Aging-Related Mechanisms Contribute to Corticosteroid Insensitivity in Elderly Asthma
Abstract
1. Introduction
2. Glucocorticoid Receptor Signaling and Hormones in the Aging
3. Asthma in the Elderly Pathogenesis
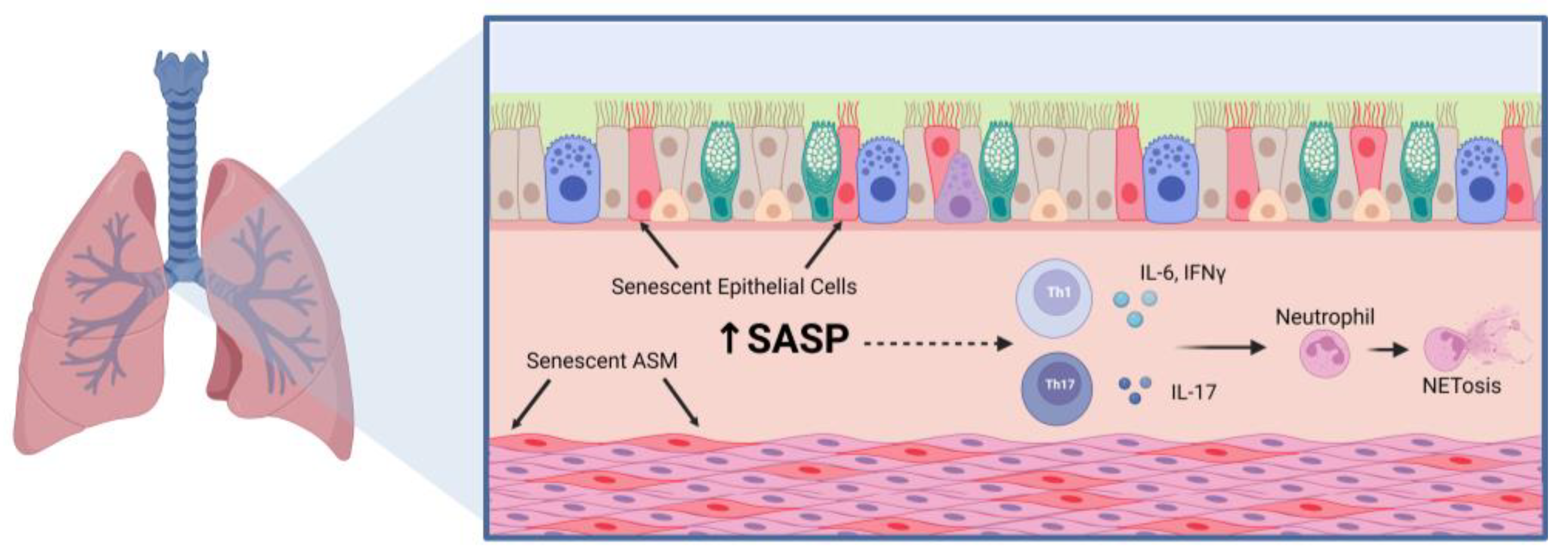
3.1. Airway Inflammation
3.2. Airway Structure and Function
4. Aging-Related Mechanisms in Asthma
4.1. Cellular Senescence
4.2. CD38 and NAD+ Metabolism
5. Factors and Comorbidities That May Influence Asthma in the Elderly
6. Conclusions
| Mechanisms | Characteristics | References |
|---|---|---|
| Hormone Production |
| [25,26,27,28,29] |
| Immune Cell Infiltration |
| [38,45,48] |
| Lung Function |
| [82,108,109] |
| Cellular Senescence |
| [39,52,59,63,64] |
| NAD+ Metabolism |
| [68,69,79,80] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thannickal, V.J.; Murthy, M.; Balch, W.E.; Chandel, N.S.; Meiners, S.; Eickelberg, O.; Selman, M.; Pardo, A.; White, E.S.; Levy, B.D.; et al. Blue journal conference. Aging and susceptibility to lung disease. Am. J. Respir. Crit. Care Med. 2015, 191, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.T.; Guppy, M.; Straus, S.E.; Bell, K.J.L.; Glasziou, P. Rate of normal lung function decline in ageing adults: A systematic review of prospective cohort studies. BMJ Open 2019, 9, e028150. [Google Scholar] [CrossRef] [PubMed]
- Dunn, R.M.; Busse, P.J.; Wechsler, M.E. Asthma in the elderly and late-onset adult asthma. Allergy 2018, 73, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Boulet, L.P. Irreversible airway obstruction in asthma. Curr. Allergy Asthma Rep. 2009, 9, 168–173. [Google Scholar] [CrossRef]
- Skloot, G.S.; Busse, P.J.; Braman, S.S.; Kovacs, E.J.; Dixon, A.E.; Vaz Fragoso, C.A.; Scichilone, N.; Prakash, Y.S.; Pabelick, C.M.; Mathur, S.K.; et al. An Official American Thoracic Society Workshop Report: Evaluation and Management of Asthma in the Elderly. Ann. Am. Thorac. Soc. 2016, 13, 2064–2077. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, M.; Caballero, A.; Jaramillo, C.; Maldonado, D.; Torres-Duque, C.A. Prevalence, risk factors and underdiagnosis of asthma and wheezing in adults 40 years and older: A population-based study. J. Asthma 2015, 52, 823–830. [Google Scholar] [CrossRef]
- Tsai, C.L.; Delclos, G.L.; Huang, J.S.; Hanania, N.A.; Camargo, C.A., Jr. Age-related differences in asthma outcomes in the United States, 1988–2006. Ann. Allergy Asthma Immunol. 2013, 110, 240–246.e1. [Google Scholar] [CrossRef]
- Ray, A.; Camiolo, M.; Fitzpatrick, A.; Gauthier, M.; Wenzel, S.E. Are We Meeting the Promise of Endotypes and Precision Medicine in Asthma? Physiol. Rev. 2020, 100, 983–1017. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Zhang, L.; Liu, Y.; Wang, G.; Zhang, H.P.; Wang, L.; Kang, Y.; Oliver, B.G.; Wan, H.J.; et al. Age-Related Clinical Characteristics, Inflammatory Features, Phenotypes, and Treatment Response in Asthma. J. Allergy Clin. Immunol. Pract. 2023, 11, 210–219.e3. [Google Scholar] [CrossRef]
- Choy, D.F.; Hart, K.M.; Borthwick, L.A.; Shikotra, A.; Nagarkar, D.R.; Siddiqui, S.; Jia, G.; Ohri, C.M.; Doran, E.; Vannella, K.M.; et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci. Transl. Med. 2015, 7, 301ra129. [Google Scholar] [CrossRef]
- Raundhal, M.; Morse, C.; Khare, A.; Oriss, T.B.; Milosevic, J.; Trudeau, J.; Huff, R.; Pilewski, J.; Holguin, F.; Kolls, J.; et al. High IFN-gamma and low SLPI mark severe asthma in mice and humans. J. Clin. Investig. 2015, 125, 3037–3050. [Google Scholar] [CrossRef]
- Isoyama, S.; Ishikawa, N.; Hamai, K.; Matsumura, M.; Kobayashi, H.; Nomura, A.; Ueno, S.; Tanimoto, T.; Maeda, H.; Iwamoto, H.; et al. Efficacy of mepolizumab in elderly patients with severe asthma and overlapping COPD in real-world settings: A retrospective observational study. Respir. Investig. 2021, 59, 478–486. [Google Scholar] [CrossRef]
- Patruno, C.; Napolitano, M.; Argenziano, G.; Peris, K.; Ortoncelli, M.; Girolomoni, G.; Offidani, A.; Ferrucci, S.M.; Amoruso, G.F.; Rossi, M.; et al. Dupilumab therapy of atopic dermatitis of the elderly: A multicentre, real-life study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Hinks, T.S.C.; Levine, S.J.; Brusselle, G.G. Treatment options in type-2 low asthma. Eur. Respir. J. 2021, 57, 2000528. [Google Scholar] [CrossRef] [PubMed]
- Desmet, S.J.; De Bosscher, K. Glucocorticoid receptors: Finding the middle ground. J. Clin. Investig. 2017, 127, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Bouazza, B.; Krytska, K.; Debba-Pavard, M.; Amrani, Y.; Honkanen, R.E.; Tran, J.; Tliba, O. Cytokines alter glucocorticoid receptor phosphorylation in airway cells: Role of phosphatases. Am. J. Respir. Cell Mol. Biol. 2012, 47, 464–473. [Google Scholar] [CrossRef]
- Chang, P.J.; Michaeloudes, C.; Zhu, J.; Shaikh, N.; Baker, J.; Chung, K.F.; Bhavsar, P.K. Impaired nuclear translocation of the glucocorticoid receptor in corticosteroid-insensitive airway smooth muscle in severe asthma. Am. J. Respir. Crit. Care Med. 2015, 191, 54–62. [Google Scholar] [CrossRef]
- Hamid, Q.A.; Wenzel, S.E.; Hauk, P.J.; Tsicopoulos, A.; Wallaert, B.; Lafitte, J.J.; Chrousos, G.P.; Szefler, S.J.; Leung, D.Y. Increased glucocorticoid receptor beta in airway cells of glucocorticoid-insensitive asthma. Am. J. Respir. Crit. Care Med. 1999, 159, 1600–1604. [Google Scholar] [CrossRef]
- Matthews, J.G.; Ito, K.; Barnes, P.J.; Adcock, I.M. Defective glucocorticoid receptor nuclear translocation and altered histone acetylation patterns in glucocorticoid-resistant patients. J. Allergy Clin. Immunol. 2004, 113, 1100–1108. [Google Scholar] [CrossRef]
- Veldhuis, J.D.; Sharma, A.; Roelfsema, F. Age-dependent and gender-dependent regulation of hypothalamic-adrenocorticotropic-adrenal axis. Endocrinol. Metab. Clin. N. Am. 2013, 42, 201–225. [Google Scholar] [CrossRef]
- Cho, Y.J.; Lee, K.E. Decreased glucocorticoid binding affinity to glucocorticoid receptor is important in the poor response to steroid therapy of older-aged patients with severe bronchial asthma. Allergy Asthma Proc. 2003, 24, 353–358. [Google Scholar] [PubMed]
- Nimmagadda, S.R.; Szefler, S.J.; Spahn, J.D.; Surs, W.; Leung, D.Y. Allergen exposure decreases glucocorticoid receptor binding affinity and steroid responsiveness in atopic asthmatics. Am. J. Respir. Crit. Care Med. 1997, 155, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Lightman, S.L.; Birnie, M.T.; Conway-Campbell, B.L. Dynamics of ACTH and Cortisol Secretion and Implications for Disease. Endocr. Rev. 2020, 41, bnaa002. [Google Scholar] [CrossRef] [PubMed]
- Dickmeis, T.; Weger, B.D.; Weger, M. The circadian clock and glucocorticoids--interactions across many time scales. Mol. Cell Endocrinol. 2013, 380, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Paragliola, R.M.; Papi, G.; Pontecorvi, A.; Corsello, S.M. Treatment with Synthetic Glucocorticoids and the Hypothalamus-Pituitary-Adrenal Axis. Int. J. Mol. Sci. 2017, 18, 2201. [Google Scholar] [CrossRef]
- Giri, A.; Wang, Q.; Rahman, I.; Sundar, I.K. Circadian molecular clock disruption in chronic pulmonary diseases. Trends Mol. Med. 2022, 28, 513–527. [Google Scholar] [CrossRef]
- Landstra, A.M.; Postma, D.S.; Boezen, H.M.; van Aalderen, W.M. Role of serum cortisol levels in children with asthma. Am. J. Respir. Crit. Care Med. 2002, 165, 708–712. [Google Scholar] [CrossRef]
- Kachroo, P.; Stewart, I.D.; Kelly, R.S.; Stav, M.; Mendez, K.; Dahlin, A.; Soeteman, D.I.; Chu, S.H.; Huang, M.; Cote, M.; et al. Metabolomic profiling reveals extensive adrenal suppression due to inhaled corticosteroid therapy in asthma. Nat. Med. 2022, 28, 814–822. [Google Scholar] [CrossRef]
- Seale, J.P. Is the pharmacology of corticosteroids in the lung modified by age? Med. J. Aust. 2005, 183, S47–S48. [Google Scholar] [CrossRef]
- Vermeulen, A. Dehydroepiandrosterone sulfate and aging. Ann. N. Y. Acad. Sci. 1995, 774, 121–127. [Google Scholar] [CrossRef]
- Yu, C.K.; Yang, B.C.; Lei, H.Y.; Chen, Y.C.; Liu, Y.H.; Chen, C.C.; Liu, C.W. Attenuation of house dust mite Dermatophagoides farinae-induced airway allergic responses in mice by dehydroepiandrosterone is correlated with down-regulation of TH2 response. Clin. Exp. Allergy 1999, 29, 414–422. [Google Scholar] [CrossRef]
- Dashtaki, R.; Whorton, A.R.; Murphy, T.M.; Chitano, P.; Reed, W.; Kennedy, T.P. Dehydroepiandrosterone and analogs inhibit DNA binding of AP-1 and airway smooth muscle proliferation. J. Pharmacol. Exp. Ther. 1998, 285, 876–883. [Google Scholar] [PubMed]
- Gandhi, V.D.; Cephus, J.Y.; Norlander, A.E.; Chowdhury, N.U.; Zhang, J.; Ceneviva, Z.J.; Tannous, E.; Polosukhin, V.V.; Putz, N.D.; Wickersham, N.; et al. Androgen receptor signaling promotes Treg suppressive function during allergic airway inflammation. J. Clin. Investig. 2022, 132, e153397. [Google Scholar] [CrossRef] [PubMed]
- Kalidhindi, R.S.R.; Ambhore, N.S.; Balraj, P.; Schmidt, T.; Khan, M.N.; Sathish, V. Androgen receptor activation alleviates airway hyperresponsiveness, inflammation, and remodeling in a murine model of asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L803–L818. [Google Scholar] [CrossRef] [PubMed]
- Zein, J.G.; McManus, J.M.; Sharifi, N.; Erzurum, S.C.; Marozkina, N.; Lahm, T.; Giddings, O.; Davis, M.D.; DeBoer, M.D.; Comhair, S.A.; et al. Benefits of Airway Androgen Receptor Expression in Human Asthma. Am. J. Respir. Crit. Care Med. 2021, 204, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Zein, J.; Gaston, B.; Bazeley, P.; DeBoer, M.D.; Igo, R.P., Jr.; Bleecker, E.R.; Meyers, D.; Comhair, S.; Marozkina, N.V.; Cotton, C.; et al. HSD3B1 genotype identifies glucocorticoid responsiveness in severe asthma. Proc. Natl. Acad. Sci. USA 2020, 117, 2187–2193. [Google Scholar] [CrossRef]
- Busse, P.J.; Birmingham, J.M.; Calatroni, A.; Manzi, J.; Goryachokovsky, A.; Fontela, G.; Federman, A.D.; Wisnivesky, J.P. Effect of aging on sputum inflammation and asthma control. J. Allergy Clin. Immunol. 2017, 139, 1808–1818.e6. [Google Scholar] [CrossRef]
- Ducharme, M.E.; Prince, P.; Hassan, N.; Nair, P.; Boulet, L.P. Expiratory flows and airway inflammation in elderly asthmatic patients. Respir. Med. 2011, 105, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K.; Yadav, J.; Makhija, S.; Sandey, M.; Suryawanshi, A.; Mitra, A.K.; Mishra, A. Short palate, lung, and nasal epithelial clone 1 (SPLUNC1) level determines steroid-resistant airway inflammation in aging. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 322, L102–L115. [Google Scholar] [CrossRef]
- Birmingham, J.M.; Gillespie, V.L.; Srivastava, K.; Li, X.M.; Busse, P.J. Influenza A infection enhances antigen-induced airway inflammation and hyperresponsiveness in young but not aged mice. Clin. Exp. Allergy 2014, 44, 1188–1199. [Google Scholar] [CrossRef]
- Brandenberger, C.; Li, N.; Jackson-Humbles, D.N.; Rockwell, C.E.; Wagner, J.G.; Harkema, J.R. Enhanced allergic airway disease in old mice is associated with a Th17 response. Clin. Exp. Allergy 2014, 44, 1282–1292. [Google Scholar] [CrossRef]
- Busse, P.J.; Zhang, T.F.; Srivastava, K.; Schofield, B.; Li, X.M. Effect of ageing on pulmonary inflammation, airway hyperresponsiveness and T and B cell responses in antigen-sensitized and -challenged mice. Clin. Exp. Allergy 2007, 37, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Alcorn, J.F.; Crowe, C.R.; Kolls, J.K. TH17 cells in asthma and COPD. Annu. Rev. Physiol. 2010, 72, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.W.; Eickhoff, C.S.; Meza, K.A.; Blase, J.R.; Audette, R.E.; Chan, D.H.; Bockerstett, K.A.; DiPaolo, R.J.; Hoft, D.F. Th17 Cells Provide Mucosal Protection against Gastric Trypanosoma cruzi Infection. Infect. Immun. 2021, 89, e0073820. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, G.; Manwani, D.; Mortha, A.; Xu, C.; Faith, J.J.; Burk, R.D.; Kunisaki, Y.; Jang, J.E.; Scheiermann, C.; et al. Neutrophil ageing is regulated by the microbiome. Nature 2015, 525, 528–532. [Google Scholar] [CrossRef]
- Green, R.H.; Brightling, C.E.; Woltmann, G.; Parker, D.; Wardlaw, A.J.; Pavord, I.D. Analysis of induced sputum in adults with asthma: Identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax 2002, 57, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Nyenhuis, S.M.; Schwantes, E.A.; Evans, M.D.; Mathur, S.K. Airway neutrophil inflammatory phenotype in older subjects with asthma. J. Allergy Clin. Immunol. 2010, 125, 1163–1165. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.K.; Schwantes, E.A.; Jarjour, N.N.; Busse, W.W. Age-related changes in eosinophil function in human subjects. Chest 2008, 133, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef]
- Munoz-Espin, D.; Canamero, M.; Maraver, A.; Gomez-Lopez, G.; Contreras, J.; Murillo-Cuesta, S.; Rodriguez-Baeza, A.; Varela-Nieto, I.; Ruberte, J.; Collado, M.; et al. Programmed cell senescence during mammalian embryonic development. Cell 2013, 155, 1104–1118. [Google Scholar] [CrossRef]
- Storer, M.; Mas, A.; Robert-Moreno, A.; Pecoraro, M.; Ortells, M.C.; Di Giacomo, V.; Yosef, R.; Pilpel, N.; Krizhanovsky, V.; Sharpe, J.; et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 2013, 155, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef]
- Coppe, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Munoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Guan, X.; Carraro, G.; Parimon, T.; Liu, X.; Huang, G.; Mulay, A.; Soukiasian, H.J.; David, G.; Weigt, S.S.; et al. Senescence of Alveolar Type 2 Cells Drives Progressive Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2021, 203, 707–717. [Google Scholar] [CrossRef]
- Prakash, Y.S. Emerging concepts in smooth muscle contributions to airway structure and function: Implications for health and disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L1113–L1140. [Google Scholar] [CrossRef]
- Parikh, P.; Britt, R.D., Jr.; Manlove, L.J.; Wicher, S.A.; Roesler, A.; Ravix, J.; Teske, J.; Thompson, M.A.; Sieck, G.C.; Kirkland, J.L.; et al. Hyperoxia-induced Cellular Senescence in Fetal Airway Smooth Muscle Cells. Am. J. Respir. Cell Mol. Biol. 2019, 61, 51–60. [Google Scholar] [CrossRef]
- Wang, Z.N.; Su, R.N.; Yang, B.Y.; Yang, K.X.; Yang, L.F.; Yan, Y.; Chen, Z.G. Potential Role of Cellular Senescence in Asthma. Front. Cell Dev. Biol. 2020, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Aghali, A.; Khalfaoui, L.; Lagnado, A.B.; Drake, L.Y.; Teske, J.J.; Pabelick, C.M.; Passos, J.F.; Prakash, Y.S. Cellular senescence is increased in airway smooth muscle cells of elderly persons with asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 323, L558–L568. [Google Scholar] [CrossRef] [PubMed]
- Wicher, S.A.; Roos, B.B.; Teske, J.J.; Fang, Y.H.; Pabelick, C.; Prakash, Y.S. Aging increases senescence, calcium signaling, and extracellular matrix deposition in human airway smooth muscle. PLoS ONE 2021, 16, e0254710. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The airway epithelium in asthma. Nat. Med. 2012, 18, 684–692. [Google Scholar] [CrossRef] [PubMed]
- de Vries, M.; Nwozor, K.O.; Muizer, K.; Wisman, M.; Timens, W.; van den Berge, M.; Faiz, A.; Hackett, T.L.; Heijink, I.H.; Brandsma, C.A. The relation between age and airway epithelial barrier function. Respir. Res. 2022, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Du, X.; Tang, S.; Wu, S.; Wang, L.; Xiang, Y.; Qu, X.; Liu, H.; Qin, X.; Liu, C. ITGB4 deficiency induces senescence of airway epithelial cells through p53 activation. FEBS J. 2019, 286, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Liu, H.; Du, X.; Yao, Y.; Qin, L.; Xia, Z.; Zhou, K.; Wu, X.; Yuan, Y.; Qing, B.; et al. Airway epithelial ITGB4 deficiency induces airway remodeling in a mouse model. J. Allergy Clin. Immunol. 2023, 151, 431–446.e16. [Google Scholar] [CrossRef]
- Hachim, M.Y.; Elemam, N.M.; Ramakrishnan, R.K.; Bajbouj, K.; Olivenstein, R.; Hachim, I.Y.; Al Heialy, S.; Hamid, Q.; Busch, H.; Hamoudi, R. Wnt Signaling Is Deranged in Asthmatic Bronchial Epithelium and Fibroblasts. Front. Cell Dev. Biol. 2021, 9, 641404. [Google Scholar] [CrossRef]
- Hasegawa, A.; Miki, T.; Hosokawa, H.; Hossain, M.B.; Shimizu, C.; Hashimoto, K.; Kimura, M.Y.; Yamashita, M.; Nakayama, T. Impaired GATA3-dependent chromatin remodeling and Th2 cell differentiation leading to attenuated allergic airway inflammation in aging mice. J. Immunol. 2006, 176, 2546–2554. [Google Scholar] [CrossRef]
- Piedra-Quintero, Z.L.; Wilson, Z.; Nava, P.; Guerau-de-Arellano, M. CD38: An Immunomodulatory Molecule in Inflammation and Autoimmunity. Front. Immunol. 2020, 11, 597959. [Google Scholar] [CrossRef]
- Camacho-Pereira, J.; Tarrago, M.G.; Chini, C.C.S.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.S.; Schoon, R.A.; Reid, J.M.; Galina, A.; et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016, 23, 1127–1139. [Google Scholar] [CrossRef]
- Chini, C.C.S.; Tarrago, M.G.; Chini, E.N. NAD and the aging process: Role in life, death and everything in between. Mol. Cell. Endocrinol. 2017, 455, 62–74. [Google Scholar] [CrossRef]
- Deshpande, D.A.; Guedes, A.G.P.; Lund, F.E.; Subramanian, S.; Walseth, T.F.; Kannan, M.S. CD38 in the pathogenesis of allergic airway disease: Potential therapeutic targets. Pharmacol. Ther. 2017, 172, 116–126. [Google Scholar] [CrossRef]
- Tliba, O.; Cidlowski, J.A.; Amrani, Y. CD38 expression is insensitive to steroid action in cells treated with tumor necrosis factor-alpha and interferon-gamma by a mechanism involving the up-regulation of the glucocorticoid receptor beta isoform. Mol. Pharmacol. 2006, 69, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.; Walum, J.; Banerjee, P.; Lewis, B.W.; Prakash, Y.S.; Sathish, V.; Xu, Z.; Britt, R.D., Jr. Th1 cytokines synergize to change gene expression and promote corticosteroid insensitivity in pediatric airway smooth muscle. Respir. Res. 2022, 23, 126. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.G.; Jude, J.A.; Paulin, J.; Kita, H.; Lund, F.E.; Kannan, M.S. Role of CD38 in TNF-alpha-induced airway hyperresponsiveness. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L290–L299. [Google Scholar] [CrossRef]
- Guedes, A.G.; Paulin, J.; Rivero-Nava, L.; Kita, H.; Lund, F.E.; Kannan, M.S. CD38-deficient mice have reduced airway hyperresponsiveness following IL-13 challenge. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 291, L1286–L1293. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.G.; Jude, J.A.; Paulin, J.; Rivero-Nava, L.; Kita, H.; Lund, F.E.; Kannan, M.S. Airway responsiveness in CD38-deficient mice in allergic airway disease: Studies with bone marrow chimeras. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L485–L493. [Google Scholar] [CrossRef] [PubMed]
- Gally, F.; Hartney, J.M.; Janssen, W.J.; Perraud, A.L. CD38 plays a dual role in allergen-induced airway hyperresponsiveness. Am. J. Respir. Cell Mol. Biol. 2009, 40, 433–442. [Google Scholar] [CrossRef]
- Cui, H.; Xie, N.; Banerjee, S.; Dey, T.; Liu, R.M.; Antony, V.B.; Sanders, Y.Y.; Adams, T.S.; Gomez, J.L.; Thannickal, V.J.; et al. CD38 Mediates Lung Fibrosis by Promoting Alveolar Epithelial Cell Aging. Am. J. Respir. Crit. Care Med. 2022, 206, 459–475. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, G. Roles of sirtuins in asthma. Respir. Res. 2022, 23, 251. [Google Scholar] [CrossRef]
- Rahman, I.; Kinnula, V.L.; Gorbunova, V.; Yao, H. SIRT1 as a therapeutic target in inflammaging of the pulmonary disease. Prev. Med. 2012, 54, S20–S28. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Rong, W.; Fan, L.; Cai, Y.; Qu, Q.; Gao, Y.; Zhao, H. miR-221 participates in the airway epithelial cells injury in asthma via targeting SIRT1. Exp. Lung Res. 2018, 44, 272–279. [Google Scholar] [CrossRef]
- Pal, S.; Tyler, J.K. Epigenetics and aging. Sci. Adv. 2016, 2, e1600584. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, G.; Schiavetti, I.; Ricciardolo, F.L.M. The impact of aging on outpatients with asthma in a real-world setting. Respir. Med. 2018, 136, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Hanania, N.A.; King, M.J.; Braman, S.S.; Saltoun, C.; Wise, R.A.; Enright, P.; Falsey, A.R.; Mathur, S.K.; Ramsdell, J.W.; Rogers, L.; et al. Asthma in the elderly: Current understanding and future research needs—A report of a National Institute on Aging (NIA) workshop. J. Allergy Clin. Immunol. 2011, 128, S4-24. [Google Scholar] [CrossRef] [PubMed]
- Hekking, P.P.; Amelink, M.; Wener, R.R.; Bouvy, M.L.; Bel, E.H. Comorbidities in Difficult-to-Control Asthma. J. Allergy Clin. Immunol. Pract. 2018, 6, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Wee, J.H.; Park, M.W.; Min, C.; Byun, S.H.; Park, B.; Choi, H.G. Association between asthma and cardiovascular disease. Eur. J. Clin. Investig. 2021, 51, e13396. [Google Scholar] [CrossRef]
- Tattersall, M.C.; Guo, M.; Korcarz, C.E.; Gepner, A.D.; Kaufman, J.D.; Liu, K.J.; Barr, R.G.; Donohue, K.M.; McClelland, R.L.; Delaney, J.A.; et al. Asthma predicts cardiovascular disease events: The multi-ethnic study of atherosclerosis. Arter. Thromb. Vasc. Biol. 2015, 35, 1520–1525. [Google Scholar] [CrossRef]
- Schatz, M.; Zeiger, R.S.; Yang, S.J.; Chen, W.; Sajjan, S.; Allen-Ramey, F.; Camargo, C.A., Jr. Prospective Study on the Relationship of Obesity to Asthma Impairment and Risk. J. Allergy Clin. Immunol. Pract. 2015, 3, 560–565.e1. [Google Scholar] [CrossRef]
- Wu, W.; Bang, S.; Bleecker, E.R.; Castro, M.; Denlinger, L.; Erzurum, S.C.; Fahy, J.V.; Fitzpatrick, A.M.; Gaston, B.M.; Hastie, A.T.; et al. Multiview Cluster Analysis Identifies Variable Corticosteroid Response Phenotypes in Severe Asthma. Am. J. Respir. Crit. Care Med. 2019, 199, 1358–1367. [Google Scholar] [CrossRef]
- Peters, M.C.; McGrath, K.W.; Hawkins, G.A.; Hastie, A.T.; Levy, B.D.; Israel, E.; Phillips, B.R.; Mauger, D.T.; Comhair, S.A.; Erzurum, S.C.; et al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: A cross-sectional analysis of two cohorts. Lancet Respir. Med. 2016, 4, 574–584. [Google Scholar] [CrossRef]
- Telenga, E.D.; Tideman, S.W.; Kerstjens, H.A.; Hacken, N.H.; Timens, W.; Postma, D.S.; van den Berge, M. Obesity in asthma: More neutrophilic inflammation as a possible explanation for a reduced treatment response. Allergy 2012, 67, 1060–1068. [Google Scholar] [CrossRef]
- Strunk, R.C.; Colvin, R.; Bacharier, L.B.; Fuhlbrigge, A.; Forno, E.; Arbelaez, A.M.; Tantisira, K.G.; Childhood Asthma Management Program Research, G. Airway Obstruction Worsens in Young Adults with Asthma Who Become Obese. J. Allergy Clin. Immunol. Pract. 2015, 3, 765–771.e2. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.A.; Matheson, M.C.; Diao, F.; Johns, D.P.; Erbas, B.; Lowe, A.J.; Gurrin, L.C.; Lodge, C.J.; Thomas, P.S.; Morrison, S.; et al. Bronchial hyperresponsiveness and obesity in middle age: Insights from an Australian cohort. Eur. Respir. J. 2017, 50, 1602181. [Google Scholar] [CrossRef] [PubMed]
- Orfanos, S.; Jude, J.; Deeney, B.T.; Cao, G.; Rastogi, D.; van Zee, M.; Pushkarsky, I.; Munoz, H.E.; Damoiseaux, R.; Di Carlo, D.; et al. Obesity increases airway smooth muscle responses to contractile agonists. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L673–L681. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.C.; Schiebler, M.L.; Cardet, J.C.; Johansson, M.W.; Sorkness, R.; DeBoer, M.D.; Bleecker, E.R.; Meyers, D.A.; Castro, M.; Sumino, K.; et al. The Impact of Insulin Resistance on Loss of Lung Function and Response to Treatment in Asthma. Am. J. Respir. Crit. Care Med. 2022, 206, 1096–1106. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, H.J.; Chang, Y.J.; Pichavant, M.; Shore, S.A.; Fitzgerald, K.A.; Iwakura, Y.; Israel, E.; Bolger, K.; Faul, J.; et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat. Med. 2014, 20, 54–61. [Google Scholar] [CrossRef]
- Woldhuis, R.R.; de Vries, M.; Timens, W.; van den Berge, M.; Demaria, M.; Oliver, B.G.G.; Heijink, I.H.; Brandsma, C.A. Link between increased cellular senescence and extracellular matrix changes in COPD. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L48–L60. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Hashimoto, M.; Minagawa, S.; Takasaka, N.; Ma, R.; Moermans, C.; Ito, S.; Araya, J.; Budelsky, A.; Goodsell, A.; et al. Role of IL-17A in murine models of COPD airway disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L122–L130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhu, L. Update on molecular mechanisms of corticosteroid resistance in chronic obstructive pulmonary disease. Pulm Pharmacol. Ther. 2016, 37, 1–8. [Google Scholar] [CrossRef]
- Tommola, M.; Ilmarinen, P.; Tuomisto, L.E.; Lehtimaki, L.; Haanpaa, J.; Niemela, O.; Kankaanranta, H. Differences between asthma-COPD overlap syndrome and adult-onset asthma. Eur. Respir. J. 2017, 49, 1602383. [Google Scholar] [CrossRef]
- Tu, X.; Kim, R.Y.; Brown, A.C.; de Jong, E.; Jones-Freeman, B.; Ali, M.K.; Gomez, H.M.; Budden, K.F.; Starkey, M.R.; Cameron, G.J.M.; et al. Airway and parenchymal transcriptomics in a novel model of asthma and COPD overlap. J. Allergy Clin. Immunol. 2022, 150, 817–829.e6. [Google Scholar] [CrossRef]
- Shah, R.; Newcomb, D.C. Sex Bias in Asthma Prevalence and Pathogenesis. Front. Immunol. 2018, 9, 2997. [Google Scholar] [CrossRef] [PubMed]
- Sathish, V.; Martin, Y.N.; Prakash, Y.S. Sex steroid signaling: Implications for lung diseases. Pharmacol. Ther. 2015, 150, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.J.; Walters, E.H.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Matheson, M.C.; Dharmage, S.C. Age-of-asthma onset as a determinant of different asthma phenotypes in adults: A systematic review and meta-analysis of the literature. Expert Rev. Respir. Med. 2015, 9, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Zein, J.G.; Denson, J.L.; Wechsler, M.E. Asthma over the Adult Life Course: Gender and Hormonal Influences. Clin. Chest Med. 2019, 40, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Pasha, M.A.; Sundquist, B.; Townley, R. Asthma pathogenesis, diagnosis, and management in the elderly. Allergy Asthma Proc. 2017, 38, 184–191. [Google Scholar] [CrossRef]
- Khosa, J.K.; Louie, S.; Lobo Moreno, P.; Abramov, D.; Rogstad, D.K.; Alismail, A.; Matus, M.J.; Tan, L.D. Asthma Care in the Elderly: Practical Guidance and Challenges for Clinical Management—A Framework of 5 “Ps”. J. Asthma Allergy 2023, 16, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Cho, S.H. Management of Elderly Asthma: Key Questions and Tentative Answers. Allergy Asthma Immunol. Res. 2023, 15, 8–18. [Google Scholar] [CrossRef]
- Inoue, H.; Niimi, A.; Takeda, T.; Matsumoto, H.; Ito, I.; Matsuoka, H.; Jinnai, M.; Otsuka, K.; Oguma, T.; Nakaji, H.; et al. Pathophysiological characteristics of asthma in the elderly: A comprehensive study. Ann. Allergy Asthma Immunol. 2014, 113, 527–533. [Google Scholar] [CrossRef]
- Kanazawa, H.; Tochino, Y.; Kyoh, S.; Ichimaru, Y.; Asai, K.; Hirata, K. Potential roles of pentosidine in age-related and disease-related impairment of pulmonary functions in patients with asthma. J. Allergy Clin. Immunol. 2011, 127, 899–904. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ford, M.L.; Ruwanpathirana, A.; Lewis, B.W.; Britt, R.D., Jr. Aging-Related Mechanisms Contribute to Corticosteroid Insensitivity in Elderly Asthma. Int. J. Mol. Sci. 2023, 24, 6347. https://doi.org/10.3390/ijms24076347
Ford ML, Ruwanpathirana A, Lewis BW, Britt RD Jr. Aging-Related Mechanisms Contribute to Corticosteroid Insensitivity in Elderly Asthma. International Journal of Molecular Sciences. 2023; 24(7):6347. https://doi.org/10.3390/ijms24076347
Chicago/Turabian StyleFord, Maria L., Anushka Ruwanpathirana, Brandon W. Lewis, and Rodney D. Britt, Jr. 2023. "Aging-Related Mechanisms Contribute to Corticosteroid Insensitivity in Elderly Asthma" International Journal of Molecular Sciences 24, no. 7: 6347. https://doi.org/10.3390/ijms24076347
APA StyleFord, M. L., Ruwanpathirana, A., Lewis, B. W., & Britt, R. D., Jr. (2023). Aging-Related Mechanisms Contribute to Corticosteroid Insensitivity in Elderly Asthma. International Journal of Molecular Sciences, 24(7), 6347. https://doi.org/10.3390/ijms24076347






