The Overexpression of Oryza sativa L. CYP85A1 Promotes Growth and Biomass Production in Transgenic Trees
Abstract
1. Introduction
2. Results
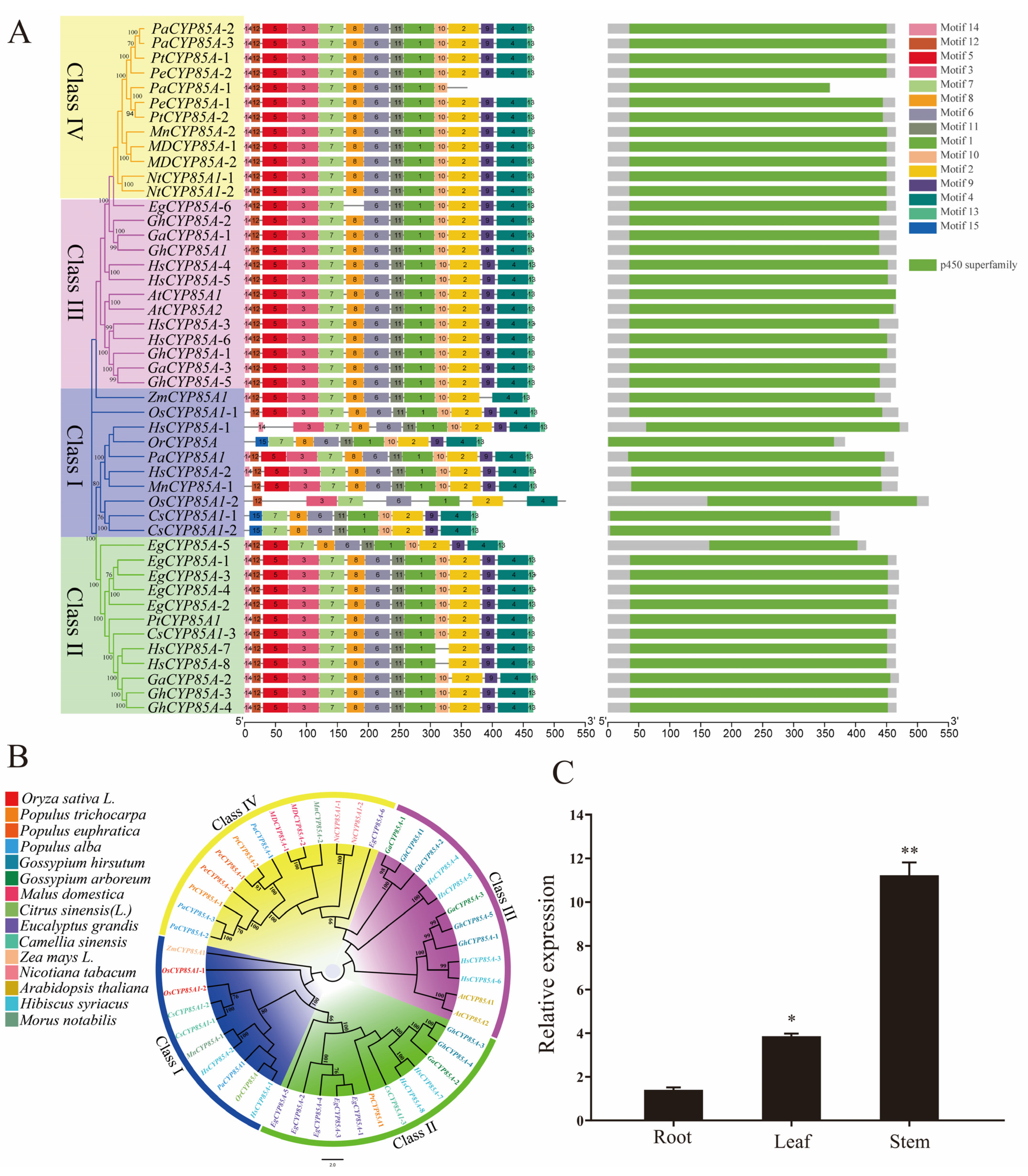
2.1. Bioinformatic Analysis of CYP85A Gene and Expression Patterns in Poplar
2.2. Overexpression of OsCYP85A1 Improves Plant Growth Development
2.2.1. Generation of OsCYP85A1 Transgenic P. tomentosa Lines
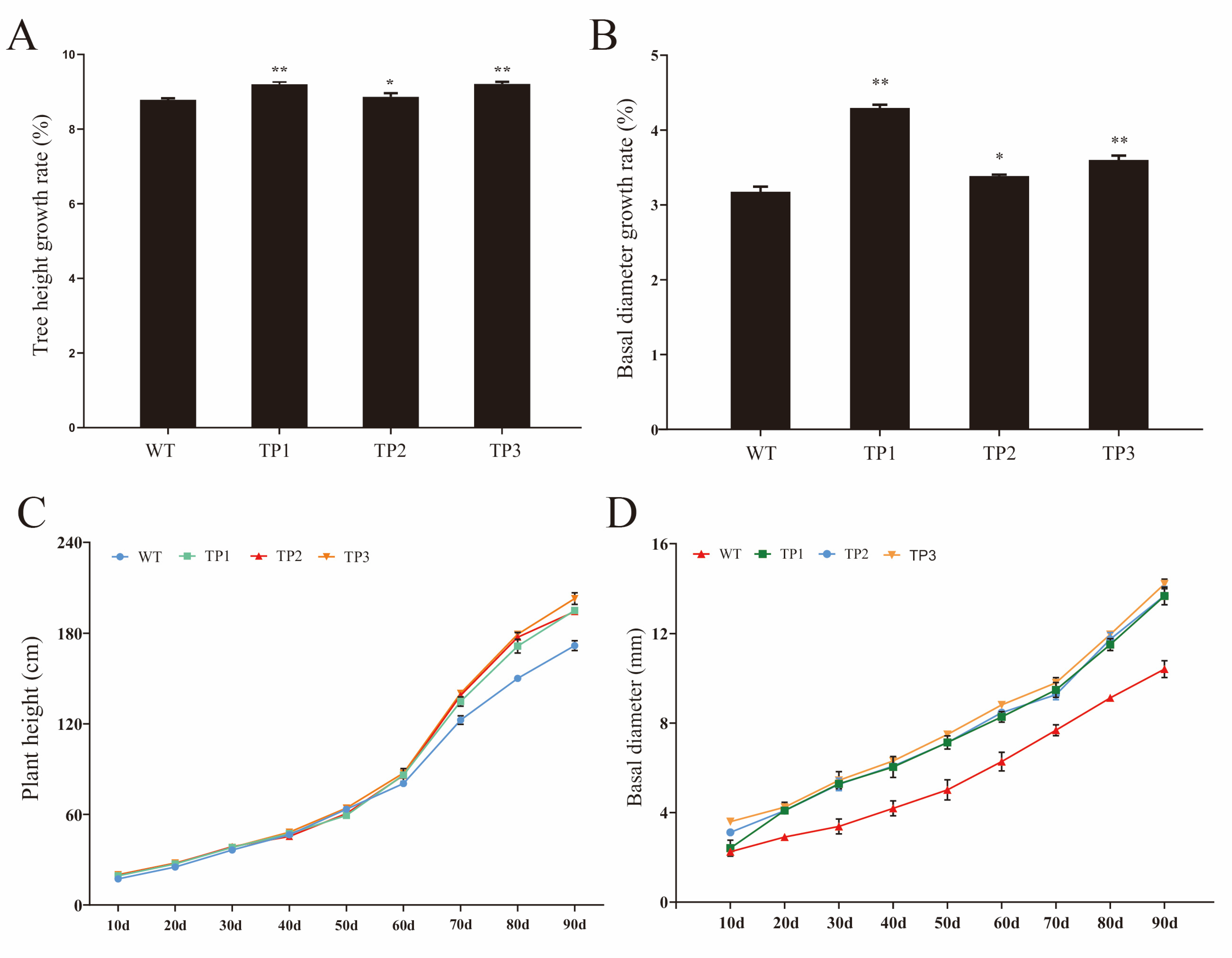
2.2.2. Growth Rate
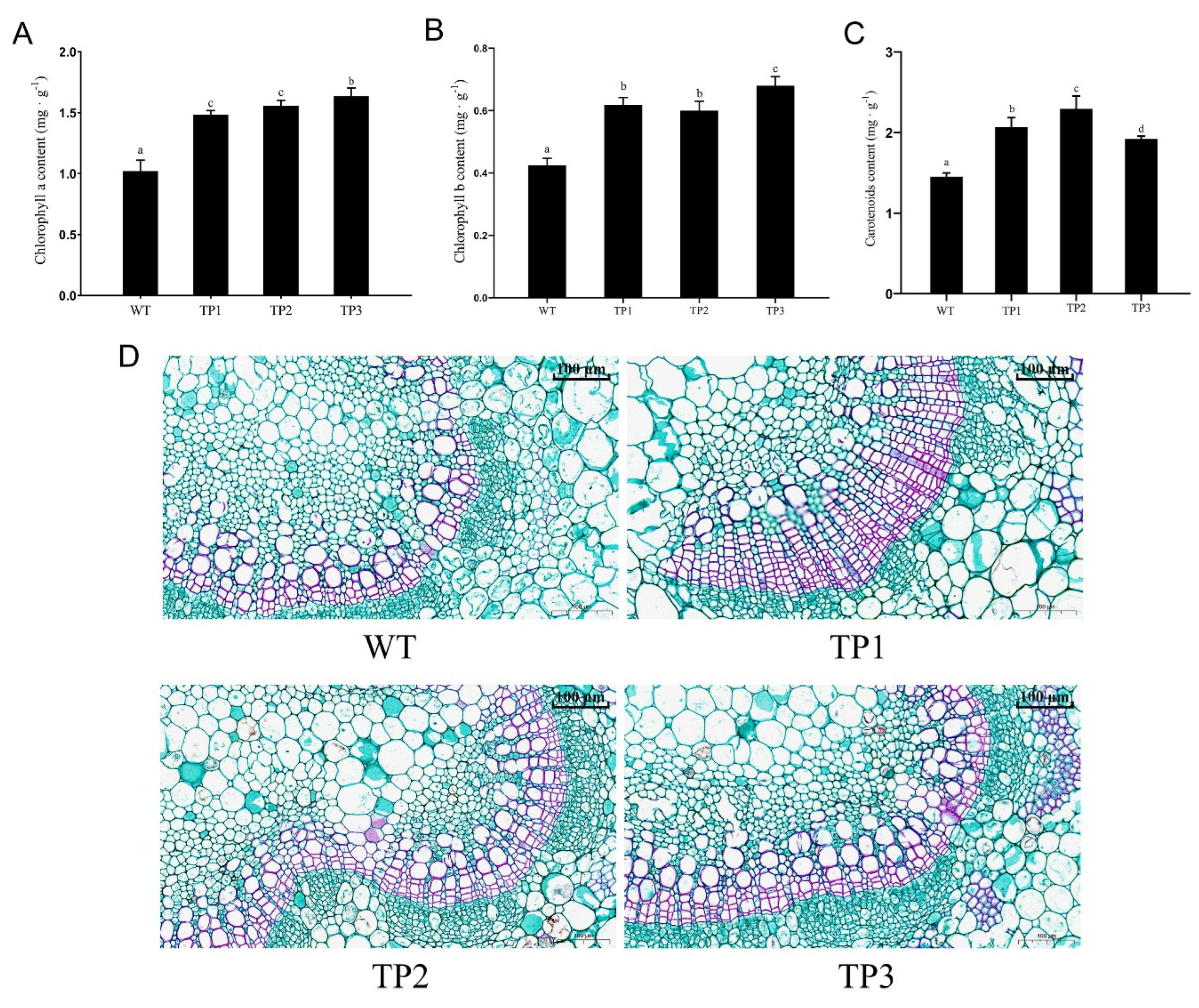
2.2.3. Effects of OsCYP85A1 on Chlorophyll Content
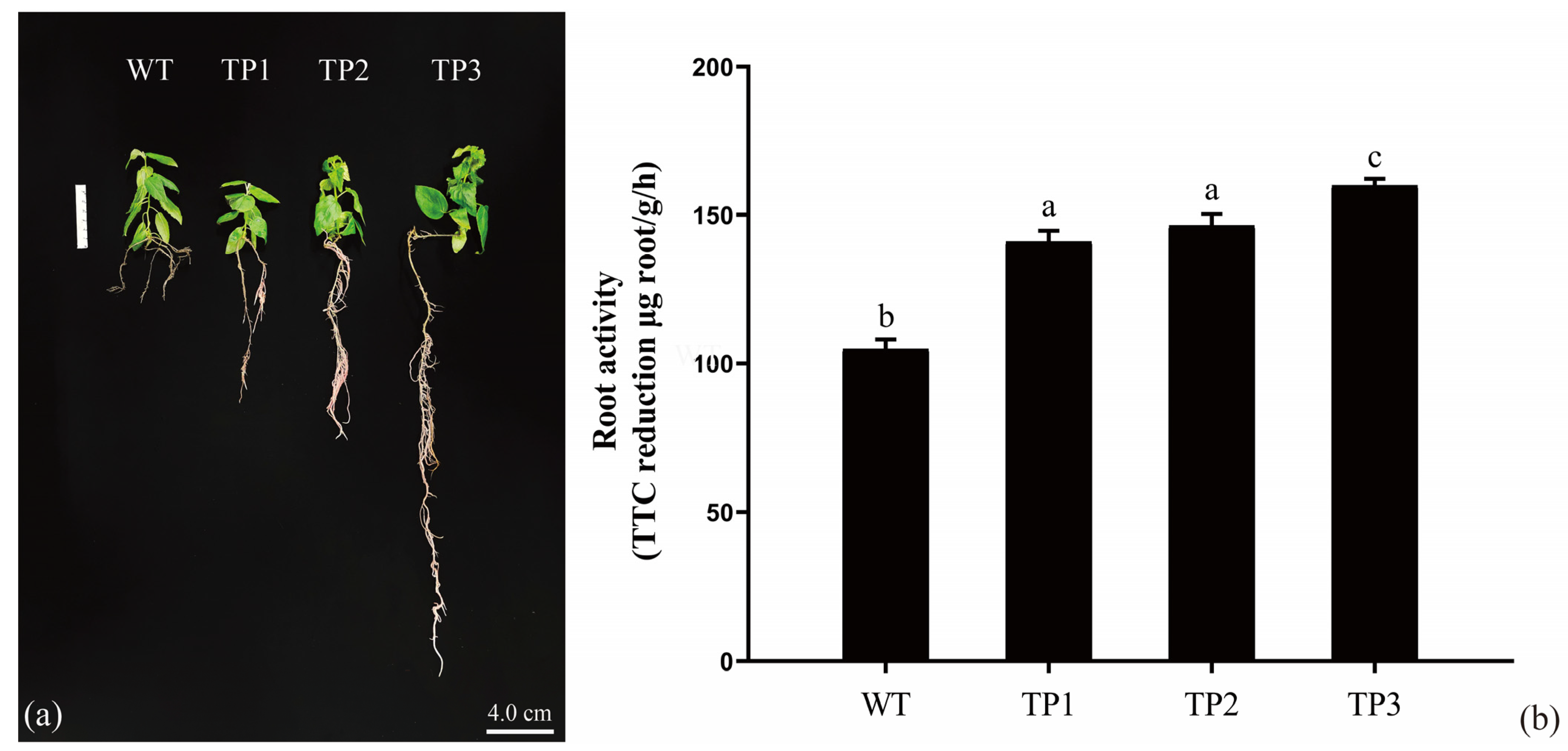
2.3. Overexpression of OsCYP85A1 Regulates Root Development
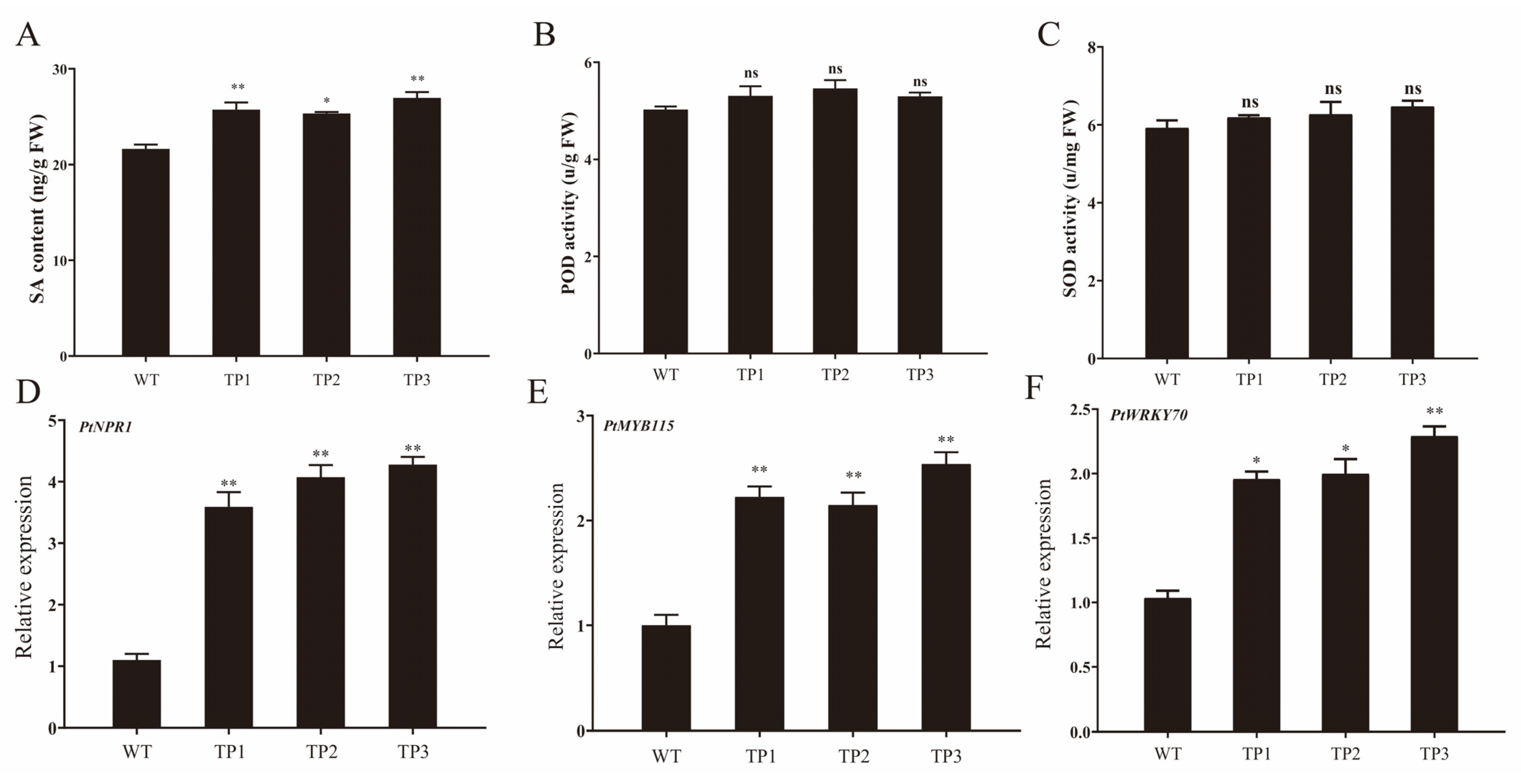
2.4. Overexpression of OsCYP85A1 Enhanced Plant Disease Resistance
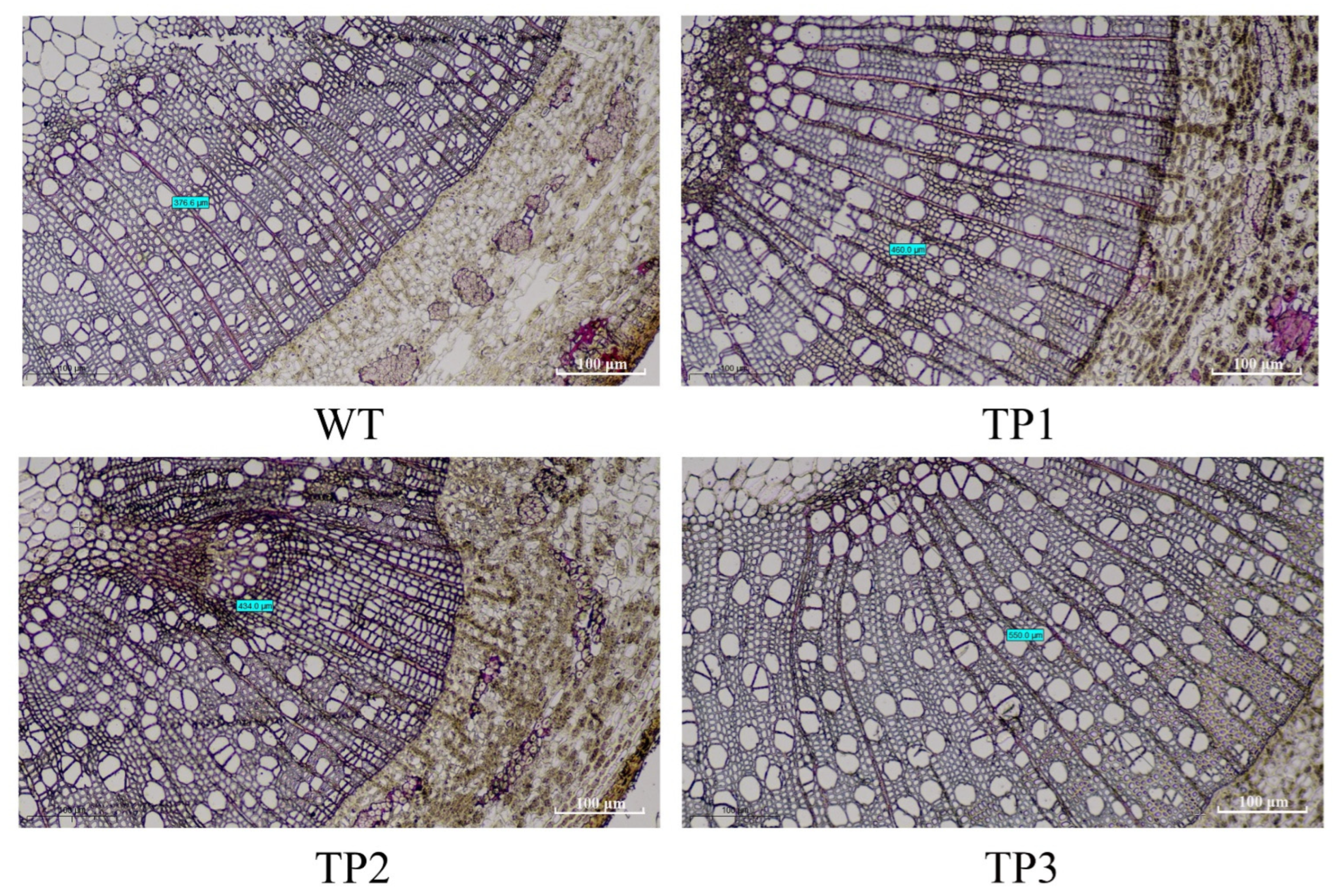
2.5. OsCYP85A1 Promotes Xylem Differentiation in Transgenic Plants
3. Discussion
4. Materials and Methods
4.1. Bioinformatics Analysis
4.2. Materials and Transformation
4.3. Quantitative PCR (qPCR) and Gus Histochemical Staining
4.4. Determination of Growth Indices
4.4.1. Growth Measurement
4.4.2. Determination of Photosynthetic Pigments
4.4.3. Lignin Content Determination
4.4.4. Determination of Cellulose, Hemicellulose, and Pectin Content
4.5. Triphenyltetrazolium Chloride (TTC) Mothed Root Activity Determination and Disease Resistance Analysis
4.6. Histological Analysis of OsCYP85A1 Transgenic Populus tomentosa and the Wild Type
4.7. Analysis of Expression by qRT-PCR
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bajguz, A.; Tretyn, A. The Chemical Characteristic and Distribution of Brassinosteroids in Plants. Phytochemistry 2003, 62, 20. [Google Scholar] [CrossRef]
- Fridman, Y.; Savaldi-Goldstein, S. Brassinosteroids in Growth Control: How, When and Where. Plant Sci. 2013, 209, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Clouse, S.D. Brassinosteroid Signal Transduction: From Receptor Kinase Activation to Transcriptional Networks Regulating Plant Development. Plant Cell 2011, 23, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, L.; Youn, J.; Sun, W.; Cheng, Z.; Jin, T.; Ma, X.; Guo, X.; Wang, J.; Zhang, X.; et al. A Comprehensive Genetic Study Reveals a Crucial Role of CYP90D2/D2 in Regulating Plant Architecture in Rice (Oryza sativa). N. Phytol. 2013, 200, 1076–1088. [Google Scholar] [CrossRef]
- Tanabe, S.; Ashikari, M.; Fujioka, S.; Takatsuto, S.; Yoshida, S.; Yano, M.; Yoshimura, A.; Kitano, H.; Matsuoka, M.; Fujisawa, Y.; et al. A Novel Cytochrome P450 Is Implicated in Brassinosteroid Biosynthesis via the Characterization of a Rice Dwarf Mutant, Dwarf11, with Reduced Seed Length. Plant Cell 2005, 17, 776–790. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) Role in Plant Development and Coping with Different Stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef]
- Watanabe, B.; Yamamoto, S.; Yokoi, T.; Sugiura, A.; Horoiwa, S.; Aoki, T.; Miyagawa, H.; Nakagawa, Y. Brassinolide-like Activity of Castasterone Analogs with Varied Side Chains against Rice Lamina Inclination. Bioorg. Med. Chem. 2017, 25, 4566–4578. [Google Scholar] [CrossRef] [PubMed]
- Northey, J.G.B.; Liang, S.; Jamshed, M.; Deb, S.; Foo, E.; Reid, J.B.; McCourt, P.; Samuel, M.A. Farnesylation Mediates Brassinosteroid Biosynthesis to Regulate Abscisic Acid Responses. Nat. Plants 2016, 2, 16114. [Google Scholar] [CrossRef]
- Sun, Y.; He, Y.; Irfan, A.R.; Liu, X.; Yu, Q.; Zhang, Q.; Yang, D. Exogenous Brassinolide Enhances the Growth and Cold Resistance of Maize (Zea mays L.) Seedlings under Chilling Stress. Agronomy 2020, 10, 488. [Google Scholar] [CrossRef]
- Li, Y.; Wei, K. Comparative Functional Genomics Analysis of Cytochrome P450 Gene Superfamily in Wheat and Maize. BMC Plant Biol. 2020, 20, 93. [Google Scholar] [CrossRef]
- Kim, B.K.; Fujioka, S.; Takatsuto, S.; Tsujimoto, M.; Choe, S. Castasterone Is a Likely End Product of Brassinosteroid Biosynthetic Pathway in Rice. Biochem. Biophys. Res. Commun. 2008, 374, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Kushiro, T.; Yokota, T.; Kamiya, Y.; Bishop, G.J.; Yamaguchi, S. The Last Reaction Producing Brassinolide Is Catalyzed by Cytochrome P-450s, CYP85A3 in Tomato and CYP85A2 in Arabidopsis. J. Biol. Chem. 2005, 280, 17873–17879. [Google Scholar] [CrossRef]
- Sakamoto, T.; Kawabe, A.; Tokida-Segawa, A.; Shimizu, B.; Takatsuto, S.; Shimada, Y.; Fujioka, S.; Mizutani, M. Rice CYP734As Function as Multisubstrate and Multifunctional Enzymes in Brassinosteroid Catabolism: Rice Brassinosteroid Catabolic Genes. Plant J. 2011, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Thornton, L.E.; Rupasinghe, S.G.; Peng, H.; Schuler, M.A.; Neff, M.M. Arabidopsis CYP72C1 Is an Atypical Cytochrome P450 That Inactivates Brassinosteroids. Plant Mol. Biol. 2010, 15, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-W.; Chang, S.C.; Lee, J.S.; Hwang, B.; Takatsuto, S.; Yokota, T.; Kim, S.-K. Cytochrome P450-Catalyzed Brassinosteroid Pathway Activation through Synthesis of Castasterone and Brassinolide in Phaseolus vulgaris. Phytochemistry 2004, 65, 679–689. [Google Scholar] [CrossRef]
- Shimada, Y.; Goda, H.; Nakamura, A.; Takatsuto, S.; Fujioka, S.; Yoshida, S. Organ-Specific Expression of Brassinosteroid-Biosynthetic Genes and Distribution of Endogenous Brassinosteroids in Arabidopsis. Plant Physiol. 2003, 131, 287–297. [Google Scholar] [CrossRef]
- Jiang, C.; Li, B.; Song, Z.; Zhang, Y.; Yu, C.; Wang, H.; Wang, L.; Zhang, H. PtBRI1.2 Promotes Shoot Growth and Wood Formation through a Brassinosteroid-Mediated PtBZR1-PtWNDs Module in Poplar. J. Exp. Bot. 2021, 72, 6350–6364. [Google Scholar] [CrossRef]
- Youn, J.-H.; Kim, M.K.; Kim, E.-J.; Son, S.-H.; Lee, J.E.; Jang, M.-S.; Kim, T.-W.; Kim, S.-K. ARF7 Increases the Endogenous Contents of Castasterone through Suppression of BAS1 Expression in Arabidopsis Thaliana. Phytochemistry 2016, 122, 34–44. [Google Scholar] [CrossRef]
- Song, W. Fangmeng Duan Overexpression of SoCYP85A1 Increases the Accumulation of Castasterone and Confers Enhanced Black Shank Tolerance in Tobacco through Modulation of the Antioxidant Enzymes’ Activities. Front. Plant Sci. 2019, 10, 10. [Google Scholar]
- Castle, J.; Szekeres, M.; Jenkins, G.; Bishop, G.J. Unique and Overlapping Expression Patterns of Arabidopsis CYP85 Genes Involved in Brassinosteroid C-6 Oxidation. Plant Mol. Biol. 2005, 57, 129–140. [Google Scholar] [CrossRef]
- Pérez-España, V.H.; Sánchez-León, N.; Vielle-Calzada, J.-P. CYP85A1 Is Required for the Initiation of Female Gametogenesis in Arabidopsis thaliana. Plant Signal. Behav. 2011, 6, 321–326. [Google Scholar] [CrossRef]
- Shimada, Y.; Fujioka, S.; Miyauchi, N.; Kushiro, M.; Takatsuto, S.; Nomura, T.; Yokota, T.; Kamiya, Y.; Bishop, G.J.; Yoshida, S. Brassinosteroid-6-Oxidases from Arabidopsis and Tomato Catalyze Multiple C-6 Oxidations in Brassinosteroid Biosynthesis. Plant Physiol. 2001, 126, 770–779. [Google Scholar] [CrossRef]
- Wang, H.; Li, W.; Qin, Y.; Pan, Y.; Wang, X.; Weng, Y.; Chen, P.; Li, Y. The Cytochrome P450 Gene CsCYP85A1 Is a Putative Candidate for Super Compact-1 (Scp-1) Plant Architecture Mutation in Cucumber (Cucumis sativus L.). Front. Plant Sci. 2017, 8, 266. [Google Scholar] [CrossRef]
- Duan, F.; Ding, J.; Lee, D.; Lu, X.; Feng, Y.; Song, W. Overexpression of SoCYP85A1, a Spinach Cytochrome P450 Gene in Transgenic Tobacco Enhances Root Development and Drought Stress Tolerance. Front. Plant Sci. 2017, 8, 1909. [Google Scholar] [CrossRef]
- Li, X.-J.; Guo, X.; Zhou, Y.-H.; Shi, K.; Zhou, J.; Yu, J.-Q.; Xia, X.-J. Overexpression of a Brassinosteroid Biosynthetic Gene Dwarf Enhances Photosynthetic Capacity through Activation of Calvin Cycle Enzymes in Tomato. BMC Plant Biol. 2016, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-L.; Tang, R.-J.; Wang, H.-H.; Jiang, C.-M.; Bao, Y.; Yang, Y.; Liang, M.-X.; Sun, Z.-C.; Kong, F.-J.; Li, B.; et al. Overexpression of Populus trichocarpa CYP85A3 Promotes Growth and Biomass Production in Transgenic Trees. Plant Biotechnol. J. 2017, 15, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, Y.; Xu, D.; Yang, C.; Li, C.; Luo, K. Molecular Cloning and Characterization of a Brassinosteriod Biosynthesis-Related Gene PtoDWF4 from Populus tomentosa. Tree Physiol. 2018, 38, 1424–1436. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Du, J.; Dong, Y.; Lu, B.; Yang, M. Cloning and Salt Tolerance Analysis of the PnHB7 Transcription Factor in Populus nigra L. Ind. Crops Prod. 2020, 158, 112943. [Google Scholar] [CrossRef]
- Fujisawa, T.; Filippakopoulos, P. Functions of Bromodomain-Containing Proteins and Their Roles in Homeostasis and Cancer. Nat. Rev. Mol. Cell Biol. 2017, 18, 246–262. [Google Scholar] [CrossRef]
- Miao, H.; Li, C.; Duan, Y.; Wei, L.; Ju, M.; Zhang, H. Identification of a Sidwf1 Gene Controlling Short Internode Length Trait in the Sesame Dwarf Mutant Dw607. Theor. Appl. Genet. 2020, 133, 73–86. [Google Scholar] [CrossRef]
- Ali, S.S.; Kumar, G.B.S.; Khan, M.; Doohan, F.M. Brassinosteroid Enhances Resistance to Fusarium Diseases of Barley. Phytopathology 2013, 103, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Bancosİ, S.; Nomura, T.; Sato, T.; Molnár, G.; Bishop, G.J.; Koncz, C.; Yokota, T.; Nagy, F.; Szekeres, M. Regulation of Transcript Levels of the Arabidopsis Cytochrome P450 Genes Involved in Brassinosteroid Biosynthesis. Plant Physiol. 2002, 130, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Zhang, K.; Ding, Z.; He, Q.; Li, W.; Zhu, S.; Cheng, W.; Zhang, K.; Li, K. Characterization of a Strong and Constitutive Promoter from the Arabidopsis Serine Carboxypeptidase-like Gene AtSCPL30 as a Potential Tool for Crop Transgenic Breeding. BMC Biotechnol. 2018, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-W.; Hwang, J.-Y.; Kim, Y.-S.; Joo, S.-H.; Chang, S.C.; Lee, J.S.; Takatsuto, S.; Kim, S.-K. Arabidopsis CYP85A2, a Cytochrome P450, Mediates the Baeyer-Villiger Oxidation of Castasterone to Brassinolide in Brassinosteroid Biosynthesis. Plant Cell 2005, 17, 2397–2412. [Google Scholar] [CrossRef]
- Vanstraelen, M.; Benková, E. Hormonal Interactions in the Regulation of Plant Development. Annu. Rev. Cell Dev. Biol. 2012, 28, 463–487. [Google Scholar] [CrossRef]
- Yamamoto, R.; Fujioka, S.; Demura, T.; Takatsuto, S.; Yoshida, S.; Fukuda, H. Brassinosteroid Levels Increase Drastically Prior to Morphogenesis of Tracheary Elements. Plant Physiol. 2001, 125, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, H.; Ayano, M.; Nakamura, A.; Fujioka, S.; Asami, T.; Takatsuto, S.; Yoshida, S.; Oka, Y.; Matsui, M.; Shimada, Y. Light Activates Brassinosteroid Biosynthesis to Promote Hook Opening and Petiole Development in Arabidopsis thaliana. Plant Cell Physiol. 2020, 61, 1239–1251. [Google Scholar] [CrossRef]
- Ohnishi, T.; Godza, B.; Watanabe, B.; Fujioka, S.; Hategan, L.; Ide, K.; Shibata, K.; Yokota, T.; Szekeres, M.; Mizutani, M. CYP90A1/CPD, a Brassinosteroid Biosynthetic Cytochrome P450 of Arabidopsis, Catalyzes C-3 Oxidation. J. Biol. Chem. 2012, 287, 31551–31560. [Google Scholar] [CrossRef]
- Clouse, S.D. A History of Brassinosteroid Research from 1970 through 2005: Thirty-Five Years of Phytochemistry, Physiology, Genes, and Mutants. J. Plant Growth Regul. 2015, 34, 828–844. [Google Scholar] [CrossRef]
- Nagata, N.; Asami, T.; Yoshida, S. Brassinazole, an Inhibitor of Brassinosteroid Biosynthesis, Inhibits Development of Secondary Xylem in Cress Plants (Lepidium sativum). Plant Cell Physiol. 2001, 42, 1006–1011. [Google Scholar] [CrossRef]
- Kim, S.; Moon, J.; Roh, J.; Kim, S.-K. Castasterone Can Be Biosynthesized from 28-Homodolichosterone in Arabidopsis thaliana. J. Plant Biol. 2018, 61, 330–335. [Google Scholar] [CrossRef]
- Feng, Q.; Ou, Y.; Han, Y.; de Dios, V.R.; Wang, L.; Zhang, Q.; Yao, Y. The Brassinosteroid Biosynthesis Enzyme Gene PeCPD Improves Plant Growth and Salt Tolerance in Populus tomentosa. Ind. Crops Prod. 2021, 162, 113218. [Google Scholar] [CrossRef]
- Liu, X.; Fu, Z.; Zhang, B.; Zhai, L.; Meng, M.; Lin, J.; Zhuang, J.; Wang, G.G.; Zhang, J. Effects of Sulfuric, Nitric, and Mixed Acid Rain on Chinese Fir Sapling Growth in Southern China. Ecotoxicol. Environ. Saf. 2018, 160, 154–161. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, W.; Huang, Y.; Zhang, Y.; Wang, J.; Yang, M. Transcriptome Analysis Reveals That Populus Tomentosa Hybrid Poplar 741 Responds to Blue Light Treatment by Regulating Growth-Related Genes and Their Metabolic Pathways. Ind. Crops Prod. 2020, 152, 112512. [Google Scholar] [CrossRef]
- Moreira-Vilar, F.C.; de Siqueira-Soares, R.C.; Finger-Teixeira, A.; de Oliveira, D.M.; Ferro, A.P.; da Rocha, G.J.; Ferrarese, M.; de, L.L.; dos Santos, W.D.; Ferrarese-Filho, O. The Acetyl Bromide Method Is Faster, Simpler and Presents Best Recovery of Lignin in Different Herbaceous Tissues than Klason and Thioglycolic Acid Methods. PLoS ONE 2014, 9, e110000. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Hocart, C.H.; Redmond, J.W.; Williamson, R.E. Fractionation of Carbohydrates in Arabidopsis Root Cell Walls Shows That Three Radial Swelling Loci Are Speci®cally Involved in Cellulose Production. Planta 2000, 211, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Cybulska, J.; Zdunek, A.; Kozioł, A. The Self-Assembled Network and Physiological Degradation of Pectins in Carrot Cell Walls. Food Hydrocoll. 2015, 43, 41–50. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and Characterization of Cellulose from Different Fruit and Vegetable Pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Berhe, A.A.; Ghezzehei, T.A. A New Method for Rapid Determination of Carbohydrate and Total Carbon Concentrations Using UV Spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, G.; Bian, X.; Zhao, Q. Effects of Root Interaction and Nitrogen Fertilization on the Chlorophyll Content, Root Activity, Photosynthetic Characteristics of Intercropped Soybean and Microbial Quantity in the Rhizosphere. Plant Soil Environ. 2013, 59, 9. [Google Scholar] [CrossRef]
- Yamagami, M.; Yanai, M. Effect of rice plant root ttc-reducing activity on the chemical form of iodine in cultivated soil solutions. Radiat. Prot. Dosim. 2022, 198, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Z.; Lu, M.; Wang, Y. ThNAC13, a NAC Transcription Factor from Tamarix Hispida, Confers Salt and Osmotic Stress Tolerance to Transgenic Tamarix and Arabidopsis. Front. Plant Sci. 2017, 8, 635. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhang, X.; Zhao, K.; Yao, W.; Li, R.; Zhou, B.; Jiang, T. Over-Expression of ERF38 Gene Enhances Salt and Osmotic Tolerance in Transgenic Poplar. Front. Plant Sci. 2019, 10, 1375. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, G.; Guan, C.; Yang, D.; Wang, Y.; Zhang, Y.; Ji, J.; Jin, C.; An, T. Overexpression of LcSABP, an Orthologous Gene for Salicylic Acid Binding Protein 2, Enhances Drought Stress Tolerance in Transgenic Tobacco. Front. Plant Sci. 2019, 10, 200. [Google Scholar] [CrossRef]
- Tsai, W.-A.; Weng, S.-H.; Chen, M.-C.; Lin, J.-S.; Tsai, W.-S. Priming of Plant Resistance to Heat Stress and Tomato Yellow Leaf Curl Thailand Virus with Plant-Derived Materials. Front. Plant Sci. 2019, 10, 906. [Google Scholar] [CrossRef]
- Ullah, C.; Tsai, C.; Unsicker, S.B.; Xue, L.; Reichelt, M.; Gershenzon, J.; Hammerbacher, A. Salicylic Acid Activates Poplar Defense against the Biotrophic Rust Fungus Melampsora larici-populina via Increased Biosynthesis of Catechin and Proanthocyanidins. N. Phytol. 2019, 221, 960–975. [Google Scholar] [CrossRef]
- Rao, X.; Chen, X.; Shen, H.; Ma, Q.; Li, G.; Tang, Y.; Pena, M.; York, W.; Frazier, T.P.; Lenaghan, S.; et al. Gene Regulatory Networks for Lignin Biosynthesis in Switchgrass (Panicum virgatum). Plant Biotechnol. J. 2019, 17, 580–593. [Google Scholar] [CrossRef]


| Usage | Primer Name | Primer Sequence |
|---|---|---|
| PCR | OsCYP85A1 | F: AACCTTCCTGGAACCAACTAC R: CGAAGATAACAGCTCGAGTGAA |
| qRT-PCR | OsCYP85A1 | F: TGGAGGAGGTAGTCGAATGT R: CCTTCTTCCTCCCATCTGTATTG |
| qRT-PCR | PtBRI1 | F: GATGTCAGAGGTGGTCAGAATG R: GGGTGGTGAGTGTGGTTAAA |
| qRT-PCR | PtBZR1 | F: GGTTAAGGGCTCAAGGGAATTA R: CTGTGTCCCTTGCGATAAGTAG |
| qRT-PCR | PtMYB2 | F: TTGGAGTGATGTAGCAAGGAA R: GATGAAGATGACAGTGACGGAT |
| qRT-PCR | PtCYP85A2 | F: GAGAGCAAGGTATGGGAGTATTT R: GCCCTTTCCCTCGTTCATTA |
| qRT-PCR | PtCYP85A3 | F: CTATCCAGAGCCTTCAACCTTC R: CCAGTTCCTTTCCAGGACATAG |
| qRT-PCR | PtNPR1 | F: GTTGACCTAAATGAGACACC R: TAATCTCAGCCTTGTCCTTG |
| qRT-PCR | PtWRKY70 | F: AATCCAAGGAGCTACTAC R: GTTACCATTGTTGTTGTGG |
| qRT-PCR | PtMYB115 | F: GCCATTGGAGGTCTTTGCC R: GGTTACCGAGGAGGGAGTGC |
| qRT-PCR | Actin | F: GCATCCACGAGACTACATACAA R: TCAGCAATACCAGGGAACATAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Yao, X.; Chen, Z.; Tian, X.; Lu, L. The Overexpression of Oryza sativa L. CYP85A1 Promotes Growth and Biomass Production in Transgenic Trees. Int. J. Mol. Sci. 2023, 24, 6480. https://doi.org/10.3390/ijms24076480
Li G, Yao X, Chen Z, Tian X, Lu L. The Overexpression of Oryza sativa L. CYP85A1 Promotes Growth and Biomass Production in Transgenic Trees. International Journal of Molecular Sciences. 2023; 24(7):6480. https://doi.org/10.3390/ijms24076480
Chicago/Turabian StyleLi, Guodong, Xinzhuan Yao, Zhouzhuoer Chen, Xingyu Tian, and Litang Lu. 2023. "The Overexpression of Oryza sativa L. CYP85A1 Promotes Growth and Biomass Production in Transgenic Trees" International Journal of Molecular Sciences 24, no. 7: 6480. https://doi.org/10.3390/ijms24076480
APA StyleLi, G., Yao, X., Chen, Z., Tian, X., & Lu, L. (2023). The Overexpression of Oryza sativa L. CYP85A1 Promotes Growth and Biomass Production in Transgenic Trees. International Journal of Molecular Sciences, 24(7), 6480. https://doi.org/10.3390/ijms24076480







