Histological and Histomorphometric Evaluation of Implanted Photodynamic Active Biomaterials for Periodontal Bone Regeneration in an Animal Study
Abstract
1. Introduction
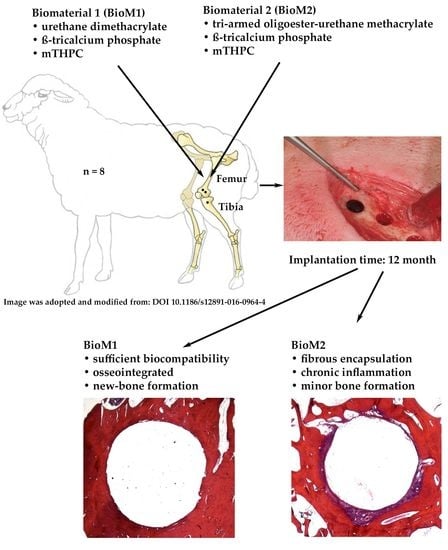
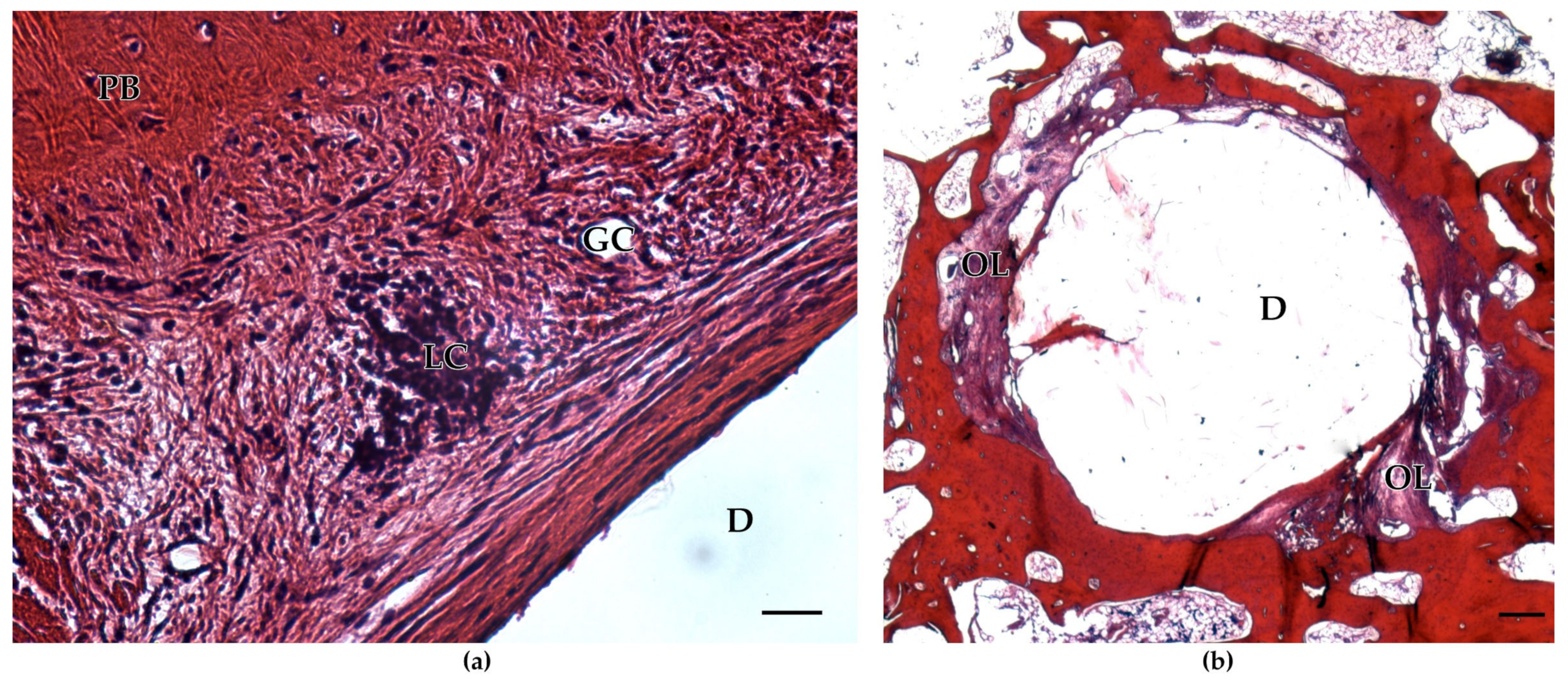
2. Results
3. Discussion
4. Materials and Methods
4.1. Characterization of the Biomaterials
4.2. Surgical Procedure and Biomaterial Application
4.3. Sample Preparation and Histological Sectioning
4.4. Histomorphometry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Polizzi, A.; Santonocito, S.; Romano, A.; Lombardi, T.; Isola, G. Impact of Oral Microbiome in Periodontal Health and Periodontitis: A Critical Review on Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 5142. [Google Scholar] [CrossRef] [PubMed]
- Plachokova, A.; Andreu-Sánchez, S.; Noz, M.; Fu, J.; Riksen, N. Oral Microbiome in Relation to Periodontitis Severity and Systemic Inflammation. Int. J. Mol. Sci. 2021, 22, 5876. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Tonetti, M.S. Diagnosis and epidemiology of periodontal osseous lesions. Periodontology 2000 2000, 22, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulos, A.; Ivanovic, A.; Chapple, I.L.C.; Nikolidakis, D.; Nikou, G.; Sculean, A. Biomaterials for promoting periodontal regeneration in human intrabony defects: A systematic review. Periodontology 2000, 68, 182–216. [Google Scholar] [CrossRef]
- Ren, Y.; Fan, L.; Alkildani, S.; Liu, L.; Emmert, S.; Najman, S.; Rimashevskiy, D.; Schnettler, R.; Jung, O.; Xiong, X.; et al. Barrier Membranes for Guided Bone Regeneration (GBR): A Focus on Recent Advances in Collagen Membranes. Int. J. Mol. Sci. 2022, 23, 14987. [Google Scholar] [CrossRef]
- Paolantonio, M.; Di Tullio, M.; Giraudi, M.; Romano, L.; Secondi, L.; Paolantonio, G.; Graziani, F.; Pilloni, A.; De Ninis, P.; Femminella, B. Periodontal regeneration by leukocyte and platelet-rich fibrin with autogenous bone graft versus enamel matrix derivative with autogenous bone graft in the treatment of periodontal intrabony defects: A randomized non-inferiority trial. J. Periodontol. 2020, 91, 1595–1608. [Google Scholar] [CrossRef]
- Sassano, P.; Gennaro, P.; Chisci, G.; Gabriele, G.; Aboh, I.V.; Mitro, V.; di Curzio, P. Calvarial onlay graft and submental incision in treatment of atrophic edentulous mandibles: An approach to reduce postoperative complications. J. Craniofac. Surg. 2014, 25, 693–697. [Google Scholar] [CrossRef]
- Jepsen, S.; Gennai, S.; Hirschfeld, J.; Kalemaj, Z.; Buti, J.; Graziani, F. Regenerative surgical treatment of furcation defects: A systematic review and Bayesian network meta-analysis of randomized clinical trials. J. Clin. Periodontol. 2019, 47, 352–374. [Google Scholar] [CrossRef]
- Chisci, G.; Fredianelli, L. Therapeutic Efficacy of Bromelain in Alveolar Ridge Preservation. Antibiotics 2022, 11, 1542. [Google Scholar] [CrossRef]
- Vahdatinia, F.; Hooshyarfard, A.; Jamshidi, S.; Shojaei, S.; Patel, K.; Moeinifard, E.; Haddadi, R.; Farhadian, M.; Gholami, L.; Tayebi, L. 3D-Printed Soft Membrane for Periodontal Guided Tissue Regeneration. Materials 2023, 16, 1364. [Google Scholar] [CrossRef]
- Ausenda, F.; Rasperini, G.; Acunzo, R.; Gorbunkova, A.; Pagni, G. New Perspectives in the Use of Biomaterials for Periodontal Regeneration. Materials 2019, 12, 2197. [Google Scholar] [CrossRef]
- Deng, Y.; Liang, Y.; Liu, X. Biomaterials for Periodontal Regeneration. Dent. Clin. N. Am. 2022, 66, 659–672. [Google Scholar] [CrossRef]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
- Tomas, M.; Čandrlić, M.; Juzbašić, M.; Ivanišević, Z.; Matijević, N.; Včev, A.; Peloza, O.C.; Matijević, M.; Kačarević, P. Synthetic Injectable Biomaterials for Alveolar Bone Regeneration in Animal and Human Studies. Materials 2021, 14, 2858. [Google Scholar] [CrossRef]
- Ruediger, T.; Berg, A.; Guellmar, A.; Rode, C.; Schnabelrauch, M.; Urbanek, A.; Wagner, K.; Wyrwa, R.; Kinne, R.W.; Sigusch, B.W. Cytocompatibility of polymer-based periodontal bone substitutes in gingival fibroblast and MC3T3 osteoblast cell cultures. Dent. Mater. 2012, 28, e239–e249. [Google Scholar] [CrossRef]
- Cheah, C.W.; Al-Namnam, N.M.; Lau, M.N.; Lim, G.S.; Raman, R.; Fairbairn, P.; Ngeow, W.C. Synthetic Material for Bone, Periodontal, and Dental Tissue Regeneration: Where Are We Now, and Where Are We Heading Next? Materials 2021, 14, 6123. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Mazzoni, E.; Manfrini, M.; D’Agostino, A.; Trevisiol, L.; Nocini, R.; Trombelli, L.; Barbanti-Brodano, G.; Martini, F.; Tognon, M. Innovative Biomaterials for Bone Regrowth. Int. J. Mol. Sci. 2019, 20, 618. [Google Scholar] [CrossRef]
- Dreifke, M.B.; Ebraheim, N.A.; Jayasuriya, A.C. Investigation of potential injectable polymeric biomaterials for bone regeneration. J. Biomed. Mater. Res. Part A 2013, 101, 2436–2447. [Google Scholar] [CrossRef]
- Mariano, L.C.; Fernandes, M.H.R.; Gomes, P.S. Antimicrobial Biomaterials for the Healing of Infected Bone Tissue: A Systematic Review of Microtomographic Data on Experimental Animal Models. J. Funct. Biomater. 2022, 13, 193. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, D.; Wang, T.-B.; Nie, X.; Chen, G.; Wang, L.-H.; You, Y.-Z.; Wang, Q. Biodegradable hydrogels with photodynamic antibacterial activity promote wound healing and mitigate scar formation. Biomater. Sci. 2022, 11, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-C.; Chiu, K.-C.; Chen, W.-C.; Lan, W.-C.; Chen, C.-Y.; Hsia, S.-M.; Wang, T.-H.; Tu, H.-F.; Shih, Y.-H.; Shieh, T.-M. Effects of Temoporfin-Based Photodynamic Therapy on the In Vitro Antibacterial Activity and Biocompatibility of Gelatin-Hyaluronic Acid Cross-Linked Hydrogel Membranes. Pharmaceutics 2022, 14, 2314. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Chu, G.; Jin, J.; Liu, C.; Cheng, L.; Guo, Y.; Deng, Z.; Wang, Y. A Combined Cyanine/Carbomer Gel Enhanced Photodynamic Antimicrobial Activity and Wound Healing. Nanomaterials 2022, 12, 2173. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Ghiladi, R.A.; Wang, Q.; Cai, Y.; Wei, Q. Protoporphyrin-IX conjugated cellulose nanofibers that exhibit high antibacterial photodynamic inactivation efficacy. Nanotechnology 2018, 29, 265601. [Google Scholar] [CrossRef]
- Nie, X.; Wu, S.; Hussain, T.; Wei, Q. PCN-224 Nanoparticle/Polyacrylonitrile Nanofiber Membrane for Light-Driven Bacterial Inactivation. Nanomaterials 2021, 11, 3162. [Google Scholar] [CrossRef]
- Pereira, A.O.; Lopes, I.M.I.; Silva, T.R.; Corrêa, T.Q.; Paschoalin, R.T.; Inada, N.M.; Iermak, I.; Neto, F.V.R.; Araujo-Chaves, J.C.; Marletta, A.; et al. Bacterial Photoinactivation Using PLGA Electrospun Scaffolds. ACS Appl. Mater. Interfaces 2021, 13, 31406–31417. [Google Scholar] [CrossRef]
- Jiang, S.; Ma, B.C.; Huang, W.; Kaltbeitzel, A.; Kizisavas, G.; Crespy, D.; Zhang, K.A.I.; Landfester, K. Visible light active nanofibrous membrane for antibacterial wound dressing. Nanoscale Horiz. 2018, 3, 439–446. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, Z.; Chen, M.; Zhang, Y.; Amagat, J.; Kang, S.; Zheng, Y.; Hu, B.; Chen, M. Co-immobilization of Ce6 Sono/photosensitizer and Protonated Graphitic-Carbon Nitride on PCL/gelation Fibrous Scaffolds for Combined Sono-photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 40728–40739. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, X.; Chan, Y.K.; Wang, Z.; Li, L.; Li, J.; Liang, K.; Deng, Y. Photo-Activated Nanofibrous Membrane with Self-Rechargeable Antibacterial Function for Stubborn Infected Cutaneous Regeneration. Small 2022, 18, 2105988. [Google Scholar] [CrossRef]
- Prieto-Montero, R.; Prieto-Castañeda, A.; Katsumiti, A.; Cajaraville, M.; Agarrabeitia, A.; Ortiz, M.; Martínez-Martínez, V. Functionalization of Photosensitized Silica Nanoparticles for Advanced Photodynamic Therapy of Cancer. Int. J. Mol. Sci. 2021, 22, 6618. [Google Scholar] [CrossRef]
- Lagos, K.J.; Buzzá, H.H.; Bagnato, V.S.; Romero, M.P. Carbon-Based Materials in Photodynamic and Photothermal Therapies Applied to Tumor Destruction. Int. J. Mol. Sci. 2021, 23, 22. [Google Scholar] [CrossRef]
- Sigusch, B.; Dietsch, S.; Berg, A.; Voelpel, A.; Guellmar, A.; Rabe, U.; Schnabelrauch, M.; Steen, D.; Gitter, B.; Albrecht, V.; et al. Antimicrobial photodynamic active biomaterials for periodontal regeneration. Dent. Mater. 2018, 34, 1542–1554. [Google Scholar] [CrossRef]
- Kranz, S.; Guellmar, A.; Völpel, A.; Gitter, B.; Albrecht, V.; Sigusch, B.W. Photodynamic suppression of Enterococcus faecalis using the photosensitizer mTHPC. Lasers Surg. Med. 2011, 43, 241–248. [Google Scholar] [CrossRef]
- Ossmann, A.; Kranz, S.; Andre, G.; Völpel, A.; Albrecht, V.; Fahr, A.; Sigusch, B.W. Photodynamic killing of Enterococcus faecalis in dentinal tubules using mTHPC incorporated in liposomes and invasomes. Clin. Oral Investig. 2014, 19, 373–384. [Google Scholar] [CrossRef]
- Voss, J.O.; Kasselmann, S.; Koerdt, S.; Rendenbach, C.; Fischer, H.; Jöhrens, K.; Czabanka, M.; Schmidt-Bleek, K.; Duda, G.N.; Heiland, M.; et al. Treatment options for critical size defects—Comparison of different materials in a calvaria split model in sheep. Biomater. Adv. 2022, 136, 212788. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, X.; Bao, M.; Zhou, T.; Shi, J.; Xu, Z.; Zhou, M.; Boccaccini, A.R.; Zheng, K.; Jiang, X. Biomineralization inspired 3D printed bioactive glass nanocomposite scaffolds orchestrate diabetic bone regeneration by remodeling micromilieu. Bioact. Mater. 2023, 25, 239–255. [Google Scholar] [CrossRef]
- Tan, Q.-C.; Jiang, X.-S.; Chen, L.; Huang, J.-F.; Zhou, Q.-X.; Wang, J.; Zhao, Y.; Zhang, B.; Sun, Y.-N.; Wei, M.; et al. Bioactive graphene oxide-functionalized self-expandable hydrophilic and osteogenic nanocomposite for orthopaedic applications. Mater. Today Bio 2023, 18, 100500. [Google Scholar] [CrossRef]
- Bucciarelli, A.; Petretta, M.; Grigolo, B.; Gambari, L.; Bossi, A.M.; Grassi, F.; Maniglio, D. Methacrylated Silk Fibroin Additive Manufacturing of Shape Memory Constructs with Possible Application in Bone Regeneration. Gels 2022, 8, 833. [Google Scholar] [CrossRef]
- Celikkin, N.; Mastrogiacomo, S.; Dou, W.; Heerschap, A.; Oosterwijk, E.; Walboomers, X.F.; Święszkowski, W. In vitro and in vivo assessment of a 3D printable gelatin methacrylate hydrogel for bone regeneration applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Nuss, K.; Auer, J.A.; Boos, A.; von Rechenberg, B. An animal model in sheep for biocompatibility testing of biomaterials in cancellous bones. BMC Musculoskelet. Disord. 2006, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cells Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Atayde, L.M.; Cortez, P.; Pereira, T.; Armada-Da-Silva, P.; Afonso, A.; Lopes, M.A.; Santos, J.D.; Maurício, A.C. A new sheep model with automatized analysis of biomaterial-induced bone tissue regeneration. J. Mater. Sci. Mater. Med. 2014, 25, 1885–1901. [Google Scholar] [CrossRef]
- Boure’, L.; Zeiter, S.; Seidenglanz, U.; Leitner, M.; Van der Pol, B.; Matthys, R.; Pearce, S. A novel sheep model for evaluating biomaterials in cancellous bone. In Proceedings of the ECM IX Musculoskeletal Trauma—50 Years of AO Research, Davos, Switzerland, 15–18 June 2008; p. 16. [Google Scholar]
- Perier-Metz, C.; Cipitria, A.; Hutmacher, D.W.; Duda, G.N.; Checa, S. An in silico model predicts the impact of scaffold design in large bone defect regeneration. Acta Biomater. 2022, 145, 329–341. [Google Scholar] [CrossRef]
- Perier-Metz, C.; Duda, G.N.; Checa, S. Mechano-Biological Computer Model of Scaffold-Supported Bone Regeneration: Effect of Bone Graft and Scaffold Structure on Large Bone Defect Tissue Patterning. Front. Bioeng. Biotechnol. 2020, 8, 585799. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Permana, M.S.; Winarni, T.I.; van der Heide, E. Adopted walking condition for computational simulation approach on bearing of hip joint prosthesis: Review over the past 30 years. Heliyon 2022, 8, e12050. [Google Scholar] [CrossRef]
- Perier-Metz, C.; Duda, G.N.; Checa, S. Initial mechanical conditions within an optimized bone scaffold do not ensure bone regeneration—An in silico analysis. Biomech. Model. Mechanobiol. 2021, 20, 1723–1731. [Google Scholar] [CrossRef]
- Sideridou, I.D.; Karabela, M.M.; Bikiaris, D.N. Aging studies of light cured dimethacrylate-based dental resins and a resin composite in water or ethanol/water. Dent. Mater. 2007, 23, 1142–1149. [Google Scholar] [CrossRef]
- Walsh, W.R.; Bertollo, N.; Christou, C.; Schaffner, D.; Mobbs, R.J. Plasma-sprayed titanium coating to polyetheretherketone improves the bone-implant interface. Spine J. 2015, 15, 1041–1049. [Google Scholar] [CrossRef]
- Martini, L.; Staffa, G.; Giavaresi, G.; Salamanna, F.; Parrilli, A.; Serchi, E.; Pressato, D.; Arcangeli, E.; Fini, M. Long-Term Results following Cranial Hydroxyapatite Prosthesis Implantation in a Large Skull Defect Model. Plast. Reconstr. Surg. 2012, 129, 625e–635e. [Google Scholar] [CrossRef]
- Ghaffar, A.; Verschuren, P.; Geenevasen, J.; Handels, T.; Berard, J.; Plum, B.; Dias, A.; Schoenmakers, P.; van der Wal, S. Fast in vitro hydrolytic degradation of polyester urethane acrylate biomaterials: Structure elucidation, separation and quantification of degradation products. J. Chromatogr. A 2010, 1218, 449–458. [Google Scholar] [CrossRef]
- Alam Ansari, M.A.; Golebiowska, A.A.; Dash, M.; Kumar, P.; Jain, P.K.; Nukavarapu, S.P.; Ramakrishna, S.; Nanda, H.S. Engineering biomaterials to 3D-print scaffolds for bone regeneration: Practical and theoretical consideration. Biomater. Sci. 2022, 10, 2789–2816. [Google Scholar] [CrossRef] [PubMed]
- Fukuba, S.; Okada, M.; Nohara, K.; Iwata, T. Alloplastic Bone Substitutes for Periodontal and Bone Regeneration in Dentistry: Current Status and Prospects. Materials 2021, 14, 1096. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef]
- Jones, K.S. Effects of biomaterial-induced inflammation on fibrosis and rejection. Semin. Immunol. 2008, 20, 130–136. [Google Scholar] [CrossRef]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, J.; Chen, C.; Wang, Y.; Hao, Z.; Chen, T.; Wang, J.; Li, J. Strategies of Macrophages to Maintain Bone Homeostasis and Promote Bone Repair: A Narrative Review. J. Funct. Biomater. 2022, 14, 18. [Google Scholar] [CrossRef]
- Klopfleisch, R.; Jung, F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. Part A 2016, 105, 927–940. [Google Scholar] [CrossRef]
- Kranz, S.; Huebsch, M.; Guellmar, A.; Voelpel, A.; Tonndorf-Martini, S.; Sigusch, B.W. Antibacterial photodynamic treatment of periodontopathogenic bacteria with indocyanine green and near-infrared laser light enhanced by TroloxTM. Lasers Surg. Med. 2015, 47, 350–360. [Google Scholar] [CrossRef]
- Sigusch, B.W.; Engelbrecht, M.; Völpel, A.; Holletschke, A.; Pfister, W.; Schütze, J. Full-Mouth Antimicrobial Photodynamic Therapy in Fusobacterium nucleatum–Infected Periodontitis Patients. J. Periodontol. 2010, 81, 975–981. [Google Scholar] [CrossRef]
- Dias, L.M.; Ferrisse, T.M.; Medeiros, K.S.; Cilli, E.M.; Pavarina, A.C. Use of Photodynamic Therapy Associated with Antimicrobial Peptides for Bacterial Control: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 3226. [Google Scholar] [CrossRef] [PubMed]
- Songca, S.P.; Adjei, Y. Applications of Antimicrobial Photodynamic Therapy against Bacterial Biofilms. Int. J. Mol. Sci. 2022, 23, 3209. [Google Scholar] [CrossRef]
- Elashiry, M.; Morandini, A.; Timothius, C.C.; Ghaly, M.; Cutler, C. Selective Antimicrobial Therapies for Periodontitis: Win the “Battle and the War”. Int. J. Mol. Sci. 2021, 22, 6459. [Google Scholar] [CrossRef]
- Giannelli, M.; Lasagni, M.; Bani, D. Photonic Therapy in Periodontal Diseases an Overview with Appraisal of the Literature and Reasoned Treatment Recommendations. Int. J. Mol. Sci. 2019, 20, 4741. [Google Scholar] [CrossRef]
- Maliszewska, I.; Zdubek, A. On the Photo-Eradication of Methicillin-Resistant Staphylococcus aureus Biofilm Using Methylene Blue. Int. J. Mol. Sci. 2023, 24, 791. [Google Scholar] [CrossRef]
- Cao, H.; Qiao, S.; Qin, H.; Jandt, K.D. Antibacterial Designs for Implantable Medical Devices: Evolutions and Challenges. J. Funct. Biomater. 2022, 13, 86. [Google Scholar] [CrossRef]
- Bielenstein, J.; Radenković, M.; Najman, S.; Liu, L.; Ren, Y.; Cai, B.; Beuer, F.; Rimashevskiy, D.; Schnettler, R.; Alkildani, S.; et al. In Vivo Analysis of the Regeneration Capacity and Immune Response to Xenogeneic and Synthetic Bone Substitute Materials. Int. J. Mol. Sci. 2022, 23, 10636. [Google Scholar] [CrossRef]
- ISO 10993-6:2017; Biological Evaluation of Medical Devices—Part 6: Tests for Local Effects after Implantation. International Organization for Standardization: Geneva, Switzerland, 2017.











| Animal ID | Sample ID | Femur | Tibia | |
|---|---|---|---|---|
| BioM1 | S4 | S4FP | 2 | - |
| S4TP | - | 1 | ||
| S5 | S5FP | 2 | - | |
| S5TP | - | 1 | ||
| S6 | S6FP | 2 | - | |
| S6TP | - | 1 | ||
| S7 | S7FP | 2 | - | |
| S7TP | - | 1 | ||
| 8 | 4 | |||
| BioM2 | S12 | S12FP | 2 | - |
| S12TP | - | 1 | ||
| S13 | S13FP | 2 | - | |
| S13TP | - | 1 | ||
| S14 | S14FP | 2 | - | |
| S14TP | - | 1 | ||
| S15 | S15FP | 2 | - | |
| S15TP | - | 1 | ||
| 8 | 4 |
| Score | Definition |
|---|---|
| 1 | Completely mineralized bone with the presence of osteoblasts and/or osteocytes |
| 2 | Deposited connective tissue within the bone matrix |
| 3 | Connective tissue without signs of bone in the ROI |
| 4 | Additional appearance of univacular fat cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigusch, B.; Kranz, S.; von Hohenberg, A.C.; Wehle, S.; Guellmar, A.; Steen, D.; Berg, A.; Rabe, U.; Heyder, M.; Reise, M. Histological and Histomorphometric Evaluation of Implanted Photodynamic Active Biomaterials for Periodontal Bone Regeneration in an Animal Study. Int. J. Mol. Sci. 2023, 24, 6200. https://doi.org/10.3390/ijms24076200
Sigusch B, Kranz S, von Hohenberg AC, Wehle S, Guellmar A, Steen D, Berg A, Rabe U, Heyder M, Reise M. Histological and Histomorphometric Evaluation of Implanted Photodynamic Active Biomaterials for Periodontal Bone Regeneration in an Animal Study. International Journal of Molecular Sciences. 2023; 24(7):6200. https://doi.org/10.3390/ijms24076200
Chicago/Turabian StyleSigusch, Bernd, Stefan Kranz, Andreas Clemm von Hohenberg, Sabine Wehle, André Guellmar, Dorika Steen, Albrecht Berg, Ute Rabe, Markus Heyder, and Markus Reise. 2023. "Histological and Histomorphometric Evaluation of Implanted Photodynamic Active Biomaterials for Periodontal Bone Regeneration in an Animal Study" International Journal of Molecular Sciences 24, no. 7: 6200. https://doi.org/10.3390/ijms24076200
APA StyleSigusch, B., Kranz, S., von Hohenberg, A. C., Wehle, S., Guellmar, A., Steen, D., Berg, A., Rabe, U., Heyder, M., & Reise, M. (2023). Histological and Histomorphometric Evaluation of Implanted Photodynamic Active Biomaterials for Periodontal Bone Regeneration in an Animal Study. International Journal of Molecular Sciences, 24(7), 6200. https://doi.org/10.3390/ijms24076200