Cheminformatics-Based Study Identifies Potential Ebola VP40 Inhibitors
Abstract
1. Introduction
2. Results and Discussion
2.1. Protein Extraction and Preparation
2.1.1. Structure Remodeling
2.1.2. Energy Minimization of Structure
2.2. Binding Site Determination
2.3. Validation of Docking Protocol
2.4. Molecular Docking Studies
2.5. Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) Profiles of the Shortlisted Compounds
2.6. Protein–Ligand Interactions of Lead Compounds
2.7. Prediction of Biological Activities of Lead Compounds
2.7.1. PASS Predictions
2.7.2. Structural Similarity Search
2.7.3. Anti-Ebola Activity Prediction
2.8. Molecular Dynamics Simulations of Some Selected Compounds
2.8.1. Root-Mean-Squared Deviation (RMSD)
2.8.2. Root Mean Square Fluctuations (RMSF)
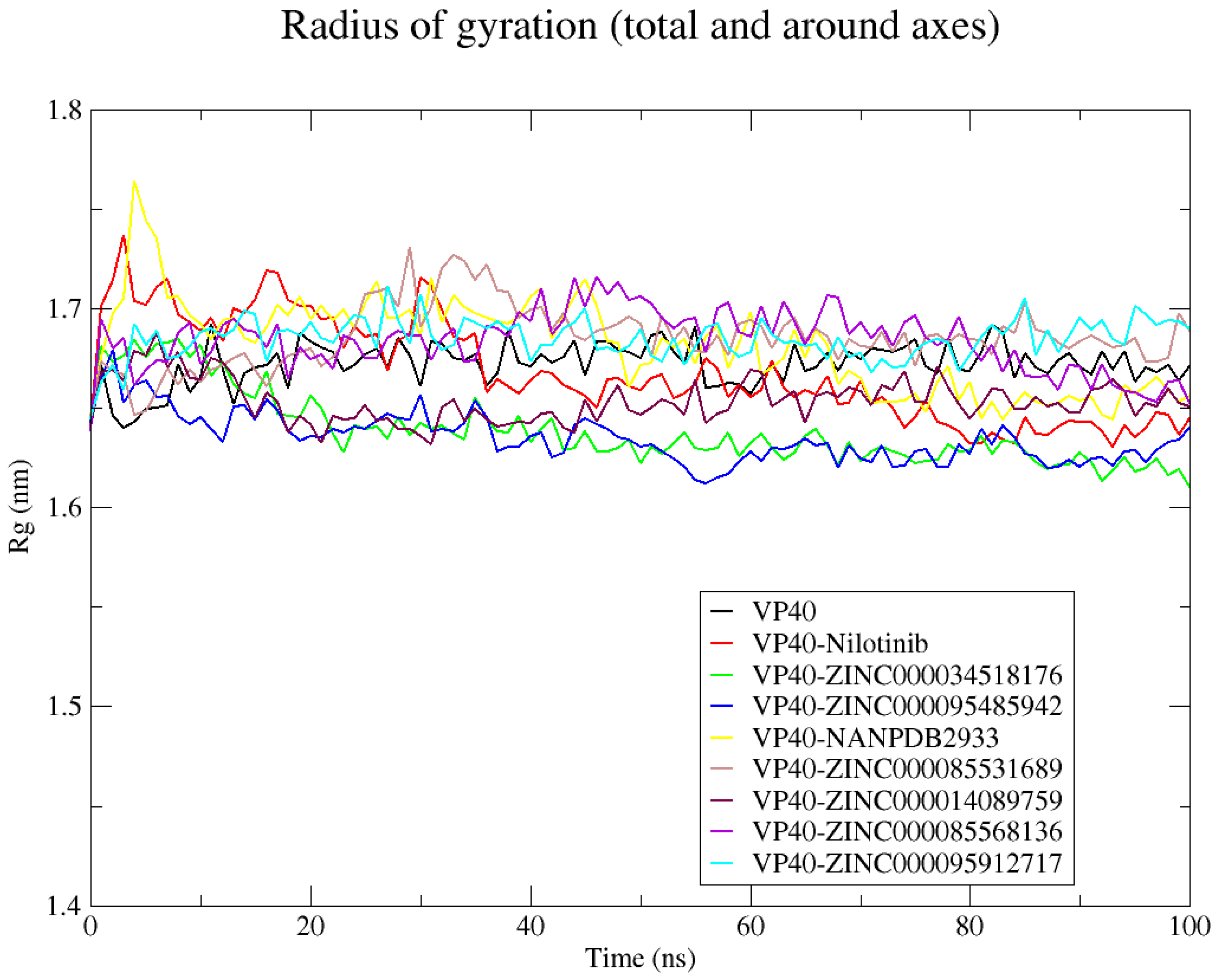
2.8.3. Radius of Gyration (Rg)
2.8.4. Hydrogen Bond Analysis
2.9. Evaluation of Lead Compounds Using MM/PBSA Computations
2.9.1. Contributing Energy Terms
2.9.2. Energy Decomposition per Residue
2.10. Origin and Sources of the Potential Lead Compounds
2.11. Limitations of the Study
3. Materials and Methods
3.1. Target Retrieval and Preparation
3.2. Binding Site Determination
3.3. Obtaining and Preparing Ligand Libraries
3.4. Docking Validation
3.5. Molecular Docking
3.6. Pharmacological and Toxicity Profiling of Molecules
3.7. Protein–Ligand Interaction
3.8. Prediction of Biological Activities of Lead Compounds
3.9. MD Simulations of VP40 and VP40–Ligand Complexes
3.10. Evaluation of Lead Compounds Using MM/PBSA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koch, L.K.; Cunze, S.; Kochmann, J.; Klimpel, S. Bats as putative Zaire ebolavirus reservoir hosts and their habitat suitability in Africa. Sci. Rep. 2020, 10, 14268. [Google Scholar] [CrossRef] [PubMed]
- Eloy, P.; Laouénan, C.; Beavogui, A.H.; Keita, S.; Manchon, P.; Etard, J.-F.; Sissoko, D.; Mentré, F.; Malvy, D. High doses of favipiravir in two men survivors of Ebola virus disease carrying Ebola virus in semen in Guinea. Idcases 2022, 27, e01412. [Google Scholar] [CrossRef] [PubMed]
- Purpura, L.J.; Rogers, E.; Baller, A.; White, S.; Soka, M.; Choi, M.J.; Mahmoud, N.; Wasunna, C.; Massaquoi, M.; Kollie, J.; et al. Ebola Virus RNA in Semen from an HIV-Positive Survivor of Ebola. Emerg. Infect. Dis. 2017, 23, 714–715. [Google Scholar] [CrossRef] [PubMed]
- Uyeki, T.M.; Erickson, B.R.; Brown, S.; McElroy, A.; Cannon, D.; Gibbons, A.; Sealy, T.; Kainulainen, M.H.; Schuh, A.J.; Kraft, C.S.; et al. Ebola Virus Persistence in Semen of Male Survivors. Clin. Infect. Dis. 2016, 62, 1552–1555. [Google Scholar] [CrossRef]
- Deen, G.F.; Broutet, N.; Xu, W.; Knust, B.; Sesay, F.R.; McDonald, S.L.; Ervin, E.; Marrinan, J.E.; Gaillard, P.; Habib, N.; et al. Ebola RNA Persistence in Semen of Ebola Virus Disease Survivors—Final Report. N. Engl. J. Med. 2017, 377, 1428–1437. [Google Scholar] [CrossRef]
- Fischer, R.J.; Judson, S.; Miazgowicz, K.; Bushmaker, T.; Munster, V.J. Ebola Virus Persistence in Semen Ex Vivo. Emerg. Infect. Dis. 2016, 22, 289–291. [Google Scholar] [CrossRef]
- Jacob, S.T.; Crozier, I.; Fischer, W.A., 2nd; Hewlett, A.; Kraft, C.S.; Vega, M.A.; Soka, M.J.; Wahl, V.; Griffiths, A.; Bollinger, L.; et al. Ebola virus disease. Nat. Rev. Dis. Prim. 2020, 6, 13. [Google Scholar] [CrossRef]
- Geisbert, T.W.; Jahrling, P.B. Differentiation of filoviruses by electron microscopy. Virus Res. 1995, 39, 129–150. [Google Scholar] [CrossRef]
- Karthick, V.; Nagasundaram, N.; Doss, C.G.P.; Chakraborty, C.; Siva, R.; Lu, A.; Zhang, G.; Zhu, H. Virtual screening of the inhibitors targeting at the viral protein 40 of Ebola virus. Infect. Dis. Poverty 2016, 5, 12. [Google Scholar] [CrossRef]
- Aleksandrowicz, P.; Marzi, A.; Biedenkopf, N.; Beimforde, N.; Becker, S.; Hoenen, T.; Feldmann, H.; Schnittler, H.-J. Ebola Virus Enters Host Cells by Macropinocytosis and Clathrin-Mediated Endocytosis. J. Infect. Dis. 2011, 204, S957–S967. [Google Scholar] [CrossRef]
- Bharat, T.A.; Noda, T.; Riches, J.D.; Kraehling, V.; Kolesnikova, L.; Becker, S.; Kawaoka, Y.; Briggs, J.A. Structural dissection of Ebola virus and its assembly determinants using cryo-electron tomography. Proc. Natl. Acad. Sci. USA 2012, 109, 4275–4280. [Google Scholar] [CrossRef]
- Billioux, B.J.; Smith, B.; Nath, A. Neurological Complications of Ebola Virus Infection. Neurotherapeutics 2016, 13, 461–470. [Google Scholar] [CrossRef]
- MacDermott, N.; Herberg, J. Ebola: Lessons learned. Paediatr. Child Health 2016, 27, 128–134. [Google Scholar] [CrossRef]
- Johnson, R.F.; McCarthy, S.E.; Godlewski, P.J.; Harty, R.N. Ebola Virus VP35-VP40 Interaction Is Sufficient for Packaging 3E-5E Minigenome RNA into Virus-Like Particles. J. Virol. 2006, 80, 5135–5144. [Google Scholar] [CrossRef]
- Timmins, J.; Scianimanico, S.; Schoehn, G.; Weissenhorn, W. Vesicular Release of Ebola Virus Matrix Protein VP40. Virology 2001, 283, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Adu-Gyamfi, E.; Digman, M.A.; Gratton, E.; Stahelin, R.V. Investigation of Ebola VP40 Assembly and Oligomerization in Live Cells Using Number and Brightness Analysis. Biophys. J. 2012, 102, 2517–2525. [Google Scholar] [CrossRef]
- Stahelin, R.V. Membrane binding and bending in Ebola VP40 assembly and egress. Front. Microbiol. 2014, 5, 300. [Google Scholar] [CrossRef]
- Noda, T.; Sagara, H.; Suzuki, E.; Takada, A.; Kida, H.; Kawaoka, Y. Ebola Virus VP40 Drives the Formation of Virus-Like Filamentous Particles Along with GP. J. Virol. 2002, 76, 4855–4865. [Google Scholar] [CrossRef] [PubMed]
- Bornholdt, Z.A.; Noda, T.; Abelson, D.M.; Halfmann, P.; Wood, M.R.; Kawaoka, Y.; Saphire, E.O. Structural Rearrangement of Ebola Virus VP40 Begets Multiple Functions in the Virus Life Cycle. Cell 2013, 154, 763–774. [Google Scholar] [CrossRef]
- Gomis-Ruth, F.X.; Dessen, A.; Timmins, J.; Bracher, A.; Kolesnikowa, L.; Becker, S.; Klenk, H.-D.; Weissenhorn, W. The Matrix Protein VP40 from Ebola Virus Octamerizes into Pore-like Structures with Specific RNA Binding Properties. Structure 2003, 11, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Saxena, D.; Kaul, G.; Dasgupta, A.; Chopra, S. Atoltivimab/maftivimab/odesivimab (Inmazeb) combination to treat infection caused by Zaire ebolavirus. Drugs Today 2021, 57, 483. [Google Scholar] [CrossRef]
- Markham, A. REGN-EB3: First Approval. Drugs 2021, 81, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Hansen, F.; Feldmann, H.; Jarvis, M.A. Targeting Ebola virus replication through pharmaceutical intervention. Expert Opin. Investig. Drugs 2021, 30, 201–226. [Google Scholar] [CrossRef]
- Lee, A. Ansuvimab: First Approval. Drugs 2021, 81, 595–598. [Google Scholar] [CrossRef]
- Aschenbrenner, D.S. Monoclonal Antibody Approved to Treat Ebola. AJN Am. J. Nurs. 2021, 121, 22. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.X.; Garneau-Tsodikova, S. What are the drugs of the future? MedChemComm 2018, 9, 757–758. [Google Scholar] [CrossRef] [PubMed]
- Iversen, P.L.; Kane, C.D.; Zeng, X.; Panchal, R.G.; Warren, T.K.; Radoshitzky, S.R.; Kuhn, J.H.; Mudhasani, R.R.; Cooper, C.L.; Shurtleff, A.C.; et al. Recent successes in therapeutics for Ebola virus disease: No time for complacency. Lancet Infect. Dis. 2020, 20, e231–e237. [Google Scholar] [CrossRef]
- Krishnasamy, L.; Saikumar, C. Updates on Treatment of Ebola Virus Disease. Malays. J. Med Sci. 2015, 22, 54–57. [Google Scholar]
- Thi, E.P.; Mire, C.E.; Lee, A.C.H.; Geisbert, J.B.; Zhou, J.Z.; Agans, K.N.; Snead, N.M.; Deer, D.J.; Barnard, T.R.; Fenton, K.A.; et al. Lipid nanoparticle siRNA treatment of Ebola-virus-Makona-infected nonhuman primates. Nature 2015, 521, 362–365. [Google Scholar] [CrossRef]
- Taylor, R.; Kotian, P.; Warren, T.; Panchal, R.; Bavari, S.; Julander, J.; Dobo, S.; Rose, A.; El-Kattan, Y.; Taubenheim, B.; et al. BCX4430—A broad-spectrum antiviral adenosine nucleoside analog under development for the treatment of Ebola virus disease. J. Infect. Public Health 2016, 9, 220–226. [Google Scholar] [CrossRef]
- Warren, T.K.; Wells, J.; Panchal, R.G.; Stuthman, K.S.; Garza, N.L.; Van Tongeren, S.A.; Dong, L.; Retterer, C.J.; Eaton, B.P.; Pegoraro, G.; et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature 2014, 508, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Takahashi, K.; Fukuda, Y.; Kuno, M.; Kamiyama, T.; Kozaki, K.; Nomura, N.; Egawa, H.; Minami, S.; Watanabe, Y.; et al. In Vitro and In Vivo Activities of Anti-Influenza Virus Compound T-705. Antimicrob. Agents Chemother. 2002, 46, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Spurgers, K.B.; Sharkey, C.M.; Warfield, K.L.; Bavari, S. Oligonucleotide antiviral therapeutics: Antisense and RNA interference for highly pathogenic RNA viruses. Antivir. Res. 2008, 78, 26–36. [Google Scholar] [CrossRef]
- Xie, T.; Song, S.; Li, S.; Ouyang, L.; Xia, L.; Huang, J. Review of natural product databases. Cell Prolif. 2015, 48, 398–404. [Google Scholar] [CrossRef]
- Rose, P.W.; Prlić, A.; Altunkaya, A.; Bi, C.; Bradley, A.R.; Christie, C.H.; Di Costanzo, L.; Duarte, J.M.; Dutta, S.; Feng, Z.; et al. The RCSB protein data bank: Integrative view of protein, gene and 3D structural information. Nucleic Acids Res. 2016, 45, D271–D281. [Google Scholar] [CrossRef]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Di Costanzo, L.; Duarte, J.M.; et al. RCSB Protein Data Bank: Powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2020, 49, D437–D451. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Landeras-Bueno, S.; Wasserman, H.; Oliveira, G.; VanAernum, Z.L.; Busch, F.; Salie, Z.L.; Wysocki, V.H.; Andersen, K.; Saphire, E.O. Cellular mRNA triggers structural transformation of Ebola virus matrix protein VP40 to its essential regulatory form. Cell Rep. 2021, 35, 108986. [Google Scholar] [CrossRef]
- Khan, S.; Fakhar, Z.; Ahmad, A. Targeting ebola virus VP40 protein through novel inhibitors: Exploring the structural and dynamic perspectives on molecular landscapes. J. Mol. Model. 2021, 27, 49. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y. I-TASSER server: New development for protein structure and function predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef]
- Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Dessen, A.; Volchkov, V.; Dolnik, O.; Klenk, H.; Weissenhorn, W. Crystal structure of the matrix protein VP40 from Ebola virus. EMBO J. 2000, 19, 4228–4236. [Google Scholar] [CrossRef]
- Tsai, H.-H.; Tsai, C.-J.; Ma, B.; Nussinov, R. In silico protein design by combinatorial assembly of protein building blocks. Protein Sci. 2009, 13, 2753–2765. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera?A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Adu-Gyamfi, E.; Soni, S.P.; Jee, C.S.; Digman, M.A.; Gratton, E.; Stahelin, R.V. A Loop Region in the N-Terminal Domain of Ebola Virus VP40 Is Important in Viral Assembly, Budding, and Egress. Viruses 2014, 6, 3837–3854. [Google Scholar] [CrossRef] [PubMed]
- Odhar, H.A.; Rayshan, A.M.; Ahjel, S.W.; Hashim, A.A.; Albeer, A.A.M.A. Molecular docking enabled updated screening of the matrix protein VP40 from Ebola virus with millions of compounds in the MCULE database for potential inhibitors. Bioinformation 2019, 15, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Nasution, M.A.F.; Alkaff, A.H.; Tambunan, U.S.F. Discovery of Indonesian natural products as potential inhibitor of Ebola virus VP40 through molecular docking simulation. AIP Conf. Proc. 2018, 2023, 020055. [Google Scholar] [CrossRef]
- Licata, J.M.; Simpson-Holley, M.; Wright, N.T.; Han, Z.; Paragas, J.; Harty, R.N. Overlapping Motifs (PTAP and PPEY) within the Ebola Virus VP40 Protein Function Independently as Late Budding Domains: Involvement of Host Proteins TSG101 and VPS-4. J. Virol. 2003, 77, 1812–1819. [Google Scholar] [CrossRef]
- Han, Z.; Ruthel, G.; Dash, S.; Berry, C.T.; Freedman, B.D.; Harty, R.N.; Shtanko, O. Angiomotin regulates budding and spread of Ebola virus. J. Biol. Chem. 2020, 295, 8596–8601. [Google Scholar] [CrossRef]
- Yasuda, J.; Nakao, M.; Kawaoka, Y.; Shida, H. Nedd4 Regulates Egress of Ebola Virus-Like Particles from Host Cells. J. Virol. 2003, 77, 9987–9992. [Google Scholar] [CrossRef]
- Han, Z.; Lu, J.; Liu, Y.; Davis, B.; Lee, M.S.; Olson, M.A.; Ruthel, G.; Freedman, B.D.; Schnell, M.J.; Wrobel, J.E.; et al. Small-Molecule Probes Targeting the Viral PPxY-Host Nedd4 Interface Block Egress of a Broad Range of RNA Viruses. J. Virol. 2014, 88, 7294–7306. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Bexiga, M.; Palencia, A.; Corbi-Verge, C.; Martin-Malpartida, P.; Blanco, F.J.; Macias, M.J.; Cobos, E.S.; Luque, I. Binding site plasticity in viral PPxY Late domain recognition by the third WW domain of human NEDD4. Sci. Rep. 2019, 9, 15076. [Google Scholar] [CrossRef]
- Johnson, K.A.; Pokhrel, R.; Budicini, M.R.; Gerstman, B.S.; Chapagain, P.P.; Stahelin, R.V. A Conserved Tryptophan in the Ebola Virus Matrix Protein C-Terminal Domain Is Required for Efficient Virus-Like Particle Formation. Pathogens 2020, 9, 402. [Google Scholar] [CrossRef]
- Chukwuemeka, P.O.; Umar, H.I.; Iwaloye, O.; Oretade, O.M.; Olowosoke, C.B.; Elabiyi, M.O.; Igbe, F.O.; Oretade, O.J.; Eigbe, J.O.; Adeojo, F.J. Targeting p53-MDM2 interactions to identify small molecule inhibitors for cancer therapy: Beyond “Failure to rescue”. J. Biomol. Struct. Dyn. 2021, 40, 9158–9176. [Google Scholar] [CrossRef] [PubMed]
- Dankwa, B.; Broni, E.; Enninful, K.S.; Kwofie, S.K.; Wilson, M.D. Consensus docking and MM-PBSA computations identify putative furin protease inhibitors for developing potential therapeutics against COVID-19. Struct. Chem. 2022, 33, 2221–2241. [Google Scholar] [CrossRef] [PubMed]
- Cleves, M.A. Comparative Assessment of Three Common Algorithms for Estimating the Variance of the Area under the Nonparametric Receiver Operating Characteristic Curve. Stata J. 2002, 2, 280–289. [Google Scholar] [CrossRef]
- Sun, X.; Xu, W. Fast Implementation of DeLong’s Algorithm for Comparing the Areas Under Correlated Receiver Operating Characteristic Curves. IEEE Signal Process. Lett. 2014, 21, 1389–1393. [Google Scholar] [CrossRef]
- Empereur-Mot, C.; Zagury, J.-F.; Montes, M. Screening Explorer–An Interactive Tool for the Analysis of Screening Results. J. Chem. Inf. Model. 2016, 56, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Doan, H.T.T.; Noh, J.H.; Kim, Y.H.; Yoo, M.S.; Reddy, K.E.; Jung, S.C.; Kang, S.W. The efficacy of avermectins (ivermectin, doramectin and abamectin) as treatments for infestation with the tick Haemaphysalis longicornis on rabbits in Korea. Vet. Parasitol. 2013, 198, 406–409. [Google Scholar] [CrossRef]
- Pereira, J.R. The efficiency of avermectins (abamectin, doramectin and ivermectin) in the control of Boophilus microplus, in artificially infested bovines kept in field conditions. Vet. Parasitol. 2009, 162, 116–119. [Google Scholar] [CrossRef]
- Varghese, F.S.; Kaukinen, P.; Gläsker, S.; Bespalov, M.; Hanski, L.; Wennerberg, K.; Kümmerer, B.M.; Ahola, T. Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antivir. Res. 2016, 126, 117–124. [Google Scholar] [CrossRef]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef]
- Heidary, F.; Gharebaghi, R. Ivermectin: A systematic review from antiviral effects to COVID-19 complementary regimen. J. Antibiot. 2020, 73, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.-L.; Han, Y.; Liu, W.; Pang, X.-Y.; Zheng, B.; Zhang, Y.; Zhou, X.-N. Antivirus effectiveness of ivermectin on dengue virus type 2 in Aedes albopictus. PLoS Negl. Trop. Dis. 2018, 12, e0006934. [Google Scholar] [CrossRef]
- Afzal, S.; Raza, S.; Rabbani, M.; Firyal, S.; Altaf, I.; Naeem, Z. Antiviral Potential of Ivermectin against Peste des Petits Ruminants Virus (PPRV). Pak. J. Zool. 2021, 53, 1575–1578. [Google Scholar] [CrossRef]
- Kinobe, R.T.; Owens, L. A systematic review of experimental evidence for antiviral effects of ivermectin and an in silico analysis of ivermectin’s possible mode of action against SARS-CoV-2. Fundam. Clin. Pharmacol. 2021, 35, 260–276. [Google Scholar] [CrossRef]
- Basler, C.F. Molecular pathogenesis of viral hemorrhagic fever. Semin. Immunopathol. 2017, 39, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Kesel, A.J. A system of protein target sequences for anti-RNA-viral chemotherapy by a vitamin B6-Derived zinc-Chelating trioxa-adamantane-triol. Bioorganic Med. Chem. 2003, 11, 4599–4613. [Google Scholar] [CrossRef] [PubMed]
- Afanasieva, T.A.; Wahl-Jensen, V.; Seebach, J.; Schillers, H.; Nikova, D.; Ströher, U.; Feldmann, H.; Schnittler, H.-J. Hemor-rhagic Fevers: Endothelial Cells and Ebola-Virus Hemorrhagic Fever. In Endothelial Biomedicine; Cambridge University Press: Cambridge, UK, 2007; pp. 1311–1319. ISBN 9780511546198. [Google Scholar]
- Geddawy, A.; Ibrahim, Y.F.; Elbahie, N.M.; Ibrahim, M.A. Direct acting anti-hepatitis C virus drugs: Clinical pharmacology and future direction. J. Transl. Intern. Med. 2017, 5, 8–17. [Google Scholar] [CrossRef]
- Vallet-Pichard, A.; Pol, S. Grazoprevir/elbasvir combination therapy for HCV infection. Ther. Adv. Gastroenterol. 2016, 10, 155–167. [Google Scholar] [CrossRef]
- Ng, T.I.; Tripathi, R.; Reisch, T.; Lu, L.; Middleton, T.; Hopkins, T.A.; Pithawalla, R.; Irvin, M.; Dekhtyar, T.; Krishnan, P.; et al. In Vitro Antiviral Activity and Resistance Profile of the Next-Generation Hepatitis C Virus NS3/4A Protease Inhibitor Glecaprevir. Antimicrob. Agents Chemother. 2018, 62, e01620-17. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.G.; Jensen, D.M. Glecaprevir/pibrentasvir for the treatment of chronic hepatitis C: Design, development, and place in therapy. Drug Des. Dev. Ther. 2019, ume13, 2565–2577. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. Glecaprevir/Pibrentasvir: First Global Approval. Drugs 2017, 77, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Pollyea, D.A.; Amaya, M.; Strati, P.; Konopleva, M.Y. Venetoclax for AML: Changing the treatment paradigm. Blood Adv. 2019, 3, 4326–4335. [Google Scholar] [CrossRef] [PubMed]
- Cang, S.; Iragavarapu, C.; Savooji, J.; Song, Y.; Liu, D. ABT-199 (venetoclax) and BCL-2 inhibitors in clinical development. J. Hematol. Oncol. 2015, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Assouline, S.; Owen, C.; Gerecitano, J.; Robak, T.; De la Serna, J.; et al. Venetoclax–Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2018, 378, 1107–1120. [Google Scholar] [CrossRef] [PubMed]
- Korycka-Wolowiec, A.; Wolowiec, D.; Kubiak-Mlonka, A.; Robak, T. Venetoclax in the treatment of chronic lymphocytic leukemia. Expert Opin. Drug Metab. Toxicol. 2019, 15, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Alto, A.; Natesampillai, S.; Chandrasekar, A.P.; Krogman, A.; Misra, A.; Shweta, F.; VanLith, C.; Yao, J.D.; Cummins, N.W.; Badley, A.D. The Combination of Venetoclax and Ixazomib Selectively and Efficiently Kills HIV-Infected Cell Lines but Has Unacceptable Toxicity in Primary Cell Models. J. Virol. 2021, 95, e00138-21. [Google Scholar] [CrossRef]
- Whitmer, S.L.; Ladner, J.T.; Wiley, M.; Patel, K.; Dudas, G.; Rambaut, A.; Sahr, F.; Prieto, K.; Shepard, S.S.; Carmody, E.; et al. Active Ebola Virus Replication and Heterogeneous Evolutionary Rates in EVD Survivors. Cell Rep. 2018, 22, 1159–1168. [Google Scholar] [CrossRef]
- García, M.; Cooper, A.; Shi, W.; Bornmann, W.; Carrion, R.; Kalman, D.; Nabel, G.J. Productive Replication of Ebola Virus Is Regulated by the c-Abl1 Tyrosine Kinase. Sci. Transl. Med. 2012, 4, 123ra24. [Google Scholar] [CrossRef] [PubMed]
- Capuzzi, S.J.; Sun, W.; Muratov, E.N.; Martínez-Romero, C.; He, S.; Zhu, W.; Li, H.; Tawa, G.; Fisher, E.G.; Xu, M.; et al. Computer-Aided Discovery and Characterization of Novel Ebola Virus Inhibitors. J. Med. Chem. 2018, 61, 3582–3594. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Barret, R. Medicinal Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 97–100. ISBN 978-1-78548-288-5. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Sander, T.; Freyss, J.; Von Korff, M.; Rufener, C. DataWarrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef]
- Poroikov, V.V.; Filimonov, D.A.; Ihlenfeldt, W.-D.; Gloriozova, T.A.; Lagunin, A.A.; Borodina, Y.V.; Stepanchikova, A.V.; Nicklaus, M.C. PASS Biological Activity Spectrum Predictions in the Enhanced Open NCI Database Browser. J. Chem. Inf. Comput. Sci. 2003, 43, 228–236. [Google Scholar] [CrossRef]
- Parasuraman, S. Prediction of activity spectra for substances. J. Pharmacol. Pharmacother. 2011, 2, 52–53. [Google Scholar] [CrossRef]
- Filimonov, D.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the Biological Activity Spectra of Organic Compounds Using the Pass Online Web Resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Lagunin, A.; Stepanchikova, A.; Filimonov, D.; Poroikov, V. PASS: Prediction of activity spectra for biologically active substances. Bioinformatics 2000, 16, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Kumar, M. Anti-Ebola: An initiative to predict Ebola virus inhibitors through machine learning. Mol. Divers. 2021, 26, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Plech, T.; Kaproń, B.; Łuszczki, J.J.; Wujec, M.; Paneth, A.; Siwek, A.; Kolaczkowski, M.; Żołnierek, M.; Nowak, G. Studies on the Anticonvulsant Activity and Influence on GABA-ergic Neurotransmission of 1,2,4-Triazole-3-thione- Based Compounds. Molecules 2014, 19, 11279–11299. [Google Scholar] [CrossRef]
- Torres-García, I.; López-Martínez, J.L.; Muñoz-Dorado, M.; Rodríguez-García, I.; Álvarez-Corral, M. Marine Terpenic Endoperoxides. Mar. Drugs 2021, 19, 661. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, K.; Daikoku, T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol. Ther. 2020, 209, 107512. [Google Scholar] [CrossRef]
- Łagocka, R.; Dziedziejko, V.; Kłos, P.; Pawlik, A. Favipiravir in Therapy of Viral Infections. J. Clin. Med. 2021, 10, 273. [Google Scholar] [CrossRef]
- Bixler, S.L.; Bocan, T.M.; Wells, J.; Wetzel, K.S.; Van Tongeren, S.A.; Dong, L.; Garza, N.L.; Donnelly, G.; Cazares, L.H.; Nuss, J.; et al. Efficacy of favipiravir (T-705) in nonhuman primates infected with Ebola virus or Marburg virus. Antivir. Res. 2018, 151, 97–104. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, Y.; Zhang, Z.; Lv, X.; Gao, G.F.; Shao, Y.; Ma, L.; Li, X. Enfuvirtide−PEG conjugate: A potent HIV fusion inhibitor with improved pharmacokinetic properties. Eur. J. Med. Chem. 2016, 121, 232–237. [Google Scholar] [CrossRef]
- A Cooper, D.; Lange, J.M. Peptide inhibitors of virus—cell fusion: Enfuvirtide as a case study in clinical discovery and development. Lancet Infect. Dis. 2004, 4, 426–436. [Google Scholar] [CrossRef]
- Wolf, M.C.; Freiberg, A.N.; Zhang, T.; Akyol-Ataman, Z.; Grock, A.; Hong, P.W.; Li, J.; Watson, N.F.; Fang, A.Q.; Aguilar, H.C.; et al. A broad-spectrum antiviral targeting entry of enveloped viruses. Proc. Natl. Acad. Sci. USA 2010, 107, 3157–3162. [Google Scholar] [CrossRef]
- Vernet, M.-A.; Reynard, S.; Fizet, A.; Schaeffer, J.; Pannetier, D.; Guedj, J.; Rives, M.; Georges, N.; Garcia-Bonnet, N.; Sylla, A.I.; et al. Clinical, virological, and biological parameters associated with outcomes of Ebola virus infection in Macenta, Guinea. J. Clin. Investig. 2017, 2, e88864. [Google Scholar] [CrossRef] [PubMed]
- Martines, R.B.; Ng, D.; Greer, P.W.; Rollin, P.; Zaki, S.R. Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses. J. Pathol. 2014, 235, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Scoon, W.A.; Mancio-Silva, L.; Suder, E.L.; Villacorta-Martin, C.; Lindstrom-Vautrin, J.; Bernbaum, J.G.; Mazur, S.; Johnson, R.F.; Olejnik, J.; Flores, E.Y.; et al. Ebola virus infection induces a delayed type I IFN response in bystander cells and the shutdown of key liver genes in human iPSC-derived hepatocytes. Stem Cell Rep. 2022, 17, 2286–2302. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Cheng, D.; Shrivastava, S.; Tzur, D.; Gautam, B.; Hassanali, M. DrugBank: A knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008, 36, D901–D906. [Google Scholar] [CrossRef] [PubMed]
- Kachur, K.; Suntres, Z.E. The antimicrobial properties of ginseng and ginseng extracts. Expert Rev. Anti-Infect. Ther. 2015, 14, 81–94. [Google Scholar] [CrossRef]
- Im, K.; Kim, J.; Min, H. Ginseng, the natural effectual antiviral: Protective effects of Korean Red Ginseng against viral infection. J. Ginseng Res. 2016, 40, 309–314. [Google Scholar] [CrossRef]
- Yoo, Y.C.; Lee, J.; Park, S.R.; Nam, K.Y.; Cho, Y.H.; Choi, J.E. Protective effect of ginsenoside-Rb2 from Korean red ginseng on the lethal infection of haemagglutinating virus of Japan in mice. J. Ginseng Res. 2013, 37, 80–86. [Google Scholar] [CrossRef]
- Tan, B.; Giangaspero, M.; Sun, N.; Jin, Y.; Liu, K.; Wang, Q.; Cheng, S.; Wang, Y.; Zhang, S. Antiviral Effect of Ginsenoside Rb2 and Rb3 Against Bovine Viral Diarrhea Virus and Classical Swine Fever Virus in vitro. Front. Vet. Sci. 2021, 8, 1413. [Google Scholar] [CrossRef]
- Dong, W.; Farooqui, A.; Leon, A.J.; Kelvin, D.J. Inhibition of influenza A virus infection by ginsenosides. PLoS ONE 2017, 12, e0171936. [Google Scholar] [CrossRef]
- Chen, C.; Shen, J.-L.; Liang, C.-S.; Sun, Z.-C.; Jiang, H.-F. First Discovery of Beta-Sitosterol as a Novel Antiviral Agent against White Spot Syndrome Virus. Int. J. Mol. Sci. 2022, 23, 10448. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-López, T.; Borges-Argáez, R.; Ayora-Talavera, G.; Canto-Ramírez, E.; Cetina-Montejo, L.; May-May, A.; Escalante-Erosa, F.; Cáceres-Farfán, M. Bioassay-Guided Fractionation of Erythrostemon yucatanensis (Greenm.) Gagnon & GP Lewis Components with Anti-hemagglutinin Binding Activity against Influenza A/H1N1 Virus. Molecules 2022, 27, 5494. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Giniyatullina, G.V.; Yamansarov, E.Y.; Tolstikov, G.A. Betulin and ursolic acid synthetic derivatives as inhibitors of Papilloma virus. Bioorganic Med. Chem. Lett. 2010, 20, 4088–4090. [Google Scholar] [CrossRef]
- Li, B.-Y.; Hu, Y.; Li, J.; Shi, K.; Shen, Y.-F.; Zhu, B.; Wang, G.-X. Ursolic acid from Prunella vulgaris L. efficiently inhibits IHNV infection in vitro and in vivo. Virus Res. 2019, 273, 197741. [Google Scholar] [CrossRef] [PubMed]
- Tohmé, M.; Giménez, M.; Peralta, A.; Colombo, M.; Delgui, L. Ursolic acid: A novel antiviral compound inhibiting rotavirus infection in vitro. Int. J. Antimicrob. Agents 2019, 54, 601–609. [Google Scholar] [CrossRef]
- Kong, L.; Li, S.; Liao, Q.; Zhang, Y.; Sun, R.; Zhu, X.; Zhang, Q.; Wang, J.; Wu, X.; Fang, X.; et al. Oleanolic acid and ursolic acid: Novel hepatitis C virus antivirals that inhibit NS5B activity. Antivir. Res. 2013, 98, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Jaisi, A.; Prema, P.; Madla, S.; Lee, Y.; Septama, A.W.; Morita, H. Investigation of HIV-1 Viral Protein R Inhibitory Activities of Twelve Thai Medicinal Plants and Their Commercially Available Major Constituents. Chem. Biodivers. 2021, 18, e2100540. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yu, L.; Zhang, Y.; Liu, Z.; Zhang, H.; Zhang, Y.; Liu, P.; Du, P. Glycyrrhetinic acid alleviates hepatic inflammation injury in viral hepatitis disease via a HMGB1-TLR4 signaling pathway. Int. Immunopharmacol. 2020, 84, 106578. [Google Scholar] [CrossRef]
- Wang, L.-J.; Geng, C.-A.; Ma, Y.-B.; Huang, X.-Y.; Luo, J.; Chen, H.; Zhang, X.-M.; Chen, J.-J. Synthesis, biological evaluation and structure–activity relationships of glycyrrhetinic acid derivatives as novel anti-hepatitis B virus agents. Bioorganic Med. Chem. Lett. 2012, 22, 3473–3479. [Google Scholar] [CrossRef]
- Yi, Y.; Li, J.; Lai, X.; Zhang, M.; Kuang, Y.; Bao, Y.-O.; Yu, R.; Hong, W.; Muturi, E.; Xue, H.; et al. Natural triterpenoids from licorice potently inhibit SARS-CoV-2 infection. J. Adv. Res. 2021, 36, 201–210. [Google Scholar] [CrossRef]
- Bailly, C.; Vergoten, G. Glycyrrhizin: An alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome? Pharmacol. Ther. 2020, 214, 107618. [Google Scholar] [CrossRef]
- Elebeedy, D.; Badawy, I.; Elmaaty, A.A.; Saleh, M.M.; Kandeil, A.; Ghanem, A.; Kutkat, O.; Alnajjar, R.; El Maksoud, A.I.A.; Al-Karmalawy, A.A. In vitro and computational insights revealing the potential inhibitory effect of Tanshinone IIA against influenza A virus. Comput. Biol. Med. 2021, 141, 105149. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-C.; Cherng, J.-M.; Hung, M.-S.; Baltina, L.A.; Baltina, L.; Kondratenko, R. Inhibitory effects of some derivatives of glycyrrhizic acid against Epstein-Barr virus infection: Structure–activity relationships. Antivir. Res. 2008, 79, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Loe, M.W.C.; Hao, E.; Chen, M.; Li, C.; Lee, R.C.H.; Zhu, I.X.Y.; Teo, Z.Y.; Chin, W.-X.; Hou, X.; Deng, J.; et al. Betulinic acid exhibits antiviral effects against dengue virus infection. Antivir. Res. 2020, 184, 104954. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.-H.; Song, J.H.; Bin Kang, K.; Sung, S.H.; Ko, H.-J.; Yang, H. Anti-Influenza Activity of Betulinic Acid from Zizyphus jujuba on Influenza A/PR/8 Virus. Biomol. Ther. 2015, 23, 345–349. [Google Scholar] [CrossRef]
- Alhadrami, H.; Sayed, A.; Sharif, A.; Azhar, E.; Rateb, M. Olive-Derived Triterpenes Suppress SARS CoV-2 Main Protease: A Promising Scaffold for Future Therapeutics. Molecules 2021, 26, 2654. [Google Scholar] [CrossRef]
- Yao, D.; Li, H.; Gou, Y.; Zhang, H.; Vlessidis, A.G.; Zhou, H.; Evmiridis, N.P.; Liu, Z. Betulinic acid-mediated inhibitory effect on hepatitis B virus by suppression of manganese superoxide dismutase expression. FEBS J. 2009, 276, 2599–2614. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-K.; Tseng, C.-K.; Chen, K.-H.; Wu, S.-H.; Liaw, C.-C.; Lee, J.-C. Betulinic acid exerts anti-hepatitis C virus activity via the suppression of NF-κB- and MAPK-ERK1/2-mediated COX-2 expression. Br. J. Pharmacol. 2015, 172, 4481–4492. [Google Scholar] [CrossRef]
- Bellampalli, S.S.; Ji, Y.; Moutal, A.; Cai, S.; Wijeratne, E.K.; Gandini, M.A.; Yu, J.; Chefdeville, A.; Dorame, A.; Chew, L.A.; et al. Betulinic acid, derived from the desert lavender Hyptis emoryi, attenuates paclitaxel-, HIV-, and nerve injury–associated peripheral sensory neuropathy via block of N- and T-type calcium channels. Pain 2018, 160, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Theo, A.; Masebe, T.; Suzuki, Y.; Kikuchi, H.; Wada, S.; Obi, C.L.; Bessong, P.O.; Usuzawa, M.; Oshima, Y.; Hattori, T. Peltophorum Africanum, a Traditional South African Medicinal Plant, Contains an Anti HIV-1 Constituent, Betulinic Acid. Tohoku J. Exp. Med. 2009, 217, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Navid, M.H.; Laszczyk-Lauer, M.; Reichling, J.; Schnitzler, P. Pentacyclic triterpenes in birch bark extract inhibit early step of herpes simplex virus type 1 replication. Phytomedicine 2014, 21, 1273–1280. [Google Scholar] [CrossRef]
- Hu, A.; Li, J.; Tang, W.; Liu, G.; Zhang, H.; Liu, C.; Chen, X. Anthralin Suppresses the Proliferation of Influenza Virus by Inhibiting the Cap-Binding and Endonuclease Activity of Viral RNA Polymerase. Front. Microbiol. 2020, 11, 178. [Google Scholar] [CrossRef]
- Richard, K.; Schonhofer, C.; Giron, L.B.; Rivera-Ortiz, J.; Read, S.; Kannan, T.; Kinloch, N.N.; Shahid, A.; Feilcke, R.; Wappler, S.; et al. The African natural product knipholone anthrone and its analogue anthralin (dithranol) enhance HIV-1 latency reversal. J. Biol. Chem. 2020, 295, 14084–14099. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.N.; Braga, A.C.S.; Campos, G.R.F.; Souza, M.M.; de Matos, R.P.A.; Lopes, T.Z.; Candido, N.M.; Lima, M.L.D.; Machado, F.C.; de Andrade, S.T.Q.; et al. Natural Products Isolated from Oriental Medicinal Herbs Inactivate Zika Virus. Viruses 2019, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Bei, Y.; Tia, B.; Li, Y.; Guo, Y.; Deng, S.; Huang, R.; Zeng, H.; Li, R.; Wang, G.-F.; Dai, J. Anti-influenza A Virus Effects and Mechanisms of Emodin and Its Analogs via Regulating PPARα/γ-AMPK-SIRT1 Pathway and Fatty Acid Metabolism. BioMed Res. Int. 2021, 2021, 9066938. [Google Scholar] [CrossRef]
- Lin, C.-J.; Lin, H.-J.; Chen, T.-H.; Hsu, Y.-A.; Liu, C.-S.; Hwang, G.-Y.; Wan, L. Polygonum cuspidatum and Its Active Components Inhibit Replication of the Influenza Virus through Toll-Like Receptor 9-Induced Interferon Beta Expression. PLoS ONE 2015, 10, e0117602. [Google Scholar] [CrossRef]
- Dai, J.-P.; Wang, Q.-W.; Su, Y.; Gu, L.-M.; Zhao, Y.; Chen, X.-X.; Chen, C.; Li, W.-Z.; Wang, G.-F.; Li, K.-S. Emodin Inhibition of Influenza A Virus Replication and Influenza Viral Pneumonia via the Nrf2, TLR4, p38/JNK and NF-kappaB Pathways. Molecules 2017, 22, 1754. [Google Scholar] [CrossRef] [PubMed]
- Alam, Z.; Al-Mahdi, Z.; Zhu, Y.; McKee, Z.; Parris, D.S.; Parikh, H.I.; Kellogg, G.E.; Kuchta, A.; McVoy, M.A. Anti-cytomegalovirus activity of the anthraquinone atanyl blue PRL. Antivir. Res. 2015, 114, 86–95. [Google Scholar] [CrossRef]
- Dang, S.-S.; Jia, X.-L.; Song, P.; Cheng, Y.-A.; Zhang, X.; Sun, M.-Z.; Liu, E.-Q. Inhibitory effect of emodin and Astragalus polysaccharideon the replication of HBV. World J. Gastroenterol. 2009, 15, 5669–5673. [Google Scholar] [CrossRef]
- Anantpadma, M.; Lane, T.; Zorn, K.M.; Lingerfelt, M.A.; Clark, A.M.; Freundlich, J.S.; Davey, R.; Madrid, P.B.; Ekins, S. Ebola Virus Bayesian Machine Learning Models Enable New in Vitro Leads. ACS Omega 2019, 4, 2353–2361. [Google Scholar] [CrossRef]
- Zhao, Z.; Martin, C.; Fan, R.; Bourne, P.E.; Xie, L. Drug repurposing to target Ebola virus replication and virulence using structural systems pharmacology. BMC Bioinform. 2016, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, G.-L.; Ming, S.-L.; Wang, C.-F.; Shi, L.-J.; Su, B.-Q.; Wu, H.-T.; Zeng, L.; Han, Y.-Q.; Liu, Z.-H.; et al. BRD4 inhibition exerts anti-viral activity through DNA damage-dependent innate immune responses. PLoS Pathog. 2020, 16, e1008429. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.; Gallo, G.; Hardy, L.; Bottazzi, M.E.; Campos, C.; Würtele, M. Biochemical Screening of Potent Zika Virus Protease Inhibitors. Chemmedchem 2022, 17, e202100695. [Google Scholar] [CrossRef]
- Esposito, F.; Carli, I.; Del Vecchio, C.; Xu, L.; Corona, A.; Grandi, N.; Piano, D.; Maccioni, E.; Distinto, S.; Parolin, C.; et al. Sennoside A, derived from the traditional chinese medicine plant Rheum L., is a new dual HIV-1 inhibitor effective on HIV-1 replication. Phytomedicine 2016, 23, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Nesměrák, K.; Kudláček, K.; Čambal, P.; Štícha, M.; Kozlik, P.; Červený, V. Authentication of senna extract from the eighteenth century and study of its composition by HPLC–MS. Mon. Chem. -Chem. Mon. 2020, 151, 1241–1248. [Google Scholar] [CrossRef]
- Cheung, Y.Y.; Chen, K.C.; Chen, H.; Seng, E.K.; Chu, J.J.H. Antiviral activity of lanatoside C against dengue virus infection. Antivir. Res. 2014, 111, 93–99. [Google Scholar] [CrossRef]
- Miao, M.; Jing, X.; De Clercq, E.; Li, G. Danoprevir for the Treatment of Hepatitis C Virus Infection: Design, Development, and Place in Therapy. Drug Des. Dev. Ther. 2020, ume14, 2759–2774. [Google Scholar] [CrossRef]
- Rong, L.; Guedj, J.; Dahari, H.; Coffield, D.J., Jr.; Levi, M.; Smith, P.; Perelson, A.S. Analysis of Hepatitis C Virus Decline during Treatment with the Protease Inhibitor Danoprevir Using a Multiscale Model. PLoS Comput. Biol. 2013, 9, e1002959. [Google Scholar] [CrossRef]
- Jiang, Y.; Andrews, S.W.; Condroski, K.R.; Buckman, B.; Serebryany, V.; Wenglowsky, S.; Kennedy, A.L.; Madduru, M.R.; Wang, B.; Lyon, M.; et al. Discovery of Danoprevir (ITMN-191/R7227), a Highly Selective and Potent Inhibitor of Hepatitis C Virus (HCV) NS3/4A Protease. J. Med. Chem. 2013, 57, 1753–1769. [Google Scholar] [CrossRef]
- Elbedewy, T.A.; Elsebaey, M.A.; Elshweikh, S.A.; Elashry, H.; Abd-Elsalam, S. Predictors for eltrombopag response in patients with hepatitis C virus-associated thrombocytopenia. Ther. Clin. Risk Manag. 2019, ume15, 269–274. [Google Scholar] [CrossRef]
- Danish, F.-I.; Yasmin, S. The role of eltrombopag in the management of hepatitis C virus-related thrombocytopenia. Hepatic Med. Evid. Res. 2013, 5, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Hunt, L.; Gupta-Wright, A.; Simms, V.; Tamba, F.; Knott, V.; Tamba, K.; Heisenberg-Mansaray, S.; Tamba, E.; Sheriff, A.; Conteh, S.; et al. Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: An observational cohort study. Lancet Infect. Dis. 2015, 15, 1292–1299. [Google Scholar] [CrossRef]
- Tapia, M.D.; Sow, S.O.; Mbaye, K.D.; Thiongane, A.; Ndiaye, B.P.; Ndour, C.T.; Mboup, S.; Keshinro, B.; Kinge, T.N.; Vernet, G.; et al. Safety, reactogenicity, and immunogenicity of a chimpanzee adenovirus vectored Ebola vaccine in children in Africa: A randomised, observer-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 2020, 20, 719–730. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Matsuura, T.; Aoyagi, H.; Matsuda, M.; Hmwe, S.S.; Date, T.; Watanabe, N.; Watashi, K.; Suzuki, R.; Ichinose, S.; et al. Antiviral Activity of Glycyrrhizin against Hepatitis C Virus In Vitro. PLoS ONE 2013, 8, e68992. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, M.; Geiler, J.; Naczk, P.; Sithisarn, P.; Ogbomo, H.; Altenbrandt, B.; Leutz, A.; Doerr, H.W.; Cinatl, J., Jr. Glycyrrhizin inhibits highly pathogenic H5N1 influenza A virus-induced pro-inflammatory cytokine and chemokine expression in human macrophages. Med. Microbiol. Immunol. 2010, 199, 291–297. [Google Scholar] [CrossRef]
- Wolkerstorfer, A.; Kurz, H.; Bachhofner, N.; Szolar, O.H. Glycyrrhizin inhibits influenza A virus uptake into the cell. Antivir. Res. 2009, 83, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef]
- Sakai-Sugino, K.; Uematsu, J.; Kamada, M.; Taniguchi, H.; Suzuki, S.; Yoshimi, Y.; Kihira, S.; Yamamoto, H.; Kawano, M.; Tsurudome, M.; et al. Glycyrrhizin inhibits human parainfluenza virus type 2 replication by the inhibition of genome RNA, mRNA and protein syntheses. Drug Discov. Ther. 2017, 11, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Singh, W.; Karabencheva-Christova, T.G.; Black, G.W.; Ainsley, J.; Dover, L.; Christov, C.Z. Conformational Dynamics, Ligand Binding and Effects of Mutations in NirE an S-Adenosyl-L-Methionine Dependent Methyltransferase. Sci. Rep. 2016, 6, 20107. [Google Scholar] [CrossRef]
- Lobanov, M.Y.; Bogatyreva, N.S.; Galzitskaya, O.V. Radius of gyration as an indicator of protein structure compactness. Mol. Biol. 2008, 42, 623–628. [Google Scholar] [CrossRef]
- Wen, C.-C.; Kuo, Y.-H.; Jan, J.-T.; Liang, P.-H.; Wang, S.-Y.; Liu, H.-G.; Lee, C.-K.; Chang, S.-T.; Kuo, C.-J.; Lee, S.-S.; et al. Specific Plant Terpenoids and Lignoids Possess Potent Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus. J. Med. Chem. 2007, 50, 4087–4095. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Kumar, R.; Lynn, A. g_mmpbsa—A GROMACS tool for high-throughput MM-PBSA calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef]
- Poli, G.; Granchi, C.; Rizzolio, F.; Tuccinardi, T. Application of MM-PBSA Methods in Virtual Screening. Molecules 2020, 25, 1971. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Aparoy, P. Application of per-residue energy decomposition to identify the set of amino acids critical for in silico prediction of COX-2 inhibitory activity. Heliyon 2020, 6, e04944. [Google Scholar] [CrossRef]
- Kwofie, S.K.; Dankwa, B.; Enninful, K.S.; Adobor, C.; Broni, E.; Ntiamoah, A.; Wilson, M.D. Molecular Docking and Dynamics Simulation Studies Predict Munc18b as a Target of Mycolactone: A Plausible Mechanism for Granule Exocytosis Impairment in Buruli Ulcer Pathogenesis. Toxins 2019, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.D.; Huggins, D.J. Optimization of Protein–Ligand Electrostatic Interactions Using an Alchemical Free-Energy Method. J. Chem. Theory Comput. 2019, 15, 6504–6512. [Google Scholar] [CrossRef]
- Sims, P.A.; Wong, C.F.; McCammon, J.A. Charge optimization of the interface between protein kinases and their ligands. J. Comput. Chem. 2004, 25, 1416–1429. [Google Scholar] [CrossRef]
- Sulea, T.; Purisima, E.O. Optimizing Ligand Charges for Maximum Binding Affinity. A Solvated Interaction Energy Approach. J. Phys. Chem. B 2001, 105, 889–899. [Google Scholar] [CrossRef]
- Sterling, T.; Irwin, J.J. ZINC 15—Ligand Discovery for Everyone. J. Chem. Inf. Model. 2015, 55, 2324–2337. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef] [PubMed]
- Mendez, D.; Gaulton, A.; Bento, A.P.; Chambers, J.; de Veij, M.; Félix, E.; Magariños, M.P.; Mosquera, J.F.; Mutowo, P.; Nowotka, M.; et al. ChEMBL: Towards direct deposition of bioassay data. Nucleic Acids Res. 2019, 47, D930–D940. [Google Scholar] [CrossRef]
- Gaulton, A.; Bellis, L.J.; Bento, A.P.; Chambers, J.; Davies, M.; Hersey, A.; Light, Y.; McGlinchey, S.; Michalovich, D.; Al-Lazikani, B.; et al. ChEMBL: A large-scale bioactivity database for drug discovery. Nucleic Acids Res. 2012, 40, D1100–D1107. [Google Scholar] [CrossRef]
- Bento, A.P.; Gaulton, A.; Hersey, A.; Bellis, L.J.; Chambers, J.; Davies, M.; Krüger, F.A.; Light, Y.; Mak, L.; McGlinchey, S.; et al. The ChEMBL bioactivity database: An update. Nucleic Acids Res. 2013, 42, D1083–D1090. [Google Scholar] [CrossRef] [PubMed]
- Rutz, A.; Sorokina, M.; Galgonek, J.; Mietchen, D.; Willighagen, E.; Gaudry, A.; Graham, J.G.; Stephan, R.; Page, R.; Vondrášek, J.; et al. The LOTUS initiative for knowledge sharing in Natural Products research. In Proceedings of the GA–69th Annual Meeting 2021, Virtual Conference, Bonn, Germany, 5–8 September 2021. [Google Scholar]
- Mohanraj, K.; Karthikeyan, B.S.; Vivek-Ananth, R.P.; Chand, R.P.B.; Aparna, S.R.; Mangalapandi, P.; Samal, A. IMPPAT: A curated database of Indian Medicinal Plants, Phytochemistry And Therapeutics. Sci. Rep. 2018, 8, 4329. [Google Scholar] [CrossRef] [PubMed]
- Abdelgaleil, S.A.M.; Nakatani, M. Antifeeding activity of limonoids from Khaya senegalensis (Meliaceae). J. Appl. Entomol. 2003, 127, 236–239. [Google Scholar] [CrossRef]
- Nakatani, M.; Abdelgaleil, S.A.M.; Kurawaki, J.; Okamura, H.; Iwagawa, T.; Doe, M. Antifeedant Rings B and D Opened Limonoids from Khaya senegalensis. J. Nat. Prod. 2001, 64, 1261–1265. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.; Iwagawa, T.; Doe, M.; Nakatani, M. Antifungal limonoids from the fruits of Khaya senegalensis. Fitoterapia 2004, 75, 566–572. [Google Scholar] [CrossRef]
- Thioune, O.; Ahodikpe, D.; Dieng, M.; Diop, A.B.; Ngom, S.; Lo, I. Inflammatory ointment from shea butter and hy-dro-alcoholic extract of Khaya senegalensis barks (Cailcederat). Dakar Med. 2000, 45, 113–116. [Google Scholar]
- Rabadeaux, C.; Vallette, L.; Sirdaarta, J.; Davis, C.; Cock, I.E. An examination of the Antimicrobial and Anticancer Properties of Khaya senegalensis (Desr.) A. Juss. Bark Extracts. Pharmacogn. J. 2017, 9, 504–518. [Google Scholar] [CrossRef]
- Li, B.; Meng, X.J.; Zhu, L.J.; Jiao, X.Y. Preparative Separation and Purification of Lancifodilactone C from Schisandra Chinensis (Turcz.) Baill by High-Speed Counter-Current Chromatography. Adv. Mater. Res. 2013, 634–638, 1502–1505. [Google Scholar] [CrossRef]
- Li, R.-T.; Xiang, W.; Li, S.-H.; Lin, Z.-W.; Sun, H.-D. Lancifodilactones B−E, New Nortriterpenes from Schisandra lancifolia. J. Nat. Prod. 2003, 67, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.-L.; Huang, S.-X.; Zhang, L.; Tian, R.-R.; Wu, L.; Li, X.-L.; Pu, J.-X.; Zheng, Y.-T.; Lu, Y.; Li, R.-T.; et al. Nortriterpenoids from Schisandra lancifolia. J. Nat. Prod. 2006, 69, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Borkosky, S.; Bardón, A.; Catalán, C.A.; Díaz, J.G.; Herz, W. Diterpenes from Vernonanthura amplexicaulis. Phytochemistry 1995, 40, 1477–1479. [Google Scholar] [CrossRef]
- Catalán, C.A.; De Iglesias, D.I.; Kavka, J.; Sosa, V.E.; Herz, W. Glaucolides and related sesquiterpene lactones from Vernonia chamaedrys. Phytochemistry 1988, 27, 197–202. [Google Scholar] [CrossRef]
- Anjaneyulu, V.; Ravi, K.; Prasad, K.; Connolly, J. Triterpenoids from Mangifera indica. Phytochemistry 1989, 28, 1471–1477. [Google Scholar] [CrossRef]
- van Eijk, J.L.; Radema, M.H. Lupanin alkaloids and other compounds fromcadia purpurea. Planta Med. 1975, 28, 139–142. [Google Scholar] [CrossRef]
- Loggia, R.; Tubaro, A.; Sosa, S.; Becker, H.; Saar, S.; Isaac, O. The Role of Triterpenoids in the Topical Anti-Inflammatory Activity of Calendula officinalis Flowers. Planta Med. 1994, 60, 516–520. [Google Scholar] [CrossRef]
- Rangaswami, S.; Rao, E.V. Chemical components of Plumieria alba Linn. Proc. Indian Acad. Sci.—Sect. A 1960, 52, 173–181. [Google Scholar] [CrossRef]
- Catalano, S.; Marsili, A.; Morelli, I.; Pistelli, L.; Scartoni, V. Constituents of the Leaves of Ilex aquifolium L. Planta Med. 1978, 33, 416–417. [Google Scholar] [CrossRef]
- Yagishita, K.; Nishimura, M. The Chemical Structure of Neoilexonol. Biosci. Biotechnol. Biochem. 1961, 25, 844–851. [Google Scholar] [CrossRef]
- Hui, W.-H.; Li, M.-M.; Luk, K. Triterpenoids and steroids from Rhodomyrtus tomentosa. Phytochemistry 1975, 14, 833–834. [Google Scholar] [CrossRef]
- Van Veen, T. Wikidata. Inf. Technol. Libr. 2019, 38, 72–81. [Google Scholar] [CrossRef]
- Waagmeester, A.; Stupp, G.; Burgstaller-Muehlbacher, S.; Good, B.M.; Griffith, M.; Griffith, O.L.; Hanspers, K.; Hermjakob, H.; Hudson, T.S.; Hybiske, K.; et al. Wikidata as a knowledge graph for the life sciences. Elife 2020, 9, e52614. [Google Scholar] [CrossRef] [PubMed]
- Kalvatchev, Z.; Walder, R.; Garzaro, D. Anti-HIV activity of extracts from Calendula officinalis flowers. Biomed. Pharmacother. 1997, 51, 176–180. [Google Scholar] [CrossRef]
- Rahman, A.U.; Naz, H.; Fadimatou; Makhmoor, T.; Yasin, A.; Fatima, N.; Ngounou, F.N.; Kimbu, S.F.; Sondengam, B.L.; Choudhary, M.I. Bioactive Constituents from Boswellia papyrifera. J. Nat. Prod. 2005, 68, 189–193. [Google Scholar] [CrossRef]
- Al-Harrasi, A.; Ali, L.; Ceniviva, E.; Al-Rawahi, A.; Hussain, J.; Hussain, H.; Rehman, N.; Abbas, G. Antiglycation and Antioxidant Activities and HPTLC Analysis of Boswellia sacra Oleogum Resin: The Sacred Frankincense. Trop. J. Pharm. Res. 2013, 12, 597–602. [Google Scholar] [CrossRef]
- Al-Yahya, A.A.; Asad, M.; Sadaby, A.; Alhussaini, M.S. Repeat oral dose safety study of standardized methanolic extract of Boswellia sacra oleo gum resin in rats. Saudi J. Biol. Sci. 2019, 27, 117–123. [Google Scholar] [CrossRef]
- Al Amri, I.; Mabood, F.; Kadim, I.T.; Alkindi, A.; Al-Harrasi, A.; Al-Hashmi, S.; Abbas, G.; Hamaed, A.; Ahmed, B.; Al-Shuhaimi, J.; et al. Evaluation of the solubility of 11-keto-β-boswellic acid and its histological effect on the diabetic mice liver using a novel technique. Vet. World 2021, 14, 1797. [Google Scholar] [CrossRef]
- Mehta, M.; Satija, S.; Garg, M. Comparison Between HPLC and HPTLC Densitometry for the Determination of 11-keto-Beta-boswellic acid and 3- acetyl-11-keto-Beta-boswellic acid from Boswellia serrata Extract. Indian J. Pharm. Educ. Res. 2016, 50, 418–423. [Google Scholar] [CrossRef]
- Katragunta, K.; Siva, B.; Kondepudi, N.; Vadaparthi, P.R.; Rao, N.R.; Tiwari, A.K.; Babu, K.S. Estimation of boswellic acids in herbal formulations containing Boswellia serrata extract and comprehensive characterization of secondary metabolites using UPLC-Q-Tof-MSe. J. Pharm. Anal. 2019, 9, 414–422. [Google Scholar] [CrossRef] [PubMed]
- von Rhein, C.; Weidner, T.; Henß, L.; Martin, J.; Weber, C.; Sliva, K.; Schnierle, B.S. Curcumin and Boswellia serrata gum resin extract inhibit chikungunya and vesicular stomatitis virus infections in vitro. Antivir. Res. 2016, 125, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Das Mahapatra, A.; Banerjee, S.; Kar, A.; Ojha, D.; Mukherjee, P.K.; Chattopadhyay, D. Boswellia serrata oleo-gum-resin and β-boswellic acid inhibits HSV-1 infection in vitro through modulation of NF-κB and p38 MAP kinase signaling. Phytomedicine 2018, 51, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Siemoneit, U.; Pergola, C.; Jazzar, B.; Northoff, H.; Skarke, C.; Jauch, J.; Werz, O. On the interference of boswellic acids with 5-lipoxygenase: Mechanistic studies in vitro and pharmacological relevance. Eur. J. Pharmacol. 2009, 606, 246–254. [Google Scholar] [CrossRef]
- Ammon, H.P.T. Modulation of the immune system by Boswellia serrata extracts and boswellic acids. Phytomedicine 2011, 17, 862–867, Erratum in 2011, 18, 334. [Google Scholar] [CrossRef]
- Ammon, H. Boswellic extracts and 11-keto-ß-boswellic acids prevent type 1 and type 2 diabetes mellitus by suppressing the expression of proinflammatory cytokines. Phytomedicine 2019, 63, 153002. [Google Scholar] [CrossRef]
- Nouveau, L.; Buatois, V.; Cons, L.; Chatel, L.; Pontini, G.; Pleche, N.; Ferlin, W.G. Immunological analysis of the murine anti-CD3-induced cytokine release syndrome model and therapeutic efficacy of anti-cytokine antibodies. Eur. J. Immunol. 2021, 51, 2074–2085. [Google Scholar] [CrossRef]
- Banerjee, G.; Shokeen, K.; Chakraborty, N.; Agarwal, S.; Mitra, A.; Kumar, S.; Banerjee, P. Modulation of immune response in Ebola virus disease. Curr. Opin. Pharmacol. 2021, 60, 158–167. [Google Scholar] [CrossRef]
- Banadyga, L.; Ebihara, H. Epidemiology and Pathogenesis of Filovirus Infections. In Biology and Pathogenesis of Rhabdo- and Filoviruses; World Scientific: Singapore, 2015; pp. 453–486. ISBN 9789814635349. [Google Scholar]
- Broni, E.; Kwofie, S.K.; Asiedu, S.O.; Miller, W.A.; Wilson, M.D. A Molecular Modeling Approach to Identify Potential Antileishmanial Compounds Against the Cell Division Cycle (cdc)-2-Related Kinase 12 (CRK12) Receptor of Leishmania donovani. Biomolecules 2021, 11, 458. [Google Scholar] [CrossRef]
- Gc, J.B.; Johnson, K.A.; Husby, M.L.; Frick, C.T.; Gerstman, B.S.; Stahelin, R.V.; Chapagain, P.P. Interdomain salt-bridges in the Ebola virus protein VP40 and their role in domain association and plasma membrane localization. Protein Sci. 2016, 25, 1648–1658. [Google Scholar] [CrossRef]
- Deshpande, N.; Addess, K.J.; Bluhm, W.F.; Merino-Ott, J.C.; Townsend-Merino, W.; Zhang, Q.; Knezevich, C.; Xie, L.; Chen, L.; Feng, Z.; et al. The RCSB Protein Data Bank: A redesigned query system and relational database based on the mmCIF schema. Nucleic Acids Res. 2004, 33, D233–D237. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K.; Berman, H.M.; Kleywegt, G.J.; Markley, J.L.; Nakamura, H.; Velankar, S. Protein Data Bank (PDB): The single global macromolecular structure archive. Protein Crystallogr. 2017, 1067, 627–641. [Google Scholar]
- Yuan, S.; Chan, H.S.; Hu, Z. Using PyMOL as a platform for computational drug design. WIREs Comput. Mol. Sci. 2017, 7, e1298. [Google Scholar] [CrossRef]
- Lee, M.-K.; Lee, K.-H.; Yoo, S.-H.; Park, C.-Y. Impact of initial active engagement in self-monitoring with a telemonitoring device on glycemic control among patients with type 2 diabetes. Sci. Rep. 2017, 7, 3866. [Google Scholar] [CrossRef]
- Rother, K. Introduction to PyMOL. Methods Mol. Biol. Clift. Nj 2005, 635, 0–32. [Google Scholar] [CrossRef]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef]
- Eswar, N.; Eramian, D.; Webb, B.; Shen, M.-Y.; Sali, A. Protein Structure Modeling with MODELLER. Struct. Proteom. High-Throughput Methods 2008, 426, 145–159. [Google Scholar] [CrossRef]
- Fiser, A.; Šali, A. Modeller: Generation and Refinement of Homology-Based Protein Structure Models. Methods Enzymol. 2003, 374, 461–491. [Google Scholar] [CrossRef]
- Kuntal, B.K.; Aparoy, P.; Reddanna, P. EasyModeller: A graphical interface to MODELLER. BMC Res. Notes 2010, 3, 226. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Zoete, V.; Cuendet, M.A.; Grosdidier, A.; Michielin, O. SwissParam: A fast force field generation tool for small organic molecules. J. Comput. Chem. 2011, 32, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Brooks, C.L., III; MacKerell, A.D., Jr.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The biomolecular simulation program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef] [PubMed]
- Gautam, B. Energy Minimization. In Homology Molecular Modeling-Perspectives and Applications; IntechOpen: London, UK, 2021. [Google Scholar]
- Tian, W.; Chen, C.; Lei, X.; Zhao, J.; Liang, J. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res. 2018, 46, W363–W367. [Google Scholar] [CrossRef]
- Binkowski, T.A.; Naghibzadeh, S.; Liang, J. CASTp: Computed Atlas of Surface Topography of proteins. Nucleic Acids Res. 2003, 31, 3352–3355. [Google Scholar] [CrossRef] [PubMed]
- Dundas, J.; Ouyang, Z.; Tseng, J.; Binkowski, A.; Turpaz, Y.; Liang, J. CASTp: Computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Res. 2006, 34, W116–W118. [Google Scholar] [CrossRef]
- Ntie-Kang, F.; Zofou, D.; Babiaka, S.B.; Meudom, R.; Scharfe, M.; Lifongo, L.L.; Mbah, J.A.; Mbaze, L.M.; Sippl, W.; Efange, S.M.N. AfroDb: A Select Highly Potent and Diverse Natural Product Library from African Medicinal Plants. PLoS ONE 2013, 8, e78085. [Google Scholar] [CrossRef]
- Ntie-Kang, F.; Telukunta, K.K.; Döring, K.; Simoben, C.V.; Moumbock, A.F.A.; Malange, Y.I.; Njume, L.E.; Yong, J.N.; Sippl, W.; Günther, S. NANPDB: A Resource for Natural Products from Northern African Sources. J. Nat. Prod. 2017, 80, 2067–2076. [Google Scholar] [CrossRef]
- Chen, C.Y.-C. TCM Database@Taiwan: The World’s Largest Traditional Chinese Medicine Database for Drug Screening In Silico. PLoS ONE 2011, 6, e15939. [Google Scholar] [CrossRef]
- Kwofie, S.K.; Broni, E.; Yunus, F.U.; Nsoh, J.; Adoboe, D.; Miller, W.A.; Wilson, M.D. Molecular Docking Simulation Studies Identifies Potential Natural Product Derived-Antiwolbachial Compounds as Filaricides against Onchocerciasis. Biomedicines 2021, 9, 1682. [Google Scholar] [CrossRef]
- Bennett, R.P.; Finch, C.L.; Postnikova, E.N.; Stewart, R.A.; Cai, Y.; Yu, S.; Liang, J.; Dyall, J.; Salter, J.D.; Smith, H.C.; et al. A Novel Ebola Virus VP40 Matrix Protein-Based Screening for Identification of Novel Candidate Medical Countermeasures. Viruses 2020, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Kouznetsova, J.; Sum, W.; Martínez-Romero, C.; Schimmer, A.; Zheng, W.; García-Sastre, A. Identification of 53 compounds that block Ebola virus-like particle entry via a repurposing screen of approved drugs. Emerg. Microbes Infect. 2014, 3, e84. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Exploring Chemical Information in PubChem. Curr. Protoc. 2021, 1, e217. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef] [PubMed]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with pyrx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar]
- Mysinger, M.M.; Carchia, M.; Irwin, J.J.; Shoichet, B.K. Directory of Useful Decoys, Enhanced (DUD-E): Better Ligands and Decoys for Better Benchmarking. J. Med. Chem. 2012, 55, 6582–6594. [Google Scholar] [CrossRef]
- Lionta, E.; Spyrou, G.; Vassilatis, D.K.; Cournia, Z. Structure-Based Virtual Screening for Drug Discovery: Principles, Applications and Recent Advances. Curr. Top. Med. Chem. 2014, 14, 1923–1938. [Google Scholar] [CrossRef]
- Cavasotto, C.; Orry, A.J.W. Ligand Docking and Structure-based Virtual Screening in Drug Discovery. Curr. Top. Med. Chem. 2007, 7, 1006–1014. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Breznik, M.; Ge, Y.; Bluck, J.P.; Briem, H.; Hahn, D.F.; Christ, C.D.; Mortier, J.; Mobley, D.L.; Meier, K. Prioritizing Small Sets of Molecules for Synthesis through in-silico Tools: A Comparison of Common Ranking Methods. Chemmedchem 2022, 18, e202200425. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.L.; Andrews, C.W.; Capelli, A.-M.; Clarke, B.; LaLonde, J.; Lambert, M.H.; Lindvall, M.; Nevins, N.; Semus, S.F.; Senger, S.; et al. A Critical Assessment of Docking Programs and Scoring Functions. J. Med. Chem. 2005, 49, 5912–5931. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, D.; Caballero, J. Is It Reliable to Use Common Molecular Docking Methods for Comparing the Binding Affinities of Enantiomer Pairs for Their Protein Target? Int. J. Mol. Sci. 2016, 17, 525. [Google Scholar] [CrossRef] [PubMed]
- Leeson, P.D.; Springthorpe, B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat. Rev. Drug Discov. 2007, 6, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Dodda, L.; De Vaca, I.C.; Tirado-Rives, J.; Jorgensen, W.L. LigParGen web server: An automatic OPLS-AA parameter generator for organic ligands. Nucleic Acids Res. 2017, 45, W331–W336. [Google Scholar] [CrossRef]
- Lu, C.; Wu, C.; Ghoreishi, D.; Chen, W.; Wang, L.; Damm, W.; Ross, G.A.; Dahlgren, M.K.; Russell, E.; Von Bargen, C.D.; et al. OPLS4: Improving Force Field Accuracy on Challenging Regimes of Chemical Space. J. Chem. Theory Comput. 2021, 17, 4291–4300. [Google Scholar] [CrossRef]
- Robertson, M.J.; Tirado-Rives, J.; Jorgensen, W.L. Improved Peptide and Protein Torsional Energetics with the OPLS-AA Force Field. J. Chem. Theory Comput. 2015, 11, 3499–3509. [Google Scholar] [CrossRef]
- Turner, P. XMGRACE, Version 5.1 ed; Center for Coastal and Land-Margin Research, Oregon Graduate Institute of Science and Technology: Beaverton, OR, USA, 2005. [Google Scholar]
- Darko, L.K.S.; Broni, E.; Amuzu, D.S.Y.; Wilson, M.D.; Parry, C.S.; Kwofie, S.K. Computational Study on Potential Novel Anti-Ebola Virus Protein VP35 Natural Compounds. Biomedicines 2021, 9, 1796. [Google Scholar] [CrossRef]
- Kwofie, S.K.; Broni, E.; Teye, J.; Quansah, E.; Issah, I.; Wilson, M.D.; Miller, W.A., III; Tiburu, E.K.; Bonney, J.H.K. Pharmacoinformatics-based identification of potential bioactive compounds against Ebola virus protein VP24. Comput. Biol. Med. 2019, 113, 103414. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Agyenkwa-Mawuli, K.; Agyapong, O.; Wilson, M.D.; Kwofie, S.K. EBOLApred: A machine learning-based web application for predicting cell entry inhibitors of the Ebola virus. Comput. Biol. Chem. 2022, 101, 107766. [Google Scholar] [CrossRef] [PubMed]





| Compound | Source/Library | Docking Score | Hydrogen Bonds (Bond Length (Å)) | Hydrophobic Contacts |
|---|---|---|---|---|
| ZINC000034518176 | AfroDb | −8.1 | Gly139 (3.05) | Phe36, Asn43, Pro47, Thr121, Phe125, Arg134, Asn136, Arg137, and Leu138. |
| ZINC000095485942 | AfroDb | −8.1 | Thr129 (2.72) and Asn130 (2.85, 3.25) | Pro131, Gln159, Glu160, Pro165, Val166, Pro169, and Gln170. |
| NANPDB2933 | NANPDB | −8.5 | Val166 (3.23) and Gln167 (3.09, 3.26). | Ala128, Thr129, Pro131, Gln159, Glu160, Pro165, and Gln170. |
| ZINC000085531689 | TCM | −8.9 | Asn130 (2.99, 3.05), Gln159 (2.94, 3.22), and Pro169 (2.71). | Glu160, Gln167, Leu168, Gln170, and Phe172. |
| ZINC000014089759 | TCM | −8.8 | Arg21 (3.02, 3.03), Pro39 (3.1), and Lys127 (3.04). | Tyr13, Tyr18, Pro19, and Asn23. |
| ZINC000085545967 | TCM | −8.8 | Gln159 (3.16), Leu168 (3.14), and Leu169 (3.16). | Lys127, Ala128, Thr129, Pro131, Glu160, Pro165, Val166, Gln167, Gln170, and Phe172. |
| ZINC000085568136 | TCM | −8.7 | Asp45 (3.11) and Gly84 (3.13). | Val42, Gly44, Gly126, Lys127, Als128, Tyr171, and Thr173. |
| ZINC000095912717 | TCM | −8.7 | Gln159 (3.09), Val166 (3.14), and Gln170 (3.04). | Ala128, Asn130, Pro131, Glu160, Pro164, Pro165, Gln167, Leu168, and Pro169. |
| ZINC000014089743 | TCM | −8.6 | Arg21 (2.96), Ser24 (2.97), and Pro39 (3.09). | Tyr13, Tyr18, Pro19, Asn23, and Lys127. |
| ZINC000101564200 | TCM | −8.6 | Val166 (2.78, 3.09), Pro169 (2.77), and Gln170 (3.09). | Lys127, Ala128, Thr129, Pro131, Gln159, Glu160, Pro165, Gln167, and Leu168. |
| ZINC000085504890 | TCM | −8.5 | Asn130 (3.23) and Gln170 (3.16) | Lys127, Ala128, Thr129, Pro131, Gln159, Glu160, Pro165, Val166, Gln167, Leu168, and Pro169. |
| ZINC000095909661 | TCM | −8.5 | Asn130 (2.8) and Pro169 (3.18) | Ala128, Thr129, Pro131, Gln159, Glu160, Leu168, and Gln170. |
| ZINC000070454124 | TCM | −8.4 | Val42 (3.05) and Thr129 (3.19). | Val20, Arg21, Pro39, Asn43, Gly44, Lys127, Ala128, Leu132, and Tyr171. |
| Doramectin | Approved | −9.1 | Glu12 (2.72), Asn130 (3.14), Gln159 (2.8), Leu168 (3.33), and Pro169 (3.06). | Tyr13, Lys127, Ala128, Thr129, Pro131, Glu160, Phe161, Pro165, and Gln170. |
| Ledipasvir | Approved | −9 | - | Arg21, Pro39, Val42, Asn43, Gly44, Asp45, Thr46, Ser83, Gly84, Lys127, Ala128, Thr129, and Tyr171. |
| Avermectin B1 (Abamectin) | Approved | −8.7 | Glu12 (2.89), Asn130 (3.04), Gln159 (2.85), and Leu168 (3.11) | Thr8, Tyr13, Ala128, Thr129, Pro131, Glu160, Phe161, Pro165, Pro169, Gln170, and Phe172. |
| Elbasvir | Approved | −8.7 | Asp45 (3.16) | Arg21, Pro39, Val42, Asn43, Gly44, Thr46, Ser48, Gly84, Lys127, Ala128, Thr129, Tyr171, Thr173, Phe174, and Asp175. |
| Venetoclax (ABT-199, GDC-0199) | Approved | −8.5 | Gly44 (3.16), Thr46 (2.88), Gly84 (3.07), and Thr173 (2.85). | Ser48, Asn49, Ile82, Ser83, Leu168, Pro169, Gln170, Tyr171, and Asp175. |
| Revefenacin | Approved | −8.5 | Asp45 (2.9) and Lys127 (2.8) | Tyr18, Val20, Arg21, Pro39, Val42, Gly44, Ser83, Ala128, Thr129, Tyr171, and Phe172. |
| Glecaprevir | Approved | −8.4 | Asn130 (2.93), Gln159 (2.99), Val166 (2.8), and Leu168 (3.31). | Ala128, Thr129, Glu160, Phe161, Pro165, Gln167, Pro169, and Gln170. |
| Nilotinib | Inhibitor | −7.9 | - | Tyr18, Arg21, Pro39, Val42, Gly44, Gly126, Lys127, Ala128, Thr129. |
| Imatinib | Inhibitor | −7.6 | - | Pro39, Glu40, Ser41, Val42, Gly44, Asp45, Ser83, Gly84, Gly126, Lys127, and Tyr171. |
| Cepharanthine | Inhibitor | −7.3 | - | Asn130, Pro131, Gln159, Glu160, Pro165, Val166, Gln167, and Leu168. |
| Sangivamycin | Inhibitor | −6.3 | Pro169 (3.03, 3.2) and Val166 (2.82, 3.11). | Ala128, Thr129, Pro131, Gln159, Glu160, Pro165, Leu168, Gln170, and Phe172. |
| Compound | Docking Score (kcal/mol) | Molecular Weight (g/mol) | TPSA (Å2) | LogP | ESOL Solubility Class | GI Absorption | BBB Permeant | Pgp Substrate |
|---|---|---|---|---|---|---|---|---|
| AfroDb | ||||||||
| ZINC000034518176 | −8.1 | 426.72 | 20.23 | 7.03 | Poorly soluble | Low | No | No |
| ZINC000095485942 | −8.1 | 474.5 | 135.8 | 1.4 | Soluble | High | No | Yes |
| NANPDB | ||||||||
| NANPDB2933 | −8.5 | 486.51 | 132.5 | 1.6 | Soluble | High | No | Yes |
| TCM | ||||||||
| ZINC000085531689 | −8.9 | 568.66 | 105.86 | 2.57 | Moderately soluble | High | No | Yes |
| ZINC000014089759 | −8.8 | 470.68 | 74.6 | 5.36 | Poorly soluble | High | No | Yes |
| ZINC000085545967 | −8.8 | 580.79 | 118.22 | 4.14 | Poorly soluble | High | No | Yes |
| ZINC000085568136 | −8.7 | 598.66 | 100.74 | 4.98 | Poorly soluble | High | No | Yes |
| ZINC000095912717 | −8.7 | 544.59 | 134.66 | 1.59 | Soluble | High | No | Yes |
| ZINC000014089743 | −8.6 | 456.7 | 57.53 | 6.12 | Poorly soluble | Low | No | No |
| ZINC000101564200 | −8.6 | 478.49 | 115.06 | 4.69 | Poorly soluble | Low | No | No |
| ZINC000085504890 | −8.5 | 536.66 | 101.16 | 1.97 | Moderately soluble | High | No | Yes |
| ZINC000095909661 | −8.5 | 594.7 | 83.86 | 4.75 | Poorly soluble | High | No | No |
| ZINC000070454124 | −8.4 | 564.58 | 119.34 | 3.83 | Moderately soluble | High | No | Yes |
| ZINC000085530485 | −8.4 | 566.64 | 124.8 | 3.15 | Moderately soluble | High | No | Yes |
| ZINC000095911418 | −8.4 | 462.62 | 52.6 | 5.63 | Moderately soluble | High | No | No |
| Compound | vdW | Electrostatic | Polar Solvation | SASA | Binding |
|---|---|---|---|---|---|
| ZINC000034518176 | −117 ± 1.444 | −33.3 ± 2.86 | 60.3 ± 2.132 | −15.15 ± 0.164 | −105 ± 1.815 |
| ZINC000095485942 | −93.49 ± 2.464 | −80.21 ± 5.726 | 82.09 ± 6.387 | −11.64 ± 0.257 | −103.3 ± 2.217 |
| NANPDB2933 | −166.5 ± 1.572 | −50.07 ± 1.021 | 114.5 ± 1.085 | −16.84 ± 0.098 | −118.9 ± 1.838 |
| ZINC000085531689 | −108.9 ± 2.797 | −47.25 ± 4.294 | 123.2 ± 5.171 | −14.06 ± 0.333 | −46.97 ± 3.062 |
| ZINC000014089759 | −97.39 ± 3.274 | 15.76 ± 2.801 | 23.02 ± 1.793 | −12.08 ± 0.395 | −70.52 ± 2.343 |
| ZINC000085568136 | −156.5 ± 2.969 | −28.37 ± 1.995 | 110.9 ± 2.643 | −18.6 ± 0.306 | −92.71 ± 2.553 |
| ZINC000095912717 | −131.9 ± 1.218 | −21.38 ± 1.359 | 101.9 ± 1.817 | −15.76 ± 0.147 | −66.99 ± 1.73 |
| Nilotinib | −108.5 ± 3.918 | 41.83 ± 5.391 | 71.17 ± 4.661 | −15.75 ± 0.56 | −11.21 ± 3.55 |
| Compound ID (PubChem Compound ID) | Common Name/IUPAC Name | Source/Origin | 2D Structure |
|---|---|---|---|
| ZINC000034518176 (CID: 10836206) | (3S,4aR,6aR,6bS,8aR,11R,12S,12aS,14aR,14bR)-4,4,6a,6b,8a,11,12,14b-octamethyl-2,3,4a,5,6,7,8,9,10,11,12,12a,14,14a-tetradecahydro-1H-picen-3-ol | Vernonanthura chamaedrys [187,188], Mangifera indica [189], Cadia purpurea [190], Calendula officinalis [191], Pinalia leucantha [192], Ilex aquifolium [193], I. goshiensis [194], and Rhodomyrtus tomentosa [195] |  |
| ZINC000095485942 (CID: 163021364) | (1R,2R,4R,7S,8R,10R,11S,12S,13R,18R)-7-(furan-3-yl)-10,13-dihydroxy-8,12,17,17-tetramethyl-3,6,16-trioxapentacyclo [9.9.0.02,4.02,8.012,18]icosane-5,15,20-trione | - |  |
| NANPDB2933 (CID: 102019659) | 2-hydroxyseneganolide | Khaya senegalensis [179,180,181] |  |
| ZINC000085531689 (CID: 97042015) | (4S,4aR,7S,7aR,12bR)-10-[(4S,4aS,7R,7aS,12bR)-7,9-dihydroxy-3-methyl-2,4,4a,7,7a,13-hexahydro-1H-4,12-methanobenzofuro [3,2-e]isoquinolin-10-yl]-3-methyl-2,4,4a,7,7a,13-hexahydro-1H-4,12-methanobenzofuro [3,2-e]isoquinoline-7,9-diol | - |  |
| ZINC000014089759 (CID: 9847548) | 11-keto boswellic acid | Boswellia papyrifera [199], Boswellia sacra [200,201,202], and Boswellia serrata [203,204] |  |
| ZINC000085568136 (CID: Not available) | 5-[(11R,12R,17R,18S)-12,22-dihydroxy-11-(4-hydroxy-3-methoxyphenyl)-4,10-dioxahexacyclo [15.7.1.02,15.03,8.09,14.021,25]pentacosa-1(25),2,8,14,19,21,23-heptaen-18-yl]-2H-indol-2-ylium | - |  |
| ZINC000095912717 (CID: 12080815) | Lancifodilactone C | Schisandra chinensis (Turcz.) Baill [184] and Schisandra lancifolia [185] |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broni, E.; Ashley, C.; Adams, J.; Manu, H.; Aikins, E.; Okom, M.; Miller, W.A., III; Wilson, M.D.; Kwofie, S.K. Cheminformatics-Based Study Identifies Potential Ebola VP40 Inhibitors. Int. J. Mol. Sci. 2023, 24, 6298. https://doi.org/10.3390/ijms24076298
Broni E, Ashley C, Adams J, Manu H, Aikins E, Okom M, Miller WA III, Wilson MD, Kwofie SK. Cheminformatics-Based Study Identifies Potential Ebola VP40 Inhibitors. International Journal of Molecular Sciences. 2023; 24(7):6298. https://doi.org/10.3390/ijms24076298
Chicago/Turabian StyleBroni, Emmanuel, Carolyn Ashley, Joseph Adams, Hammond Manu, Ebenezer Aikins, Mary Okom, Whelton A. Miller, III, Michael D. Wilson, and Samuel K. Kwofie. 2023. "Cheminformatics-Based Study Identifies Potential Ebola VP40 Inhibitors" International Journal of Molecular Sciences 24, no. 7: 6298. https://doi.org/10.3390/ijms24076298
APA StyleBroni, E., Ashley, C., Adams, J., Manu, H., Aikins, E., Okom, M., Miller, W. A., III, Wilson, M. D., & Kwofie, S. K. (2023). Cheminformatics-Based Study Identifies Potential Ebola VP40 Inhibitors. International Journal of Molecular Sciences, 24(7), 6298. https://doi.org/10.3390/ijms24076298







