Integrated Physiological and Transcriptomic Analyses Revealed Improved Cold Tolerance in Cucumber (Cucumis sativus L.) by Exogenous Chitosan Oligosaccharide
Abstract
1. Introduction
2. Results
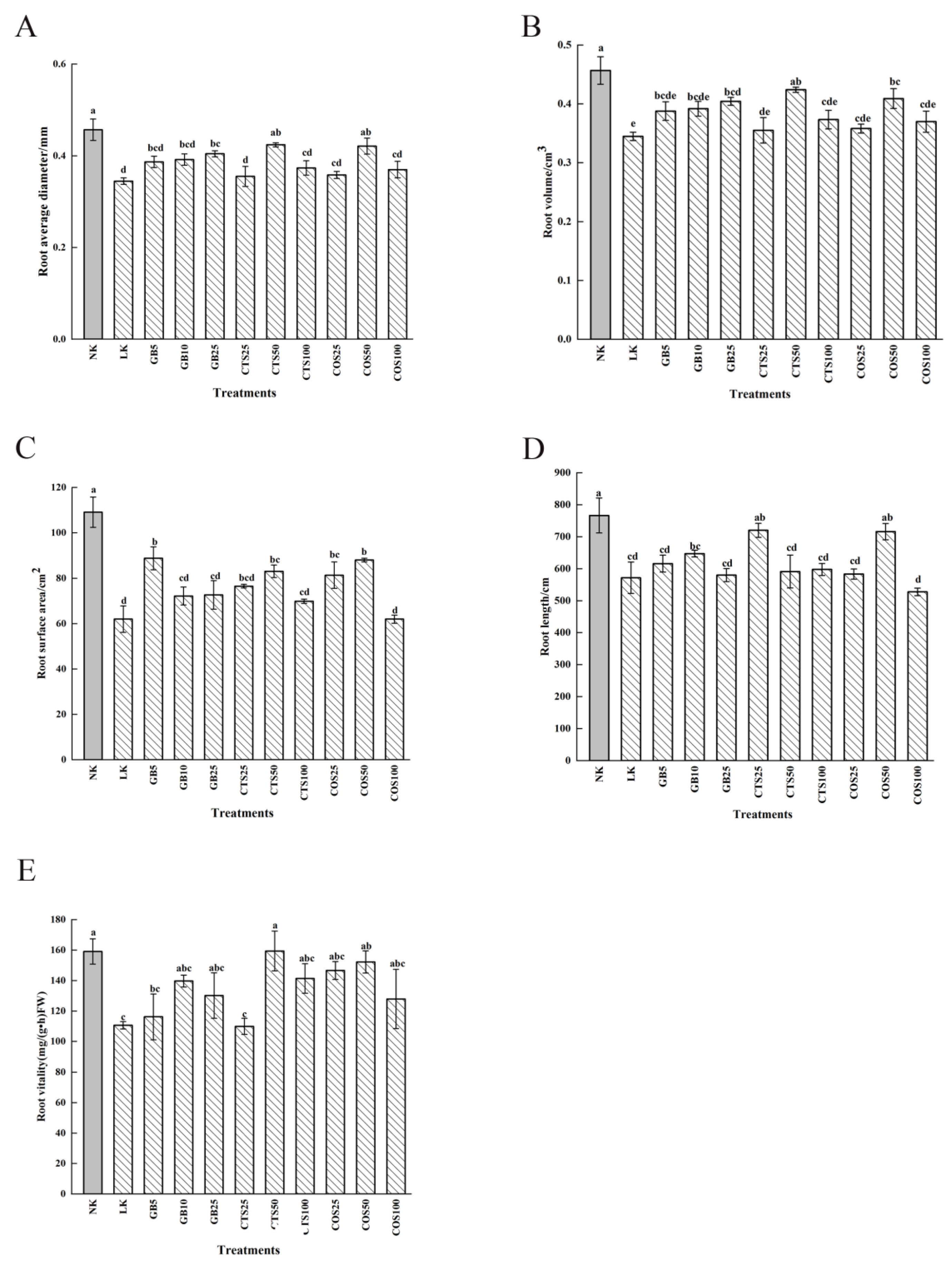
2.1. Effects of the Exogenous Substances on Cucumber Seedling Growth and Physiological Characteristics under Low-Temperature Stress
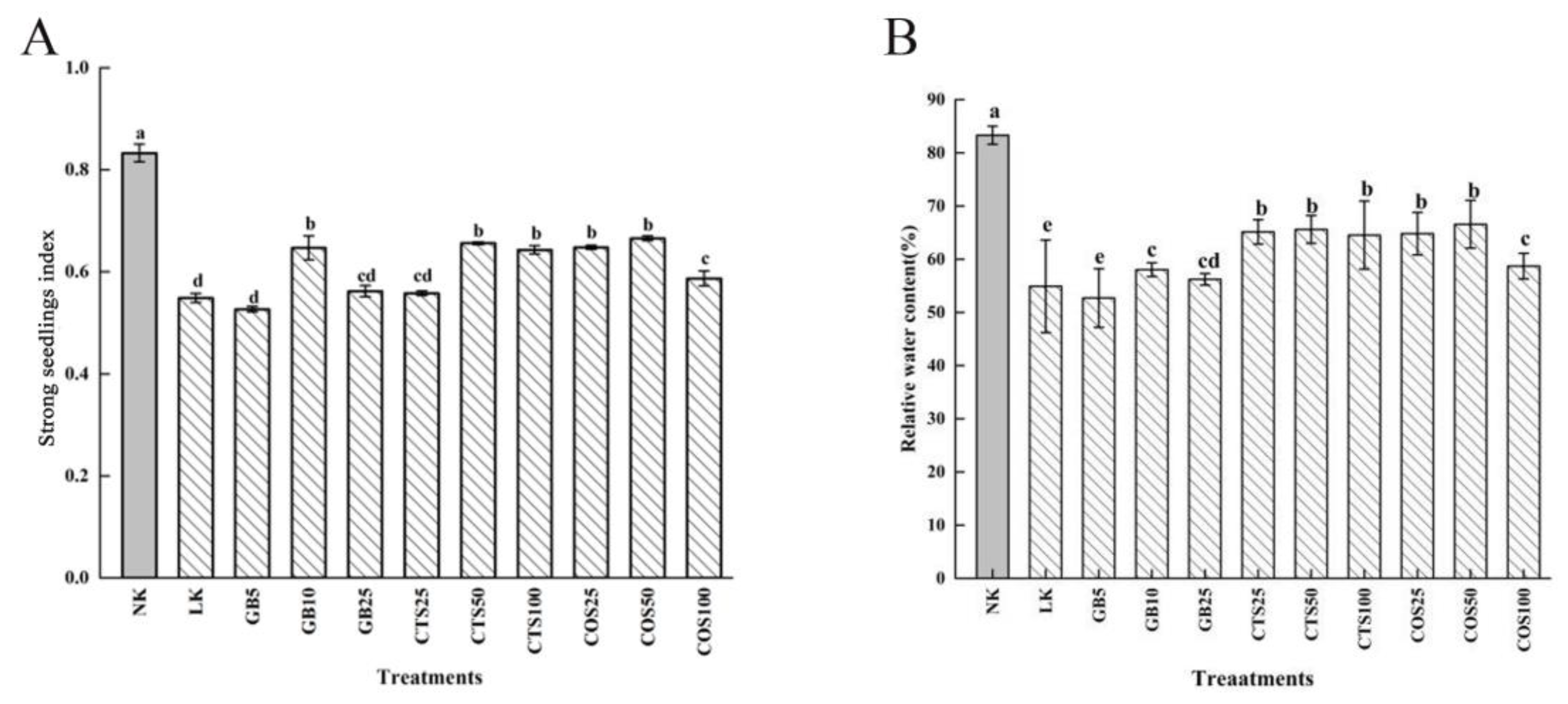
2.2. Effects of the Exogenous Substances at Optimum Concentration on Cold Tolerance of Cucumber Seedlings
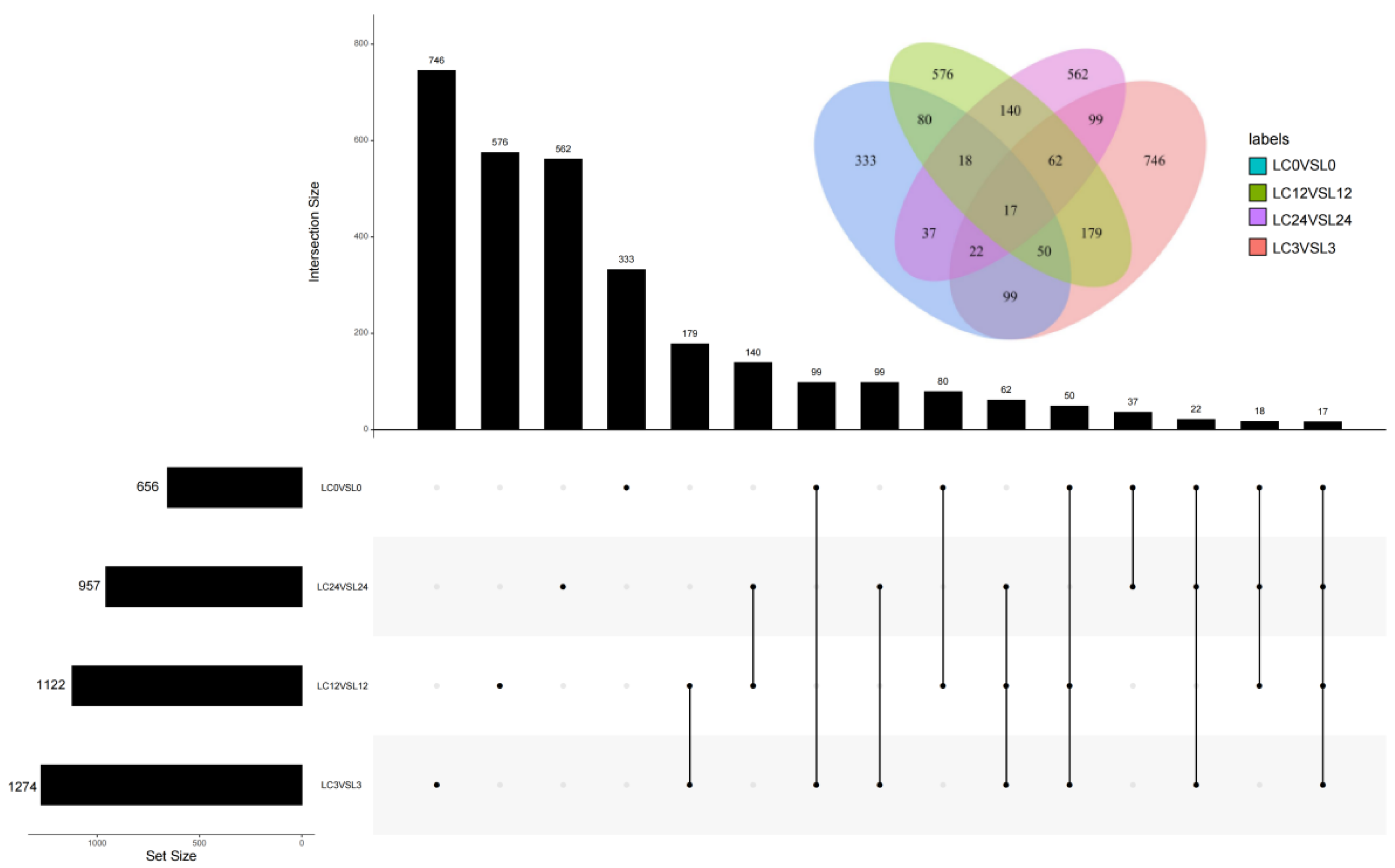
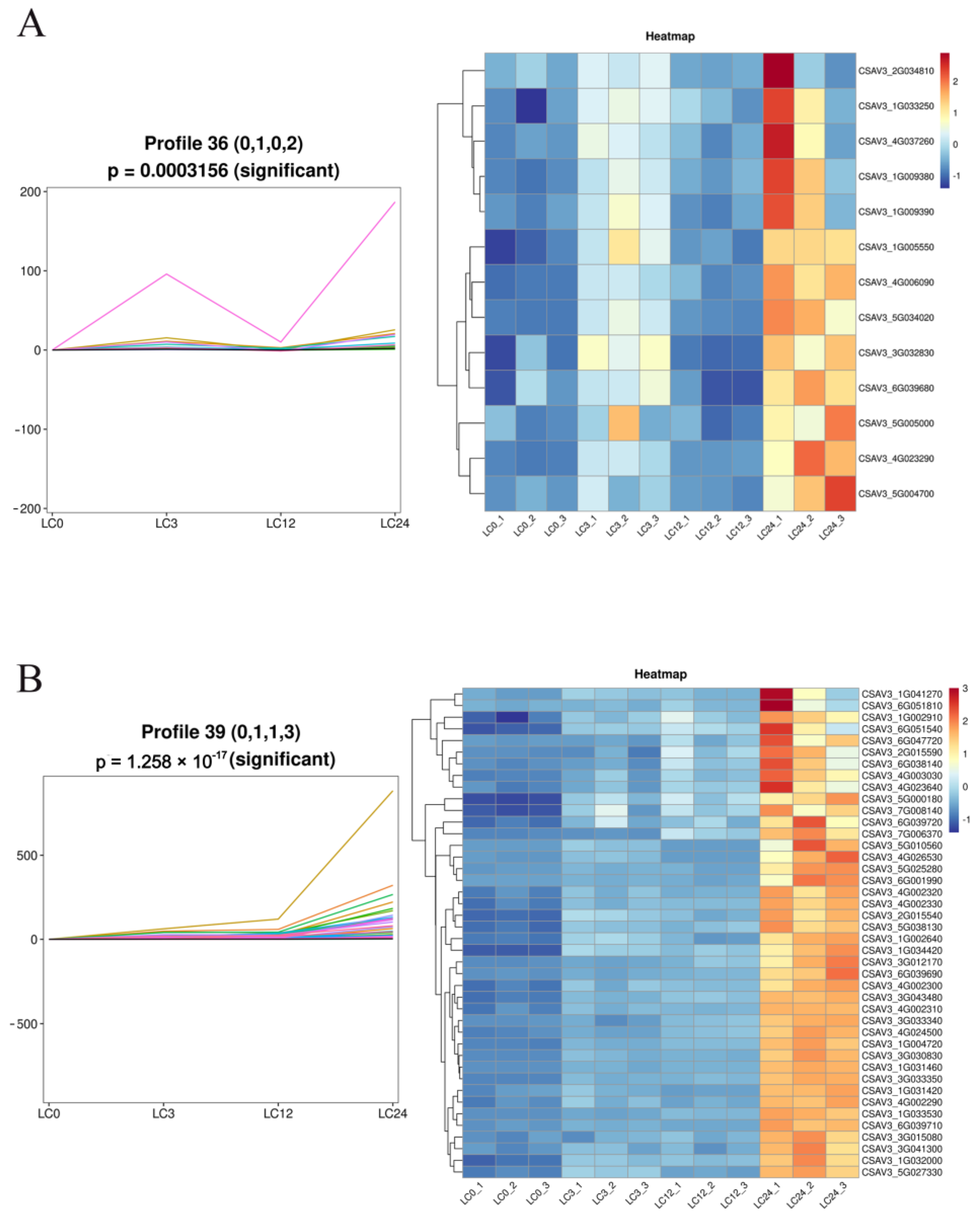
2.3. Transcriptome Analysis of Exogenous COS in Response to Cold Stress in Cucumber Seedlings
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Sample Treatments
4.3. Morphological, Physiological, and Biochemical Analyses
4.4. RNA Isolation, Library Construction, and Illumina Sequencing
4.5. Bioinformatics Analysis of RNA-Sequencing (RNASseq)
4.6. Quantitative Real-Time PCR (qRT-PCR)
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GB | Glycine betaine |
| CTS | Chitosan |
| COS | Chitosan oligosaccharide |
| ABA | Abscisic acid |
| JA | Jasmonic acid |
| MT | Melatonin |
| SA | Salicylic acid |
| PA | Polyamine |
| Ci | Intercellular CO2 |
| gsw | Stomatal conductance |
| Pn | Net photosynthesis |
| E | Transpiration rate |
| REC | Relative electrical conductivity |
| MDA | Malondialdehyde |
| SP | Soluble protein |
| SS | Soluble sugar |
| Pro | Proline |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| POD | Peroxidase |
| RNA-Seq | RNA sequencing |
| DEGs | Differentially expressed genes |
References
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Molecular regulation of plant responses to environmental temperatures. Mol. Plant. 2020, 13, 544–564. [Google Scholar] [CrossRef]
- Guo, X.Y.; Liu, D.F.; Chong, K. Cold signaling in plants: Insights into mechanisms and regulation. J. Integrative Plant Biol. 2018, 60, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Z.; Jin, Y.N.; Ding, X.H.; Wang, W.J.; Zhai, S.S.; Bai, L.P.; Guo, Z.F. Gene regulation and signal transduction in the ICE-CBF-COR signaling pathway during cold stress in plants. Biochemistry 2017, 82, 1103–1117. [Google Scholar] [CrossRef] [PubMed]
- Juurakko, C.L.; Bredow, M.; Nakayama, T.; Imai, H.; Kawamura, Y.; diCenzo, G.C.; Uemura, M.; Walker, V.K. The Brachypodium disrachyon cold-acclimated plasma membrane proteome is primed for stress resistance. G3 2021, 11, jkab198. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ding, Y.; Yang, S. Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 2018, 23, 623–637. [Google Scholar] [CrossRef]
- Browse, J.; Xin, Z. Temperature sensing and cold acclimation. Curr. Opin. Plant Biol. 2001, 4, 241–246. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, S. Surviving and thriving: How plants perceive and respond to temperature stress. Dev. Cell 2022, 57, 947–958. [Google Scholar] [CrossRef]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identifification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2003, 416, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP channel that senses cold stimuli and menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef]
- Cui, Y.; Lu, S.; Li, Z.; Cheng, J.; Hu, P.; Zhu, T.; Wang, X.; Jin, M.; Wang, X.; Li, L.; et al. CYCLIC NUCLEOTIDE-GATED ION CHANNELs 14 and 16 promote tolerance to heat and chilling in rice. Plant Physiol. 2020, 183, 1794–1808. [Google Scholar] [CrossRef]
- Mori, K.; Renhu, N.; Naito, M.; Nakamura, A.; Shiba, H.; Yamamoto, T.; Suzaki, T.; Iida, H.; Miura, K. Ca2+-permeable mechanosensitive channels MCA1 and MCA2 mediate cold-induced cytosolic Ca2+ increase and cold tolerance in Arabidopsis. Sci. Rep. 2018, 8, 550. [Google Scholar] [CrossRef]
- Liu, Q.; Ding, Y.; Shi, Y.; Ma, L.; Wang, Y.; Song, C.; Wilkins, K.A.; Davies, J.M.; Knight, H.; Knight, M.R.; et al. The calcium transporter ANNEXIN1 mediates cold-induced calcium signaling and freezing tolerance in plants. EMBO J. 2021, 40, e104559. [Google Scholar] [CrossRef]
- Kidokoro, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant cold-stress responses. Trends Plant Sci. 2022, 37, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Doherty, C.J.; Van Buskirk, H.A.; Myers, S.J.; Thomashow, M.F. Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. Plant Cell 2009, 21, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.A.; Farré, E.M.; Thomashow, M.F. Circadian clock-associated 1 and late elongated hypocotyl regulate expression of the C-repeat binding factor (CBF) pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 7241–7246. [Google Scholar] [CrossRef]
- Jiang, B.; Shi, Y.; Zhang, X.; Xin, X.; Qi, L.; Guo, H.; Li, J.; Yang, S. PIF3 is a negative regulator of the CBF pathway and freezing tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, E6695–E6702. [Google Scholar] [CrossRef]
- Kanaoka, M.M.; Pillitteri, L.J.; Fujii, H.; Yoshida, Y.; Bogenschutz, N.L.; Takabayashi, J.; Zhu, J.K.; Torii, K.U. Scream/ICE1 and SCREAM2 specify three cell-state transitional steps leading to Arabidopsis stomatal differentiation. Plant Cell 2008, 20, 1775–1785. [Google Scholar] [CrossRef]
- Kidokoro, S.; Maruyama, K.; Nakashima, K.; Imura, Y.; Narusaka, Y.; Shinwari, Z.K.; Osakabe, Y.; Fujita, Y.; Mizoi, J.; Shinozaki, K.; et al. The phytochrome-interacting factor PIF7 negatively regulates DREB1 expression under circadian control in Arabidopsis. Plant Physiol. 2009, 151, 2046–2057. [Google Scholar] [CrossRef] [PubMed]
- Kidokoro, S.; Hayashi, K.; Haraguchi, H.; Ishikawa, T.; Soma, F.; Konoura, I.; Toda, S.; Mizoi, J.; Suzuki, T.; Shinozaki, K.; et al. Posttranslational regulation of multiple clock-related transcription factors triggers cold-inducible gene expression in Arabidopsis. Proc. Natl. Acad. Sci. USA 2021, 118, e2021048118. [Google Scholar] [CrossRef]
- Lee, E.S.; Park, J.H.; Wi, S.D.; Kang, C.H.; Chi, Y.H.; Chae, H.B.; Paeng, S.K.; Ji, M.G.; Kim, W.Y.; Kim, M.G.; et al. Redox-dependent structural switch and CBF activation confer freezing tolerance in plants. Nat. Plants 2021, 7, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Jia, Y.; Shi, Y.; Zhang, X.; Song, C.; Gong, Z.; Yang, S. OST1-mediated BTF3L phosphorylation positively regulates CBFs during plant cold responses. EMBO J. 2018, 37, e98228. [Google Scholar] [CrossRef]
- Hemsley, P.A.; Hurst, C.H.; Kaliyadasa, E.; Lamb, R.; Knight, M.R.; de Cothi, E.A.; Steele, J.F.; Knight, H. The Arabidopsis mediator complex subunits MED16, MED14, and MED2 regulate mediator and RNA polymerase II recruitment to CBF-responsive coldregulated genes. Plant Cell 2014, 26, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jia, Y.; Ding, Y.; Shi, Y.; Li, Z.; Guo, Y.; Gong, Z.; Yang, S. Plasma membrane CRPK1-mediated phosphorylation of 14-3-3 proteins induces their nuclear import to fifine-tune CBF signaling during cold response. Mol. Cell 2017, 66, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.J.; Park, J.; Shen, M.; Park, H.J.; Cheong, M.S.; Park, K.S.; Baek, D.; Bae, M.J.; Ali, A.; Jan, M.; et al. The histone-modifying complex PWR/HOS15/HD2C epigenetically regulates cold tolerance. Plant Physiol. 2020, 184, 1097–1111. [Google Scholar] [CrossRef]
- Lee, C.M.; Thomashow, M.F. Photoperiodic regulation of the C-repeat binding factor (CBF) cold acclimation pathway and freezing tolerance in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2012, 109, 15054–15059. [Google Scholar] [CrossRef]
- Ohama, N.; Moo, T.L.; Chua, N.H. Differential requirement of MED14/17 recruitment for activation of heat inducible genes. New Phytol. 2021, 229, 3360–3376. [Google Scholar] [CrossRef]
- Park, J.; Lim, C.J.; Shen, M.; Park, H.J.; Cha, J.Y.; Iniesto, E.; Rubio, V.; Mengiste, T.; Zhu, J.K.; Bressan, R.A.; et al. Epigenetic switch from repressive to permissive chromatin in response to cold stress. Proc. Natl. Acad. Sci. USA 2018, 115, E5400–E5409. [Google Scholar] [CrossRef]
- Catalá, R.; Medina, J.; Salinas, J. Integration of low temperature and light signaling during cold acclimation response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 16475–16480. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ye, K.; Shi, Y.; Cheng, J.; Zhang, X.; Yang, S. BZR1 positively regulates freezing tolerance via CBF-dependent and CBF-independent pathways in Arabidopsis. Mol. Plant 2017, 10, 545–559. [Google Scholar] [CrossRef]
- Olate, E.; Jiménez-Gómez, J.M.; Holuigue, L.; Salinas, J. NPR1 mediates a novel regulatory pathway in cold acclimation by interacting with HSFA1 factors. Nat. Plants 2018, 4, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, C.M.; Doherty, C.J.; Gilmour, S.J.; Kim, Y.; Thomashow, M.F. Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. Plant J. 2015, 82, 193–207. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, X.; Liu, F.; Wang, Y.; Bi, H.; Ai, X. Hydrogen Sulfide Improves the cold stress resistance through the CsARF5-CsDREB3 module in cucumber. Int. J. Mol. Sci. 2021, 22, 13229. [Google Scholar] [CrossRef]
- Lei, Y.; He, H.; Raza, A.; Liu, Z.; Xiaoyu, D.; Guijuan, W.; Yan, L.; Yong, C.; Xiling, Z. Exogenous melatonin confers cold tolerance in rapeseed (Brassica napus L.) seedlings by improving antioxidants and genes expression. Plant Signal Behav. 2022, 17, 2129289. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Dong, D.; Li, Y.; Wang, X.; Guo, L.; Liu, L.; Dong, X.; Li, X.; Yuan, X.; Ren, S.; et al. Heat shock-induced cold acclimation in cucumber through CsHSFA1d-activated JA biosynthesis and signaling. Plant J. 2022, 111, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; An, J.; Yin, M.; Jia, X.; Guan, Y.; He, F.; Hu, J. Cold plasma treatment and exogenous salicylic acid priming enhances salinity tolerance of Oryza sativa seedlings. Protoplasma 2019, 256, 9–99. [Google Scholar]
- Zhao, H.; Zhang, K.; Zhou, X.; Xi, L.; Wang, Y.; Xu, H.; Pan, T.; Zou, Z. Melatonin alleviates chilling stress in cucumber seedlings by up-regulation of CsZat12 and modulation of polyamine and abscisic acid metabolism. Sci. Rep. 2017, 7, 4998. [Google Scholar] [CrossRef]
- Yang, N.; Sun, K.; Wang, X.; Wang, K.; Kong, X.; Gao, J.; Wen, D. Melatonin participates in selenium-enhanced cold tolerance of cucumber seedlings. Front. Plant Sci. 2021, 12, 786043. [Google Scholar] [CrossRef]
- Xu, X.Y.; Yu, J.H.; Xie, J.M.; Hu, L.L.; Li, J. [Effects of exogenous salicylic acid and brassinolide on photosynthesis of cucumber seedlings under low temperature stress]. Ying Yong Sheng Tai Xue Bao 2016, 27, 3009–3015. [Google Scholar] [PubMed]
- Khalid, M.; Rehman, H.M.; Ahmed, N.; Nawaz, S.; Saleem, F.; Ahmad, S.; Uzair, M.; Rana, I.A.; Atif, R.M.; Zaman, Q.U.; et al. Using exogenous melatonin, glutathione, proline, and glycine betaine treatments to combat abiotic stresses in crops. Int. J. Mol. Sci. 2022, 23, 12913. [Google Scholar] [CrossRef]
- Allard, F.; Houde, M.; Kröl, M.; Ivanov, A.; Huner, N.P.; Sarhan, F. Betaine improves freezing tolerance in wheat. Plant Cell Physiol. 1998, 39, 1194–1202. [Google Scholar] [CrossRef]
- Min, K.; Cho, Y.; Kim, E.; Lee, M.; Lee, S.R. Exogenous Glycine betaine application improves freezing tolerance of Cabbage (Brassica oleracea L.) leaves. Plants 2021, 10, 2821. [Google Scholar] [CrossRef]
- Park, E.J.; Jeknic, Z.; Chen, T.H. Exogenous application of glycinebetaine increases chilling tolerance in tomato plants. Plant Cell Physiol. 2006, 47, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Rajashekar, C.B. Glycine betaine involvement in freezing tolerance and water stress in Arabidopsis thaliana. Environ. Exp. Bot. 2001, 46, 21–28. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, T.; Liu, Y.; Wang, J.; Wang, Q.; Zhu, W. Effect of exogenous glycine betaine on the germination of tomato seeds under cold stress. Int. J. Mol. Sci. 2022, 23, 10474. [Google Scholar] [CrossRef]
- Huang, C.; Tian, Y.; Zhang, B.; Hassan, M.J.; Li, Z.; Zhu, Y. Chitosan (CTS) alleviates heat-Induced leaf senescence in creeping bentgrass by regulating chlorophyll metabolism, antioxidant defense, and the heat shock pathway. Molecules 2021, 26, 5337. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhang, X.; Merewitz, E.; Peng, Y.; Ma, X.; Huang, L.; Yan, Y. Metabolic pathways regulated by chitosan contributing to drought resistance in white clover. J. Proteome Res. 2017, 16, 3039–3052. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, T.; Liang, L.; Li, Z.; Hassan, M.J.; Peng, Y.; Wang, D. Enhanced photosynthesis, carbohydrates, and energy metabolism associated with chitosan-induced drought tolerance in creeping bentgrass. Crop. Sci. 2020, 60, 1064–1076. [Google Scholar] [CrossRef]
- Yang, F.; Hu, J.; Li, J.; Wu, X.; Qian, Y. Chitosan enhances leaf membrane stability and antioxidant enzyme activities in apple seedlings under drought stress. Plant Growth Regul. 2009, 58, 131–136. [Google Scholar] [CrossRef]
- ALKahtani, M.D.F.; Attia, K.A.; Hafez, Y.M.; Khan, N.; Eid, A.M.; Ali, M.A.M.; Abdelaal, K.A.A. Chlorophyll fluorescence parameters and antioxidant defense system can display salt tolerance of salt acclimated sweet pepper plants treated with chitosan and plant growth promoting rhizobacteria. Agronomy 2020, 10, 1180. [Google Scholar] [CrossRef]
- Pongprayoon, W.; Roytrakul, S.; Pichayangkura, R.; Chadchawan, S. The role of hydrogen peroxide in chitosan-induced resistance to osmotic stress in rice (Oryza sativa L.). Plant Growth Regul. 2013, 70, 159–173. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Y.; Bu, N.; Li, N.; Liu, T. Physiological characters of oligochitosan on alleviating Cd toxicity of wheat seedling. Environ. Sci. Technol. 2010, 33, 31e34. [Google Scholar]
- Xiao, L.; Kuang, Y.; Qin, C. Inflfluence of oligochitosan on some physiological and biochemical characteristics of Chinese Cabbage under the stress of cadmium. North Hortic. 2012, 17, 27e30. [Google Scholar]
- Zong, H.; Li, K.; Liu, S.; Song, L.; Xing, R.; Chen, X.; Li, P. Improvement in cadmium tolerance of edible rape (Brassica rapa L.) with exogenous application of chitooligosaccharide. Chemosphere 2017, 181, 92–100. [Google Scholar] [CrossRef]
- Zou, P.; Li, K.; Liu, S.; Xing, R.; Qin, Y.; Yu, H.; Zhou, M.; Li, P. Effect of chitooligosaccharides with different degrees of acetylation on wheat seedlings under salt stress. Carbohydr. Polym. 2015, 126, 62e69. [Google Scholar] [CrossRef]
- Chen, X.; Ding, Y.; Yang, Y.; Song, C.; Wang, B.; Yang, S.; Guo, Y.; Gong, Z. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 2021, 63, 53–78. [Google Scholar] [CrossRef]
- Yang, T.; Shad Ali, G.; Yang, L.; Du, L.; Reddy, A.S.; Poovaiah, B.W. Calcium/calmodulin regulated receptor-like kinase CRLK1 interacts with MEKK1 in plants. Plant Signal Behav. 2010, 5, 991–994. [Google Scholar] [CrossRef]
- Du, X.; Jin, Z.; Liu, D.; Yang, G.; Pei, Y. Hydrogen sulfide alleviates the cold stress through MPK4 in Arabidopsis thaliana. Plant Physiol. Biochem. 2017, 120, 112–119. [Google Scholar] [CrossRef]
- Li, H.; Ding, Y.; Shi, Y.; Zhang, X.; Zhang, S.; Gong, Z.; Yang, S. MPK3- and MPK6-mediated ICE1 phosphorylation negatively regulates ICE1 stability and freezing tolerance in Arabidopsis. Dev. Cell 2017, 43, 630–642.e4. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, P.; Si, T.; Hsu, C.C.; Wang, L.; Zayed, O.; Yu, Z.; Zhu, Y.; Dong, J.; Tao, W.A.; et al. MAP kinase cascades regulate the cold response by modulating ICE1 protein stability. Dev. Cell 2017, 43, 618–629.e5. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, C.; Wang, Y.; Ye, L.; Wu, J.; Chen, L.; Zou, T.; Lu, G. Genome-wide identification of MAPK, MAPKK, and MAPKKK gene families and transcriptional profiling analysis during development and stress response in cucumber. BMC Genom. 2015, 16, 386. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Barros, J.; Dixon, R.A. Plant phenylalanine/tyrosine ammonia-lyases. Trends Plant Sci. 2020, 25, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, Q.; Liu, G.; Xu, N.; Yang, Y.; Zeng, W.; Chen, A.; Wang, S. Integrated analysis of transcriptomic andmetabolomic data reveals critical metabolic pathways involved in polyphenol biosynthesis in Nicotiana tabacumunder chilling stress. Funct. Plant Biol. 2018, 46, 30–43. [Google Scholar] [CrossRef]
- Sun, F.; Chen, Z.; Zhang, Q.; Wan, Y.; Hu, R.; Shen, S.; Chen, S.; Yin, N.; Tang, Y.; Liang, Y.; et al. Genome-wide identification of the TIFY gene family in Brassiceae and its potential association with heavy metal stress in rapeseed. Plants 2022, 11, 667. [Google Scholar] [CrossRef]
- Huang, Z.; Jin, S.H.; Guo, H.D.; Zhong, X.J.; He, J.; Li, X.; Jiang, M.Y.; Yu, X.F.; Long, H.; Ma, M.D.; et al. Genome-wide identification and characterization of TIFY family genes in Moso Bamboo (Phyllostachys edulis) and expression profiling analysis under dehydration and cold stresses. PeerJ 2016, 4, e2620. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zheng, L.; Jin, L.G.; Liu, Y.X.; Kong, Y.N.; Wang, Y.X.; Yu, T.F.; Chen, J.; Zhou, Y.B.; Chen, M.; et al. Genome-wide analysis of the soybean TIFY family and identification of GmTIFY10e and GmTIFY10g response to salt stress. Front. Plant Sci. 2022, 13, 845314. [Google Scholar] [CrossRef]
- Singh, P.; Mukhopadhyay, K. Comprehensive molecular dissection of TIFY Transcription factors reveal their dynamic responses to biotic and abiotic tress in wheat (Triticum aestivum L.). Sci. Rep. 2021, 11, 9739. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.H.; Lee, S.C. Function of ABA in stomatal defense against biotic and drought stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Jian, C.; Cheng, X.; Chen, B.; Mei, F.; Li, F.; Zhang, Y.; Li, S.; Du, L.; Li, T.; et al. The wheat ABA receptor gene TaPYL1-1B contributes to drought tolerance and grain yield by increasing water-use efficiency. Plant Biotechnol. J. 2022, 20, 846–861. [Google Scholar] [CrossRef] [PubMed]
- Nian, L.; Zhang, X.; Yi, X.; Liu, X.; Ain, N.U.; Yang, Y.; Li, X.; Haider, F.U.; Zhu, X. Genome-wide identification of ABA receptor PYL/RCAR gene family and their response to cold stress in Medicago sativa L. Physiol. Mol. Biol. Plants 2021, 27, 1979–1995. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Kuang, Y.; Lin, Y.; Guo, Y.; Li, H.; Fan, P.; Li, S.; Liang, Z. Overexpression of grape ABA receptor gene VaPYL4 enhances tolerance to multiple abiotic stresses in Arabidopsis. BMC Plant Biol. 2022, 22, 271. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Z.; Li, X.; Lv, T.; Liu, H.; Wang, L.; Niu, H.; Bu, Q. Characterization and functional analysis of pyrabactin resistance-like abscisic acid receptor family in rice. Rice 2015, 8, 28. [Google Scholar] [CrossRef]
- Yu, J.; Ge, H.; Wang, X.; Tang, R.; Wang, Y.; Zhao, F.; Lan, W.; Luan, S.; Yang, L. Overexpression of pyrabactin resistance-like abscisic acid receptors enhances drought, osmotic, and cold tolerance in transgenic poplars. Front. Plant Sci. 2017, 8, 752. [Google Scholar] [CrossRef]
- Zhao, Y.; Chan, Z.; Gao, J.; Xing, L.; Cao, M.; Yu, C.; Hu, Y.; You, J.; Shi, H.; Zhu, Y.; et al. ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc. Natl. Acad. Sci. USA 2016, 113, 1949–1954. [Google Scholar] [CrossRef]
- Zhang, Q.; Kong, X.; Yu, Q.; Ding, Y.; Li, X.; Yang, Y. Responses of PYR/PYL/RCAR ABA receptors to contrasting stresses, heat and cold in Arabidopsis. Plant Signal Behav. 2019, 14, 1670596. [Google Scholar] [CrossRef]
- Anwar, A.; Bai, L.; Miao, L.; Liu, Y.; Li, S.; Yu, X.; Li, Y. 24-epibrassinolide ameliorates endogenous hormone levels to enhance low-temperature stress tolerance in cucumber seedlings. Int. J. Mol. Sci. 2018, 19, 2497. [Google Scholar] [CrossRef]
- Yousaf, M.F.; Demirel, U.; Naeem, M.; Çalışkan, M.E. Association mapping reveals novel genomic regions controlling some root and stolon traits in tetraploid potato (Solanum tuberosum L.). 3 Biotech 2021, 11, 174. [Google Scholar] [CrossRef]
- Jungklang, J.; Saengnil, K.; Uthaibutra, J. Effects of water-deficit stress and paclobutrazol on growth, relative water content, electrolyte leakage, proline content and some antioxidant changes in Curcuma alismatifolia Gagnep. cv. Chiang Mai Pink. Saudi. J. Biol. Sci. 2017, 24, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The gene ontology consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 3, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]



| Treatments | Plant Height (cm) | Stem Diameter (mm) | Ground Fresh Weight (g) | Ground Dry Weight (g) | Underground Fresh Weight (g) | Underground Dry Weight (g) |
|---|---|---|---|---|---|---|
| NK | 16.57 ± 0.29 a | 4.27 ± 0.09 a | 7.90 ± 0.36 a | 0.68 ± 0.01 a | 1.45 ± 0.07 a | 0.10 ± 0.058 a |
| LK | 13.30 ± 0.38 e | 3.24 ± 0.05 e | 5.31 ± 0.15 bcd | 0.53 ± 0.01 bc | 1.03 ± 0.11 c | 0.07 ± 0.001 bc |
| GB5 | 14.57 ± 0.52 cd | 3.83 ± 0.06 bcd | 5.23 ± 0.09 cde | 0.53 ± 0.06 bc | 1.25 ± 0.03 abc | 0.07 ± 0.003 bc |
| GB10 | 16.00 ± 0.52 ab | 3.94 ± 0.02 bc | 5.99 ± 0.13 bc | 0.61 ± 0.04 abc | 1.30 ± 0.14 abc | 0.09 ± 0.06 ab |
| GB25 | 14.37 ± 0.37 cd | 3.66 ± 0.03 cd | 5.41 ± 0.16 bcd | 0.56 ± 0.04 bc | 1.25 ± 0.04 abc | 0.07 ± 003 bc |
| CTS25 | 13.87 ± 0.43 cde | 3.82 ± 0.07 bc | 5.11 ± 0.02 de | 0.51 ± 0.06 c | 1.17 ± 0.03 bc | 0.06 ± 0.005 c |
| CTS50 | 13.97 ± 0.20 cde | 3.95 ± 0.13 bc | 5.43 ± 0.28 bcd | 0.55 ± 0.03 bc | 1.30 ± 0.06 abc | 0.09 ± 0.01 ab |
| CTS100 | 13.97 ± 0.58 cde | 3.67 ± 0.10 cd | 4.50 ± 0.32 e | 0.53 ± 0.02 bc | 1.20 ± 0.09 bc | 0.08 ± 0.05 abc |
| COS25 | 14.23 ± 0.62 cde | 3.59 ± 0.21 d | 5.63 ± 0.09 bcd | 0.57 ± 0.02 bc | 1.17 ± 0.07 abc | 0.07 ± 0.003 bc |
| COS50 | 15.07 ± 0.47 bc | 3.99 ± 0.09 ab | 6.06 ± 0.18 b | 0.62 ± 0.03 ab | 1.40 ± 0.07 ab | 0.09 ± 0.006 ab |
| COS100 | 13.53 ± 0.23 de | 3.94 ± 0.04 bc | 5.87 ± 0.43 bcd | 0.60 ± 0.03 abc | 1.11 ± 0.01 c | 0.08 ± 0.008 ab |
| Sample | Raw Data | Valid Data | Valid Ratio | Q20% | Q30% | GC Content% | Mapped Reads | Unique Mapped Reads |
|---|---|---|---|---|---|---|---|---|
| LC0_1 | 42,126,804 | 41,188,626 | 97.77 | 99.99 | 98.72 | 43.50 | 40,359,532 | 34,784,941 |
| LC0_2 | 43,493,534 | 42,687,216 | 98.15 | 99.99 | 98.73 | 44.00 | 41,817,716 | 35,960,624 |
| LC0_3 | 50,654,312 | 49,472,472 | 97.67 | 99.99 | 98.70 | 44.00 | 48,569,549 | 41,786,964 |
| LC3_1 | 40,601,628 | 39,681,714 | 97.73 | 99.99 | 98.64 | 42.50 | 38,854,745 | 33,562,509 |
| LC3_2 | 39,968,552 | 39,014,400 | 97.61 | 99.99 | 98.58 | 43.00 | 38,175,803 | 33,057,422 |
| LC3_3 | 45,104,026 | 43,962,284 | 97.47 | 99.99 | 98.58 | 42.50 | 42,832,193 | 36,969,503 |
| LC12_1 | 40,735,332 | 39,647,658 | 97.33 | 99.97 | 97.83 | 43.50 | 38,561,473 | 32,787,604 |
| LC12_2 | 46,820,294 | 45,474,216 | 97.13 | 99.99 | 98.70 | 44.00 | 44,594,127 | 38,804,478 |
| LC12_3 | 40,971,728 | 40,014,628 | 97.66 | 99.98 | 98.58 | 43.50 | 39,128,642 | 33,963,021 |
| LC24_1 | 50,827,142 | 49,679,218 | 97.74 | 99.97 | 97.83 | 43.00 | 48,135,263 | 40,328,014 |
| LC24_2 | 49,186,364 | 47,684,894 | 96.95 | 99.97 | 97.81 | 42.50 | 45,853,350 | 38,741,200 |
| LC24_3 | 46,031,094 | 44,790,962 | 97.31 | 99.97 | 97.90 | 42.50 | 43,332,675 | 36,565,195 |
| L0_1 | 41,077,610 | 40,257,174 | 98.00 | 99.99 | 98.55 | 43.00 | 39,308,565 | 33,687,253 |
| L0_2 | 43,754,034 | 42,721,610 | 97.64 | 99.99 | 98.63 | 43.00 | 41,684,077 | 35,793,435 |
| L0_3 | 44,196,938 | 43,303,516 | 97.98 | 99.99 | 98.58 | 43.50 | 42,289,716 | 36,321,136 |
| L3_1 | 39,312,430 | 38,223,758 | 97.23 | 99.99 | 98.73 | 44.00 | 37,459,192 | 32,346,810 |
| L3_2 | 38,853,612 | 37,929,152 | 97.62 | 99.99 | 98.70 | 44.00 | 37,171,141 | 32,123,659 |
| L3_3 | 41,441,694 | 40,526,978 | 97.79 | 99.99 | 98.60 | 44.00 | 39,660,858 | 34,218,611 |
| L12_1 | 39,318,436 | 38,390,548 | 97.64 | 99.99 | 98.70 | 44.00 | 37,604,628 | 32,743,831 |
| L12_2 | 36,926,050 | 36,050,098 | 97.63 | 99.98 | 97.99 | 44.50 | 35,133,412 | 29,903,919 |
| L12_3 | 39,152,442 | 38,130,792 | 97.39 | 99.98 | 97.94 | 44.50 | 37,170,463 | 31,603,798 |
| L24_1 | 37,801,080 | 36,925,402 | 97.68 | 99.97 | 98.60 | 44.00 | 36,196,280 | 31,135,293 |
| L24_2 | 49,462,012 | 48,542,266 | 98.14 | 99.98 | 98.69 | 44.00 | 47,587,324 | 41,034,627 |
| L24_3 | 52,902,880 | 51,657,496 | 97.65 | 99.97 | 97.90 | 43.50 | 50,157,853 | 42,120,150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, C.; Li, N.; Wang, Y.; Yu, X.; Yang, L.; Cao, R.; Ye, X. Integrated Physiological and Transcriptomic Analyses Revealed Improved Cold Tolerance in Cucumber (Cucumis sativus L.) by Exogenous Chitosan Oligosaccharide. Int. J. Mol. Sci. 2023, 24, 6202. https://doi.org/10.3390/ijms24076202
Tan C, Li N, Wang Y, Yu X, Yang L, Cao R, Ye X. Integrated Physiological and Transcriptomic Analyses Revealed Improved Cold Tolerance in Cucumber (Cucumis sativus L.) by Exogenous Chitosan Oligosaccharide. International Journal of Molecular Sciences. 2023; 24(7):6202. https://doi.org/10.3390/ijms24076202
Chicago/Turabian StyleTan, Chong, Na Li, Yidan Wang, Xuejing Yu, Lu Yang, Ruifang Cao, and Xueling Ye. 2023. "Integrated Physiological and Transcriptomic Analyses Revealed Improved Cold Tolerance in Cucumber (Cucumis sativus L.) by Exogenous Chitosan Oligosaccharide" International Journal of Molecular Sciences 24, no. 7: 6202. https://doi.org/10.3390/ijms24076202
APA StyleTan, C., Li, N., Wang, Y., Yu, X., Yang, L., Cao, R., & Ye, X. (2023). Integrated Physiological and Transcriptomic Analyses Revealed Improved Cold Tolerance in Cucumber (Cucumis sativus L.) by Exogenous Chitosan Oligosaccharide. International Journal of Molecular Sciences, 24(7), 6202. https://doi.org/10.3390/ijms24076202





