Inhibitory Effects of Thermolysis Transformation Products of Rotenone on Nitric Oxide Production
Abstract
:1. Introduction
2. Results and Discussion
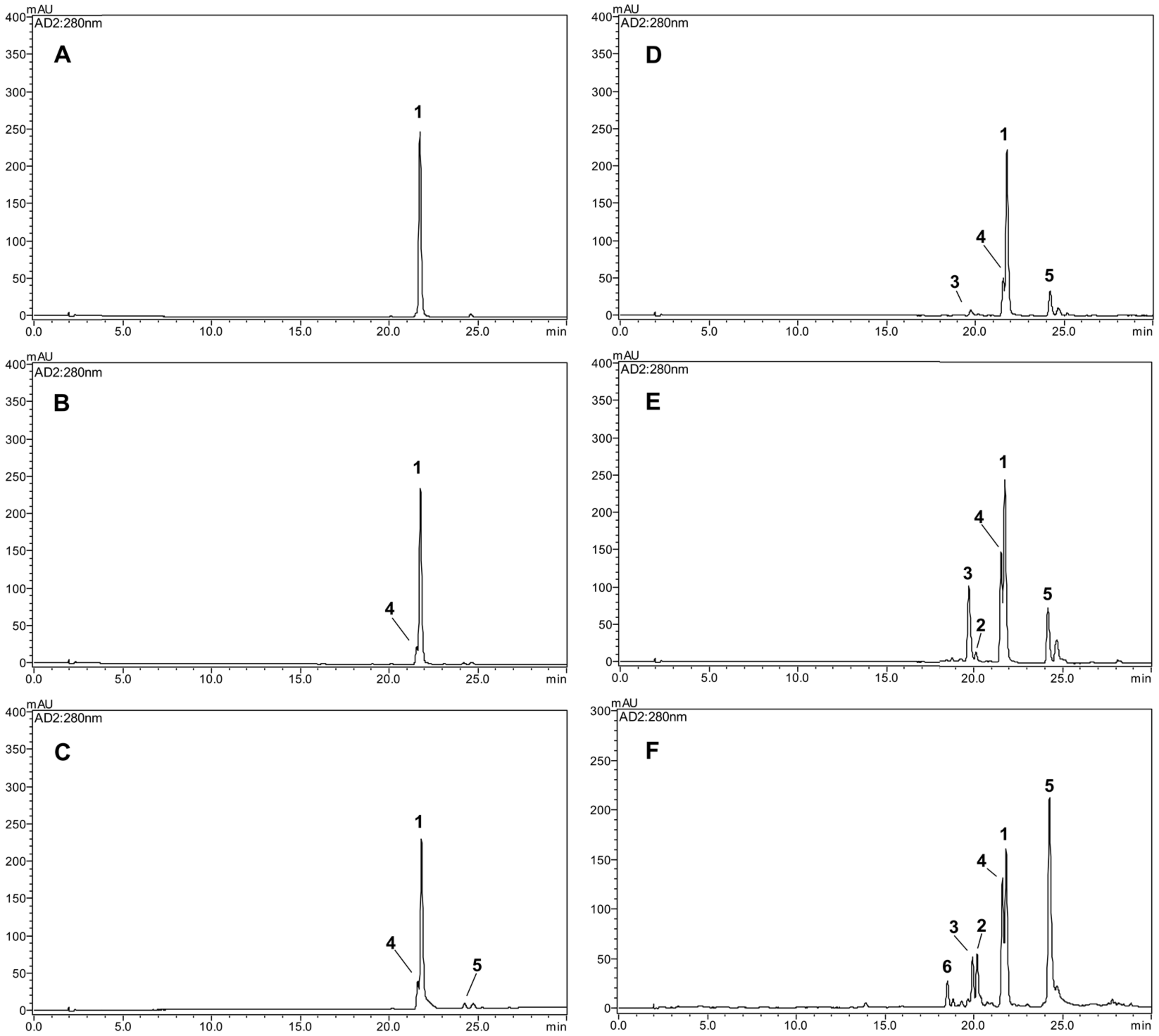
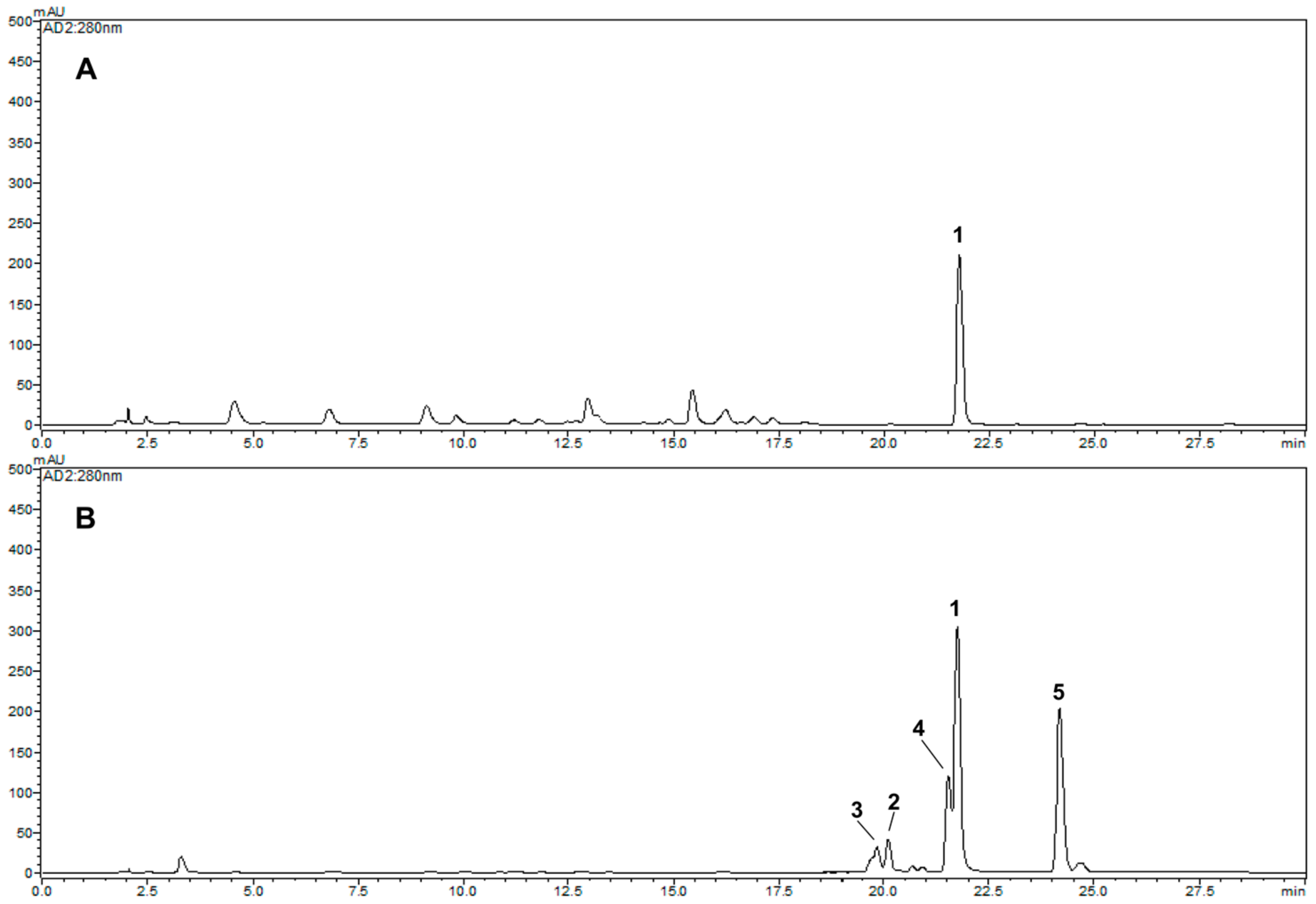
2.1. Characterization of Newly Formed Products
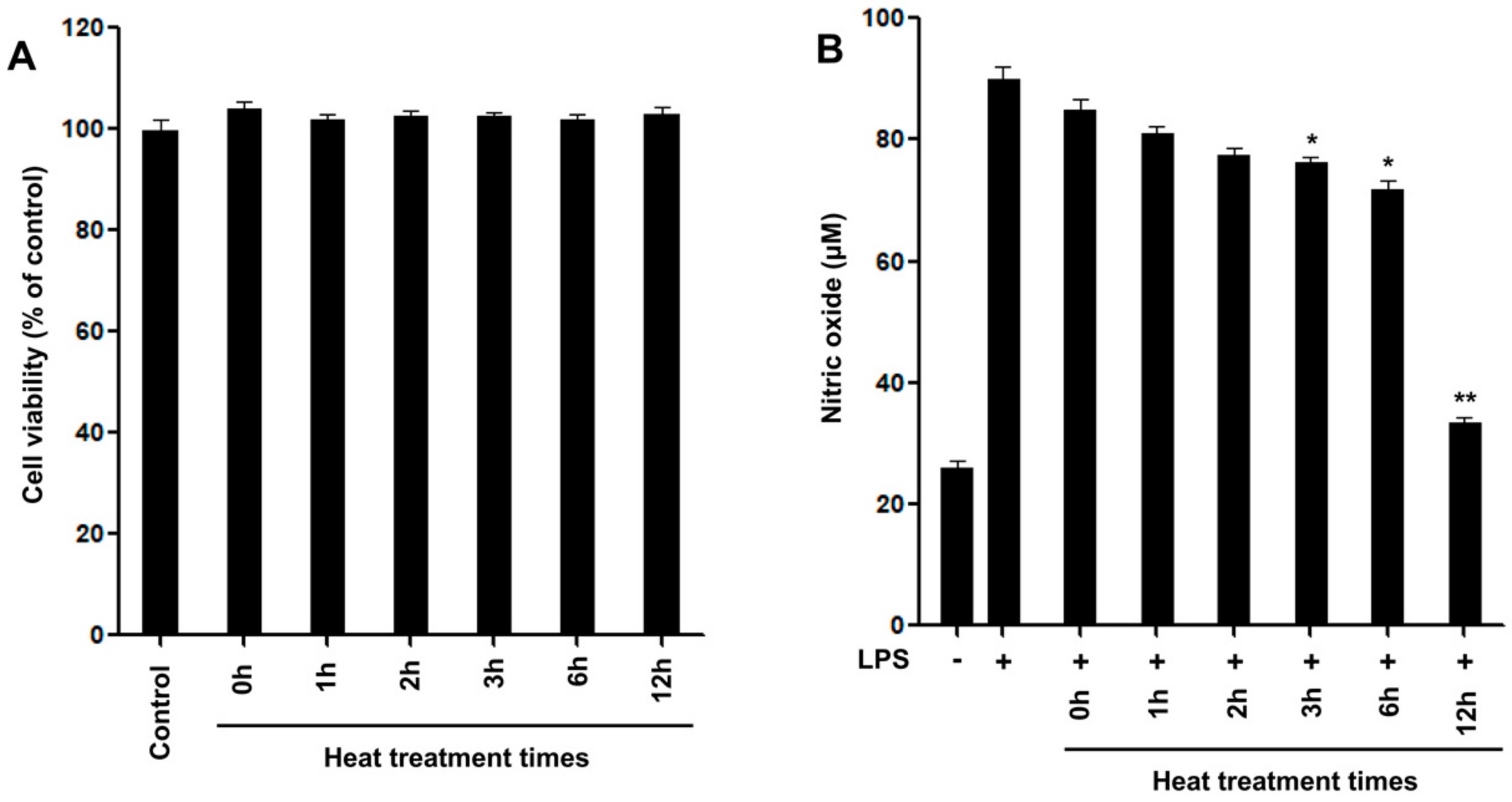
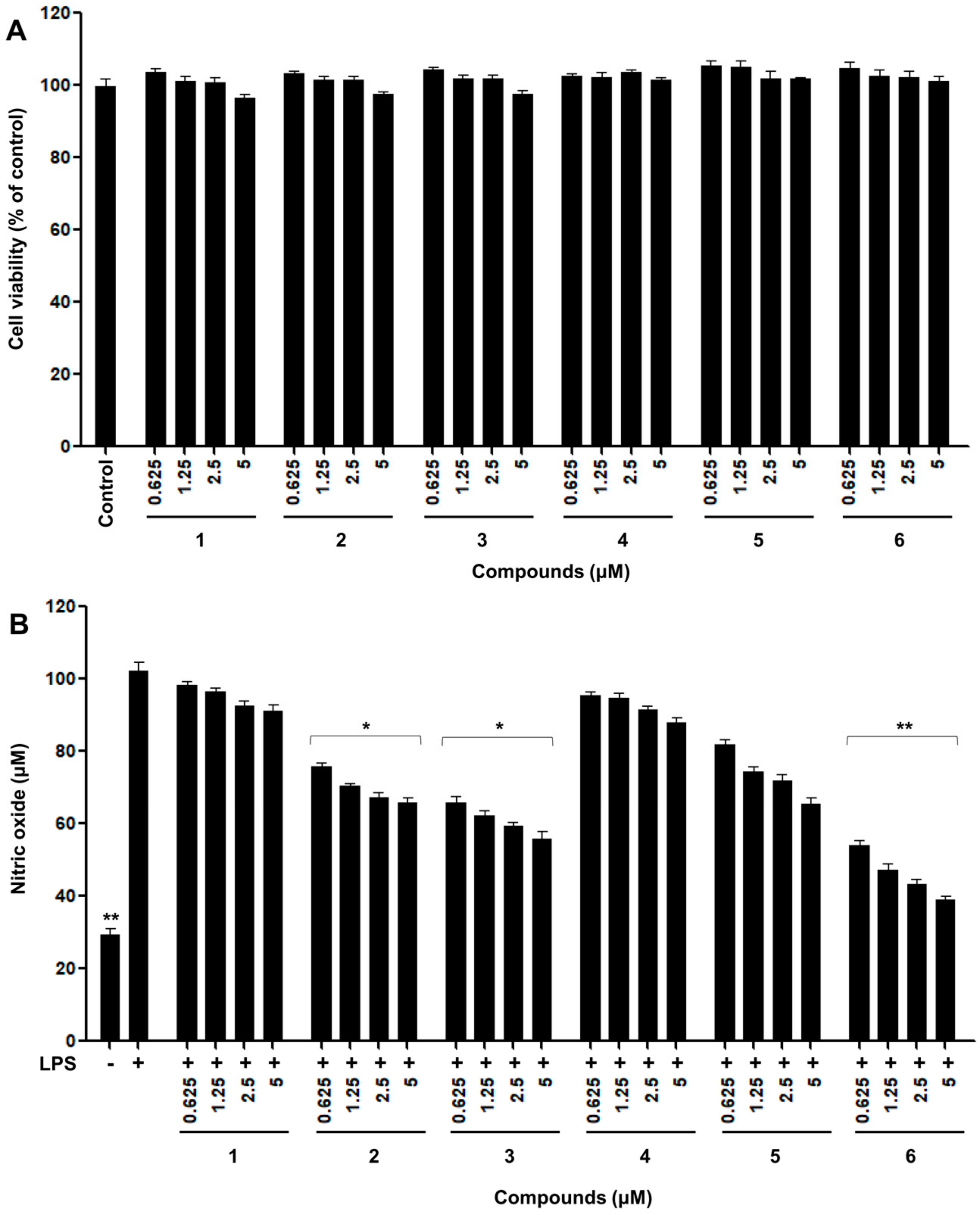
2.2. Inhibitory Effects of Nitric Oxide Production
2.3. Comparative HPLC Analysis of the Major Products Formed from Thermolysis Rotenone
2.4. Comparative HPLC Analysis of the Major Products Formed from Thermolysis Rotenone
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Preparation of Thermolysis Samples
3.3. Isolation of Degraded Compounds
3.4. Cell Culture
3.5. Cell Viability Assay
3.6. Nitric Oxide Production
3.7. Extraction of Derris Root and LC-MS Analysis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Orr, C.; Rowe, D.; Halliday, G. An inflammatory review of Parkinson’s disease. Prog. Neurobiol. 2002, 68, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Al-Omran, A.; Parvathy, S. Role of nitric oxide in inflammatory diseases. Inflammopharm 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Attur, M.; Patel, R.; Thakker, G.; Vyas, P.; Levartovsky, D.; Patel, P.; Naqvi, S.; Raza, R.; Patel, K.; Abramson, D. Differential anti-inflammatory effects of immunosuppressive drugs: Cyclosporin, rapamycin and FK-506 on inducible nitric oxide synthase, nitric oxide, cyclooxygenase-2 and PGE2 production. Inflamm. Res. 2000, 49, 20–26. [Google Scholar] [CrossRef]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef] [Green Version]
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef]
- Sherer, T.B.; Betarbet, R.; Testa, C.M.; Seo, B.B.; Richardson, J.R.; Kim, J.H.; Miller, G.W.; Yagi, T.; Matsuno-Yagi, A.; Greenamyre, J.T. Mechanism of toxicity in rotenone models of Parkinson’s disease. J. Neurosci. 2003, 23, 10756–10764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.T.; Huang, H.C.; Lin, J.K. Rotenone induces apoptosis in MCF-7 human breast cancer cell-mediated ROS through JNK and p38 signaling. Mol. Carcinog. 2010, 49, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Upegui, Y.; Gil, J.F.; Quiñones, W.; Torres, F.; Escobar, G.; Robledo, S.M.; Echeverri, F. Preparation of rotenone derivatives and in vitro analysis of their antimalarial, antileishmanial and selective cytotoxic activities. Molecules 2014, 19, 18911–18922. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Chung, B.Y.; Lee, S.S.; Bai, H.W.; Cho, J.Y.; Jo, C.; Kim, T.H. Radiolytic transformation of rotenone with potential anti-adipogenic activity. Bioorg. Med. Chem. Lett. 2013, 23, 1099–1103. [Google Scholar] [CrossRef]
- Badaboina, S.; Bai, H.W.; Na, Y.H.; Park, C.H.; Kim, T.H.; Lee, T.H.; Chung, B.Y. Novel radiolytic rotenone derivative, rotenoisin B with potent anti-carcinogenic activity in hepatic cancer cells. Int. J. Mol. Sci. 2015, 16, 16806–16815. [Google Scholar] [CrossRef] [Green Version]
- Bak, D.H.; Kang, S.H.; Park, C.H.; Chung, B.Y.; Bai, H.W. A novel radiolytic rotenone derivative, rotenoisin A, displays potent anticarcinogenic activity in breast cancer cells. J. Radiat. Res. 2021, 62, 249–258. [Google Scholar] [CrossRef]
- Cho, H.H.; Park, H.S.; Jang, S.H.; Won, C.; Kim, H.D.; Kim, T.H.; Cho, J.H. Rotenoisin A is a novel anti-adipogenic compound. Bioorg. Med. Chem. Lett. 2019, 29, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Sariaslani, F.S.; Rosazza, J. Microbial transformations of natural antitumor agents: Products of rotenone and dihydrorotenone transformation by Cunninghamella blakesleeana. Appl. Environ. Microb. 1983, 45, 616–621. [Google Scholar] [CrossRef] [Green Version]
- Cavoski, I.; Caboni, P.; Sarais, G.; Miano, T. Degradation and persistence of rotenone in soils and influence of temperature variations. J. Agric. Food Chem. 2008, 56, 8066–8073. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Miyoshi, H.; Ebisui, K.; Iwamura, H. Comparison of the inhibitory action of natural rotenone and its stereoisomers with various NADH−ubiquinone reductases. Eur. J. Biochem. 1994, 225, 411–417. [Google Scholar] [CrossRef]
- Krupadanam, G.D.; Sarma, P.; Srimannarayana, G.; Rao, N.S. New C-6 oxygenated rotenoids from Tephrosia villosa-villosin, villosone, villol and villinol. Tetrahedron Lett. 1977, 18, 2125–2128. [Google Scholar] [CrossRef]
- Oberholzer, M.; Rall, G.; Roux, D. The concurrence of 12a-hydroxy-and 12a-O-methylrotenoids. Isolation of the first natural 12a-O-methylrotenoids. Tetrahedron Lett. 1974, 15, 2211–2214. [Google Scholar] [CrossRef]
- Fang, N.; Casida, J.E. Novel bioactive cube insecticide constituents: Isolation and preparation of 13-homo-13-oxa-6a, 12a-dehydrorotenoids. J. Org. Chem. 1997, 62, 350–353. [Google Scholar] [CrossRef]
- Obara, Y.; Matsubara, H.; Munakata, K. Isolation and identification of tubaic acid and β-tubaic acid from Derris roots. Agric. Biol. Chem. 1976, 40, 1245–1246. [Google Scholar]
- Parmar, V.; Jain, R.; Gupta, S.; Boll, P.; Mikkelsen, J. Phytochemical investigation of Tephrosia candida: HPLC separation of tephrosin and 12a-hydroxyrotenone. J. Nat. Prod. 1988, 51, 185. [Google Scholar] [CrossRef]
- Dagne, E.; Yenesew, A.; Waterman, P.G. Flavonoids and isoflavonoids from Tephrosia fulvinervis and Tephrosia pentaphylla. Phytochemistry 1989, 28, 3207–3210. [Google Scholar] [CrossRef]
- Haller, H.; LaForge, F. Rotenone. VII. The structure of tubanol and tubaic acid. J. Am. Chem. Soc. 1930, 52, 3207–3212. [Google Scholar] [CrossRef]
- Kim, T.; Choi, H.J.; Eom, S.H.; Lee, J.; Kim, T.H. Potential α-glucosidase inhibitors from thermal transformation of (+)-catechin. Bioorg. Med. Chem. Lett. 2014, 24, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, H.; Okuda, K.; Kunihira, Y.; Inada, A.; Miyagi, C.; Matsuo, Y.; Saito, Y.; Tanaka, T. Oligomerization mechanism of tea catechins during tea roasting. Food Chem. 2019, 285, 252–259. [Google Scholar] [CrossRef]
- Lai, N.Y.; Mills, K.; Chiu, I.M. Sensory neuron regulation of gastrointestinal inflammation and bacterial host defence. J. Int. Med. 2017, 282, 5–23. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases. Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef] [Green Version]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Escribano-Ferrer, E.; Queralt Regue, J.; Garcia-Sala, X.; Boix Montanes, A.; Lamuela-Raventos, R.M. In vivo anti-inflammatory and antiallergic activity of pure naringenin, naringenin chalcone, and quercetin in mice. J. Nat. Prod. 2019, 82, 177–182. [Google Scholar] [CrossRef]
- Rao, M. Nitric oxide scavenging by curcuminoids. J. Pharm. Pharmacol. 1997, 49, 105–107. [Google Scholar]
- Li, J.; Yuan, C.; Pan, L.; Benatrehina, P.A.; Chai, H.; Keller, W.J.; Naman, C.B.; Kinghorn, A.D. Bioassay-guided isolation of antioxidant and cytoprotective constituents from a maqui berry (Aristotelia chilensis) dietary supplement ingredient as markers for qualitative and quantitative analysis. J. Agric. Food Chem. 2017, 65, 8634–8642. [Google Scholar] [CrossRef]
- Zhang, H.X.; Wang, Z.Z.; Du, Z.Z. Sensory-guided isolation and identification of new sweet-tasting dammarane-type saponins from Jiaogulan (Gynostemma pentaphyllum) herbal tea. Food Chem. 2022, 388, 132981. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C.; Blázquez-Castro, A.; Cañete, M.; Horobin, R.W.; Villanueva, Á. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef] [PubMed]

| Compounds | tR (min) | Absolute Content (mg/g) 1 | ||||
|---|---|---|---|---|---|---|
| 1 h Reactant | 2 h Reactant | 3 h Reactant | 6 h Reactant | 12 h Reactant | ||
| Rotenone (1) | 21.8 | 952.3 ± 3.2 a | 892.6 ± 2.9 a | 805.3 ± 2.7 a | 477.6 ± 1.8 ab | 204.1 ± 1.6 b |
| 2 | 20.1 | ND 2 | ND | ND | 13.5 ± 0.3 f | 108.9 ± 0.8 c |
| 3 | 19.8 | ND | ND | 10.7 ± 0.3 f | 99.7 ± 0.8 c | 105.4 ± 0.9 c |
| 4 | 21.6 | 12.3 ± 0.2 f | 30.1 ± 0.2 e | 42.3 ± 0.5 d | 165.6 ± 1.0 bc | 186.9 ± 1.0 bc |
| 5 | 24.2 | ND | 8.3 ± 0.1 f | 39.7 ± 0.5 d | 87.0 ± 0.7 cd | 258.8 ± 1.5 b |
| 6 | 18.5 | ND | ND | ND | ND | 40.3 ± 0.5 d |
| tR (min) | UV λmax (nm) | MF | [M + H]+ | [M − H]− | Identification |
|---|---|---|---|---|---|
| 19.8 | 242, 303 | C23H22O7 | 411 | 409 | (+)-Epirotenone (3) |
| 20.1 | 240, 305 | C23H22O7 | 411 | 409 | (−)-Rotenolone (2) |
| 21.6 | 218, 230, 294 | C23H22O6 | 395 | 393 | (+)-Epirotenolone (4) |
| 21.8 | 217, 234, 290 | C23H22O6 | 395 | 393 | Rotenone (1) |
| 24.2 | 228, 280, 310 | C23H20O6 | 393 | 392 | Dehydrorotenone (5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, G.H.; Lee, H.; Lee, S.S.; Chung, B.Y.; Bai, H.-W.; Kim, T.H. Inhibitory Effects of Thermolysis Transformation Products of Rotenone on Nitric Oxide Production. Int. J. Mol. Sci. 2023, 24, 6095. https://doi.org/10.3390/ijms24076095
Jeong GH, Lee H, Lee SS, Chung BY, Bai H-W, Kim TH. Inhibitory Effects of Thermolysis Transformation Products of Rotenone on Nitric Oxide Production. International Journal of Molecular Sciences. 2023; 24(7):6095. https://doi.org/10.3390/ijms24076095
Chicago/Turabian StyleJeong, Gyeong Han, Hanui Lee, Seung Sik Lee, Byung Yeoup Chung, Hyoung-Woo Bai, and Tae Hoon Kim. 2023. "Inhibitory Effects of Thermolysis Transformation Products of Rotenone on Nitric Oxide Production" International Journal of Molecular Sciences 24, no. 7: 6095. https://doi.org/10.3390/ijms24076095
APA StyleJeong, G. H., Lee, H., Lee, S. S., Chung, B. Y., Bai, H.-W., & Kim, T. H. (2023). Inhibitory Effects of Thermolysis Transformation Products of Rotenone on Nitric Oxide Production. International Journal of Molecular Sciences, 24(7), 6095. https://doi.org/10.3390/ijms24076095





