Combined lncRNA and mRNA Expression Profiles Identified the lncRNA–miRNA–mRNA Modules Regulating the Cold Stress Response in Ammopiptanthus nanus
Abstract
1. Introduction
2. Results
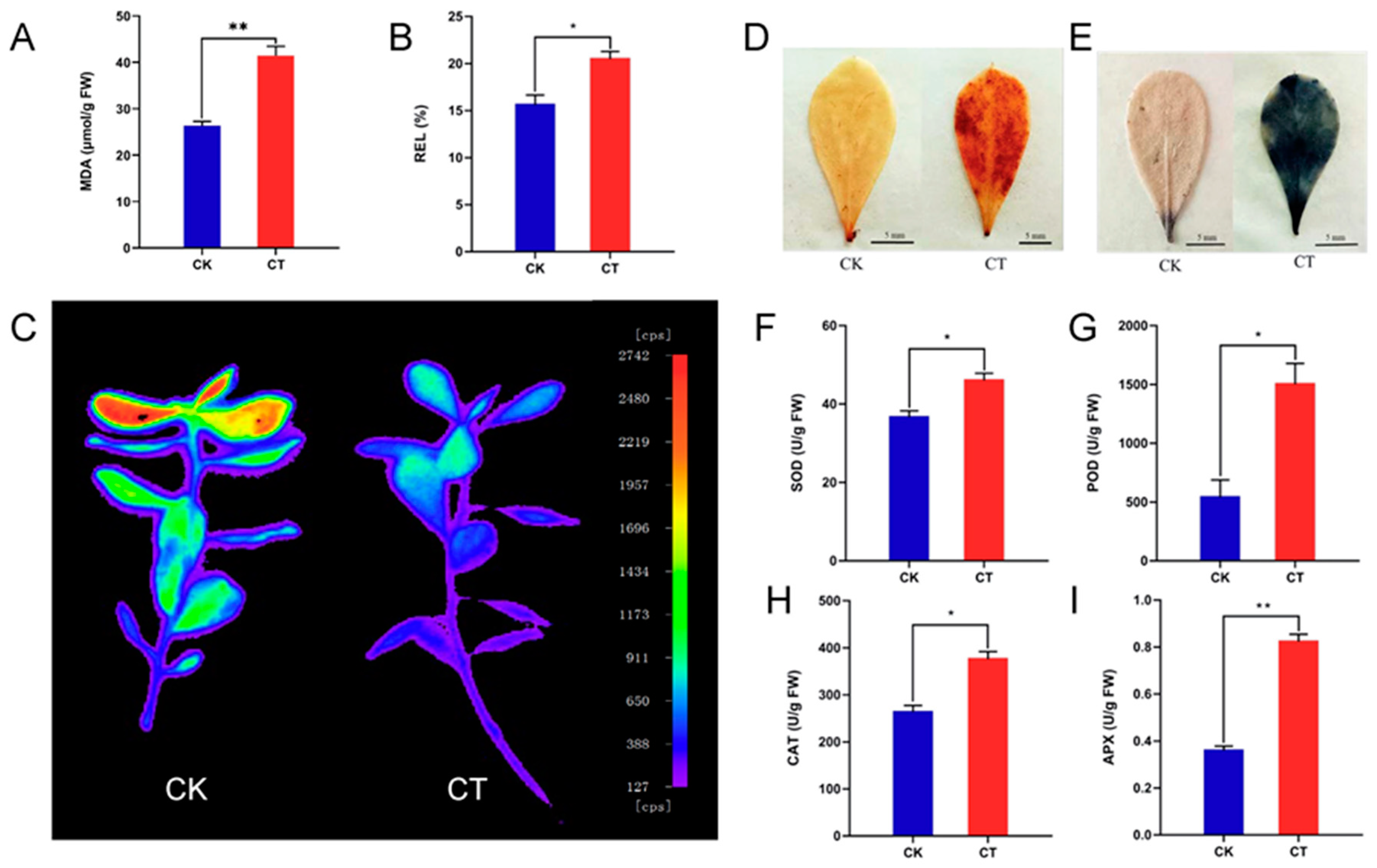
2.1. Physiological and Biochemical Indexes of A. nanus Leaves under Cold Stress
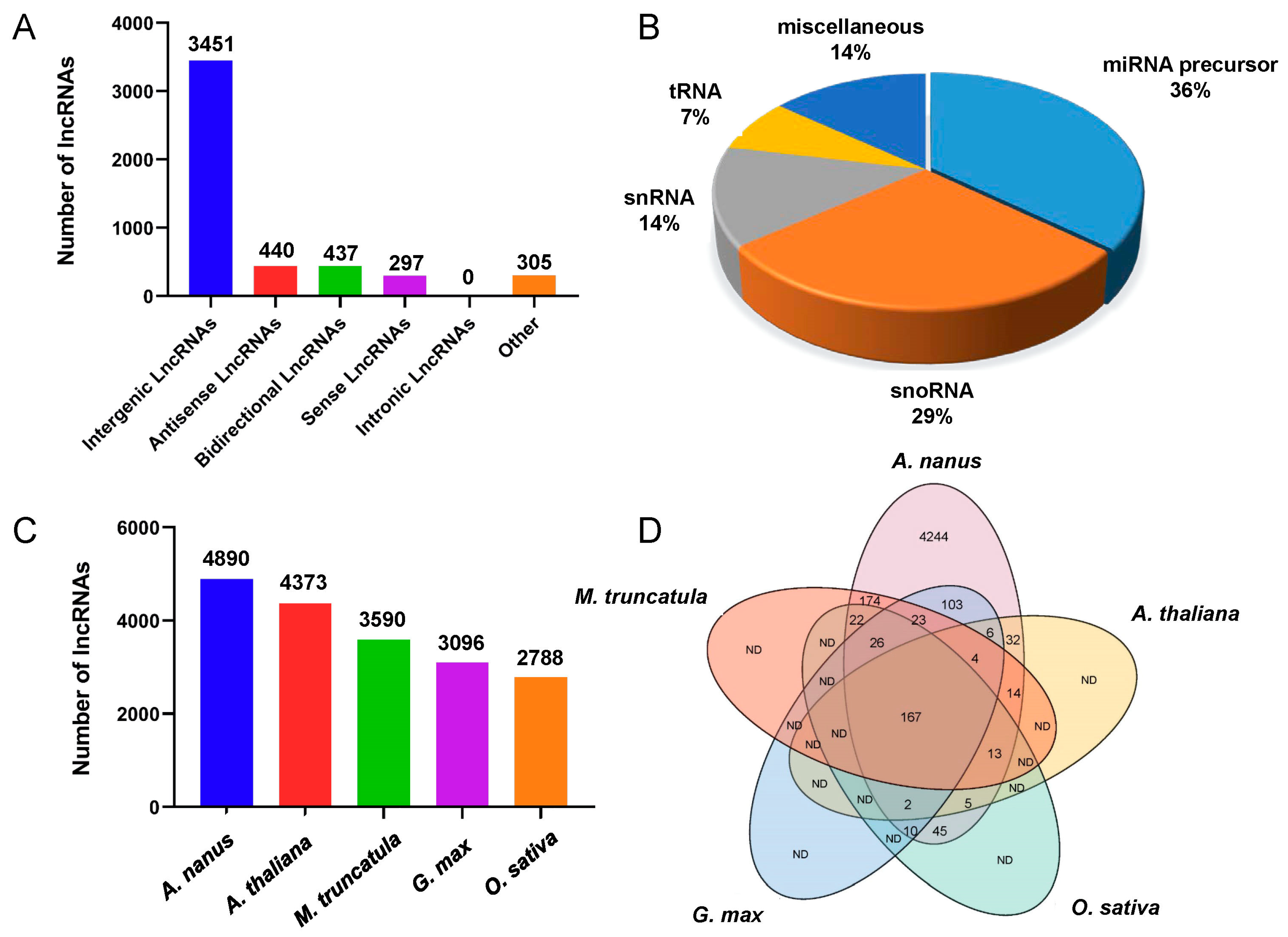
2.2. Genome-Wide Identification of lncRNAs in A. nanus
2.3. Target Prediction for lncRNAs Acting in Cis in A. nanus
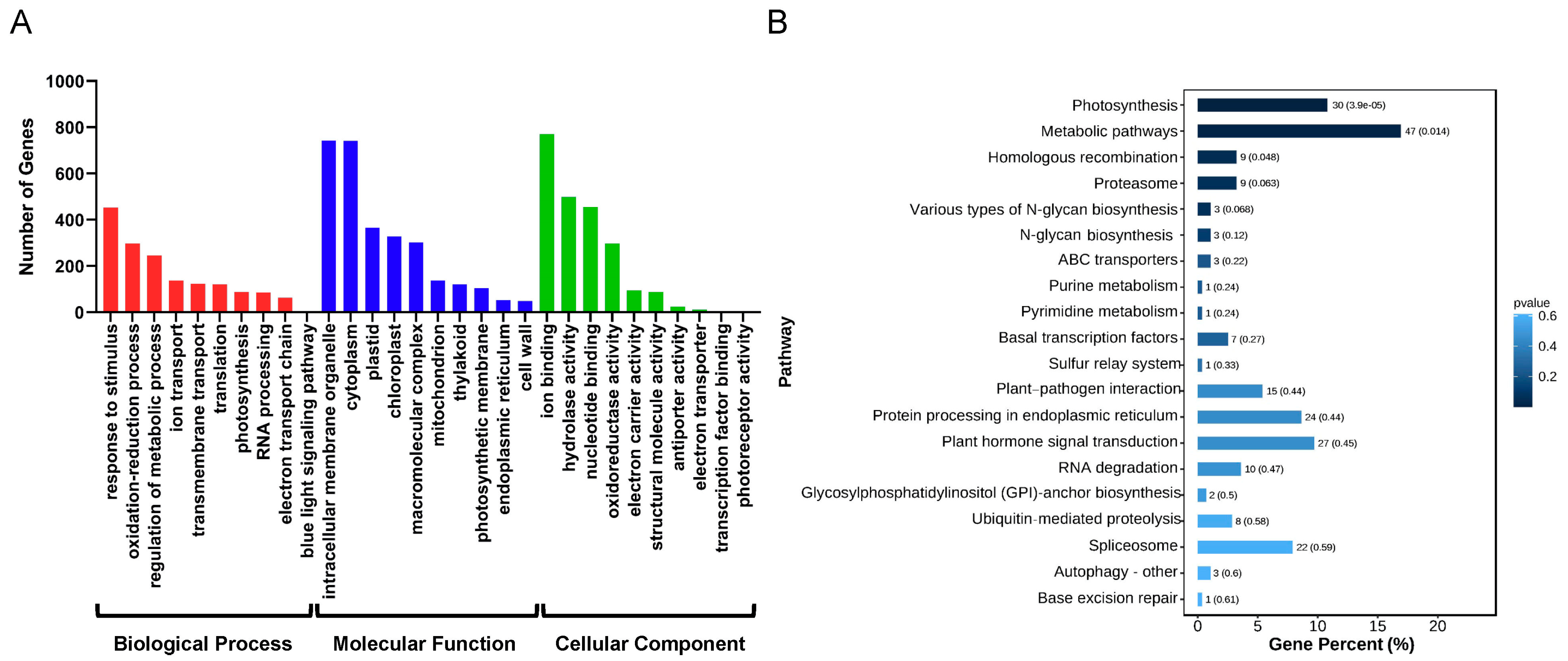
2.4. Ammopiptanthus nanus lncRNAs Regulate a Variety of Biological Processes by Affecting miRNAs
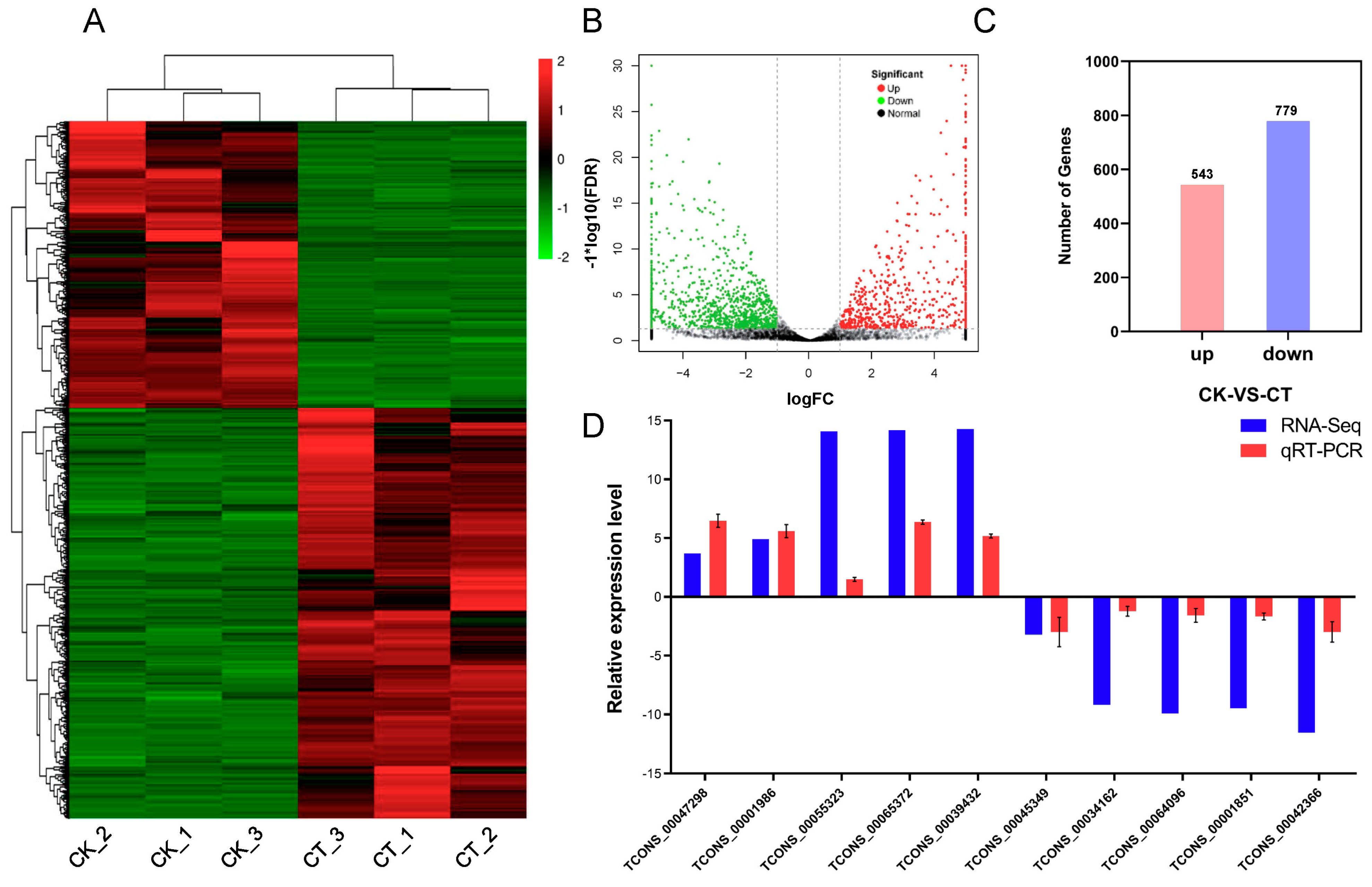
2.5. Responses of lncRNAs to Cold Stress in A. nanus
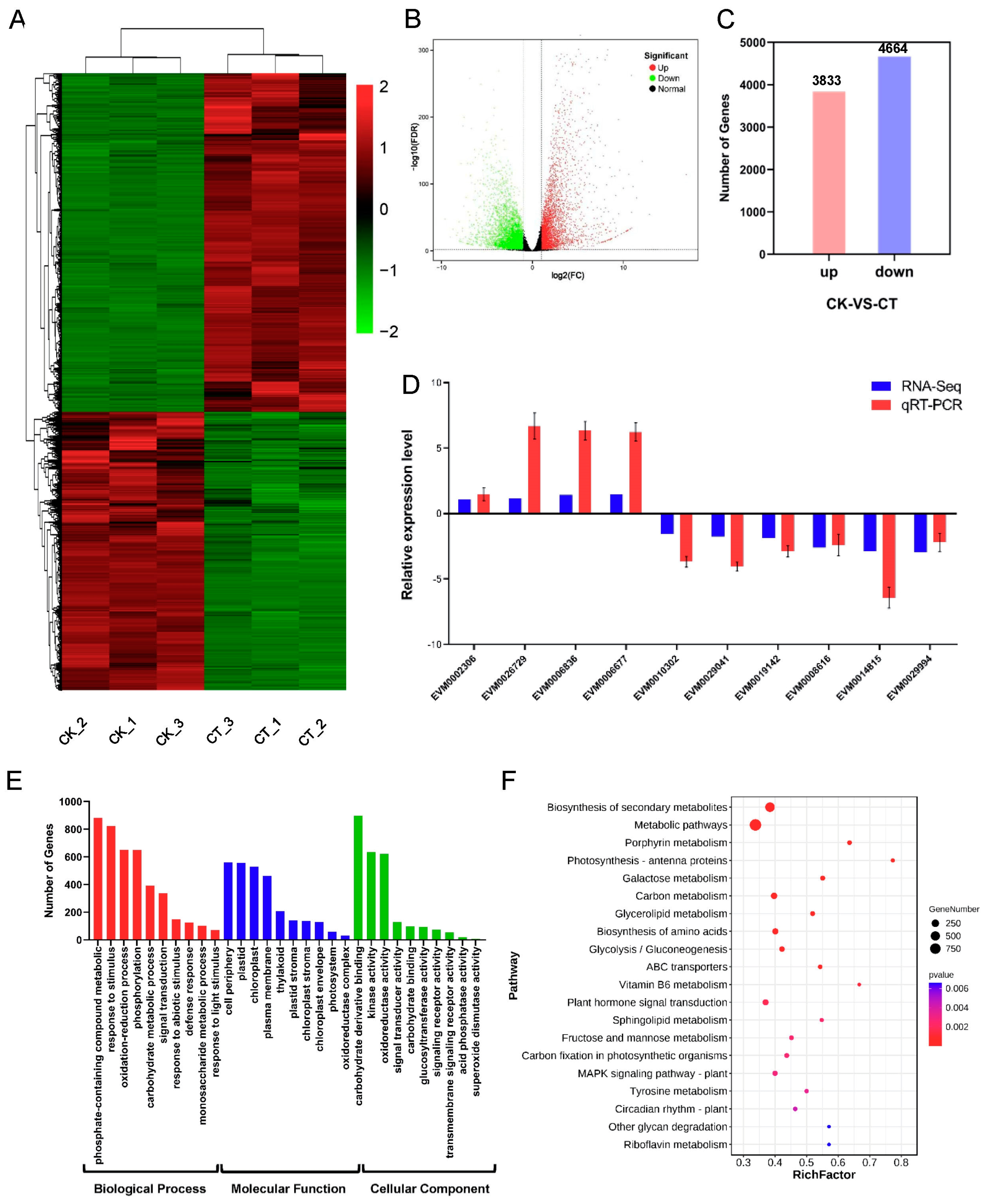
2.6. Response of mRNAs to Cold Stress in A. nanus
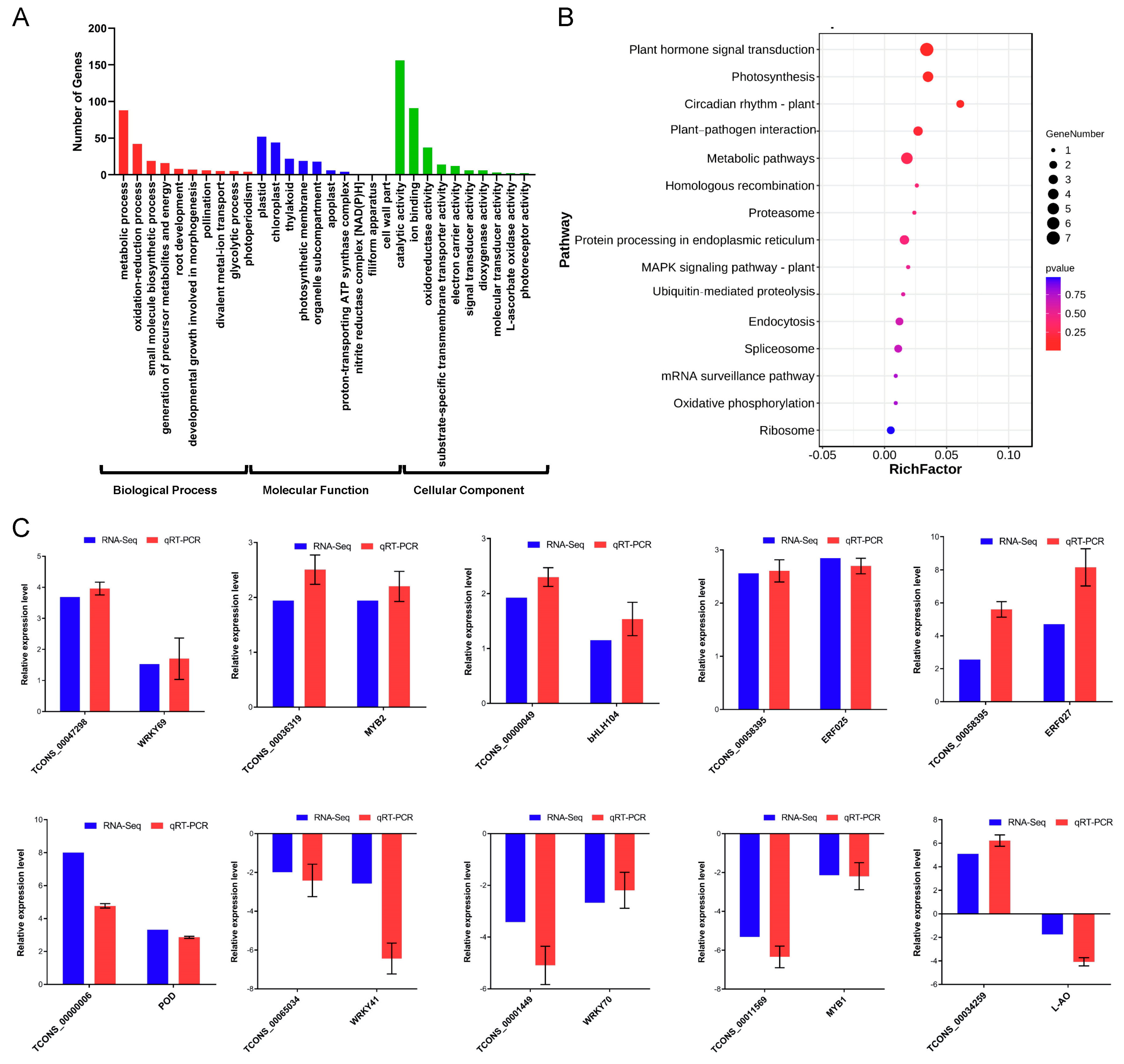
2.7. lncRNAs Participate in Cold Stress Response through Regulating mRNAs in Cis
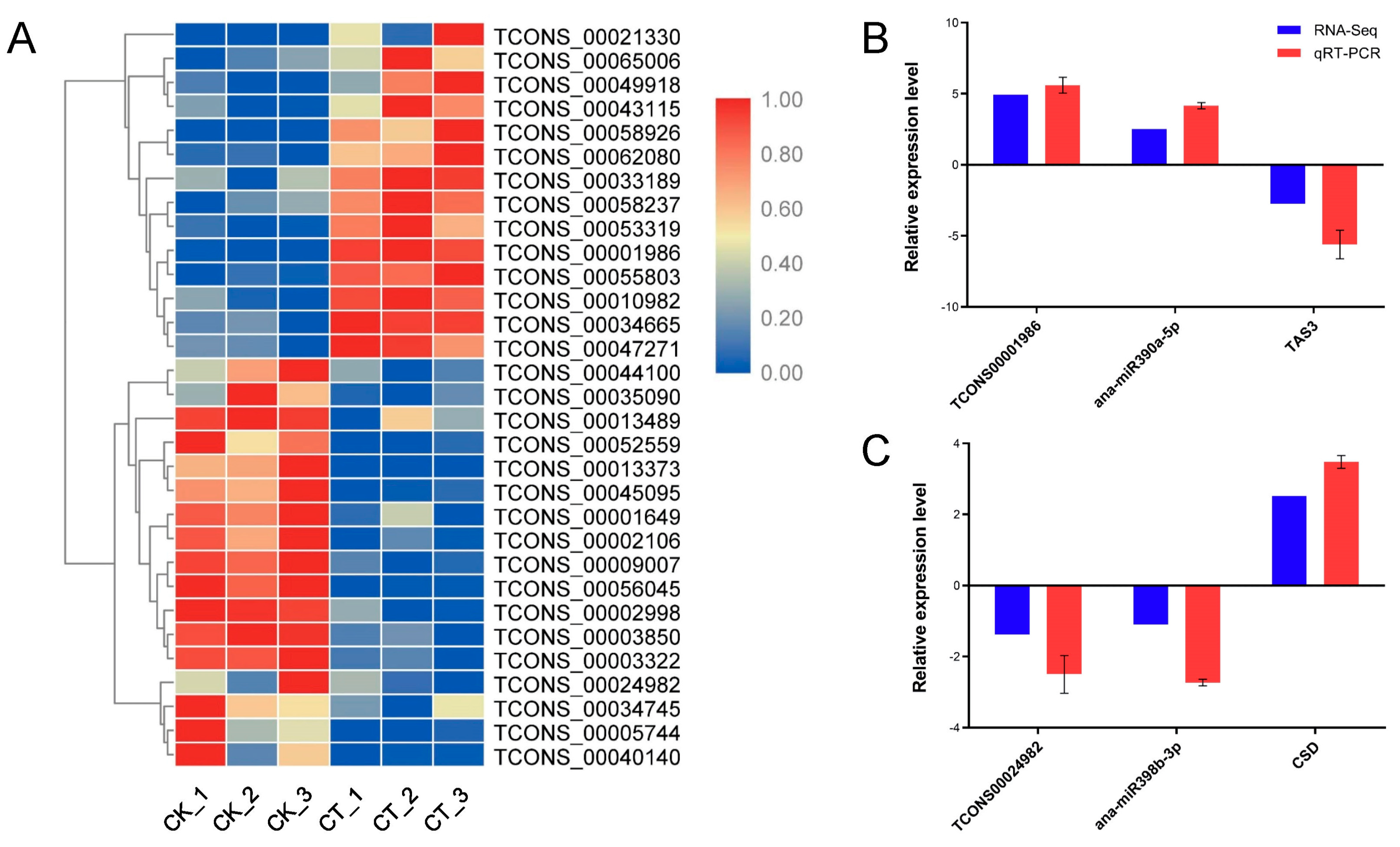
2.8. Cold Stress-Responsive lncRNAs Representing miRNA Precursors
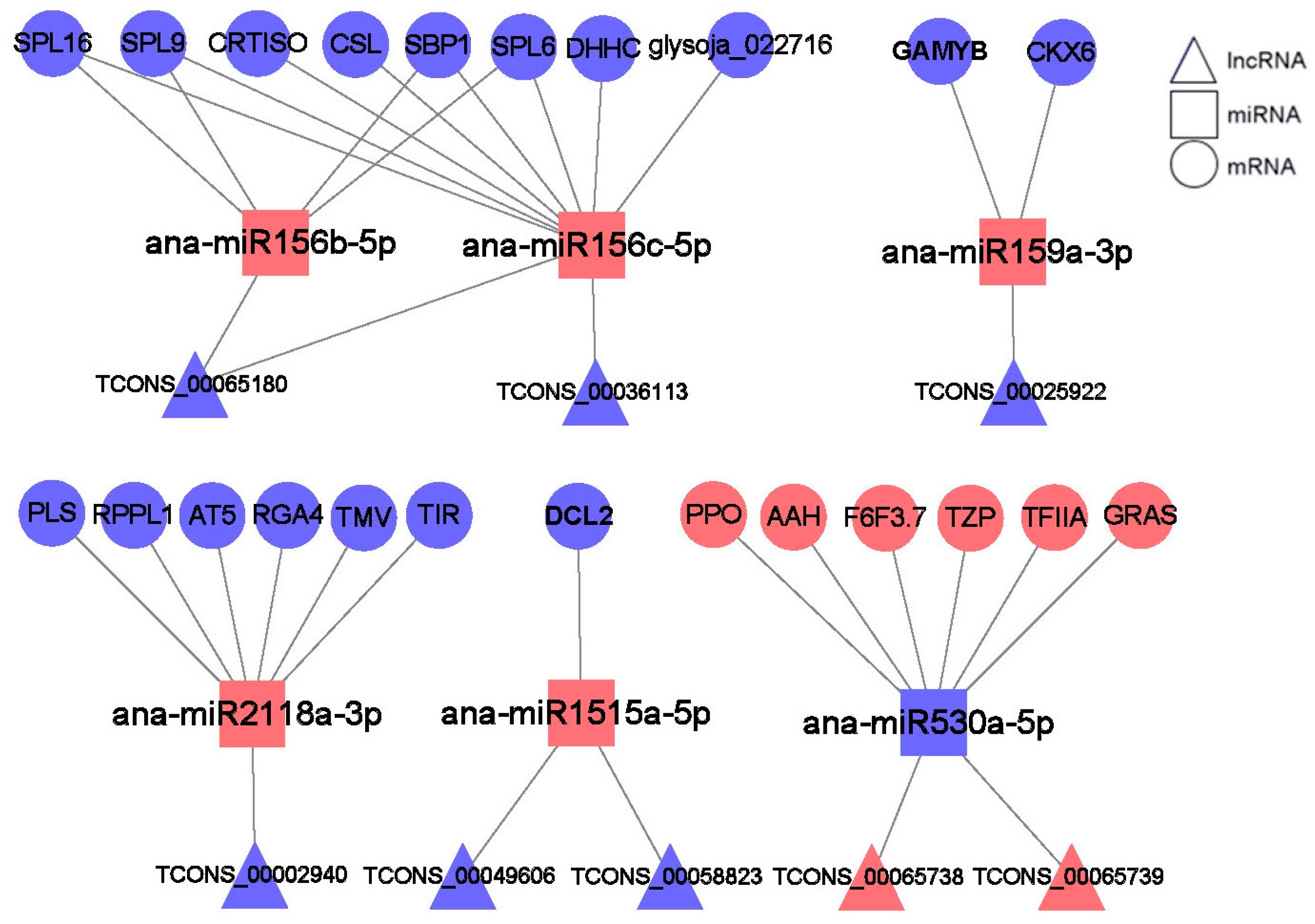
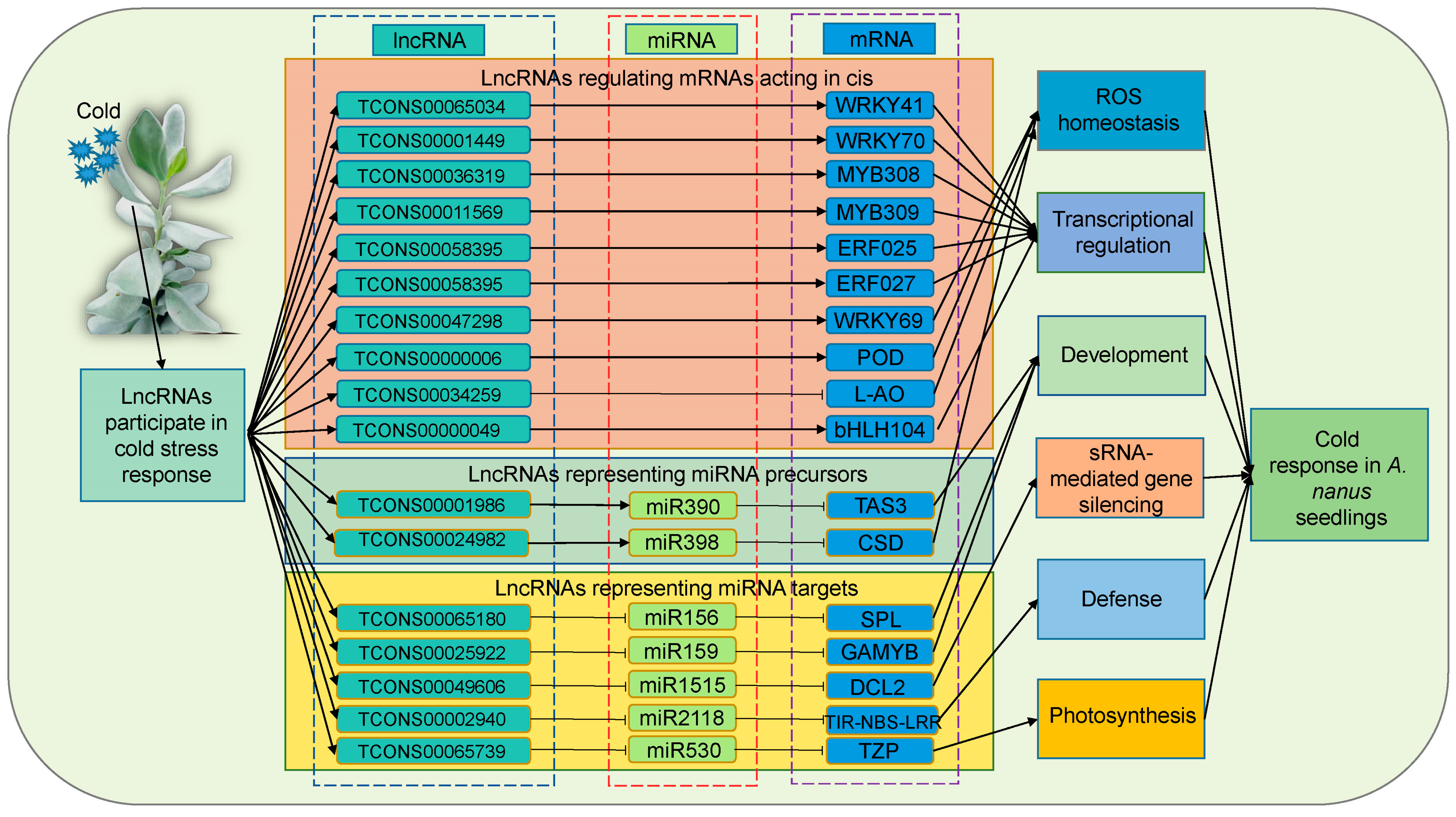
2.9. lncRNA–miRNA–mRNA Networks Construction and Analysis
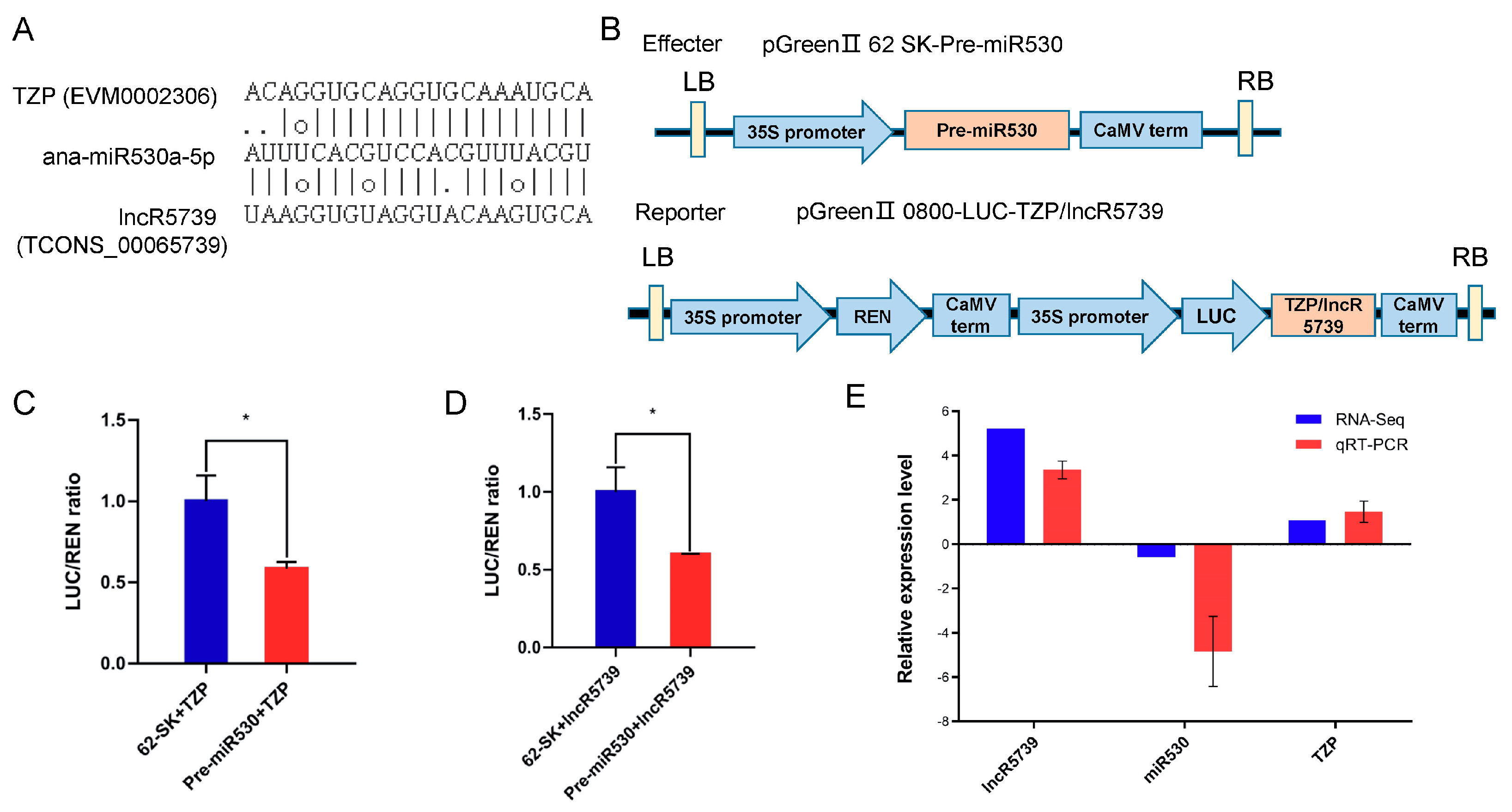
2.10. miR530 Targets the lncR5739 (TCONS00065739) and the TZP
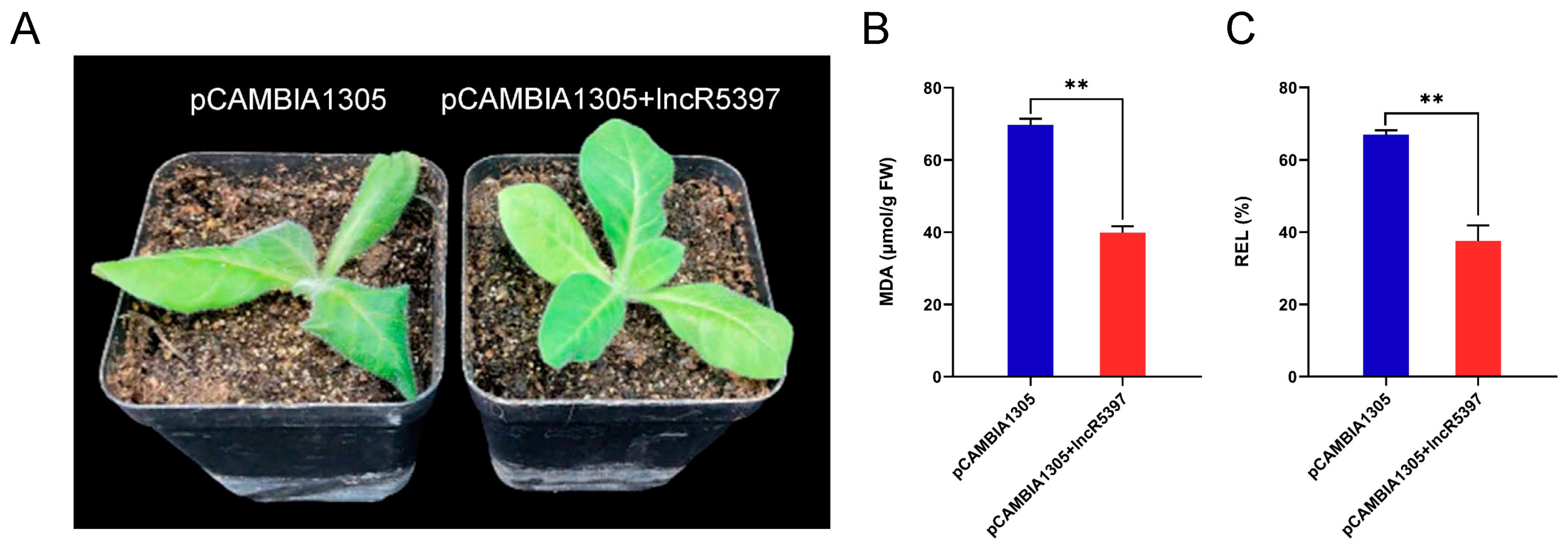
2.11. Overexpression of lncR5739 Enhanced the Cold Stress Tolerance in Tobacco
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Cold Stress Treatment
4.2. Delayed Fluorescence Imaging, Physiological and Biochemical Analysis of A. nanus Seedlings
4.3. lncRNA and Transcriptome Sequencing
4.4. lncRNA Identification
4.5. Differential Expression Analysis of lncRNAs and mRNAs, and GO and KEGG Pathway Analysis
4.6. qRT-PCR Analysis of lncRNAs, miRNAs and mRNAs
4.7. Dual-Luciferase Reporter Assay
4.8. The Transient Expression of lncRNA in Nicotiana tabacum
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomashow, M.F. Molecular basis of plant cold acclimation: Insights gained from studying the CBF cold response pathway. Plant Physiol. 2010, 154, 571–577. [Google Scholar] [CrossRef]
- Knight, M.R.; Knight, H. Low-temperature perception leading to gene expression and cold tolerance in higher plants. New Phytol. 2012, 195, 737–751. [Google Scholar] [CrossRef]
- Furtauer, L.; Weiszmann, J.; Weckwerth, W.; Nagele, T. Dynamics of plant metabolism during cold acclimation. Int. J. Mol. Sci. 2019, 20, 5411. [Google Scholar] [CrossRef] [PubMed]
- Kidokoro, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant cold-stress responses. Trends. Plant Sci. 2022, 27, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Mahpour, A.; Mullen, A.C. Our emerging understanding of the roles of long non-coding RNAs in normal liver function, disease, and malignancy. JHEP Rep. 2021, 3, 100177. [Google Scholar] [CrossRef] [PubMed]
- Hermans-Beijnsberger, S.; van Bilsen, M.; Schroen, B. Long non-coding RNAs in the failing heart and vasculature. Noncoding RNA Res. 2018, 3, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Y.; Chen, X.; Chen, Y. Plant noncoding RNAs: Hidden players in development and stress responses. Annu. Rev. Cell Dev. Biol. 2019, 35, 407–431. [Google Scholar] [CrossRef]
- Wang, L.; Han, J.; Lu, K.; Li, M.; Gao, M.; Cao, Z.; Zhao, T.; Chen, X.; Tao, X.; Chen, Q. Functional examination of lncRNAs in allotetraploid Gossypium hirsutum. BMC Genom. 2021, 22, 443. [Google Scholar] [CrossRef]
- Li, J.; Hull, J.J.; Liang, S.; Wang, Q.; Chen, L.; Zhang, Q.; Wang, M.; Mansoor, S.; Zhang, X.; Jin, S. Genome-wide analysis of cotton miRNAs during whitefly infestation offers new insights into plant-herbivore interaction. Int. J. Mol. Sci. 2019, 20, 5357. [Google Scholar] [CrossRef]
- Lu, Q.; Guo, F.; Xu, Q.; Cang, J. LncRNA improves cold resistance of winter wheat by interacting with miR398. Funct. Plant Biol. 2020, 47, 544–557. [Google Scholar] [CrossRef]
- Zhou, R.; Sanz-Jimenez, P.; Zhu, X.T.; Feng, J.W.; Shao, L.; Song, J.M.; Chen, L.L. Analysis of rice transcriptome reveals the lncRNA/circRNA regulation in tissue development. Rice 2021, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhao, T.; Wang, L.; Han, J.; Chen, J.; Hao, Y.; Guan, X. The lincRNA XH123 is involved in cotton cold-stress regulation. Plant Mol. Biol. 2021, 106, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, J.; Lian, B.; Gu, H.; Li, Y.; Qi, Y. Global identification of Arabidopsis lncRNAs reveals the regulation of MAF4 by a natural antisense RNA. Nat. Commun. 2018, 9, 5056. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhong, Y.; Qi, X. LncRNA TCONS_00021861 is functionally associated with drought tolerance in rice (Oryza sativa L.) via competing endogenous RNA regulation. BMC Plant Biol. 2021, 21, 410. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, J.; Deng, F.; Wang, W.; Cheng, Y.; Song, L.; Hu, M.; Shen, J.; Xu, Q.; Shen, F. The long non-coding RNA lncRNA973 is involved in cotton response to salt stress. BMC Plant Biol. 2019, 19, 459. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, M.; Gross-Hardt, R.; Schoffl, F. Heat shock factor HSFB2a involved in gametophyte development of Arabidopsis and its expression is controlled by a heat-inducible long non-coding antisense RNA. Plant Mol. Biol. 2014, 85, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liu, F.; Wang, Y.; Liu, X. A novel long noncoding RNA CIL1 enhances cold stress tolerance in Arabidopsis. Plant Sci. 2022, 323, 111370. [Google Scholar] [CrossRef]
- Aslam, M.M.; Waseem, M.; Xu, W.; Ying, L.; Zhang, J.; Yuan, W. Global identification of white lupin lncRNAs reveals their role in cluster roots under phosphorus deficiency. Int. J. Mol. Sci. 2022, 23, 9012. [Google Scholar] [CrossRef]
- Deng, F.; Zhang, X.; Wang, W.; Yuan, R.; Shen, F. Identification of Gossypium hirsutum long non-coding RNAs (lncRNAs) under salt stress. BMC Plant Biol. 2018, 18, 23. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, R.; Zhang, M.; Xia, G.; Liu, S. Functional analysis of long non-coding RNAs involved in alkaline stress responses in wheat. J. Exp. Bot. 2022, 73, 5698–5714. [Google Scholar] [CrossRef]
- Cao, S.; Wang, Y.; Abla, M.; Gao, F.; Zhou, Y.; Feng, J. Genome-wide identification and expression analyses of the Chitinases in Ammopiptanthus nanus under cold and osmotic stress. Genes 2019, 10, 472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, S.; Sui, X.; Wang, J.; Gao, F.; Zhou, Y. Genome-wide characterization, evolution, and expression analysis of the ascorbate peroxidase and glutathione peroxidase gene families in response to cold and osmotic stress in Ammopiptanthus nanus. J. Plant Growth Regul. 2023, 42, 502–522. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, F.; Liu, R.; Feng, J.; Li, H. De novo sequencing and analysis of root transcriptome using 454 pyrosequencing to discover putative genes associated with drought tolerance in Ammopiptanthus mongolicus. BMC Genom. 2012, 13, 266. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Wang, N.; Li, H.; Liu, J.; Fu, C.; Xiao, Z.; Wei, C.; Lu, X.; Feng, J.; Zhou, Y. Identification of drought-responsive microRNAs and their targets in Ammopiptanthus mongolicus by using high-throughput sequencing. Sci. Rep. 2016, 6, 34601. [Google Scholar] [CrossRef]
- Gao, F.; Li, H.; Xiao, Z.; Wei, C.; Feng, J.; Zhou, Y. De novo transcriptome analysis of Ammopiptanthus nanus and its comparative analysis with A. mongolicus. Trees 2018, 32, 287–300. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, Q.; Liu, F.; Bing, J.; Zhou, Y.; Gao, F. Gene profiling of the ascorbate oxidase family genes under osmotic and cold stress reveals the role of AnAO5 in cold adaptation in Ammopiptanthus nanus. Plants 2023, 12, 677. [Google Scholar] [CrossRef]
- Liu, Q.; Sui, X.; Wang, Y.; Zhu, M.; Zhou, Y.; Gao, F. Genome-wide analyses of thaumatin-like protein family genes reveal the involvement in the response to low temperature stress in Ammopiptanthus nanus. IJMS 2023, 24, 2209. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Wang, J.; Wei, S.; Li, Z.; Wang, N.; Li, H.; Feng, J.; Li, H.; Zhou, Y.; Zhang, F. Transcriptomic analysis of drought stress responses in Ammopiptanthus mongolicus leaves using the RNA-Seq technique. PLoS ONE 2015, 29, e0124382. [Google Scholar] [CrossRef]
- Cao, S.; Wang, Y.; Li, X.; Gao, F.; Feng, J.; Zhou, Y. Characterization of the AP2/ERF transcription factor family and expression profiling of DREB Subfamily under cold and osmotic stresses in Ammopiptanthus nanus. Plants 2020, 9, 455. [Google Scholar] [CrossRef]
- Sun, H.; Xia, B.; Wang, X.; Gao, F.; Zhou, Y. Quantitative phosphoproteomic analysis provides insight into the response to short-term drought stress in Ammopiptanthus mongolicus roots. IJMS 2017, 18, 2158. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, X.; Zhou, Y.; Tan, J.; Zhou, Y.; Gao, F. Small RNA sequencing revealed that miR4415, a legume-specific miRNA, was involved in the cold acclimation of Ammopiptanthus nanus by targeting an L-Ascorbate Oxidase gene and regulating the redox state of apoplast. Front. Genet. 2022, 13, 870446. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Chan, C.K. Analysis of RNA-Seq data using TopHat and Cufflinks. Methods Mol. Biol. 2016, 1374, 339–361. [Google Scholar] [PubMed]
- Gao, F.; Wang, X.; Li, X.; Xu, M.; Li, H.; Abla, M.; Sun, H.; Wei, S.; Feng, J.; Zhou, Y. Long-read sequencing and de novo genome assembly of Ammopiptanthus nanus, a desert shrub. Gigascience 2018, 7, giy074. [Google Scholar] [CrossRef] [PubMed]
- Hasiow-Jaroszewska, B.; Borodynko, N.; Rymelska, N.; Pospieszny, H. First report of papaya ringspot virus infecting zucchini plants in poland. Plant Dis. 2010, 94, 633. [Google Scholar] [CrossRef]
- Pang, T.; Ye, C.Y.; Xia, X.; Yin, W. De novo sequencing and transcriptome analysis of the desert shrub, Ammopiptanthus mongolicus, during cold acclimation using Illumina/Solexa. BMC Genom. 2013, 14, 488. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, W.; Pang, X.; Wang, X.; Zhang, H.; Dong, B.; Xing, Y.; Li, X.; Wang, M. Comparative transcriptome profiling of a desert evergreen shrub, Ammopiptanthus mongolicus, in response to drought and cold stresses. BMC Genom. 2014, 15, 671. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Chen, S. Physiological and molecular mechanism involved in cold stress tolerance in plants. Plants 2020, 9, 560. [Google Scholar] [CrossRef]
- Jarroux, J.; Morillon, A.; Pinskaya, M. History, discovery, and classification of lncRNAs. Adv. Exp. Med. Biol. 2017, 1008, 1–46. [Google Scholar]
- Ferre, F.; Colantoni, A.; Helmer-Citterich, M. Revealing protein-lncRNA interaction. Brief Bioinform 2016, 17, 106–116. [Google Scholar] [CrossRef]
- Ulitsky, I. Interactions between short and long noncoding RNAs. FEBS Lett. 2018, 592, 2874–2883. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Tang, W. MicroRNA156 amplifies transcription factor-associated cold stress tolerance in plant cells. Mol. Genet Genom. 2019, 294, 379–393. [Google Scholar] [CrossRef]
- Garighan, J.; Dvorak, E.; Estevan, J.; Loridon, K.; Huettel, B.; Sarah, G.; Farrera, I.; Leclercq, J.; Grynberg, P.; Coiti Togawa, R. The identification of small RNAs differentially expressed in apple buds reveals a potential role of the miR159-MYB regulatory module during dormancy. Plants 2021, 10, 2665. [Google Scholar] [CrossRef]
- Jodder, J.; Das, R.; Sarkar, D.; Bhattacharjee, P.; Kundu, P. Distinct transcriptional and processing regulations control miR167a level in tomato during stress. RNA Biol. 2018, 15, 130–143. [Google Scholar] [CrossRef]
- Mathieu, J.; Yant, L.J.; Murdter, F.; Kuttner, F.; Schmid, M. Repression of flowering by the miR172 target SMZ. PLoS Biol. 2009, 7, e1000148. [Google Scholar] [CrossRef] [PubMed]
- Abla, M.; Sun, H.; Li, Z.; Wei, C.; Gao, F.; Zhou, Y.; Feng, J. Identification of miRNAs and their response to cold stress in Astragalus Membranaceus. Biomolecules 2019, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, K.; Li, D.; Yan, J.; Zhang, W. Enhanced cold tolerance and tillering in switchgrass (Panicum virgatum L.) by heterologous expression of osa-miR393a. Plant Cell Physiol. 2017, 58, 2226–2240. [Google Scholar] [CrossRef]
- Chen, L.; Luan, Y.; Zhai, J. Sp-miR396a-5p acts as a stress-responsive genes regulator by conferring tolerance to abiotic stresses and susceptibility to Phytophthora nicotianae infection in transgenic tobacco. Plant Cell Rep. 2015, 34, 2013–2025. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, J.; Song, A.; Chen, S.; Shan, H.; Luo, H.; Gu, C.; Sun, J.; Zhu, L.; Fang, W. Ambient temperature enhanced freezing tolerance of Chrysanthemum dichrum CdICE1 Arabidopsis via miR398. BMC Biol. 2013, 11, 121. [Google Scholar] [CrossRef]
- Yamamura, S.; Imai-Sumida, M.; Tanaka, Y.; Dahiya, R. Interaction and cross-talk between non-coding RNAs. Cell Mol. Life Sci. 2018, 75, 467–484. [Google Scholar] [CrossRef]
- Lin, K.Y.; Wu, S.Y.; Hsu, Y.H.; Lin, N.S. MiR398-regulated antioxidants contribute to Bamboo mosaic virus accumulation and symptom manifestation. Plant Physiol. 2022, 188, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liao, X.; Yang, X.; Luo, Y.; Lin, P.; Zeng, Q.; Bai, H.; Jiang, B.; Pan, Y.; Zhang, F. Lysine crotonylation of DgTIL1 at K72 modulates cold tolerance by enhancing DgnsLTP stability in chrysanthemum. Plant Biotechnol. J. 2021, 19, 1125–1140. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Jiang, W.; Yu, D. Male gametophyte-specific WRKY34 transcription factor mediates cold sensitivity of mature pollen in Arabidopsis. J. Exp. Bot. 2010, 61, 3901–3914. [Google Scholar] [CrossRef] [PubMed]
- An, J.P.; Li, R.; Qu, F.J.; You, C.X.; Wang, X.F.; Hao, Y.J. R2R3-MYB transcription factor MdMYB23 is involved in the cold tolerance and proanthocyanidin accumulation in apple. Plant J. 2018, 96, 562–577. [Google Scholar] [CrossRef]
- Lv, K.; Li, J.; Zhao, K.; Chen, S.; Nie, J.; Zhang, W.; Liu, G.; Wei, H. Overexpression of an AP2/ERF family gene, BpERF13, in birch enhances cold tolerance through upregulating CBF genes and mitigating reactive oxygen species. Plant Sci. 2020, 292, 110375. [Google Scholar] [CrossRef]
- Min, J.H.; Park, C.R.; Jang, Y.H.; Ju, H.W.; Lee, K.H.; Lee, S.; Kim, C.S. A basic helix-loop-helix 104 (bHLH104) protein functions as a transcriptional repressor for glucose and abscisic acid signaling in Arabidopsis. Plant Physiol. Biochem. 2019, 136, 34–42. [Google Scholar] [CrossRef]
- Prerostova, S.; Dobrev, P.I.; Knirsch, V.; Jarosova, J.; Gaudinova, A.; Zupkova, B.; Prasil, I.T.; Janda, T.; Brzobohaty, B.; Skalak, J. Light quality and intensity modulate cold acclimation in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 2736. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Zhou, Y.; Wang, X.; Li, H.; Feng, Z.; Chen, H.; Qin, G.; Jin, D.; Terzaghi, W. TANDEM ZINC-FINGER/PLUS3 is a key component of phytochrome a signaling. Plant Cell 2018, 30, 835–852. [Google Scholar] [CrossRef]
- Gould, P.D.; Diaz, P.; Hogben, C.; Kusakina, J.; Salem, R.; Hartwell, J.; Hall, A. Delayed fluorescence as a universal tool for the measurement of circadian rhythms in higher plants. Plant J. 2009, 58, 893–901. [Google Scholar] [CrossRef]
- Fryer, M.J.; Oxborough, K.; Mullineaux, P.M.; Baker, N.R. Imaging of photo-oxidative stress responses in leaves. J. Exp. Bot. 2002, 53, 1249–1254. [Google Scholar]
- Korhonen, A.; Lehto, T.; Heinonen, J.; Repo, T. Whole-plant frost hardiness of mycorrhizal (Hebeloma sp. or Suillus luteus) and non-mycorrhizal Scots pine seedlings. Tree Physiol. 2019, 39, 526–535. [Google Scholar] [CrossRef]
- Trapnell, C.; Hendrickson, D.G.; Sauvageau, M.; Goff, L.; Rinn, J.L.; Pachter, L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 2013, 31, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhang, Y.; Ye, Z.Q.; Liu, X.Q.; Zhao, S.Q.; Wei, L.; Gao, G. CPC: Assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007, 35, W345–W349. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]
- Varkonyi-Gasic, E.; Wu, R.; Wood, M.; Walton, E.F.; Hellens, R.P. Protocol: A highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 2007, 3, 12. [Google Scholar] [CrossRef]
- Clement, T.; Salone, V.; Rederstorff, M. Dual luciferase gene reporter assays to study miRNA function. Methods Mol. Biol. 2015, 1296, 187–198. [Google Scholar]
- Yang, Y.; Zhang, X.; Su, Y.; Zou, J.; Wang, Z.; Xu, L.; Que, Y. miRNA alteration is an important mechanism in sugarcane response to low-temperature environment. BMC Genom. 2017, 18, 833. [Google Scholar] [CrossRef] [PubMed]

| Library | Raw Reads Number | Clean Reads Number after Filtering Low-Quality Reads (%) | Clean Reads Obtained after Filtering rRNA (%) |
|---|---|---|---|
| CK_1 | 85,744,166 | 84,218,844 (98.22%) | 84,174,080 (99.95%) |
| CK_2 | 91,203,058 | 89,512,572 (98.15%) | 89,370,830 (99.84%) |
| CK_3 | 81,083,996 | 79,176,154 (97.65%) | 79,090,710 (99.89%) |
| CT_1 | 83,086,322 | 81,076,694 (97.58%) | 80,973,362 (99.87%) |
| CT_2 | 78,783,216 | 76,828,286 (97.52%) | 76,785,718 (99.94%) |
| CT_3 | 74,450,226 | 73,215,036 (98.34%) | 73,179,680 (99.95%) |
| Library Name | Known mRNA Number | New mRNA Number | Total mRNA Number | New lncRNA Number |
|---|---|---|---|---|
| CK_1 | 28,216 | 12,494 | 40,710 | 4012 |
| CK_2 | 28,373 | 12,621 | 40,994 | 4078 |
| CK_3 | 27,920 | 12,506 | 40,426 | 3998 |
| CT_1 | 26,958 | 12,080 | 39,038 | 3803 |
| CT_2 | 26,741 | 12,007 | 38,748 | 3784 |
| CT_3 | 28,132 | 12,307 | 40,439 | 4069 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Dong, Q.; Bing, J.; Songbuerbatu; Zheng, L.; Dorjee, T.; Liu, Q.; Zhou, Y.; Gao, F. Combined lncRNA and mRNA Expression Profiles Identified the lncRNA–miRNA–mRNA Modules Regulating the Cold Stress Response in Ammopiptanthus nanus. Int. J. Mol. Sci. 2023, 24, 6502. https://doi.org/10.3390/ijms24076502
Zhu M, Dong Q, Bing J, Songbuerbatu, Zheng L, Dorjee T, Liu Q, Zhou Y, Gao F. Combined lncRNA and mRNA Expression Profiles Identified the lncRNA–miRNA–mRNA Modules Regulating the Cold Stress Response in Ammopiptanthus nanus. International Journal of Molecular Sciences. 2023; 24(7):6502. https://doi.org/10.3390/ijms24076502
Chicago/Turabian StyleZhu, Ming, Qianshi Dong, Jie Bing, Songbuerbatu, Lamei Zheng, Tashi Dorjee, Qi Liu, Yijun Zhou, and Fei Gao. 2023. "Combined lncRNA and mRNA Expression Profiles Identified the lncRNA–miRNA–mRNA Modules Regulating the Cold Stress Response in Ammopiptanthus nanus" International Journal of Molecular Sciences 24, no. 7: 6502. https://doi.org/10.3390/ijms24076502
APA StyleZhu, M., Dong, Q., Bing, J., Songbuerbatu, Zheng, L., Dorjee, T., Liu, Q., Zhou, Y., & Gao, F. (2023). Combined lncRNA and mRNA Expression Profiles Identified the lncRNA–miRNA–mRNA Modules Regulating the Cold Stress Response in Ammopiptanthus nanus. International Journal of Molecular Sciences, 24(7), 6502. https://doi.org/10.3390/ijms24076502






