Improved Real-Time Quaking Induced Conversion for Early Diagnostics of Creutzfeldt–Jakob Disease in Denmark
Abstract
1. Introduction
2. Results
2.1. rHaTrPrP Quality Assurance
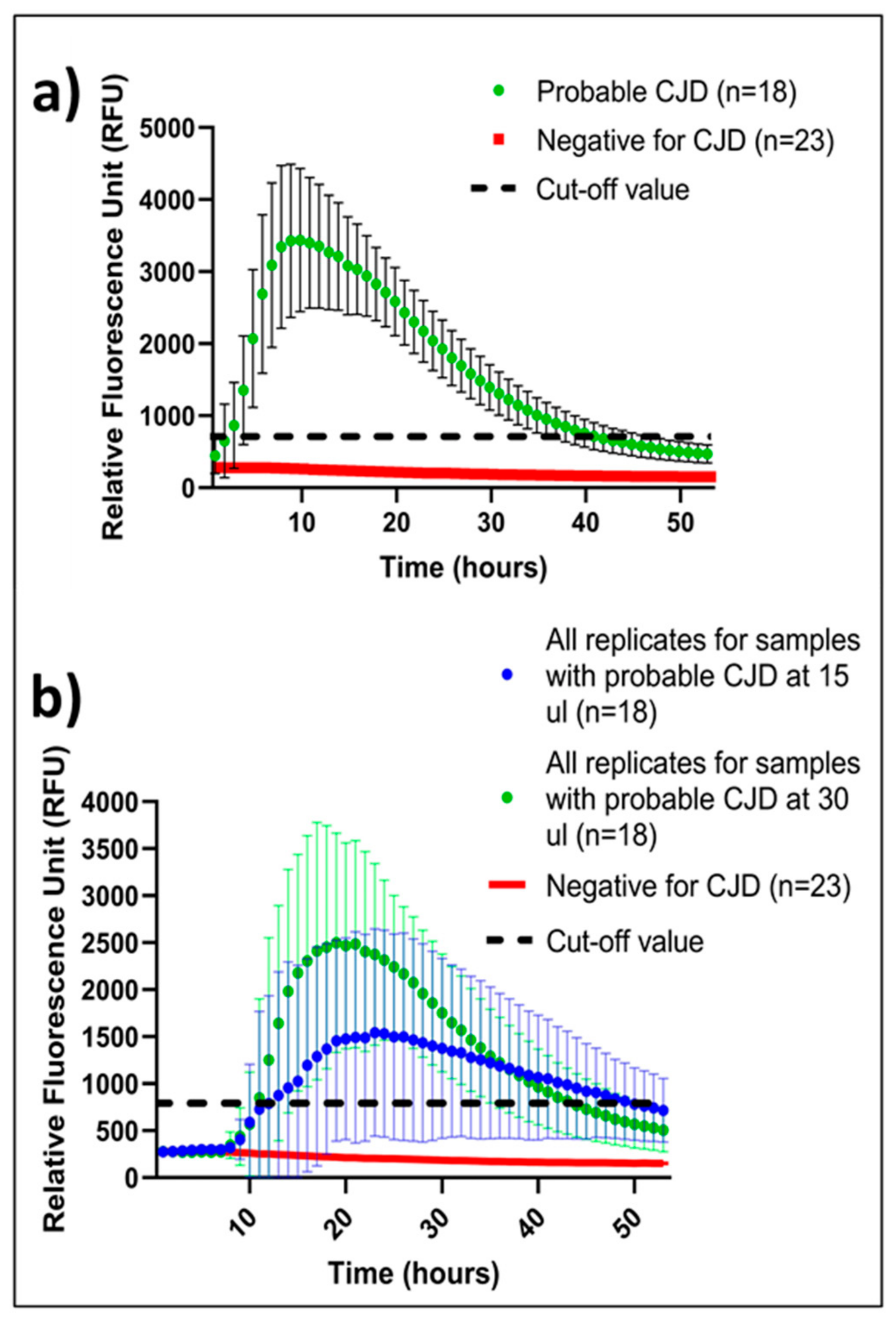
2.2. Threshold for a Positive IQ
2.3. Mean of Positive IQ Peaks
2.4. CSF Volume-Dependent IQ Sensitivity
2.5. 100% Concordance between DRCPD and NCJDRSU
3. Discussion
3.1. rHaTrPrP Validation, Preservation, and Stability over Time
3.2. IQ at DRCPD
3.3. Uniform IQ Controls
3.4. Concluding Remarks
4. Materials and Methods
4.1. Production of Syrian Hamster Truncated Recombinant Prion Protein (rHaTrPrP) 90-231 aa
4.2. Coomassie SDS-Page Analysis
4.3. HaTrPrP Stability and Sensitivity Evaluation
4.4. IQ RT-QuIC Plate Outline, Instrument Parameters and Interpretation of Results
4.5. Brain Samples and Brain Homogenate Control Preparation
4.6. CSF Samples
4.7. IQ Mastermix Preparation
4.8. Software and Statistical Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilham, J.M.; Orrú, C.D.; Bessen, R.A.; Atarashi, R.; Sano, K.; Race, B.; Meade-White, K.D.; Taubner, L.M.; Timmes, A.; Caughey, B. Rapid End-Point Quantitation of Prion Seeding Activity with Sensitivity Comparable to Bioassays. PLoS Pathog. 2010, 6, e1001217. [Google Scholar] [CrossRef] [PubMed]
- Hermann, P.; Appleby, B.; Brandel, J.P.; Caughey, B.; Collins, S.; Geschwind, M.D.; Green, A.; Haïk, S.; Kovacs, G.G.; Ladogana, A.; et al. Biomarkers and diagnostic guidelines for sporadic Creutzfeldt-Jakob disease. Lancet Neurol. 2021, 20, 235–246, Erratum in Lancet Neurol. 2021, 20, e3. [Google Scholar] [CrossRef] [PubMed]
- Hermann, P.; Laux, M.; Glatzel, M.; Matschke, J.; Knipper, T.; Goebel, S.; Treig, J.; Schulz-Schaeffer, W.; Cramm, M.; Schmitz, M.; et al. Validation and utilization of amended diagnostic criteria in Creutzfeldt-Jakob disease surveillance. Neurology 2018, 91, e331–e338. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, N.; Piconi, G.; Culeux, A.; Hammarin, A.; Stergiou, C.; Tzartos, S.; Versleijen, A.A.M.; van de Geer, J.; Cras, P.; Cardone, F.; et al. Concordance of cerebrospinal fluid real-time quaking-induced conversion across the European Creutzfeldt–Jakob Disease Surveillance Network. Eur. J. Neurol. 2022, 29, 2431–2438. [Google Scholar] [CrossRef] [PubMed]
- Groveman, B.R.; Orrú, C.D.; Hughson, A.G.; Bongianni, M.; Fiorini, M.; Imperiale, D.; Ladogana, A.; Pocchiari, M.; Zanusso, G.; Caughey, B. Extended and direct evaluation of RT-QuIC assays for Creutzfeldt-Jakob disease diagnosis. Ann. Clin. Transl. Neurol. 2016, 4, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Orrú, C.D.; Hughson, A.G.; Groveman, B.R.; Campbell, K.J.; Anson, K.J.; Manca, M.; Kraus, A.; Caughey, B. Factors That Improve RT-QuIC Detection of Prion Seeding Activity. Viruses 2016, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Orrú, C.D.; Groveman, B.R.; Foutz, A.; Bongianni, M.; Cardone, F.; McKenzie, N.; Culeux, A.; Poleggi, A.; Grznarova, K.; Perra, D.; et al. Ring trial of 2nd generation RT-QuIC diagnostic tests for sporadic CJD. Ann. Clin. Transl. Neurol. 2020, 7, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, A.; Baiardi, S.; Hughson, A.G.; McKenzie, N.; Moda, F.; Rossi, M.; Capellari, S.; Green, A.; Giaccone, G.; Caughey, B.; et al. High diagnostic value of second generation CSF RT-QuIC across the wide spectrum of CJD prions. Sci. Rep. 2017, 7, 10655. [Google Scholar] [CrossRef] [PubMed]
- Parchi, P.; Giese, A.; Capellari, S.; Brown, P.; Schulz-Schaeffer, W.; Windl, O.; Zerr, I.; Budka, H.; Kopp, N.; Piccardo, P.; et al. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann. Neurol. 1999, 46, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, A.E.; Appleby, B.S.; Cali, I.; Okhravi, H.R. Atypical Case of VV1 Creutzfeldt–Jakob Disease Subtype: Case Report. Front. Neurol. 2022, 13, 875370. [Google Scholar] [CrossRef] [PubMed]
- Zanusso, G.; Bongianni, M.; Caughey, B. A test for Creutzfeldt-Jakob disease using nasal brushings. N. Engl. J. Med. 2014, 371, 1842–1843, Erratum in N. Engl. J. Med. 2014, 371, 1852. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Yang, X.; Zhou, W.; Chen, C.; Shi, Q.; Dong, X. Validation and Application of Skin RT-QuIC to Patients in China with Probable CJD. Pathogens 2021, 10, 1642. [Google Scholar] [CrossRef] [PubMed]
- Green, A.J.E. RT-QuIC: A new test for sporadic CJD. Pract. Neurol. 2019, 19, 49–55. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, N.; McGuire, L.; Green, A.; European Centre for Disease Prevention and Control. Laboratory Standard Operating Procedure for Detecting Sporadic Creuzfeldt-Jakob Disease Using Real-Time Quaking-Induced Conversion (RT-QuIC) Assay; ECDC: Stockholm, Sweden, 2021. [CrossRef]
- Hwang, S.; Tatum, T.; Lebepe-Mazur, S.; Nicholson, E.M. Preparation of lyophilized recombinant prion protein for TSE diagnosis by RT-QuIC. BMC Res. Notes 2018, 11, 895. [Google Scholar] [CrossRef] [PubMed]
- Groveman, B.R.; Orrù, C.D.; Hughson, A.G.; Raymond, L.D.; Zanusso, G.; Ghetti, B.; Campbell, K.J.; Safar, J.; Galasko, D.; Caughey, B. Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol. Commun. 2018, 6, 7, Erratum in Acta Neuropathol. Commun. 2020, 8, 180. [Google Scholar] [CrossRef] [PubMed]
- Bargar, C.; Wang, W.; Gunzler, S.A.; LeFevre, A.; Wang, Z.; Lerner, A.J.; Singh, N.; Tatsuoka, C.; Appleby, B.; Zhu, X.; et al. Streamlined alpha-synuclein RT-QuIC assay for various biospecimens in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol. Commun. 2021, 9, 62. [Google Scholar] [CrossRef] [PubMed]
| DRCPD | NCJDRSU | |||
|---|---|---|---|---|
| Probable CJD (n = 18) Sample No. | Positive Replicate/ Total No. of Replicates; Sample Volume 15 µL | Positive Replicate/ Total No. of Replicates; Sample Volume 30 µL | Positive Replicate/ Total No. of Replicates; Sample Volume 15 µL | Positive Replicate/ Total No. of Replicates; Sample Volume 30 µL |
| 1 | 4/4 | 4/4 | 4/4 | 4/4 |
| 2 | 2/4 | 4/4 | 4/4 | 4/4 |
| 3 | 2/4 | 4/4 | 2/4 | 2/4 |
| 4 | 0/4 | 2/4 | 4/4 | 4/4 |
| 5 | 1/4 | 4/4 | 3/4 | 1/4 |
| 6 | 2/4 | 4/4 | 4/4 | 4/4 |
| 7 | 4/4 | 4/4 | 3/4 | 4/4 |
| 8 | 4/4 | 4/4 | 4/4 | 4/4 |
| 9 | 1/4 | 3/4 | 3/4 | 1/4 |
| 10 | 1/4 | 4/4 | 1/4 | 3/4 |
| 11 | 2/4 | 4/4 | 4/4 | 4/4 |
| 12 | 4/4 | 3/4 | 4/4 | 3/4 |
| 13 | 3/4 | 4/4 | 3/4 | 4/4 |
| 14 | 4/4 | 4/4 | 4/4 | 4/4 |
| 15 | 3/4 | 3/4 | 4/4 | 4/4 |
| 16 | 3/4 | 2/4 | 4/4 | 3/4 |
| 17 | 4/4 | 4/4 | 3/4 | 3/4 |
| 18 | 2/4 | 3/4 | 1/4 | 4/4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bsoul, R.; Lund, E.L.; Burns, K.; Andrews, M.; McKenzie, N.; Green, A.; Areškevičiūtė, A. Improved Real-Time Quaking Induced Conversion for Early Diagnostics of Creutzfeldt–Jakob Disease in Denmark. Int. J. Mol. Sci. 2023, 24, 6098. https://doi.org/10.3390/ijms24076098
Bsoul R, Lund EL, Burns K, Andrews M, McKenzie N, Green A, Areškevičiūtė A. Improved Real-Time Quaking Induced Conversion for Early Diagnostics of Creutzfeldt–Jakob Disease in Denmark. International Journal of Molecular Sciences. 2023; 24(7):6098. https://doi.org/10.3390/ijms24076098
Chicago/Turabian StyleBsoul, Remarh, Eva Løbner Lund, Kimberley Burns, Mary Andrews, Neil McKenzie, Alison Green, and Aušrinė Areškevičiūtė. 2023. "Improved Real-Time Quaking Induced Conversion for Early Diagnostics of Creutzfeldt–Jakob Disease in Denmark" International Journal of Molecular Sciences 24, no. 7: 6098. https://doi.org/10.3390/ijms24076098
APA StyleBsoul, R., Lund, E. L., Burns, K., Andrews, M., McKenzie, N., Green, A., & Areškevičiūtė, A. (2023). Improved Real-Time Quaking Induced Conversion for Early Diagnostics of Creutzfeldt–Jakob Disease in Denmark. International Journal of Molecular Sciences, 24(7), 6098. https://doi.org/10.3390/ijms24076098






