CsCIPK11-Regulated Metalloprotease CsFtsH5 Mediates the Cold Response of Tea Plants
Abstract
1. Introduction
2. Results
2.1. Sequence and Phylogenetic Analyses of CsFtsH5 in Tea Plants
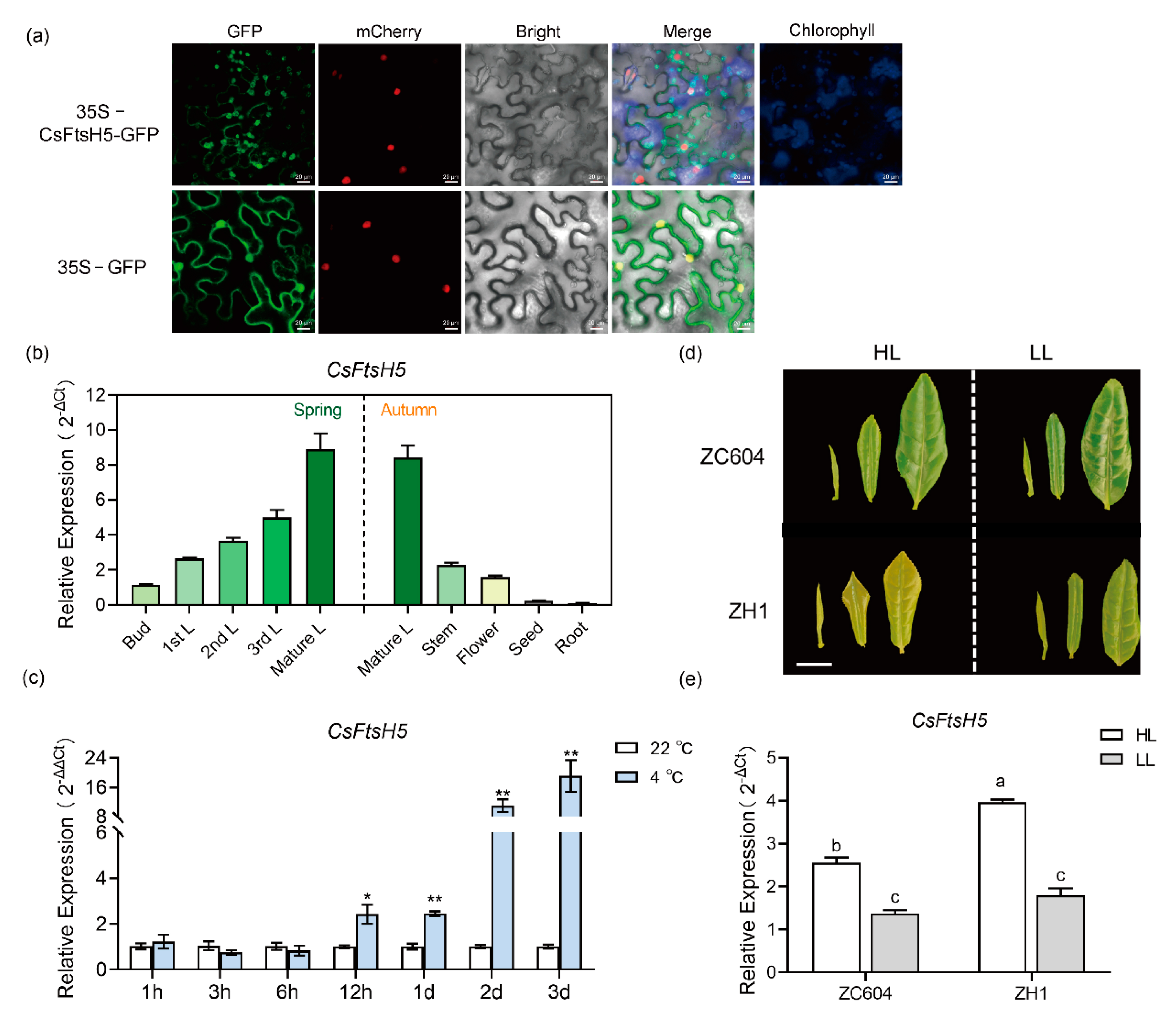
2.2. Expression Pattern and Sub-Cellular Localization of CsFtsH5
2.3. CsFtsH5 Down-Regulation Causes Tea Leaf Hypersensitivity to Cold Stress
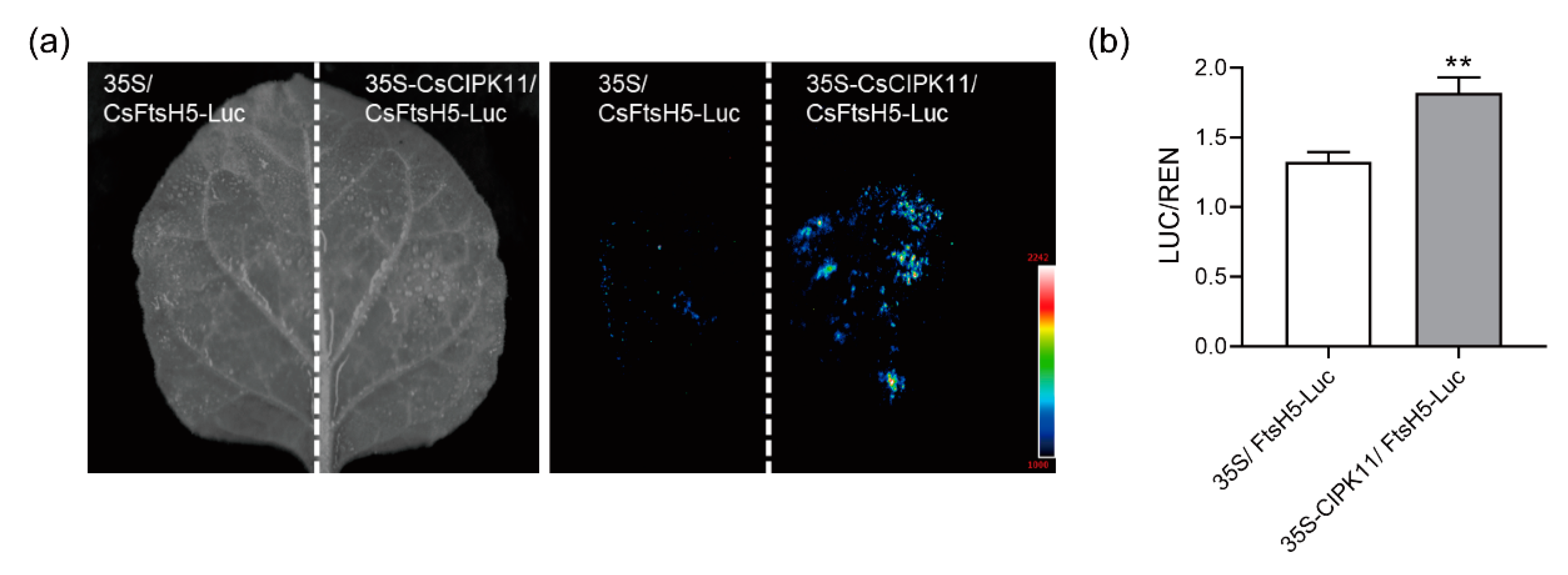
2.4. Interaction between CsFtsH5 and CsCIPK11
2.5. CsCIPK11 May Contribute to CsFtsH5 Protein Stability
3. Discussion
3.1. Metalloprotease CsFtsH5 of Tea Plant Is Homologous to FtsH5
3.2. Repressing CsFtsH5 Facilitated the Cold Sensitivity of Tea Plants
3.3. Regulation of CsFtsH5 by CsCIPK11 Likely Mediates the Cold Response of Tea Plants
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. RNA Extraction and RT-qPCR Reactions
4.3. Sequence Alignment and Phylogenetic Tree Analysis
4.4. Subcellular Localization of CsFtsH5
4.5. Down-Regulation of CsFtsH5 Gene in Tea Plant
4.6. Low-Temperature Treatment and Measurement of Fv/Fm and REL
4.7. Y2H
4.8. LCI Analysis
4.9. Dual-Luc Assays
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zimorski, V.; Ku, C.; Martin, W.F.; Gould, S.B. Endosymbiotic theory for organelle origins. Curr. Opin. Microbiol. 2014, 22, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Leister, D. Chloroplast research in the genomic age. Trends Genet. 2003, 19, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.P.; Francke, C.; van Gorkom, H.J.; Ghanotakis, D.F. Destructive role of singlet oxygen during aerobic illumi-nation of the photosystem II core complex. Biochim. Biophys. Acta 1994, 1186, 81–90. [Google Scholar] [CrossRef]
- Nixon, P.J.; Michoux, F.; Yu, J.; Boehm, M.; Komenda, J. Recent advances in understanding the assembly and repair of photosystem II. Ann. Bot. 2010, 106, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Järvi, S.; Suorsa, M.; Aro, E.-M. Photosystem II repair in plant chloroplasts—Regulation, assisting proteins and shared components with photosystem II biogenesis. Biochim. Biophys. Acta (BBA) Bioenerg. 2015, 1847, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Sakamoto, W. Protein quality control in chloroplasts: A current model of D1 protein degradation in the photo-system II repair cycle. J. Biochem. 2009, 146, 463–469. [Google Scholar] [CrossRef]
- Bailey, S.; Thompson, E.; Nixon, P.J.; Horton, P.; Mullineaux, C.W.; Robinson, C.; Mann, N.H. A Critical Role for the Var2 FtsH Homologue of Arabidopsis thaliana in the Photosystem II Repair Cycle in Vivo. J. Biol. Chem. 2002, 277, 2006–2011. [Google Scholar] [CrossRef]
- Kato, Y.; Miura, E.; Ido, K.; Ifuku, K.; Sakamoto, W. The Variegated Mutants Lacking Chloroplastic FtsHs Are Defective in D1 Degradation and Accumulate Reactive Oxygen Species. Plant Physiol. 2009, 151, 1790–1801. [Google Scholar] [CrossRef]
- Suno, R.; Niwa, H.; Tsuchiya, D.; Zhang, X.; Yoshida, M.; Morikawa, K. Structure of the Whole Cytosolic Region of ATP-Dependent Protease FtsH. Mol. Cell 2006, 22, 575–585. [Google Scholar] [CrossRef]
- Santos, D.; Almeida, D.F.D. Isolation and characterization of a new temperature-sensitive cell division mutant of esch-erichia coli K-12. J. Bacteriol. 1975, 124, 1502–1507. [Google Scholar] [CrossRef]
- Adam, Z.; Zaltsman, A.; Sinvany-Villalobo, G.; Sakamoto, W. FtsH proteases in chloroplasts and cyanobacteria. Physiol. Plant. 2005, 123, 386–390. [Google Scholar] [CrossRef]
- Tatsuta, T.; Langer, T. AAA proteases in mitochondria: Diverse functions of membrane-bound proteolytic machines. Res. Microbiol. 2009, 160, 711–717. [Google Scholar] [CrossRef]
- Sakamoto, W.; Zaltsman, A.; Adam, Z.; Takahashi, Y. Coordinated regulation and complex formation of yellow varie-gated1 and yellow variegated2, chloroplastic FtsH metalloproteases involved in the repair cycle of photosystem II in Ara-bidopsis thylakoid membranes. Plant Cell 2003, 15, 2843–2855. [Google Scholar] [CrossRef] [PubMed]
- Zaltsman, A.; Ori, N.; Adam, Z. Two types of FtsH protease subunits are required for chloroplast biogenesis and pho-tosystem II repair in Arabidopsis. Plant Cell 2005, 17, 2782–2790. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, W.; Tamura, T.; Hanba-Tomita, Y.; Murata, M. The VAR1 locus of Arabidopsis encodes a chloroplastic FtsH and is responsible for leaf variegation in the mutant alleles. Genes Cells Devoted Mol. Cell. Mech. 2002, 7, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Lee, K.P.; Dogra, V.; Zhang, S.; Liu, K.; Caceres-Moreno, C.; Lv, S.; Xing, W.; Kato, Y.; Sakamoto, W.; et al. Impaired PSII Proteostasis Promotes Retrograde Signaling via Salicylic Acid. Plant Physiol. 2019, 180, 2182–2197. [Google Scholar] [CrossRef]
- Wang, Q.; Sullivan, R.W.; Kight, A.; Henry, R.L.; Huang, J.; Jones, A.M.; Korth, K.L. Deletion of the Chloroplast-Localized Thylakoid Formation1 Gene Product in Arabidopsis Leads to Deficient Thylakoid Formation and Variegated Leaves. Plant Physiol. 2004, 136, 3594–3604. [Google Scholar] [CrossRef]
- Huang, W.; Chen, Q.; Zhu, Y.; Hu, F.; Zhang, L.; Ma, Z.; He, Z.; Huang, J. Arabidopsis thylakoid formation 1 is a criti-cal regulator for dynamics of PSII–LHCII complexes in leaf senescence and excess light. Mol. Plant 2013, 6, 1673–1691. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, Q.; Wu, W.; Cheng, Y.; Hu, G.; Hu, F.; Sun, Y.; Zhu, Y.; Sakamoto, W.; Huang, J. Activation of the heterotrimeric G protein alpha-subunit GPA1 suppresses the ftsh-mediated inhibition of chloroplast development in Arabidop-sis. Plant J. Cell Mol. Biol. 2009, 58, 1041–1053. [Google Scholar] [CrossRef]
- Kato, Y.; Hyodo, K.; Sakamoto, W. The Photosystem II Repair Cycle Requires FtsH Turnover through the EngA GTPase. Plant Physiol. 2018, 178, 596–611. [Google Scholar] [CrossRef]
- Lopes, K.L.; Rodrigues, R.A.O.; Silva, M.C.; Braga, W.G.S.; Silva-Filho, M.C. The Zinc-Finger Thylakoid-Membrane Protein FIP Is Involved with Abiotic Stress Response in Arabidopsis thaliana. Front. Plant Sci. 2018, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Stael, S.; Rocha, A.G.; Wimberger, T.; Anrather, D.; Vothknecht, U.C.; Teige, M. Cross-talk between calcium signalling and protein phosphorylation at the thylakoid. J. Exp. Bot. 2011, 63, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Schliebner, I.; Pribil, M.; Zühlke, J.; Dietzmann, A.; Leister, D. A Survey of chloroplast protein kinases and phospha-tases in Arabidopsis thaliana. Curr. Genom. 2008, 9, 184–190. [Google Scholar] [CrossRef]
- Kato, Y.; Sakamoto, W. Phosphorylation of the Chloroplastic Metalloprotease FtsH in Arabidopsis Characterized by Phos-Tag SDS-PAGE. Front. Plant Sci. 2019, 10, 1080. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-C.; Zhao, Q.-Y.; Ma, C.-L.; Zhang, Z.-H.; Cao, H.-L.; Kong, Y.-M.; Yue, C.; Hao, X.-Y.; Chen, L.; Ma, J.-Q.; et al. Global transcriptome profiles of Camellia sinensis during cold acclimation. BMC Genom. 2013, 14, 415. [Google Scholar] [CrossRef]
- Wang, L.; Feng, X.; Yao, L.; Ding, C.; Lei, L.; Hao, X.; Li, N.; Zeng, J.; Yang, Y.; Wang, X. Characterization of CBL–CIPK signaling complexes and their involvement in cold response in tea plant. Plant Physiol. Biochem. 2020, 154, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Tomoyasu, T.; Yuki, T.; Morimura, S.; Mori, H.; Yamanaka, K.; Niki, H.; Hiraga, S.; Ogura, T. The Escherichia coli FtsH protein is a prokaryotic member of a protein family of putative ATPases involved in membrane functions, cell cycle control, and gene expression. J. Bacteriol. 1993, 175, 1344–1351. [Google Scholar] [CrossRef]
- Li, F.; Zhao, N.; Li, Z.; Xu, X.; Wang, Y.; Yang, X.; Liu, S.-S.; Wang, A.; Zhou, X. A calmodulin-like protein suppresses RNA silencing and promotes geminivirus infection by degrading SGS3 via the autophagy pathway in Nicotiana benthamiana. PLoS Pathog. 2017, 13, e1006213. [Google Scholar] [CrossRef]
- Wang, L.; Cao, H.; Chen, C.; Yue, C.; Hao, X.; Yang, Y.; Wang, X. Complementary transcriptomic and proteomic analyses of a chlorophyll-deficient tea plant cultivar reveal multiple metabolic pathway changes. J. Proteom. 2016, 130, 160–169. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, N.; Gao, T.; Jin, J.; Jing, T.; Wang, J.; Wu, Y.; Wan, X.; Schwab, W.; Song, C. Sesquiterpene glucosyla-tion mediated by glucosyltransferase UGT91Q2 is involved in the modulation of cold stress tolerance in tea plants. New Phytol. 2020, 226, 362–372. [Google Scholar] [CrossRef]
- Wang, L.; Di, T.; Peng, J.; Li, Y.; Li, N.; Hao, X.; Ding, C.; Huang, J.; Zeng, J.; Yang, Y.; et al. Comparative metabolomic analysis reveals the involvement of catechins in adaptation mechanism to cold stress in tea plant (Camellia sinensis var. sinensis). Environ. Exp. Bot. 2022, 201, 104978. [Google Scholar] [CrossRef]
- Komenda, J.; Tichy, M.; Prásil, O.; Knoppová, J.; Kuviková, S.; de Vries, R.; Nixon, P.J. The exposed N-terminal tail of the D1 subunit is required for rapid D1 degradation during photosystem II repair in Synechocystis sp PCC 6803. Plant Cell 2007, 19, 2839–2854. [Google Scholar] [CrossRef] [PubMed]
- Okuno, T.; Ogura, T. FtsH protease-mediated regulation of various cellular functions. Regul. Proteolysis Microorg. 2013, 66, 53–69. [Google Scholar] [CrossRef]
- Xu, K.; Zhu, J.; Zhai, H.; Wu, H.; Gao, Y.; Li, Y.; Zhu, X.; Xia, Z. A critical role of PvFtsH2 in the degradation of pho-todamaged D1 protein in common bean. Hortic. Res. 2021, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka-Nishimura, M.; Yamamoto, Y. Quality control of Photosystem II: The molecular basis for the action of FtsH protease and the dynamics of the thylakoid membranes. J. Photochem. Photobiol. B Biol. 2014, 137, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Chen, S.; Chen, Y.; Deng, Y.; Chen, F.; Zhang, F.; Wang, S. Anatomical and Physiological Differences and Differentially Expressed Genes Between the Green and Yellow Leaf Tissue in a Variegated Chrysanthemum Variety. Mol. Biotechnol. 2012, 54, 393–411. [Google Scholar] [CrossRef]
- Wagner, R.; Aigner, H.; Pružinská, A.; Jänkänpää, H.J.; Jansson, S.; Funk, C. Fitness analyses of Arabidopsis thaliana mutants depleted of FtsH metalloproteases and characterization of three FtsH6 deletion mutants exposed to high light stress, senescence and chilling. New Phytol. 2011, 191, 449–458. [Google Scholar] [CrossRef]
- Chen, J.; Burke, J.J.; Velten, J.; Xin, Z. FtsH11 protease plays a critical role in Arabidopsis thermotolerance. Plant J. 2006, 48, 73–84. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.; Yang, Z.; Li, W.; Hu, D.; Yu, H.; Li, X.; Cheng, H.; Kan, G.; Che, Z.; et al. GmFtsH25 overexpression increases soybean seed yield by enhancing photosynthesis and photo-synthates. J. Integr. Plant Biol. 2022. ahead of print. [Google Scholar]
- Yue, X.; Ke, X.; Shi, Y.; Li, Y.; Zhang, C.; Wang, Y.; Hou, X. Chloroplast inner envelope protein FtsH11 is involved in the adjustment of assembly of chloroplast ATP synthase under heat stress. Plant Cell Environ. 2023, 46, 850–864. [Google Scholar] [CrossRef]
- Hepler, P.K. Calcium: A Central Regulator of Plant Growth and Development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Horvath, D.P.; Chao, W.S.; Yang, Y.; Wang, X.; Xiao, B. Identification and Evaluation of Reliable Reference Genes for Quantitative Real-Time PCR Analysis in Tea Plant (Camellia sinensis (L.) O. Kuntze). Int. J. Mol. Sci. 2014, 15, 22155–22172. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Ding, C.; Hao, X.; Zeng, J.; Yang, Y.; Wang, X.; Wang, L. CsSWEET1a and CsSWEET17 Mediate Growth and Freezing Tolerance by Promoting Sugar Transport across the Plasma Membrane. Plant Cell Physiol. 2020, 61, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yao, L.; Hao, X.; Li, N.; Qian, W.; Yue, C.; Ding, C.; Zeng, J.; Yang, Y.; Wang, X. Tea plant SWEET transporters: Expression profiling, sugar transport, and the involvement of CsSWEET16 in modifying cold tolerance in Arabidopsis. Plant Mol. Biol. 2018, 96, 577–592. [Google Scholar] [CrossRef]
- Xu, F.; He, S.; Zhang, J.; Mao, Z.; Wang, W.; Li, T.; Hua, J.; Du, S.; Xu, P.; Li, L.; et al. Photoactivated CRY1 and phyB Interact Directly with AUX/IAA Proteins to Inhibit Auxin Signaling in Arabidopsis. Mol. Plant 2017, 11, 523–541. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, N.; Di, T.; Ding, C.; Li, X.; Wu, Y.; Hao, X.; Wang, Y.; Yang, Y.; Wang, X.; et al. The interaction of CsWRKY4 and CsOCP3 with CsICE1 regulates CsCBF1/3 and mediates stress response in tea plant (Camellia sinensis). Environ. Exp. Bot. 2022, 199, 104892. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di, T.; Wu, Y.; Peng, J.; Wang, J.; Wang, H.; He, M.; Li, N.; Hao, X.; Yang, Y.; Ni, D.; et al. CsCIPK11-Regulated Metalloprotease CsFtsH5 Mediates the Cold Response of Tea Plants. Int. J. Mol. Sci. 2023, 24, 6288. https://doi.org/10.3390/ijms24076288
Di T, Wu Y, Peng J, Wang J, Wang H, He M, Li N, Hao X, Yang Y, Ni D, et al. CsCIPK11-Regulated Metalloprotease CsFtsH5 Mediates the Cold Response of Tea Plants. International Journal of Molecular Sciences. 2023; 24(7):6288. https://doi.org/10.3390/ijms24076288
Chicago/Turabian StyleDi, Taimei, Yedie Wu, Jing Peng, Jie Wang, Haoqian Wang, Mingming He, Nana Li, Xinyuan Hao, Yajun Yang, Dejiang Ni, and et al. 2023. "CsCIPK11-Regulated Metalloprotease CsFtsH5 Mediates the Cold Response of Tea Plants" International Journal of Molecular Sciences 24, no. 7: 6288. https://doi.org/10.3390/ijms24076288
APA StyleDi, T., Wu, Y., Peng, J., Wang, J., Wang, H., He, M., Li, N., Hao, X., Yang, Y., Ni, D., Wang, L., & Wang, X. (2023). CsCIPK11-Regulated Metalloprotease CsFtsH5 Mediates the Cold Response of Tea Plants. International Journal of Molecular Sciences, 24(7), 6288. https://doi.org/10.3390/ijms24076288






