High-Performance Potassium-Selective Biosensor Platform Based on Resistive Coupling of a-IGZO Coplanar-Gate Thin-Film Transistor
Abstract
:1. Introduction
2. Results and Discussion
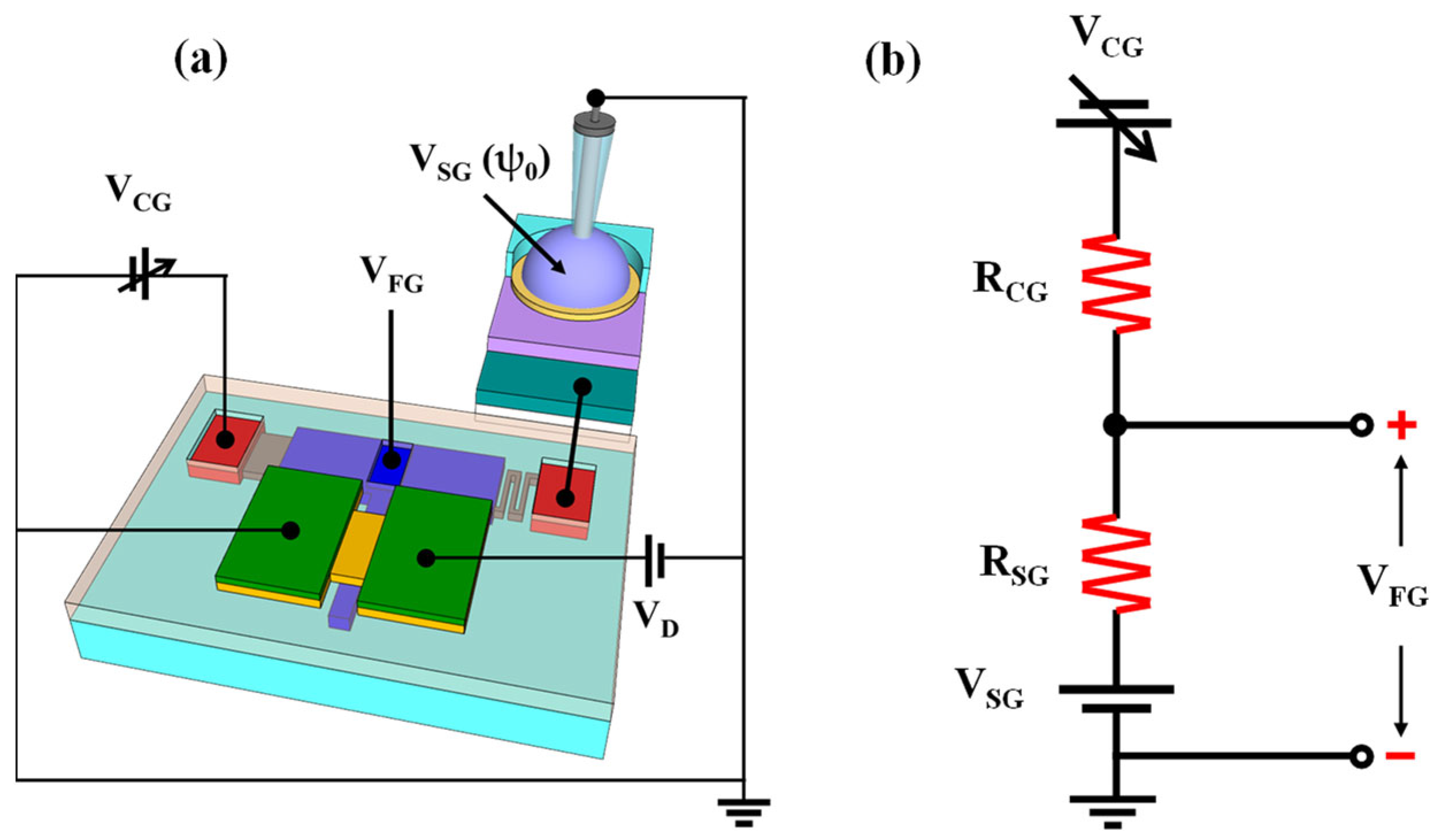
2.1. Electrical Characteristics of the Resistively Coupled TFT Transducer
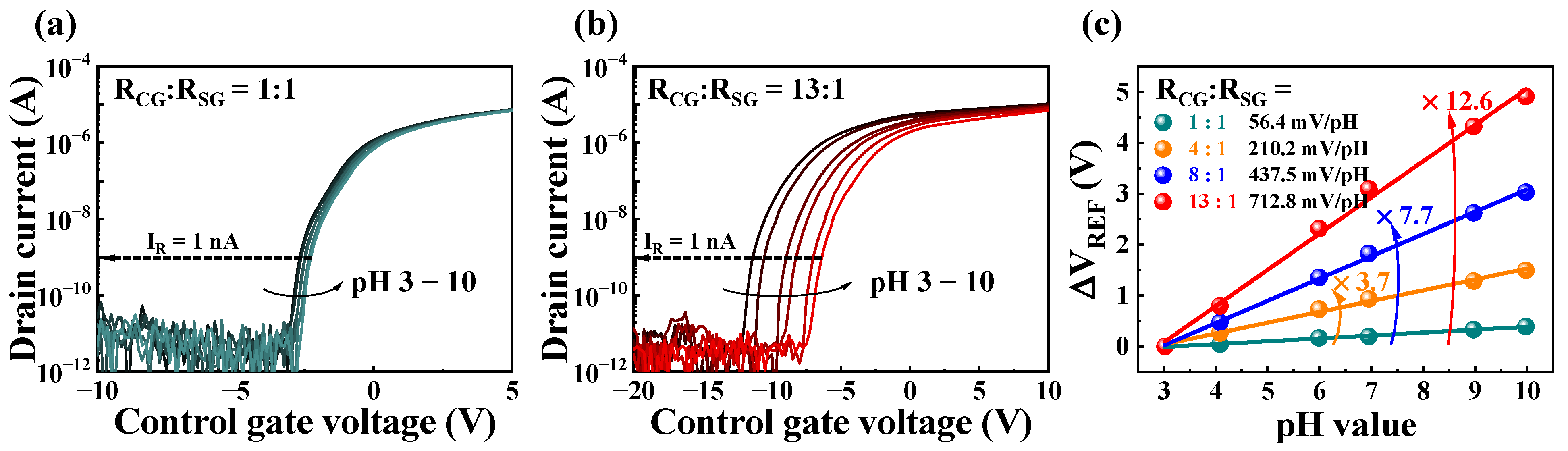
2.2. Self-Amplification of Low Sensitivity Based on Resistive Coupling
2.3. pH-Sensing of the Resistively Coupled Coplanar-Gate EGFET Biosensors
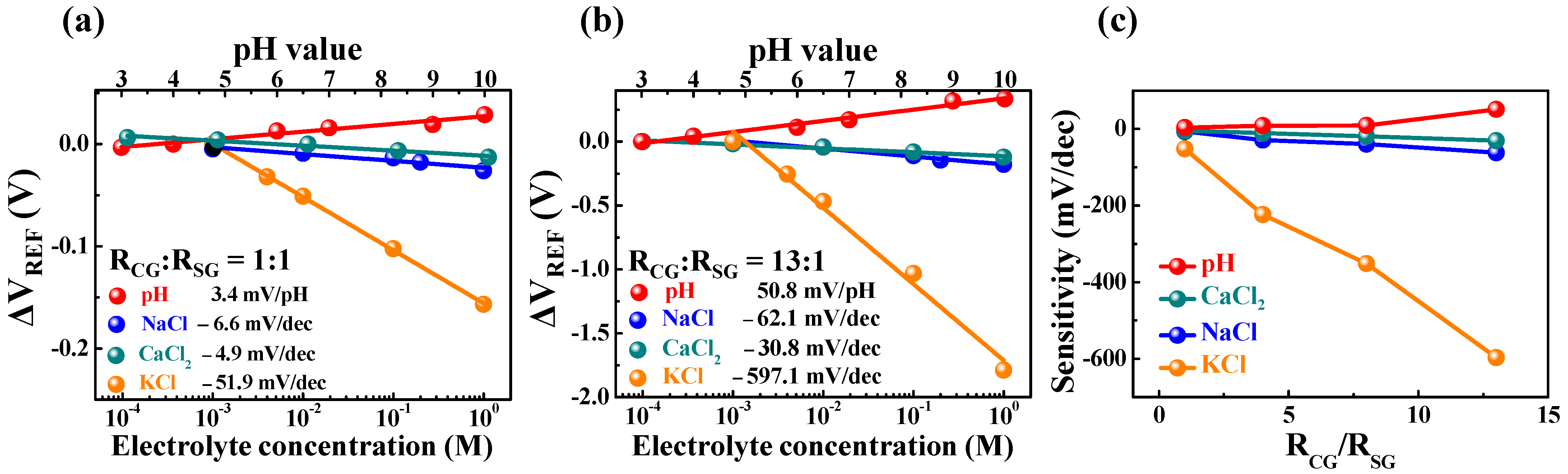
2.4. Potassium-Selective Sensing by the Resistively Coupled Coplanar-Gate EGFET Biosensors
3. Materials and Methods
3.1. Materials
3.2. Fabrication of the Resistively Coupled TFT Transducer Unit
3.3. Fabrication of the Potassium-Selective EG-Sensing Unit
3.4. Device Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zieg, J.; Gonsorcikova, L.; Landau, D. Current views on the diagnosis and management of hypokalaemia in children. Acta Paediatr. 2016, 105, 762–772. [Google Scholar] [CrossRef]
- Weaver, C.M. Potassium and health. Adv. Nutr. 2013, 4, 368–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depauw, A.; Dossi, E.; Kumar, N.; Fiorini-Debuisschert, C.; Huberfeld, G.; Ha-Thi, M.H.; Rouach, N.; Leray, I. A highly selective potassium sensor for the detection of potassium in living tissues. Chem. Eur. J. 2016, 22, 14902–14911. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, X.; Li, Y.; Fan, L.; Kraatz, H.B. A novel colorimetric potassium sensor based on the substitution of lead from G-quadruplex. Analyst 2013, 138, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, H.; Takagi, M.; Takenaka, S. A novel potassium sensing in aqueous media with a synthetic oligonucleotide derivative. fluorescence resonance energy transfer associated with guanine quartet−potassium ion complex formation. J. Am. Chem. Soc. 2002, 124, 14286–14287. [Google Scholar] [CrossRef]
- Bergveld, P. Development of an ion-sensitive solid-state device for neurophysiological measurements. IEEE Trans. Biomed. Eng. 1970, 1, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Huang, N.T. Dual Ion-Selective Membrane Deposited Ion-Sensitive Field-Effect Transistor Integrating a Whole Blood Processing Microchamber for In situ Blood Ion Testing. ACS Sens. 2023, in press. [CrossRef]
- Kim, D.H.; Cho, H.S.; Kim, J.H.; Jo, D.A.; Oh, H.G.; Jang, B.K.; Song, K.S. The integration of reference electrode for ISFET ion sensors using fluorothiophenol-treated RGO. Biosensors 2023, 13, 89. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Huang, N.T. A proton-selective membrane (PSM)-deposited dual-gate ion-sensitive field-effect transistor (DG-ISFET) integrating a microchamber-embedded filter membrane for bacterial enrichment and antimicrobial susceptibility test. Sens. Actuators B Chem. 2022, 359, 131580. [Google Scholar] [CrossRef]
- Dhar, R.; Kumar, N.; Garcia, C.P.; Georgiev, V. Deriving a novel methodology for nano-BioFETs and analysing the effect of high-k oxides on the amino-acids sensing application. Solid-State Electron. 2023, 200, 108525. [Google Scholar] [CrossRef]
- Van der Spiegel, J.; Lauks, I.; Chan, P.; Babic, D. The extended gate chemical sensitive field effect transistor as multi-species microprobe. Sens. Actuators 1983, 4, 291–298. [Google Scholar] [CrossRef]
- Chen, J.; Chou, J.; Sun, T.; Hsiung, S. Portable urea biosensor based on the extended-gate field effect transistor. Sens. Actuators B Chem. 2003, 91, 180–186. [Google Scholar] [CrossRef]
- Chi, L.; Chou, J.; Chung, W.; Sun, T.; Hsiung, S. Study on extended gate field effect transistor with tin oxide sensing membrane. Mater. Chem. Phys. 2000, 63, 19–23. [Google Scholar] [CrossRef]
- Rosdan, M.A.; Herman, S.H.; Abdullah, W.F.H.; Kamarozaman, N.S.; Syono, M.I. Sputtered titanium dioxide thin film for Extended-Gate FET sensor application. In Proceedings of the RSM 2013 IEEE Regional Symposium on Micro and Nanoelectronics, Daerah Langkawi, Malaysia, 25–27 September 2013. [Google Scholar]
- Cho, S.K.; Cho, W.J. Highly sensitive and- transparent urea-EnFET based point-of-care diagnostic test sensor with a triple-gate a-IGZO TFT. Sensors 2021, 21, 4748. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.Y.; Chang, C.H.; Chen, Y.Y.; Lai, W.H. An EGFET based common source amplifier as a low-frequency instrumentation amplifier for sensitivity measurement on RuO2 lactic acid biosensor. IEEE Access 2022, 10, 67605–67614. [Google Scholar] [CrossRef]
- Pullano, S.A.; Critello, C.D.; Mahbub, I.; Tasneem, N.T.; Shamsir, S.; Islam, S.K.; Greco, M.; Fiorillo, A.S. EGFET-based sensors for bioanalytical applications: A review. Sensors 2018, 18, 4042. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.K.; Jarwal, D.K.; Mukherjee, B.; Jit, S. CuO nanoparticles decorated ZnO nanorods based extended-gate field-effect-transistor (EGFET) for enzyme-free glucose sensing application. IEEE Trans. Nanobiosci. 2022, 21, 3–9. [Google Scholar] [CrossRef]
- Könemund, L.; Neumann, L.; Hirschberg, F.; Biedendieck, R.; Jahn, D.; Johannes, H.H.; Kowalsky, W. Functionalization of an extended-gate field-effect transistor (EGFET) for bacteria detection. Sci. Rep. 2022, 12, 4397. [Google Scholar] [CrossRef]
- Kuo, P.Y.; Chen, Y.Y.; Lai, W.H.; Chang, C.H. An extended-gate field-effect transistor applied to resistive divider integrated with the readout circuit using 180nm CMOS process for uric acid detection. IEEE Sens. J. 2021, 21, 20229–20238. [Google Scholar] [CrossRef]
- Dwivedi, P.; Singh, R.; Chauhan, Y.S. Crossing the Nernst limit (59 mV/pH) of sensitivity through tunneling transistor-based biosensor. IEEE Sens. J. 2021, 21, 3233–3240. [Google Scholar] [CrossRef]
- Jung, S.H.; Seo, Y.M.; Gu, T.; Jang, W.; Kang, S.G.; Hyeon, Y.; Hyun, S.H.; Lee, J.H.; Whang, D. Super-Nernstian pH sensor based on anomalous charge transfer doping of defect-engineered graphene. Nano Lett. 2021, 21, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Sanjay, S.; Hossain, M.; Rao, A.; Bhat, N. Super-Nernstian ion sensitive field-effect transistor exploiting charge screening in WSe2/MoS2 heterostructure. NPJ 2D Mater. Appl. 2021, 5, 93. [Google Scholar] [CrossRef]
- Madeira, G.D.M.; Dias Mello, H.J.N.P.; Faleiros, M.C.; Mulato, M. Model improvement for super-Nernstian pH sensors: The effect of surface hydration. J. Mater. Sci. 2021, 56, 2738–2747. [Google Scholar] [CrossRef]
- Cho, S.K.; Cho, W.J. High-sensitivity pH sensor based on coplanar gate AlGaN/GaN metal-oxide-semiconductor high electron mobility transistor. Chemosensors 2021, 9, 42. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, S.; Jang, Y.; Lim, K.; Lee, W.H. Attomolar detection of virus by liquid coplanar-gate graphene transistor on plastic. Nanotechnology 2019, 30, 345502. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.; McCarthy, M.; Hebard, A.F. Electric field gating with ionic liquids. Appl. Phys. Lett. 2007, 90, 052905. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, J.Y.; Seol, M.L.; Baek, D.J.; Guo, Z.; Kim, C.H.; Choi, S.J.; Choi, Y.K. A pH sensor with a double-gate silicon nanowire field-effect transistor. Appl. Phys. Lett. 2013, 102, 083701. [Google Scholar] [CrossRef]
- Bousse, L.; Bergveld, P. The role of buried OH sites in the response mechanism of inorganic-gate pH-sensitive ISFETs. Sens. Actuators 1984, 6, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Chou, J.C.; Wang, Y.F. Preparation and study on the drift and hysteresis properties of the tin oxide gate ISFET by the sol–gel method. Sens. Actuators B Chem. 2002, 86, 58–62. [Google Scholar] [CrossRef]
- Chou, J.C.; Weng, C.Y. Sensitivity and hysteresis effect in Al2O3 gate pH-ISFET. Mater. Chem. Phys. 2001, 71, 120–124. [Google Scholar] [CrossRef]
- Jamasb, S.; Collins, S.; Smith, R.L. A physical model for drift in pH ISFETs. Sens. Actuators B Chem. 1998, 49, 146–155. [Google Scholar] [CrossRef]
- Eyal, E.; Rechnitz, G.A. Mechanistic studies on the valinomycin-based potassium electrode. Anal. Chem. 1971, 43, 1090–1093. [Google Scholar] [CrossRef]
- Seki, A.; Motoya, K.; Watanabe, S.; Kubo, I. Novel sensors for potassium, calcium and magnesium ions based on a silicon transducer as a light-addressable potentiometric sensor. Anal. Chim. Acta 1999, 382, 131–136. [Google Scholar] [CrossRef]
- Zhang, J.; Rupakula, M.; Bellando, F.; Garcia Cordero, E.; Longo, J.; Wildhaber, F.; Herment, G.; Guérin, H.; Ionescu, A.M. Sweat biomarker sensor incorporating picowatt, three-dimensional extended metal gate ion sensitive field effect transistors. ACS Sens. 2019, 4, 2039–2047. [Google Scholar] [CrossRef]
- Kabaa, E.A.; Abdulateef, S.A.; Ahmed, N.M.; Hassan, Z.; Sabah, F.A. A novel porous silicon multi-ions selective electrode based extended gate field effect transistor for sodium, potassium, calcium, and magnesium sensor. Appl. Phys. A 2019, 125, 753. [Google Scholar] [CrossRef]
- Melzer, K.; Bhatt, V.D.; Schuster, T.; Jaworska, E.; Maksymiuk, K.; Michalska, A.; Lugli, P.; Scarpa, G. Flexible electrolyte-gated ion-selective sensors based on carbon nanotube networks. IEEE Sens. J. 2015, 15, 3127–3134. [Google Scholar] [CrossRef]
- Al Baroot, A.F.; Grell, M. Comparing electron- and hole transporting semiconductors in ion sensitive water-gated transistors. Mater. Sci. Semicond. Process 2019, 89, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Cordero, E.; Bellando, F.; Zhang, J.; Wildhaber, F.; Longo, J.; Guérin, H.; Ionescu, A.M. Three-dimensional integrated ultra-low-volume passive microfluidics with ion-sensitive field-effect transistors for multiparameter wearable sweat analyzers. ACS Nano 2018, 12, 12646–12656. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.H. Development of EGFET-Based Sodium/Calcium/Chloride/Ammonium/Potassium Multi-Ion Sensing Microsystem with a Solid-State Reference Electrode. Ph.D. Thesis, Sun Yat-Sen University, Kaohsiung, Taiwan, 2015. [Google Scholar]


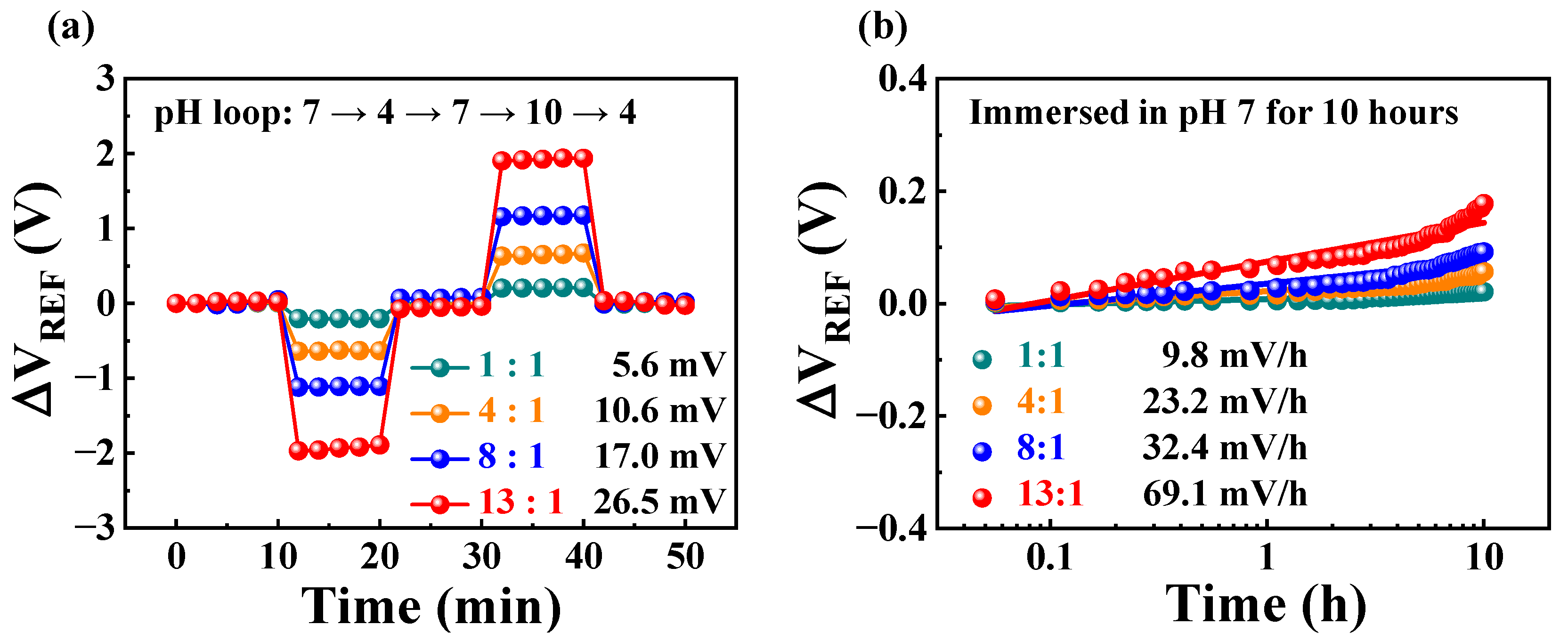
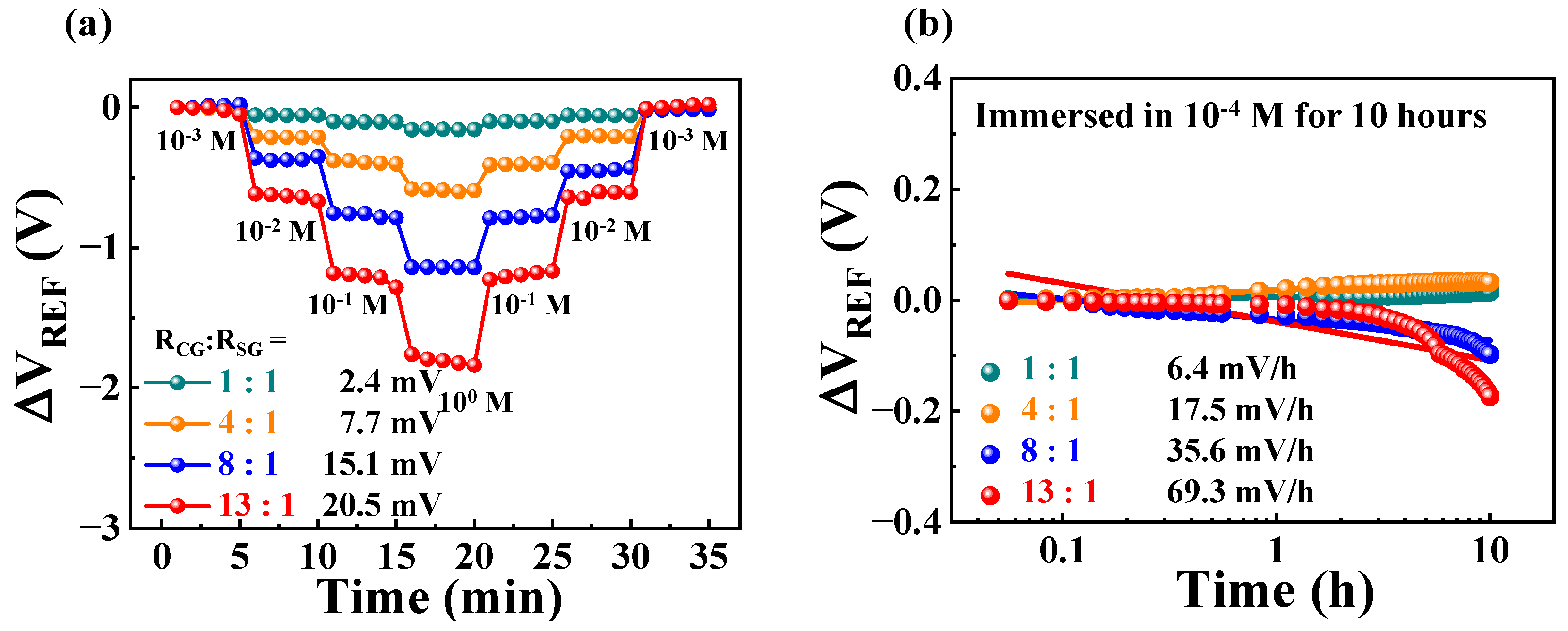
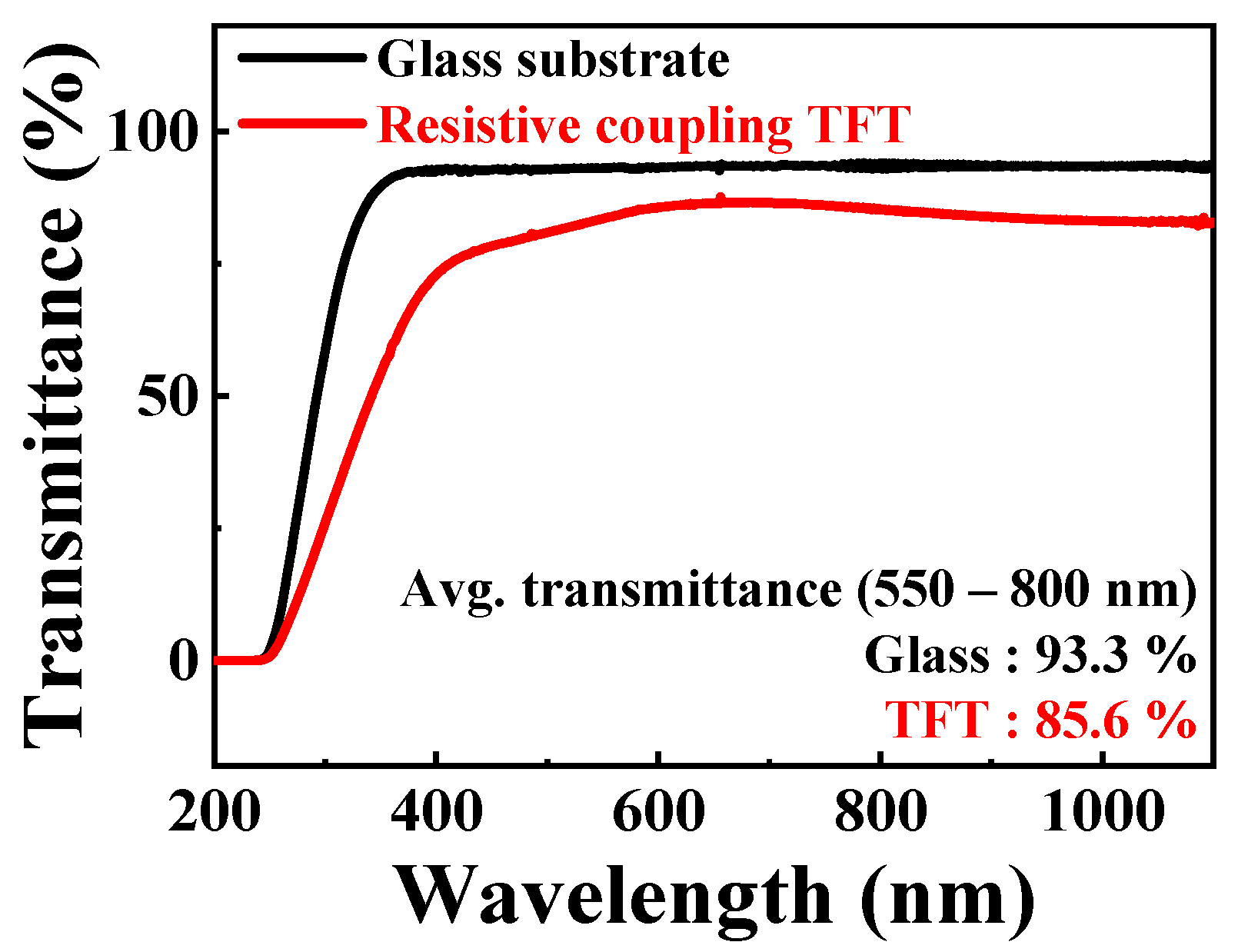
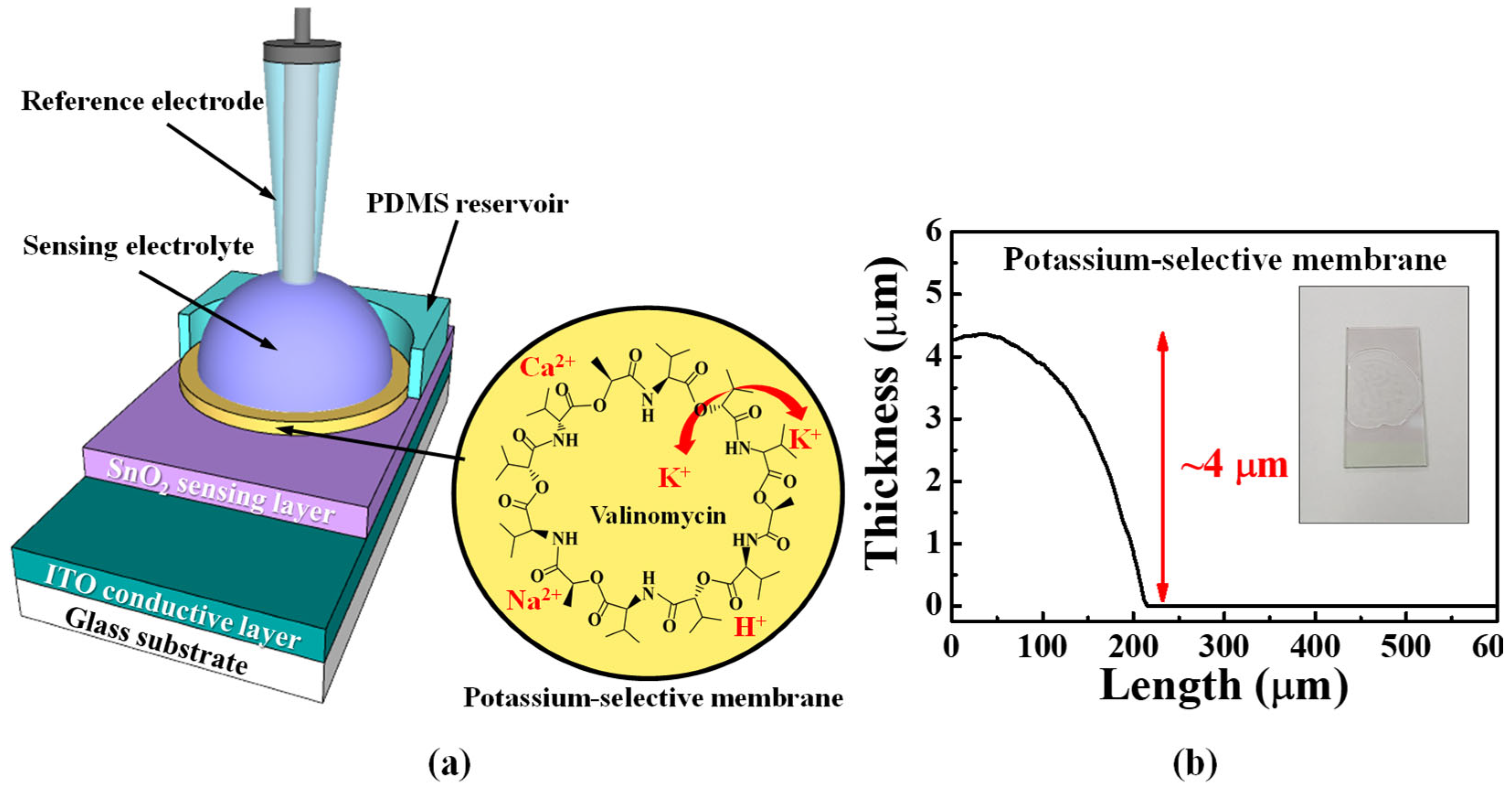
| RCG:RSG | Sensitivity (mV/pH) | VH (mV) | RD (mV/h) | VH to Sensitivity (%) | RD to Sensitivity (%) |
|---|---|---|---|---|---|
| 1:1 | 56.4 | 5.6 | 9.8 | 10.0 | 17.3 |
| 4:1 | 210.2 | 10.6 | 23.2 | 5.0 | 11.0 |
| 8:1 | 437.5 | 17.6 | 32.4 | 4.0 | 7.4 |
| 13:1 | 712.8 | 26.5 | 69.1 | 3.7 | 9.7 |
| RCG:RSG | H+ Sensitivity (mV/pH) | Na+ Sensitivity (mV/dec) | Ca2+ Sensitivity (mV/dec) | K+ Sensitivity (mV/dec) |
|---|---|---|---|---|
| 1:1 | 3.4 | 6.6 | 4.9 | 51.9 |
| 4:1 | 8.3 | 28.9 | 4.4 | 223.6 |
| 8:1 | 9.1 | 39.7 | 19.2 | 351.5 |
| 13:1 | 50.8 | 62.1 | 30.8 | 597.1 |
| Sensing Membrane | Platform | Sensitivity (mV/dec) | Reference Electrode | Test Solution | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Ca2+ | H+ | |||||
| Al2O3 | LAPS | - | 50 | 16 | 43 | Ag/AgCl | Tris-HCl | [34] |
| Al2O3 | ISFET | 56.9 | 48.1 | 25.7 | 57.2 | Ag/AgCl | DI water | [35] |
| PS (111) | EGFET | 58.76 | 57.9 | 34.9 | - | Ag/AgCl | DI water | [36] |
| CNT | ISFET | - | 62.8 | 26.2 | - | Pt | DI water | [37] |
| rrPHHT | ISFET | 47 | 77 | - | - | Au | DI water | [38] |
| Au | ISFET | 62 | 55 | - | 36 | Ag/AgCl | DI water, artificial sweat | [39] |
| Au | EGFET | 64.2 | 61.3 | - | - | Ag/AgCl | PBS | [40] |
| Ti/Ag/AgCl | EGFET | 38 | 48 | 36.7 | - | Ti/Ag/AgCl | DI water | [41] |
| SnO2 | EGFET | 62.1 | 597.1 | 30.8 | 50.8 | Ag/AgCl | DI water | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyun, T.-H.; Cho, W.-J. High-Performance Potassium-Selective Biosensor Platform Based on Resistive Coupling of a-IGZO Coplanar-Gate Thin-Film Transistor. Int. J. Mol. Sci. 2023, 24, 6164. https://doi.org/10.3390/ijms24076164
Hyun T-H, Cho W-J. High-Performance Potassium-Selective Biosensor Platform Based on Resistive Coupling of a-IGZO Coplanar-Gate Thin-Film Transistor. International Journal of Molecular Sciences. 2023; 24(7):6164. https://doi.org/10.3390/ijms24076164
Chicago/Turabian StyleHyun, Tae-Hwan, and Won-Ju Cho. 2023. "High-Performance Potassium-Selective Biosensor Platform Based on Resistive Coupling of a-IGZO Coplanar-Gate Thin-Film Transistor" International Journal of Molecular Sciences 24, no. 7: 6164. https://doi.org/10.3390/ijms24076164





