Oral Cladribine Impairs Intermediate, but Not Conventional, Monocyte Transmigration in Multiple Sclerosis Patients across a Model Blood-Brain Barrier
Abstract
1. Introduction
2. Results
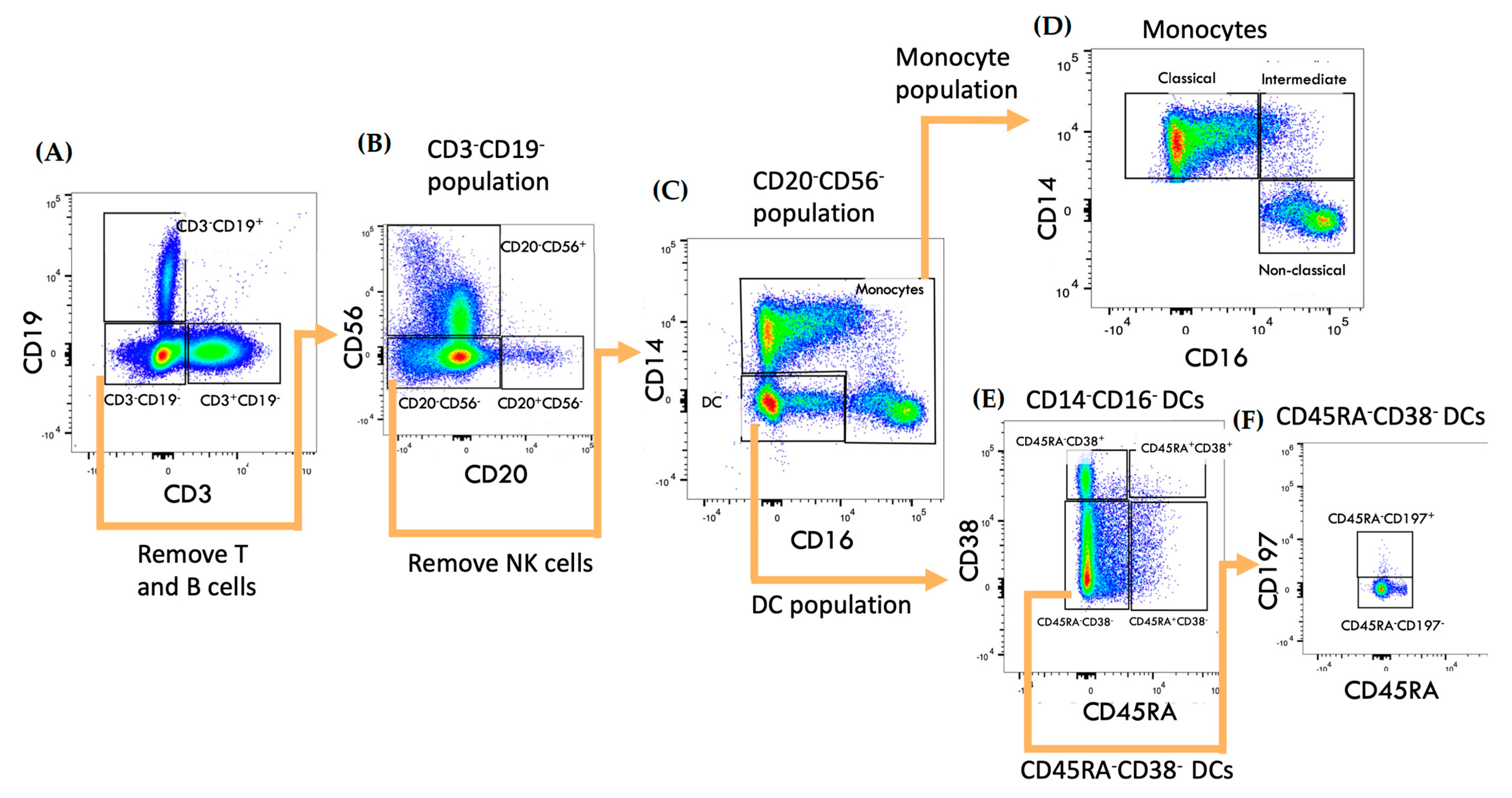
2.1. Oral Cladribine Has No Effect on Circulating Monocyte or DC Levels
2.2. DC Subset Transmigration Is Unaltered following Cladribine
2.3. Oral Cladribine Inhibits the Transmigration of Intermediate Monocytes
3. Discussion
4. Methods and Materials
4.1. Patient Characteristics
4.2. Isolation of PBMCs from Whole Blood
- A fresh cell sample consisting of PBMCs after immediate isolation from whole blood;
- Migrated cells consisting of PBMCs in the lower chamber of the transmigration assay;
- Non-migrated cells consisting of PBMC in the upper chamber of the transmigration assay.
4.3. Transmigration Assay—An In Vitro Model of the Human BBB
4.4. Spectral Flow Cytometry
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bernard, C.C.A.; de Rosbo, N.K. Multiple sclerosis: An autoimmune disease of multifactorial etiology. Curr. Opin. Immunol. 1992, 4, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Katz Sand, I. Classification, diagnosis, and differential diagnosis of multiple sclerosis. Curr. Opin. Neurol. 2015, 28, 193–205. [Google Scholar] [CrossRef]
- Ransohoff, R.; Kivisäkk, P.; Kidd, G. Three or more routes for leukocyte migration into the CNS. Nat. Rev. Immunol. 2003, 3, 569–581. [Google Scholar] [CrossRef]
- Palmer, A.M. Multiple Sclerosis and the Blood-Central Nervous System Barrier. Cardiovasc. Psychiatry Neurol. 2013, 2013, 530356. [Google Scholar] [CrossRef]
- Tajes, M.; Ramos-Fernández, E.; Weng-Jiang, X.; Bosch-Morató, M.; Guivernau, B.; Eraso-Pichot, A.; Salvador, B.; Fernàndez-Busquets, X.; Roquer, J.; Muñoz, F. The blood-brain barrier: Structure, function and therapeutic approaches to cross it. Mol. Membr. Biol. 2014, 31, 152–167. [Google Scholar] [CrossRef]
- Dargahi, N.; Katsara, M.; Tselios, T.; Androutsou, M.E.; de Courten, M.; Matsoukas, J.; Apostolopoulos, V. Multiple Sclerosis: Immunopathology and Treatment Update. Brain Sci. 2017, 7, 78. [Google Scholar] [CrossRef]
- Weiss, N.; Miller, F.; Cazaubon, S.; Couraud, P.O. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim. Biophys. Acta 2009, 1788, 842–857. [Google Scholar] [CrossRef]
- Boyden, S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J. Exp. Med. 1962, 115, 453–466. [Google Scholar] [CrossRef]
- Prat, A.; Biernacki, K.; Lavoie, J.-F.; Poirier, J.; Duquette, P.; Antel, J.P. Migration of Multiple Sclerosis Lymphocytes Through Brain Endothelium. Arch. Neurol. 2002, 59, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Reder, A.T.; Genç, K.; Byskosh, P.V.; Porrini, A.M. Monocyte activation in multiple sclerosis. Mult. Scler. 1998, 4, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Karni, A.; Abraham, M.; Monsonego, A.; Cai, G.; Freeman, G.J.; Hafler, D.; Khoury, S.J.; Weiner, H.L. Innate immunity in multiple sclerosis: Myeloid dendritic cells in secondary progressive multiple sclerosis are activated and drive a proinflammatory immune response. J. Immunol. 2006, 177, 4196–4202. [Google Scholar] [CrossRef] [PubMed]
- Trebst, C.; Lykke Sørensen, T.; Kivisäkk, P.; Cathcart, M.K.; Hesselgesser, J.; Horuk, R.; Sellebjerg, F.; Lassmann, H.; Ransohoff, R.M. CCR1+/CCR5+ Mononuclear Phagocytes Accumulate in the Central Nervous System of Patients with Multiple Sclerosis. Am. J. Pathol. 2001, 159, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Gjelstrup, M.C.; Stilund, M.; Petersen, T.; Møller, H.J.; Petersen, E.L.; Christensen, T. Subsets of activated monocytes and markers of inflammation in incipient and progressed multiple sclerosis. Immunol. Cell Biol. 2018, 96, 160–174. [Google Scholar] [CrossRef]
- Nuyts, A.H.; Lee, W.P.; Bashir-Dar, R.; Berneman, Z.N.; Cools, N. Dendritic cells in multiple sclerosis: Key players in the immunopathogenesis, key players for new cellular immunotherapies? Mult. Scler. 2013, 19, 995–1002. [Google Scholar] [CrossRef]
- Lande, R.; Gafa, V.; Serafini, B.; Giacomini, E.; Visconti, A.; Remoli, M.E.; Severa, M.; Parmentier, M.; Ristori, G.; Salvetti, M.; et al. Plasmacytoid dendritic cells in multiple sclerosis: Intracerebral recruitment and impaired maturation in response to interferon-beta. J. Neuropathol. Exp. Neurol. 2008, 67, 388–401. [Google Scholar] [CrossRef]
- Serafini, B.; Rosicarelli, B.; Magliozzi, R.; Stigliano, E.; Capello, E.; Mancardi, G.L.; Aloisi, F. Dendritic cells in multiple sclerosis lesions: Maturation stage, myelin uptake, and interaction with proliferating T cells. J. Neuropathol. Exp. Neurol. 2006, 65, 124–141. [Google Scholar] [CrossRef]
- Stasiolek, M.; Bayas, A.; Kruse, N.; Wieczarkowiecz, A.; Toyka, K.V.; Gold, R.; Selmaj, K. Impaired maturation and altered regulatory function of plasmacytoid dendritic cells in multiple sclerosis. Brain 2006, 129 Pt 5, 1293–1305. [Google Scholar] [CrossRef]
- Dhib-Jalbut, S.; Marks, S. Interferon-β mechanisms of action in multiple sclerosis. Neurology 2010, 74, S17–S24. [Google Scholar] [CrossRef]
- Chun, J.; Hartung, H.P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neuropharmacol. 2010, 33, 91–101. [Google Scholar] [CrossRef]
- Giovannoni, G.; Soelberg Sorensen, P.; Cook, S.; Rammohan, K.; Rieckmann, P.; Comi, G.; Dangond, F.; Adeniji, A.; Vermersch, P. Safety and efficacy of cladribine tablets in patients with relapsing–remitting multiple sclerosis: Results from the randomized extension trial of the CLARITY study. Mult. Scler. 2018, 24, 1594–1604. [Google Scholar] [CrossRef]
- Leist, T.P.; Weissert, R. Cladribine: Mode of action and implications for treatment of multiple sclerosis. Clin. Neuropharmacol. 2011, 34, 28–35. [Google Scholar] [CrossRef]
- Fissolo, N.; Calvo-Barreiro, L.; Eixarch, H.; Boschert, U.; Espejo, C.; Montalban, X.; Comabella, M. Immunomodulatory Effects Associated with Cladribine Treatment. Cells 2021, 10, 3488. [Google Scholar] [CrossRef]
- Giovannoni, G. Cladribine to Treat Relapsing Forms of Multiple Sclerosis. Neurotherapeutics 2017, 14, 874–887. [Google Scholar] [CrossRef]
- Sorensen, P.S.; Sellebjerg, F. Pulsed immune reconstitution therapy in multiple sclerosis. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419836913. [Google Scholar] [CrossRef]
- Hawke, S.; Zinger, A.; Juillard, P.G.; Holdaway, K.; Byrne, S.N.; Grau, G.E. Selective modulation of trans-endothelial migration of lymphocyte subsets in multiple sclerosis patients under fingolimod treatment. J. Neuroimmunol. 2020, 349, 577392. [Google Scholar] [CrossRef]
- Ford, R.K.; Juillard, P.; Hawke, S.; Grau, G.E.; Marsh-Wakefield, F. Cladribine Reduces Trans-Endothelial Migration of Memory T Cells across an In Vitro Blood–Brain Barrier. J. Clin. Med. 2022, 11, 6006. [Google Scholar] [CrossRef]
- Nguyen, K.; Juillard, P.; Hawke, S.; Grau, G.E.; Marsh-Wakefield, F. Trans-Endothelial Migration of Memory T Cells Is Impaired in Alemtuzumab-Treated Multiple Sclerosis Patients. J. Clin. Med. 2022, 11, 6266. [Google Scholar] [CrossRef]
- Soelberg-Sorensen, P.; Dangond, F.; Hicking, C.; Giovannoni, G. Innate immune cell counts in patients with Relapsing-Remitting Multiple Sclerosis (RRMS) treated with cladribine tablets 3.5 mg/kg in CLARITY and CLARITY extension. Eur. J. Neurol. 2018, 25, 528. [Google Scholar]
- Singh, V.; Prajeeth, C.K.; Gudi, V.; Bénardais, K.; Voss, E.V.; Stangel, M. 2-Chlorodeoxyadenosine (cladribine) induces apoptosis in human monocyte-derived dendritic cells. Clin. Exp. Immunol. 2013, 173, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Moser, T.; Schwenker, K.; Seiberl, M.; Feige, J.; Akgün, K.; Haschke-Becher, E.; Ziemssen, T.; Sellner, J. Long-term peripheral immune cell profiling reveals further targets of oral cladribine in MS. Ann. Clin. Transl. Neurol. 2020, 7, 2199–2212. [Google Scholar] [CrossRef] [PubMed]
- Waschbisch, A.; Schroeder, S.; Lee, D.-H.; Pfeifenbring, S.; Stadelmann-Nessler, C.; Linker, R. Non-Classical and Intermediate Monocytes in the Peripheral Blood and Cerebrospinal Fluid of Multiple Sclerosis Patients (P03.228). Neurology 2013, 80 (Suppl. 7), P03.228. [Google Scholar]
- Savinetti, I.; Papagna, A.; Foti, M. Human Monocytes Plasticity in Neurodegeneration. Biomedicines 2021, 9, 717. [Google Scholar] [CrossRef] [PubMed]
- Waschbisch, A.; Schröder, S.; Schraudner, D.; Sammet, L.; Weksler, B.; Melms, A.; Pfeifenbring, S.; Stadelmann, C.; Schwab, S.; Linker, R.A. Pivotal Role for CD16+ Monocytes in Immune Surveillance of the Central Nervous System. J. Immunol. 2016, 196, 1558–1567. [Google Scholar] [CrossRef]
- Troncoso, L.L.; Pontillo, A.; Oliveira, E.M.L.; Finkelszteijn, A.; Schneider, S.; Chies, J.A.B. CCR5Δ32—A piece of protection in the inflammatory puzzle of multiple sclerosis susceptibility. Hum. Immunol. 2018, 79, 621–626. [Google Scholar] [CrossRef]
- Bartosik-Psujek, H.; Belniak, E.; Mitosek-Szewczyk, K.; Dobosz, B.; Stelmasiak, Z. Interleukin-8 and RANTES levels in patients with relapsing-remitting multiple sclerosis (RR-MS) treated with cladribine. Acta Neurol. Scand. 2004, 109, 390–392. [Google Scholar] [CrossRef]
- Bar-Or, A.; Nuttall, R.K.; Duddy, M.; Alter, A.; Kim, H.J.; Ifergan, I.; Pennington, C.J.; Bourgoin, P.; Edwards, D.R.; Yong, V.W. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain 2003, 126, 2738–2749. [Google Scholar] [CrossRef]
- Huang, W.C.; Sala-Newby, G.B.; Susana, A.; Johnson, J.L.; Newby, A.C. Classical macrophage activation up-regulates several matrix metalloproteinases through mitogen activated protein kinases and nuclear factor-κB. PLoS ONE 2012, 7, e42507. [Google Scholar] [CrossRef]
- Chuluundorj, D.; Harding, S.A.; Abernethy, D.; La Flamme, A.C. Expansion and preferential activation of the CD14+CD16+ monocyte subset during multiple sclerosis. Immunol. Cell Biol. 2014, 92, 509–517. [Google Scholar] [CrossRef]
- Mair, F.; Prlic, M. OMIP-044: 28-color immunophenotyping of the human dendritic cell compartment. Cytom. Part A 2018, 93, 402–405. [Google Scholar] [CrossRef]
- De Laere, M.; Berneman, Z.N.; Cools, N. To the Brain and Back: Migratory Paths of Dendritic Cells in Multiple Sclerosis. J. Neuropathol. Exp. Neurol. 2018, 77, 178–192. [Google Scholar] [CrossRef]
- Longhini, A.L.; von Glehn, F.; Brandão, C.O.; de Paula, R.F.; Pradella, F.; Moraes, A.S.; Farias, A.S.; Oliveira, E.C.; Quispe-Cabanillas, J.G.; Abreu, C.H.; et al. Plasmacytoid dendritic cells are increased in cerebrospinal fluid of untreated patients during multiple sclerosis relapse. J. Neuroinflamm. 2011, 8, 2. [Google Scholar] [CrossRef]
- Von Glehn, F.; Santos, L.M.; Balashov, K.E. Plasmacytoid dendritic cells and immunotherapy in multiple sclerosis. Immunotherapy 2012, 4, 1053–1061. [Google Scholar] [CrossRef]
- Schweingruber, N.; Fischer, H.J.; Fischer, L.; Van Den Brandt, J.; Karabinskaya, A.; Labi, V.; Villunger, A.; Kretzschmar, B.; Huppke, P.; Simons, M.; et al. Chemokine-Mediated Redirection Of T Cells Constitutes A Critical Mechanism Of Glucocorticoid Therapy In Autoimmune Cns Responses. Acta Neuropathol. 2014, 127, 713–729. [Google Scholar] [CrossRef]
- Sheikh, M.H.; Henson, S.M.; Loiola, R.A.; Mercurio, S.; Colamatteo, A.; Maniscalco, G.T.; De Rosa, V.; Mcarthur, S.; Solito, E. Immuno-Metabolic Impact Of The Multiple Sclerosis Patients’ Sera On Endothelial Cells Of The Blood-Brain Barrier. J. Neuroinflamm. 2020, 17, 153. [Google Scholar] [CrossRef]
- Van Langelaar, J.; Van Der Vuurst De Vries, R.M.; Janssen, M.; Wierenga-Wolf, A.F.; Spilt, I.M.; Siepman, T.A.; Dankers, W.; Verjans, G.M.G.M.; De Vries, H.E.; Lubberts, E.; et al. T Helper 17.1 Cells Associate with Multiple Sclerosis Disease Activity: Perspectives For Early Intervention. Brain 2018, 141, 1334–1349. [Google Scholar] [CrossRef]
- Burgoyne, P.; Hayes, A.J.; Cooper, R.S.; Le Brocq, M.L.; Hansell, C.A.; Campbell, J.D.; Graham, G.J. CCR7(+) dendritic cells sorted by binding of CCL19 show enhanced Ag-presenting capacity and antitumor potency. J. Leukoc. Biol. 2022, 111, 1243–1251. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Biari Ke Gaudioso, Á.; Fernández-Alonso, M.C.; Jiménez-Barbero, J.; Cañada, F.J. Peptidoglycan Recognition by Wheat Germ Agglutinin. A View by NMR. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.Y.; Juillard, P.; Hawke, S.; Marsh-Wakefield, F.; Grau, G.E. Oral Cladribine Impairs Intermediate, but Not Conventional, Monocyte Transmigration in Multiple Sclerosis Patients across a Model Blood-Brain Barrier. Int. J. Mol. Sci. 2023, 24, 6487. https://doi.org/10.3390/ijms24076487
Lin LY, Juillard P, Hawke S, Marsh-Wakefield F, Grau GE. Oral Cladribine Impairs Intermediate, but Not Conventional, Monocyte Transmigration in Multiple Sclerosis Patients across a Model Blood-Brain Barrier. International Journal of Molecular Sciences. 2023; 24(7):6487. https://doi.org/10.3390/ijms24076487
Chicago/Turabian StyleLin, Linda Y., Pierre Juillard, Simon Hawke, Felix Marsh-Wakefield, and Georges E. Grau. 2023. "Oral Cladribine Impairs Intermediate, but Not Conventional, Monocyte Transmigration in Multiple Sclerosis Patients across a Model Blood-Brain Barrier" International Journal of Molecular Sciences 24, no. 7: 6487. https://doi.org/10.3390/ijms24076487
APA StyleLin, L. Y., Juillard, P., Hawke, S., Marsh-Wakefield, F., & Grau, G. E. (2023). Oral Cladribine Impairs Intermediate, but Not Conventional, Monocyte Transmigration in Multiple Sclerosis Patients across a Model Blood-Brain Barrier. International Journal of Molecular Sciences, 24(7), 6487. https://doi.org/10.3390/ijms24076487






