrossRef]
- Raeissadat, S.A.; Ghazi Hosseini, P.; Bahrami, M.H.; Salman Roghani, R.; Fathi, M.; Gharooee Ahangar, A.; Darvish, M. The comparison effects of intra-articular injection of Platelet Rich Plasma (PRP), Plasma Rich in Growth Factor (PRGF), Hyaluronic Acid (HA), and ozone in knee osteoarthritis; a one year randomized clinical trial. BMC Musculoskelet. Disord. 2021, 22, 134. [Google Scholar] [CrossRef]
- Raeissadat, S.A.; Ghorbani, E.; Sanei Taheri, M.; Soleimani, R.; Rayegani, S.M.; Babaee, M.; Payami, S. MRI Changes After Platelet Rich Plasma Injection in Knee Osteoarthritis (Randomized Clinical Trial). J. Pain Res. 2020, 13, 65–73. [Google Scholar] [CrossRef]
- Raeissadat, S.A.; Rayegani, S.M.; Forogh, B.; Abadi, P.H.; Moridnia, M.; Rahimi-Dehgolan, S. Intra-articular ozone or hyaluronic acid injection:Which one is superior in patients with knee osteoarthritis? A 6-month randomized clinical trial. J. Pain Res. 2018, 11, 111–117. [Google Scholar] [CrossRef]
- Raeissadat, S.A.; Rayegani, S.M.; Hassanabadi, H.; Fathi, M.; Ghorbani, E.; Babaee, M.; Azma, K. Knee osteoarthritis injection choices: Platelet-rich plasma (PRP) versus hyaluronic acid (A one-year randomized clinical trial). Clin. Med. Insights Arthritis Musculoskelet. Disord. 2015, 8, 1–8. [Google Scholar] [CrossRef]
- Rayegani, S.M.; Raeissadat, S.A.; Taheri, M.S.; Babaee, M.; Bahrami, M.H.; Eliaspour, D.; Ghorbani, E. Does intra articular platelet rich plasma injection improve function, pain and quality of life in patients with osteoarthritis of the knee? A randomized clinical trial. Orthop. Rev. 2014, 6, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Raynauld, J.P.; Torrance, G.W.; Band, P.A.; Goldsmith, C.H.; Tugwell, P.; Walker, V.; Schultz, M.; Bellamy, N. A prospective, randomized, pragmatic, health outcomes trial evaluating the incorporation of hylan G-F 20 into the treatment paradigm for patients with knee osteoarthritis (Part 1 of 2): Clinical results. Osteoarthr. Cartil. 2002, 10, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Rezasoltani, Z.; Azizi, S.; Najafi, S.; Sanati, E.; Dadarkhah, A.; Abdorrazaghi, F. Physical therapy, intra-articular dextrose prolotherapy, botulinum neurotoxin, and hyaluronic acid for knee osteoarthritis: Randomized clinical trial. Int. J. Rehabil. Res. 2020, 43, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Rezasoltani, Z.; Dadarkhah, A.; Tabatabaee, S.M.; Abdorrazaghi, F.; Kazempour Mofrad, M.; Kazempour Mofrad, R. Therapeutic Effects of Intra-articular Botulinum Neurotoxin Versus Physical Therapy in Knee Osteoarthritis. Anesth. Pain Med. 2021, 11, e112789. [Google Scholar] [CrossRef] [PubMed]
- Saccomanno, M.F.; Donati, F.; Careri, S.; Bartoli, M.; Severini, G.; Milano, G. Efficacy of intra-articular hyaluronic acid injections and exercise-based rehabilitation programme, administered as isolated or integrated therapeutic regimens for the treatment of knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1686–1694. [Google Scholar] [CrossRef]
- Sadat-Ali, M.; AlOmran, A.S.; AlMousa, S.A.; AlSayed, H.N.; AlTabash, K.W.; Azam, M.Q.; Hegazi, T.M.; Acharya, S. Autologous Bone Marrow-Derived Chondrocytes for Patients with Knee Osteoarthritis: A Randomized Controlled Trial. Adv. Orthop. 2021, 2021, 2146722. [Google Scholar] [CrossRef]
- Sert, A.T.; Sen, E.I.; Esmaeilzadeh, S.; Ozcan, E. The Effects of Dextrose Prolotherapy in Symptomatic Knee Osteoarthritis: A Randomized Controlled Study. J. Altern. Complement. Med. 2020, 26, 409–417. [Google Scholar] [CrossRef]
- Sezgin, M.; Demirel, A.C.; Karaca, C.; Ortancil, O.; Ulkar, G.B.; Kanik, A.; Cakçi, A. Does hyaluronan affect inflammatory cytokines in knee osteoarthritis? Rheumatol. Int. 2005, 25, 264–269. [Google Scholar] [CrossRef]
- Shrestha, R.; Shrestha, R.; Thapa, S.; Khadka, S.K.; Shrestha, D. Clinical Outcome following Intra-articular Triamcinolone Injection in Osteoarthritic Knee at the Community: A Randomized Double Blind Placebo Controlled Trial. Kathmandu Univ. Med. J. KUMJ 2018, 16, 175–180. [Google Scholar]
- Sit, R.W.S.; Wu, R.W.K.; Rabago, D.; Reeves, K.D.; Chan, D.C.C.; Yip, B.H.K.; Chung, V.C.H.; Wong, S.Y.S. Efficacy of Intra-Articular Hypertonic Dextrose (Prolotherapy) for Knee Osteoarthritis: A Randomized Controlled Trial. Ann. Fam. Med. 2020, 18, 235–242. [Google Scholar] [CrossRef]
- Soliman, D.M.I.; Sherif, N.M.; Omar, O.H.; El Zohiery, A.K. Healing effects of prolotherapy in treatment of knee osteoarthritis healing effects of prolotherapy in treatment of knee osteoarthritis. Egypt. Rheumatol. Rehabil. 2016, 43, 47–52. [Google Scholar] [CrossRef]
- Su, W.; Lin, Y.; Wang, G.; Geng, Z.; Wang, Z.; Hou, D.; Suo, B. Prospective clinical study on extracorporeal shock wave therapy combined with platelet-rich plasma injection for knee osteoarthritis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2019, 33, 1527–1531. [Google Scholar] [CrossRef]
- Subazwari, S.A.B.; Qayyum, S.; Ahmad, Z.; Ishfaq, N.; Khalid, A.; Awais, L.; Anwar, I. Level of Pain and Physical Function of Patients with Knee Osteoarthritis Receiving Physiotherapy with and without Intra-Articular Injection in Pakistan: A Qusai Experimental Study. Health Sci. J. 2020, 14, 1–5. [Google Scholar] [CrossRef]
- Taftain, E.; Azizi, S.; Dadarkhah, A.; Maghbouli, N.; Najafi, S.; Soltani, Z.R.; Khavandegar, A. A Single-blind Randomised Trial of Intra-Articular Hyaluronic Acid, Hypertonic Saline, and Physiotherapy in Knee Osteoarthritis. Muscles Ligaments Tendons J. 2021, 11, 416–426. [Google Scholar] [CrossRef]
- Tucker, J.D.; Goetz, L.L.; Duncan, M.B.; Gilman, J.B.; Elmore, L.W.; Sell, S.A.; McClure, M.J.; Quagliano, P.V.; Martin, C.C. Randomized, Placebo-Controlled Analysis of the Knee Synovial Environment Following Platelet-Rich Plasma Treatment for Knee Osteoarthritis. PMR 2021, 13, 707–719. [Google Scholar] [CrossRef]
- Uslu Güvendi, E.; Aşkin, A.; Güvendi, G.; Koçyiğit, H. Comparison of Efficiency Between Corticosteroid and Platelet Rich Plasma Injection Therapies in Patients With Knee Osteoarthritis. Arch. Rheumatol. 2018, 33, 273–281. [Google Scholar] [CrossRef]
- Chang, K.V.; Hung, C.Y.; Aliwarga, F.; Wang, T.G.; Han, D.S.; Chen, W.S. Comparative effectiveness of platelet-rich plasma injections for treating knee joint cartilage degenerative pathology: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2014, 95, 562–575. [Google Scholar] [CrossRef]
- Tan, J.; Chen, H.; Zhao, L.; Huang, W. Platelet-Rich Plasma Versus Hyaluronic Acid in the Treatment of Knee Osteoarthritis: A Meta-analysis of 26 Randomized Controlled Trials. Arthroscopy 2021, 37, 309–325. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef]
- Burdakov, D.; Jensen, L.T.; Alexopoulos, H.; Williams, R.H.; Fearon, I.M.; O’Kelly, I.; Gerasimenko, O.; Fugger, L.; Verkhratsky, A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron 2006, 50, 711–722. [Google Scholar] [CrossRef]
- Hassan, F.; Trebinjac, S.; Murrell, W.D.; Maffulli, N. The effectiveness of prolotherapy in treating knee osteoarthritis in adults: A systematic review. Br. Med. Bull. 2017, 122, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.T.; Rabago, D.P.; Best, T.M.; Patterson, J.J.; Vanderby, R., Jr. Early inflammatory response of knee ligaments to prolotherapy in a rat model. J. Orthop. Res. 2008, 26, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.T.; Rabago, D.P.; Best, T.M.; Patterson, J.J.; Vanderby, R., Jr. Response of knee ligaments to prolotherapy in a rat injury model. Am. J. Sports Med. 2008, 36, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Manjoo, A.; Fierlinger, A.; Niazi, F.; Nicholls, M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: A systematic review. BMC Musculoskelet. Disord. 2015, 16, 321. [Google Scholar] [CrossRef]
- Wang, L.T.; Ting, C.H.; Yen, M.L.; Liu, K.J.; Sytwu, H.K.; Wu, K.K.; Yen, B.L. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: Review of current clinical trials. J. Biomed. Sci. 2016, 23, 76. [Google Scholar] [CrossRef]
- ter Huurne, M.; Schelbergen, R.; Blattes, R.; Blom, A.; de Munter, W.; Grevers, L.C.; Jeanson, J.; Noel, D.; Casteilla, L.; Jorgensen, C.; et al. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012, 64, 3604–3613. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, C.; Tuan, R.S. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res. Ther. 2014, 16, 204. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Lane, N.E.; Brandt, K.; Hawker, G.; Peeva, E.; Schreyer, E.; Tsuji, W.; Hochberg, M.C. OARSI-FDA initiative: Defining the disease state of osteoarthritis. Osteoarthr. Cartil. 2011, 19, 478–482. [Google Scholar] [CrossRef]
- Liu, C.J.; Latham, N.K. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst. Rev. 2009, 2009, CD002759. [Google Scholar] [CrossRef]
- Lange, A.K.; Vanwanseele, B.; Fiatarone Singh, M.A. Strength training for treatment of osteoarthritis of the knee: A systematic review. Arthritis Rheum. 2008, 59, 1488–1494. [Google Scholar] [CrossRef]
- Shull, P.B.; Jirattigalachote, W.; Hunt, M.A.; Cutkosky, M.R.; Delp, S.L. Quantified self and human movement: A review on the clinical impact of wearable sensing and feedback for gait analysis and intervention. Gait Posture 2014, 40, 11–19. [Google Scholar] [CrossRef]
- Cheung, R.T.H.; Ho, K.K.W.; Au, I.P.H.; An, W.W.; Zhang, J.H.W.; Chan, Z.Y.S.; Deluzio, K.; Rainbow, M.J. Immediate and short-term effects of gait retraining on the knee joint moments and symptoms in patients with early tibiofemoral joint osteoarthritis: A randomized controlled trial. Osteoarthr. Cartil. 2018, 26, 1479–1486. [Google Scholar] [CrossRef]
- Baki, N.M.A.; Nawito, Z.O.; Abdelsalam, N.M.S.; Sabry, D.; Elashmawy, H.; Seleem, N.A.; Taha, A.A.A.; El Ghobashy, M. Does Intra-Articular Injection of Platelet-Rich Plasma Have an Effect on Cartilage Thickness in Patients with Primary Knee Osteoarthritis? Curr. Rheumatol. Rev. 2021, 17, 294–302. [Google Scholar] [CrossRef]
- Hegaze, A.H.; Hamdi, A.S.; Alqrache, A.; Hegazy, M. Efficacy of Platelet-Rich Plasma on Pain and Function in the Treatment of Knee Osteoarthritis: A Prospective Cohort Study. Cureus 2021, 13, e13909. [Google Scholar] [CrossRef]
- Kon, E.; Mandelbaum, B.; Buda, R.; Filardo, G.; Delcogliano, M.; Timoncini, A.; Fornasari, P.M.; Giannini, S.; Marcacci, M. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: From early degeneration to osteoarthritis. Arthroscopy 2011, 27, 1490–1501. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Di Martino, A.; Di Matteo, B.; Merli, M.L.; Cenacchi, A.; Fornasari, P.M.; Marcacci, M. Platelet-rich plasma vs. hyaluronic acid to treat knee degenerative pathology: Study design and preliminary results of a randomized controlled trial. BMC Musculoskelet. Disord. 2012, 13, 229. [Google Scholar] [CrossRef]
- Cerza, F.; Carnì, S.; Carcangiu, A.; Di Vavo, I.; Schiavilla, V.; Pecora, A.; De Biasi, G.; Ciuffreda, M. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am. J. Sports Med. 2012, 40, 2822–2827. [Google Scholar] [CrossRef]
- Gobbi, A.; Dallo, I.; Rogers, C.; Striano, R.D.; Mautner, K.; Bowers, R.; Rozak, M.; Bilbool, N.; Murrell, W.D. Two-year clinical outcomes of autologous microfragmented adipose tissue in elderly patients with knee osteoarthritis: A multi-centric, international study. Int. Orthop. 2021, 45, 1179–1188. [Google Scholar] [CrossRef]
- Sucuoglu, H.; Ustunsoy, S. The short-term effect of PRP on chronic pain in knee osteoarthritis. Agri Agri (Algoloji) Dern. Yayin Organidir = J. Turk. Soc. Algol. 2019, 31, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Wang, H.; Li, G.; Yao, P.; Ding, Y. Systematic Review and Meta-Analysis of 12 Randomized Controlled Trials Evaluating the Efficacy of Invasive Radiofrequency Treatment for Knee Pain and Function. BioMed Res. Int. 2019, 2019, 9037510. [Google Scholar] [CrossRef] [PubMed]
- Witt, C.M.; Vertosick, E.A.; Foster, N.E.; Lewith, G.; Linde, K.; MacPherson, H.; Sherman, K.J.; Vickers, A.J. The Effect of Patient Characteristics on Acupuncture Treatment Outcomes: An Individual Patient Data Meta-Analysis of 20,827 Chronic Pain Patients in Randomized Controlled Trials. Clin. J. Pain 2019, 35, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.S.; Callahan, D.M.; Tourville, T.W.; Slauterbeck, J.R.; Kaplan, A.; Fiske, B.R.; Savage, P.D.; Ades, P.A.; Beynnon, B.D.; Toth, M.J. Moderate-intensity resistance exercise alters skeletal muscle molecular and cellular structure and function in inactive older adults with knee osteoarthritis. J. Appl. Physiol. 2017, 122, 775–787. [Google Scholar] [CrossRef]
- Deveza, L.A.; Melo, L.; Yamato, T.P.; Mills, K.; Ravi, V.; Hunter, D.J. Knee osteoarthritis phenotypes and their relevance for outcomes: A systematic review. Osteoarthr. Cartil. 2017, 25, 1926–1941. [Google Scholar] [CrossRef]
- Tschon, M.; Contartese, D.; Pagani, S.; Borsari, V.; Fini, M. Gender and Sex Are Key Determinants in Osteoarthritis Not Only Confounding Variables. A Systematic Review of Clinical Data. J. Clin. Med. 2021, 10, 3178. [Google Scholar] [CrossRef]
- Jones, M.D.; Wewege, M.A.; Hackett, D.A.; Keogh, J.W.L.; Hagstrom, A.D. Sex Differences in Adaptations in Muscle Strength and Size Following Resistance Training in Older Adults: A Systematic Review and Meta-analysis. Sports Med. 2021, 51, 503–517. [Google Scholar] [CrossRef]
- Da Boit, M.; Sibson, R.; Meakin, J.R.; Aspden, R.M.; Thies, F.; Mangoni, A.A.; Gray, S.R. Sex differences in the response to resistance exercise training in older people. Physiol. Rep. 2016, 4, e12834. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef]
- Georgiev, T. Multimodal approach to intraarticular drug delivery in knee osteoarthritis. Rheumatol. Int. 2020, 40, 1763–1769. [Google Scholar] [CrossRef]
- Roemer, F.W.; Jarraya, M.; Collins, J.E.; Kwoh, C.K.; Hayashi, D.; Hunter, D.J.; Guermazi, A. Structural phenotypes of knee osteoarthritis: Potential clinical and research relevance. Skelet. Radiol. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- van der Esch, M.; Knoop, J.; van der Leeden, M.; Roorda, L.D.; Lems, W.F.; Knol, D.L.; Dekker, J. Clinical phenotypes in patients with knee osteoarthritis: A study in the Amsterdam osteoarthritis cohort. Osteoarthr. Cartil. 2015, 23, 544–549. [Google Scholar] [CrossRef]
- Dell’Isola, A.; Steultjens, M. Classification of patients with knee osteoarthritis in clinical phenotypes: Data from the osteoarthritis initiative. PLoS ONE 2018, 13, e0191045. [Google Scholar] [CrossRef]
- Mills, K.; Hübscher, M.; O’Leary, H.; Moloney, N. Current concepts in joint pain in knee osteoarthritis. Schmerz 2019, 33, 22–29. [Google Scholar] [CrossRef]
- Veronese, N.; Cooper, C.; Bruyère, O.; Al-Daghri, N.M.; Branco, J.; Cavalier, E.; Cheleschi, S.; da Silva Rosa, M.C.; Conaghan, P.G.; Dennison, E.M.; et al. Multimodal Multidisciplinary Management of Patients with Moderate to Severe Pain in Knee Osteoarthritis: A Need to Meet Patient Expectations. Drugs 2022, 82, 1347–1355. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software. Veritas Health Innovation 2022, Melbourne, Australia. Available online: www.covidence.org. (accessed on 15 March 2023).
- McKeown, S.; Mir, Z.M. Considerations for conducting systematic reviews: Evaluating the performance of different methods for de-duplicating references. Syst. Rev. 2021, 10, 38. [Google Scholar] [CrossRef]
- Chamorro-Moriana, G.; Perez-Cabezas, V.; Espuny-Ruiz, F.; Torres-Enamorado, D.; Ridao-Fernández, C. Assessing knee functionality: Systematic review of validated outcome measures. Ann. Phys. Rehabil. Med. 2022, 65, 101608. [Google Scholar] [CrossRef]
- Zanker, J.; Sim, M.; Anderson, K.; Balogun, S.; Brennan-Olsen, S.L.; Dent, E.; Duque, G.; Girgis, C.M.; Grossmann, M.; Hayes, A.; et al. Consensus guidelines for sarcopenia prevention, diagnosis and management in Australia and New Zealand. J. Cachexia Sarcopenia Muscle 2022, 14, 142–156. [Google Scholar] [CrossRef]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef]
- de Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration: 2011. Available online: www.cochrane-handbook.org (accessed on 15 March 2023).
- Higgins, J.P.T.; Li, T.; Deeks, J.J. (Eds.) Chapter 6: Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022); Cochrane: London, UK, 2022. [Google Scholar]
- Rosenthal, R. Meta-Analytic Procedures for Social Research; Sage Publications: Newbury Park, CA, USA, 1993. [Google Scholar]
- Chaimani, A.; Caldwell, D.M.; Li, T.; Higgins, J.P.T.S.G. (Eds.) Chapter 11: Undertaking network meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022); Cochrane: London, UK, 2022. [Google Scholar]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. (Eds.) Chapter 12: Network Meta-Analysis. In Doing Meta-Analysis with R: A Hands-On Guide; Chapman & Hall: London, UK; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.R.; Kim, S.-J.; Lee, J.; Rücker, G. Network meta-analysis: Application and practice using R software. Epidemiol. Health 2019, 41, e2019010–e2019013. [Google Scholar] [CrossRef]
- Brignardello-Petersen, R.; Bonner, A.; Alexander, P.E.; Siemieniuk, R.A.; Furukawa, T.A.; Rochwerg, B.; Hazlewood, G.S.; Alhazzani, W.; Mustafa, R.A.; Murad, M.H.; et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J. Clin. Epidemiol. 2018, 93, 36–44. [Google Scholar] [CrossRef]


| Injection Alone | Physical Therapy Alone | Combined Treatment | Usual Care | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trials (Groups), n a | Sample (n) | Mean (Range) a | Trials (Groups), n a | Sample (n) | Mean (Range)a | Trials (Groups), n a | Sample (n) | Mean (Range) a | Trials (Groups), n a | Sample (n) | Mean (Range) a | |
| All included trials | 23 (26) | 748 | 59 (62) | 2438 | 65 (91) | 3577 | 7 (7) | 171 | ||||
| Age, year b | 22 (25) | 738 | 60.3 (51–78) | 58 (61) | 2417 | 60.5 (42–75) | 65 (91) | 3566 | 59.4 (42–76) | 7 (7) | 171 | 57.9 (49–65) |
| BMI, kg/m2 b | 10 (14) | 490 | 28.1 (24.5–32.6) | 40 (42) | 1847 | 29.9 (24.4–34.3) | 48 (70) | 2864 | 29.2 (23.7–34.3) | 1 (1) | 28 | 32.7 |
| Gender, n (%) | ||||||||||||
| Male | 17 (19) | 180 | 28% (10–55%) | 44 (46) | 644 | 32% (3–81%) | 52 (80) | 1166 | 36% (3–87%) | 7 (7) | 70 | 38% (7–70%) |
| Female | 18 (20) | 468 | 73% (45–100%) | 46 (48) | 1402 | 70% (19–100%) | 53 (81) | 2123 | 65% (13–100%) | 7 (7) | 101 | 62% (40–93%) |
| Population area, n | ||||||||||||
| America | 3 (3) | 134 | 15 (15) | 880 | 15 (19) | 995 | 2 (2) | 57 | ||||
| Europe | 8 (9) | 184 | 6 (6) | 234 | 14 (18) | 714 | 2 (2) | 44 | ||||
| Asia | 11 (12) | 399 | 32 (35) | 1191 | 32 (47) | 1708 | 2 (2) | 50 | ||||
| Africa | 2 (3) | 60 | 4 (4) | 94 | 2 (3) | 119 | 1 (1) | 20 | ||||
| Oceania | 0 | 1 (1) | 10 | 2 (4) | 41 | 0 | ||||||
| Disease duration, month b | 11 (13) | 420 | 56 (12–85) | 26 (28) | 1158 | 76 (13–306) | 26 (39) | 1659 | 70 (10–307) | 2 (2) | 64 | 91 (5–144) |
| KL grade, n (%) | ||||||||||||
| ≤2 | 14 (15) | 294 | 51.3% (0–100%) | 46 (48) | 949 | 47.5% (0–100%) | 61 (73) | 1590 | 50.3% (0–100%) | 4 (4) | 51 | 69.2% (48–100%) |
| ≥3 | 14 (15) | 239 | 45.5% (0–100%) | 46 (48) | 1150 | 49.9% (0–100%) | 53 (73) | 1698 | 52.3% (0–100%) | 4 (4) | 63 | 55.7% (29–100%) |
| Intervention regimen, n (compliance, %) b | ||||||||||||
| Injection therapy | 22 (25) | 738 | 0 | 66 (92) | 3587 | 0 | ||||||
| CS | 5 (5) | 138 | 12 (12) | 377 | ||||||||
| BoNTA | 1 (1) | 25 | 3 (4) | 137 | ||||||||
| HA | 9 (11) | 316 | 25 (25) | 1462 | ||||||||
| OZ | 2 (2) | 44 | 9 (9) | 332 | ||||||||
| DxTP | 2 (2) | 62 | 8 (9) | 292 | ||||||||
| Autologous biotics c | 5 (5) | 163 | 26 (32) | 977 | ||||||||
| Physical therapy | 0 | 59 (62) | 2438 | 66 (92) | 3587 | 0 | ||||||
| Exercise | 28 (30) | 813 | 39 (60) | 1890 | ||||||||
| Physical agent modality | 14 (14) | 425 | 7 (7) | 241 | ||||||||
| Physical activity | 3 (3) | 54 | 7 (10) | 315 | ||||||||
| Clinical characteristics (baseline) b | ||||||||||||
| Pain status | ||||||||||||
| VAS (0–100) | 14 (16) | 461 | 65.8 (28.0–85.2) | 44 (44) | 1757 | 61.4 (30.0–93.5) | 46 (64) | 2464 | 66.8 (32.9–97.1) | 6 (6) | 142 | 54.3 (33.0–83.8) |
| WOMAC–pain (0–20) | 9 (10) | 293 | 9.4 (4.8–13.9) | 28 (29) | 1036 | 9.8 (3.6–17.3) | 35 (47) | 1873 | 9.0 (3.0–18.9) | 4 (4) | 92 | 11.1 (7.5–13.3) |
| Global function | ||||||||||||
| WOMAC–PF (0–68) | 8 (9) | 261 | 45.7 (4.8–13.9) | 30 (31) | 1067 | 39.0 (12.3–70.7) | 36 (49) | 1823 | 36.0 (17.7–79.9) | 4 (4) | 92 | 37.9 (33.4–45.3) |
| KOOS–PF (0–100) | 3 (3) | 75 | 40.7 (34.4–48.3) | 10 (10) | 370 | 52.8 (34.7–77.0) | 13 (19) | 669 | 56.8 (33.7–75.4) | 2 (2) | 43 | 46.3 (44.0–48.6) |
| Walking speed, m/s | 1 (2) | 40 | 0.54 (0.51–0.56) | 9 (10) | 330 | 0.87 (0.59–1.37) | 13 (18) | 455 | 0.93 (0.69–1.89) | 0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.-D.; Chen, H.-C.; Huang, M.-H.; Liou, T.-H.; Lin, C.-L.; Huang, S.-W. Comparative Efficacy of Intra-Articular Injection, Physical Therapy, and Combined Treatments on Pain, Function, and Sarcopenia Indices in Knee Osteoarthritis: A Network Meta-Analysis of Randomized Controlled Trials. Int. J. Mol. Sci. 2023, 24, 6078. https://doi.org/10.3390/ijms24076078
Liao C-D, Chen H-C, Huang M-H, Liou T-H, Lin C-L, Huang S-W. Comparative Efficacy of Intra-Articular Injection, Physical Therapy, and Combined Treatments on Pain, Function, and Sarcopenia Indices in Knee Osteoarthritis: A Network Meta-Analysis of Randomized Controlled Trials. International Journal of Molecular Sciences. 2023; 24(7):6078. https://doi.org/10.3390/ijms24076078
Chicago/Turabian StyleLiao, Chun-De, Hung-Chou Chen, Mao-Hua Huang, Tsan-Hon Liou, Che-Li Lin, and Shih-Wei Huang. 2023. "Comparative Efficacy of Intra-Articular Injection, Physical Therapy, and Combined Treatments on Pain, Function, and Sarcopenia Indices in Knee Osteoarthritis: A Network Meta-Analysis of Randomized Controlled Trials" International Journal of Molecular Sciences 24, no. 7: 6078. https://doi.org/10.3390/ijms24076078
APA StyleLiao, C.-D., Chen, H.-C., Huang, M.-H., Liou, T.-H., Lin, C.-L., & Huang, S.-W. (2023). Comparative Efficacy of Intra-Articular Injection, Physical Therapy, and Combined Treatments on Pain, Function, and Sarcopenia Indices in Knee Osteoarthritis: A Network Meta-Analysis of Randomized Controlled Trials. International Journal of Molecular Sciences, 24(7), 6078. https://doi.org/10.3390/ijms24076078






