α7- and α9-Containing Nicotinic Acetylcholine Receptors in the Functioning of Immune System and in Pain
Abstract
1. Introduction
- -
- nAChRs of α7 subtype are involved in anti-inflammatory pathways;
- -
- α9α10 nAChRs participate in pain relief mechanisms.
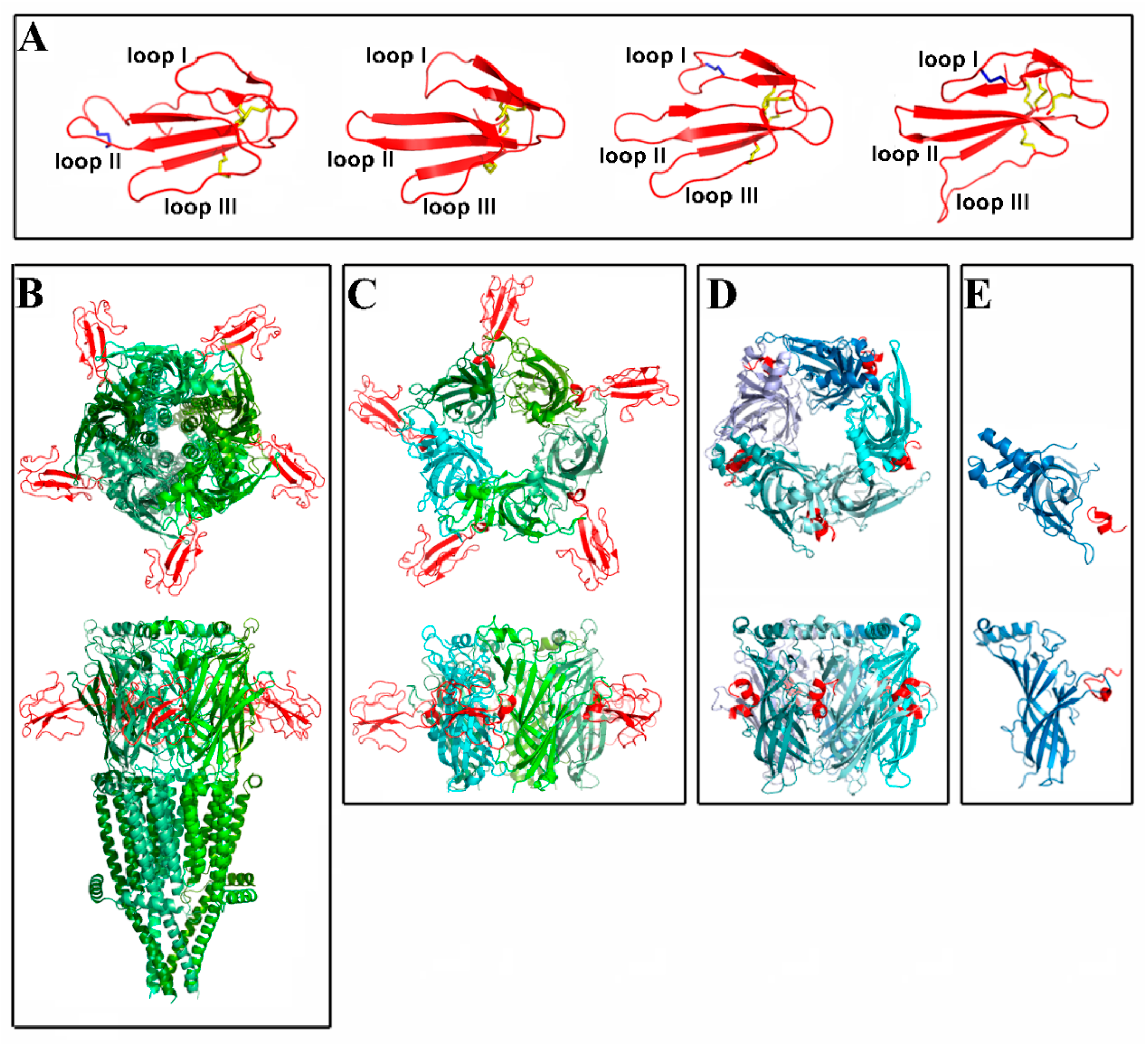
2. Spatial Structure of Nicotinic Receptors and Their Complexes with Various Ligands
3. Snake α-Neurotoxins and Ly6/uPAR Proteins as Research Tools and Potential Drugs
4. α-Conotoxins in Distinguishing the Individual nAChR Subtypes
5. Functional Roles of nAChR in Immune Cells
5.1. Non-Neuronal Cholinergic Anti-Inflammatory Reflex
5.2. T-Cells
5.3. B Cells
5.4. Dendritic Cells (DC)
5.5. Monocytes
5.6. Macrophages
5.7. Mast Cells
5.8. Neutrophils and Granulocytes
5.9. Natural Killer (NK) Cells
6. α7- and α9-Containing nAChRs in Chronic Pain
6.1. α7 nAChR in Pain
6.2. α9-Containing nAChR in Pain
6.2.1. Molecular Mechanisms of Analgesia Mediated by α9-Containing nAChRs
6.2.2. α-Conotoxin-Based Drug Development Strategies
7. α7- and α9-Containing nAChRs as Targets in Viral Infection
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE2 | angiotensin-converting enzyme 2 |
| ACh | acetylcholine |
| AChBP | acetylcholine-binding protein |
| APC | antigen-presenting cell |
| CAP | cholinergic anti-inflammatory pathway |
| CCI | chronic constriction injury |
| CCK | cholecystokinin |
| ChAT | choline acetyltransferase |
| CIA | collagen-induced arthritis |
| CIPN | chemotherapy-induced peripheral neuropathy |
| DC | dendritic cell |
| DRG | dorsal root ganglion |
| ECD | extracellular domain |
| GPI | glycosylphosphatidylinositol |
| HIV | human immunodeficiency virus |
| IFN | interferon |
| KO | knockout |
| MDM | human macrophage |
| MLA | methyllycaconitine |
| nAChR | nicotinic acetylcholine receptor |
| NK | natural killer |
| PHA | phytohemagglutinin |
| RVG | Rabies virus glycoprotein |
| RBD | receptor binding domain |
| SLURP-1 | lymphocytic antigen-6/urokinase type plasminogen activator receptor-related peptide |
| Tregs | regulatory T-cells |
| wsLynx1 | water-soluble Lynx1 |
References
- Tsetlin, V.; Kuzmin, D.; Kasheverov, I. Assembly of nicotinic and other Cys-loop receptors. J. Neurochem. 2011, 116, 734–741. [Google Scholar] [CrossRef]
- Dupont, Y.; Cohen, J.B.; Changeux, J.P. X-ray diffraction study of membrane fragments rich in acetylcholine receptor protein prepared from the electric organ of Torpedo marmorata. FEBS Lett. 1974, 40, 130–133. [Google Scholar] [CrossRef]
- Unwin, N. The nicotinic acetylcholine receptor of the Torpedo electric ray. J. Struct. Biol. 1998, 121, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Brejc, K.; van Dijk, W.J.; Klaassen, R.V.; Schuurmans, M.; van Der Oost, J.; Smit, A.B.; Sixma, T.K. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 2001, 411, 269–276. [Google Scholar] [CrossRef]
- Unwin, N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J. Mol. Biol. 2005, 346, 967–989. [Google Scholar] [CrossRef]
- Rahman, M.M.; Teng, J.; Worrell, B.T.; Noviello, C.M.; Lee, M.; Karlin, A.; Stowell, M.H.B.; Hibbs, R.E. Structure of the Native Muscle-type Nicotinic Receptor and Inhibition by Snake Venom Toxins. Neuron 2020, 106, 952–962 e5. [Google Scholar] [CrossRef]
- Nys, M.; Zarkadas, E.; Brams, M.; Mehregan, A.; Kambara, K.; Kool, J.; Casewell, N.R.; Bertrand, D.; Baenziger, J.E.; Nury, H.; et al. The molecular mechanism of snake short-chain α-neurotoxin binding to muscle-type nicotinic acetylcholine receptors. Nat. Commun. 2022, 13, 4543. [Google Scholar] [CrossRef]
- Kabbani, N.; Nichols, R.A. Beyond the Channel: Metabotropic Signaling by Nicotinic Receptors. Trends Pharmacol. Sci. 2018, 39, 354–366. [Google Scholar] [CrossRef]
- Bondarenko, V.; Wells, M.M.; Chen, Q.; Tillman, T.S.; Singewald, K.; Lawless, M.J.; Caporoso, J.; Brandon, N.; Coleman, J.A.; Saxena, S.; et al. Structures of highly flexible intracellular domain of human α7 nicotinic acetylcholine receptor. Nat. Commun. 2022, 13, 793. [Google Scholar] [CrossRef]
- Utkin, Y.N.; Kukhtina, V.V.; Kryukova, E.V.; Chiodini, F.; Bertrand, D.; Methfessel, C.; Tsetlin, V.I. “Weak toxin” from Naja kaouthia is a nontoxic antagonist of α 7 and muscle-type nicotinic acetylcholine receptors. J. Biol. Chem. 2001, 276, 15810–15815. [Google Scholar] [CrossRef]
- Shenkarev, Z.O.; Chesnokov, Y.M.; Zaigraev, M.M.; Chugunov, A.O.; Kulbatskii, D.S.; Kocharovskaya, M.V.; Paramonov, A.S.; Bychkov, M.L.; Shulepko, M.A.; Nolde, D.E.; et al. Membrane-mediated interaction of non-conventional snake three-finger toxins with nicotinic acetylcholine receptors. Commun. Biol. 2022, 5, 1344. [Google Scholar] [CrossRef]
- Nirthanan, S.; Gopalakrishnakone, P.; Gwee, M.C.; Khoo, H.E.; Kini, R.M. Non-conventional toxins from Elapid venoms. Toxicon Off. J. Int. Soc. Toxinol. 2003, 41, 397–407. [Google Scholar] [CrossRef]
- Miwa, J.M.; Ibanez-Tallon, I.; Crabtree, G.W.; Sanchez, R.; Sali, A.; Role, L.W.; Heintz, N. lynx1, an endogenous toxin-like modulator of nicotinic acetylcholine receptors in the mammalian CNS. Neuron 1999, 23, 105–114. [Google Scholar] [CrossRef]
- Adermann, K.; Wattler, F.; Wattler, S.; Heine, G.; Meyer, M.; Forssmann, W.G.; Nehls, M. Structural and phylogenetic characterization of human SLURP-1, the first secreted mammalian member of the Ly-6/uPAR protein superfamily. Protein Sci. 1999, 8, 810–819. [Google Scholar] [CrossRef]
- Chimienti, F.; Hogg, R.C.; Plantard, L.; Lehmann, C.; Brakch, N.; Fischer, J.; Huber, M.; Bertrand, D.; Hohl, D. Identification of SLURP-1 as an epidermal neuromodulator explains the clinical phenotype of Mal de Meleda. Hum. Mol. Genet. 2003, 12, 3017–3024. [Google Scholar] [CrossRef]
- Tsetlin, V.I. Three-finger snake neurotoxins and Ly6 proteins targeting nicotinic acetylcholine receptors: Pharmacological tools and endogenous modulators. Trends Pharmacol. Sci. 2015, 36, 109–123. [Google Scholar] [CrossRef]
- Miwa, J.M.; Anderson, K.R.; Hoffman, K.M. Lynx Prototoxins: Roles of Endogenous Mammalian Neurotoxin-Like Proteins in Modulating Nicotinic Acetylcholine Receptor Function to Influence Complex Biological Processes. Front. Pharmacol. 2019, 10, 343. [Google Scholar] [CrossRef]
- Tsetlin, V.I.; Kasheverov, I.E.; Utkin, Y.N. Three-finger proteins from snakes and humans acting on nicotinic receptors: Old and new. J. Neurochem. 2021, 158, 1223–1235. [Google Scholar] [CrossRef]
- Lyukmanova, E.N.; Shenkarev, Z.O.; Shulepko, M.A.; Mineev, K.S.; D’Hoedt, D.; Kasheverov, I.E.; Filkin, S.Y.; Krivolapova, A.P.; Janickova, H.; Dolezal, V.; et al. NMR structure and action on nicotinic acetylcholine receptors of water-soluble domain of human LYNX1. J. Biol. Chem. 2011, 286, 10618–10627. [Google Scholar] [CrossRef]
- Lyukmanova, E.N.; Shulepko, M.A.; Kudryavtsev, D.; Bychkov, M.L.; Kulbatskii, D.S.; Kasheverov, I.E.; Astapova, M.V.; Feofanov, A.V.; Thomsen, M.S.; Mikkelsen, J.D.; et al. Human Secreted Ly-6/uPAR Related Protein-1 (SLURP-1) Is a Selective Allosteric Antagonist of α7 Nicotinic Acetylcholine Receptor. PLoS ONE 2016, 11, e0149733. [Google Scholar] [CrossRef]
- Durek, T.; Shelukhina, I.V.; Tae, H.S.; Thongyoo, P.; Spirova, E.N.; Kudryavtsev, D.S.; Kasheverov, I.E.; Faure, G.; Corringer, P.J.; Craik, D.J.; et al. Interaction of Synthetic Human SLURP-1 with the Nicotinic Acetylcholine Receptors. Sci. Rep. 2017, 7, 16606. [Google Scholar] [CrossRef]
- Swamynathan, S.; Tiwari, A.; Loughner, C.L.; Gnalian, J.; Alexander, N.; Jhanji, V.; Swamynathan, S.K. The secreted Ly6/uPAR-related protein-1 suppresses neutrophil binding, chemotaxis, and transmigration through human umbilical vein endothelial cells. Sci. Rep. 2019, 9, 5898. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujii, T.; Moriwaki, Y.; Misawa, H.; Horiguchi, K. Non-neuronal cholinergic system in regulation of immune function with a focus on α7 nAChRs. Int. Immunopharmacol. 2015, 29, 127–134. [Google Scholar] [CrossRef]
- Chernyavsky, A.I.; Galitovskiy, V.; Shchepotin, I.B.; Grando, S.A. Anti-inflammatory effects of the nicotinergic peptides SLURP-1 and SLURP-2 on human intestinal epithelial cells and immunocytes. Biomed. Res. Int. 2014, 2014, 609086. [Google Scholar] [CrossRef]
- Campbell, G.; Swamynathan, S.; Tiwari, A.; Swamynathan, S.K. The secreted Ly-6/uPAR related protein-1 (SLURP-1) stabilizes epithelial cell junctions and suppresses TNF-α-induced cytokine production. Biochem. Biophys. Res. Commun. 2019, 517, 729–734. [Google Scholar] [CrossRef]
- Ertle, C.M.; Rommel, F.R.; Tumala, S.; Moriwaki, Y.; Klein, J.; Kruse, J.; Gieler, U.; Peters, E.M.J. New Pathways for the Skin’s Stress Response: The Cholinergic Neuropeptide SLURP-1 Can Activate Mast Cells and Alter Cytokine Production in Mice. Front. Immunol. 2021, 12, 631881. [Google Scholar] [CrossRef]
- Paramonov, A.S.; Kocharovskaya, M.V.; Tsarev, A.V.; Kulbatskii, D.S.; Loktyushov, E.V.; Shulepko, M.A.; Kirpichnikov, M.P.; Lyukmanova, E.N.; Shenkarev, Z.O. Structural Diversity and Dynamics of Human Three-Finger Proteins Acting on Nicotinic Acetylcholine Receptors. Int. J. Mol. Sci. 2020, 21, 7280. [Google Scholar] [CrossRef]
- Nissen, N.I.; Anderson, K.R.; Wang, H.; Lee, H.S.; Garrison, C.; Eichelberger, S.A.; Ackerman, K.; Im, W.; Miwa, J.M. Augmenting the antinociceptive effects of nicotinic acetylcholine receptor activity through lynx1 modulation. PLoS ONE 2018, 13, e0199643. [Google Scholar] [CrossRef]
- Shenkarev, Z.O.; Shulepko, M.A.; Bychkov, M.L.; Kulbatskii, D.S.; Shlepova, O.V.; Vasilyeva, N.A.; Andreev-Andrievskiy, A.A.; Popova, A.S.; Lagereva, E.A.; Loktyushov, E.V.; et al. Water-soluble variant of human Lynx1 positively modulates synaptic plasticity and ameliorates cognitive impairment associated with α7-nAChR dysfunction. J. Neurochem. 2020, 155, 45–61. [Google Scholar] [CrossRef]
- Kryukova, E.V.; Egorova, N.S.; Kudryavtsev, D.S.; Lebedev, D.S.; Spirova, E.N.; Zhmak, M.N.; Garifulina, A.I.; Kasheverov, I.E.; Utkin, Y.N.; Tsetlin, V.I. From Synthetic Fragments of Endogenous Three-Finger Proteins to Potential Drugs. Front. Pharmacol. 2019, 10, 748. [Google Scholar] [CrossRef]
- Mineev, K.S.; Kryukova, E.V.; Kasheverov, I.E.; Egorova, N.S.; Zhmak, M.N.; Ivanov, I.A.; Senko, D.A.; Feofanov, A.V.; Ignatova, A.A.; Arseniev, A.S.; et al. Spatial Structure and Activity of Synthetic Fragments of Lynx1 and of Nicotinic Receptor Loop C Models. Biomolecules 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Throm, V.M.; Mannle, D.; Giese, T.; Bauer, A.S.; Gaida, M.M.; Kopitz, J.; Bruckner, T.; Plaschke, K.; Grekova, S.P.; Felix, K.; et al. Endogenous CHRNA7-ligand SLURP-1 as a potential tumor suppressor and anti-nicotinic factor in pancreatic cancer. Oncotarget 2018, 9, 11734–11751. [Google Scholar] [CrossRef] [PubMed]
- Lyukmanova, E.N.; Bychkov, M.L.; Sharonov, G.V.; Efremenko, A.V.; Shulepko, M.A.; Kulbatskii, D.S.; Shenkarev, Z.O.; Feofanov, A.V.; Dolgikh, D.A.; Kirpichnikov, M.P. Human secreted proteins SLURP-1 and SLURP-2 control the growth of epithelial cancer cells via interactions with nicotinic acetylcholine receptors. Br. J. Pharmacol. 2018, 175, 1973–1986. [Google Scholar] [CrossRef]
- Upadhyay, G. Emerging Role of Lymphocyte Antigen-6 Family of Genes in Cancer and Immune Cells. Front. Immunol. 2019, 10, 819. [Google Scholar] [CrossRef]
- Shulepko, M.A.; Bychkov, M.L.; Shlepova, O.V.; Shenkarev, Z.O.; Kirpichnikov, M.P.; Lyukmanova, E.N. Human secreted protein SLURP-1 abolishes nicotine-induced proliferation, PTEN down-regulation and α7-nAChR expression up-regulation in lung cancer cells. Int. Immunopharmacol. 2020, 82, 106303. [Google Scholar] [CrossRef] [PubMed]
- Lebbe, E.K.; Peigneur, S.; Wijesekara, I.; Tytgat, J. Conotoxins targeting nicotinic acetylcholine receptors: An overview. Mar. Drugs 2014, 12, 2970–3004. [Google Scholar] [CrossRef]
- Abraham, N.; Lewis, R.J. Neuronal Nicotinic Acetylcholine Receptor Modulators from Cone Snails. Mar. Drugs 2018, 16, 208. [Google Scholar] [CrossRef]
- Kasheverov, I.; Kudryavtsev, D.; Shelukhina, I.; Nikolaev, G.; Utkin, Y.; Tsetlin, V. Marine Origin Ligands of Nicotinic Receptors: Low Molecular Compounds, Peptides and Proteins for Fundamental Research and Practical Applications. Biomolecules 2022, 12, 189. [Google Scholar] [CrossRef]
- Lin, B.; Xiang, S.; Li, M. Residues Responsible for the Selectivity of α-Conotoxins for Ac-AChBP or nAChRs. Mar. Drugs 2016, 14, 173. [Google Scholar] [CrossRef]
- Ho, T.N.T.; Abraham, N.; Lewis, R.J. Structure-Function of Neuronal Nicotinic Acetylcholine Receptor Inhibitors Derived From Natural Toxins. Front. Neurosci. 2020, 14, 609005. [Google Scholar] [CrossRef]
- Johnson, D.S.; Martinez, J.; Elgoyhen, A.B.; Heinemann, S.F.; McIntosh, J.M. α-Conotoxin ImI exhibits subtype-specific nicotinic acetylcholine receptor blockade: Preferential inhibition of homomeric α 7 and α 9 receptors. Mol. Pharmacol. 1995, 48, 194–199. [Google Scholar]
- Broxton, N.M.; Down, J.G.; Gehrmann, J.; Alewood, P.F.; Satchell, D.G.; Livett, B.G. α-conotoxin ImI inhibits the α-bungarotoxin-resistant nicotinic response in bovine adrenal chromaffin cells. J. Neurochem. 1999, 72, 1656–1662. [Google Scholar] [CrossRef]
- Ellison, M.; Gao, F.; Wang, H.L.; Sine, S.M.; McIntosh, J.M.; Olivera, B.M. α-conotoxins ImI and ImII target distinct regions of the human α7 nicotinic acetylcholine receptor and distinguish human nicotinic receptor subtypes. Biochemistry 2004, 43, 16019–16026. [Google Scholar] [CrossRef]
- Ellison, M.; McIntosh, J.M.; Olivera, B.M. α-conotoxins ImI and ImII. Similar α 7 nicotinic receptor antagonists act at different sites. J. Biol. Chem. 2003, 278, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Nicke, A.; Samochocki, M.; Loughnan, M.L.; Bansal, P.S.; Maelicke, A.; Lewis, R.J. α-conotoxins EpI and AuIB switch subtype selectivity and activity in native versus recombinant nicotinic acetylcholine receptors. FEBS Lett. 2003, 554, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Nguyen, T.A.; Cartier, G.E.; Olivera, B.M.; Yoshikami, D.; McIntosh, J.M. Single-residue alteration in α-conotoxin PnIA switches its nAChR subtype selectivity. Biochemistry 1999, 38, 14542–14548. [Google Scholar] [CrossRef] [PubMed]
- Loughnan, M.L.; Nicke, A.; Jones, A.; Adams, D.J.; Alewood, P.F.; Lewis, R.J. Chemical and functional identification and characterization of novel sulfated α-conotoxins from the cone snail Conus anemone. J. Med. Chem. 2004, 47, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Nicke, A.; Loughnan, M.L.; Millard, E.L.; Alewood, P.F.; Adams, D.J.; Daly, N.L.; Craik, D.J.; Lewis, R.J. Isolation, structure, and activity of GID, a novel α 4/7-conotoxin with an extended N-terminal sequence. J. Biol. Chem. 2003, 278, 3137–3144. [Google Scholar] [CrossRef] [PubMed]
- Talley, T.T.; Olivera, B.M.; Han, K.H.; Christensen, S.B.; Dowell, C.; Tsigelny, I.; Ho, K.Y.; Taylor, P.; McIntosh, J.M. α-conotoxin OmIA is a potent ligand for the acetylcholine-binding protein as well as α3β2 and α7 nicotinic acetylcholine receptors. J. Biol. Chem. 2006, 281, 24678–24686. [Google Scholar] [CrossRef]
- Safavi-Hemami, H.; Siero, W.A.; Kuang, Z.; Williamson, N.A.; Karas, J.A.; Page, L.R.; MacMillan, D.; Callaghan, B.; Kompella, S.N.; Adams, D.J.; et al. Embryonic toxin expression in the cone snail Conus victoriae: Primed to kill or divergent function? J. Biol. Chem. 2011, 286, 22546–22557. [Google Scholar] [CrossRef]
- Franco, A.; Kompella, S.N.; Akondi, K.B.; Melaun, C.; Daly, N.L.; Luetje, C.W.; Alewood, P.F.; Craik, D.J.; Adams, D.J.; Mari, F. RegIIA: An α4/7-conotoxin from the venom of Conus regius that potently blocks α3β4 nAChRs. Biochem. Pharmacol. 2012, 83, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Inserra, M.C.; Kompella, S.N.; Vetter, I.; Brust, A.; Daly, N.L.; Cuny, H.; Craik, D.J.; Alewood, P.F.; Adams, D.J.; Lewis, R.J. Isolation and characterization of α-conotoxin LsIA with potent activity at nicotinic acetylcholine receptors. Biochem. Pharmacol. 2013, 86, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Giribaldi, J.; Wilson, D.; Nicke, A.; El Hamdaoui, Y.; Laconde, G.; Faucherre, A.; Moha Ou Maati, H.; Daly, N.L.; Enjalbal, C.; Dutertre, S. Synthesis, Structure and Biological Activity of CIA and CIB, Two α-Conotoxins from the Predation-Evoked Venom of Conus catus. Toxins 2018, 10, 222. [Google Scholar] [CrossRef]
- Tae, H.S.; Gao, B.; Jin, A.H.; Alewood, P.F.; Adams, D.J. Globular and ribbon isomers of Conus geographus α-conotoxins antagonize human nicotinic acetylcholine receptors. Biochem. Pharmacol. 2021, 190, 114638. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.H.; Vetter, I.; Dutertre, S.; Abraham, N.; Emidio, N.B.; Inserra, M.; Murali, S.S.; Christie, M.J.; Alewood, P.F.; Lewis, R.J. MrIC, a novel α-conotoxin agonist in the presence of PNU at endogenous α7 nicotinic acetylcholine receptors. Biochemistry 2014, 53, 1–3. [Google Scholar] [CrossRef]
- Lebbe, E.K.; Peigneur, S.; Maiti, M.; Devi, P.; Ravichandran, S.; Lescrinier, E.; Ulens, C.; Waelkens, E.; D’Souza, L.; Herdewijn, P.; et al. Structure-function elucidation of a new α-conotoxin, Lo1a, from Conus longurionis. J. Biol. Chem. 2014, 289, 9573–9583. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Le Caer, J.P.; Araoz, R.; Thai, R.; Lamthanh, H.; Benoit, E.; Molgo, J. Isolation, purification and functional characterization of α-BnIA from Conus bandanus venom. Toxicon 2014, 91, 155–163. [Google Scholar] [CrossRef]
- Lebbe, E.K.; Peigneur, S.; Maiti, M.; Mille, B.G.; Devi, P.; Ravichandran, S.; Lescrinier, E.; Waelkens, E.; D’Souza, L.; Herdewijn, P.; et al. Discovery of a new subclass of α-conotoxins in the venom of Conus australis. Toxicon 2014, 91, 145–154. [Google Scholar] [CrossRef]
- Whiteaker, P.; Christensen, S.; Yoshikami, D.; Dowell, C.; Watkins, M.; Gulyas, J.; Rivier, J.; Olivera, B.M.; McIntosh, J.M. Discovery, synthesis, and structure activity of a highly selective α7 nicotinic acetylcholine receptor antagonist. Biochemistry 2007, 46, 6628–6638. [Google Scholar] [CrossRef]
- Armishaw, C.J.; Singh, N.; Medina-Franco, J.L.; Clark, R.J.; Scott, K.C.; Houghten, R.A.; Jensen, A.A. A synthetic combinatorial strategy for developing α-conotoxin analogs as potent α7 nicotinic acetylcholine receptor antagonists. J. Biol. Chem. 2010, 285, 1809–1821. [Google Scholar] [CrossRef]
- Kasheverov, I.E.; Chugunov, A.O.; Kudryavtsev, D.S.; Ivanov, I.A.; Zhmak, M.N.; Shelukhina, I.V.; Spirova, E.N.; Tabakmakher, V.M.; Zelepuga, E.A.; Efremov, R.G.; et al. High-Affinity α-Conotoxin PnIA Analogs Designed on the Basis of the Protein Surface Topography Method. Sci. Rep. 2016, 6, 36848. [Google Scholar] [CrossRef] [PubMed]
- Kasheverov, I.E.; Zhmak, M.N.; Khruschov, A.Y.; Tsetlin, V.I. Design of new α-conotoxins: From computer modeling to synthesis of potent cholinergic compounds. Mar. Drugs 2011, 9, 1698–1714. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhu, X.; Zhang, L.; Kudryavtsev, D.; Kasheverov, I.; Lei, Y.; Zhangsun, D.; Tsetlin, V.; Luo, S. Species specificity of rat and human α7 nicotinic acetylcholine receptors towards different classes of peptide and protein antagonists. Neuropharmacology 2018, 139, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, X.; Zhangsun, M.; Wu, Y.; Yu, J.; Harvey, P.J.; Kaas, Q.; Zhangsun, D.; Craik, D.J.; Luo, S. Engineered Conotoxin Differentially Blocks and Discriminates Rat and Human α7 Nicotinic Acetylcholine Receptors. J. Med. Chem. 2021, 64, 5620–5631. [Google Scholar] [CrossRef] [PubMed]
- Innocent, N.; Livingstone, P.D.; Hone, A.; Kimura, A.; Young, T.; Whiteaker, P.; McIntosh, J.M.; Wonnacott, S. α-conotoxin Arenatus IB[V11L,V16D] [corrected] is a potent and selective antagonist at rat and human native α7 nicotinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 2008, 327, 529–537. [Google Scholar] [CrossRef]
- Whiteaker, P.; Marks, M.J.; Christensen, S.; Dowell, C.; Collins, A.C.; McIntosh, J.M. Synthesis and characterization of 125I-α-conotoxin ArIB[V11L;V16A], a selective α7 nicotinic acetylcholine receptor antagonist. J. Pharmacol. Exp. Ther. 2008, 325, 910–919. [Google Scholar] [CrossRef]
- Hone, A.J.; Whiteaker, P.; Mohn, J.L.; Jacob, M.H.; McIntosh, J.M. Alexa Fluor 546-ArIB[V11L;V16A] is a potent ligand for selectively labeling α 7 nicotinic acetylcholine receptors. J. Neurochem. 2010, 114, 994–1006. [Google Scholar] [CrossRef]
- Padilla, A.; Keating, P.; Hartmann, J.X.; Mari, F. Effects of α-conotoxin ImI on TNF-α, IL-8 and TGF-β expression by human macrophage-like cells derived from THP-1 pre-monocytic leukemic cells. Sci. Rep. 2017, 7, 12742. [Google Scholar] [CrossRef]
- Grau, V.; Richter, K.; Hone, A.J.; McIntosh, J.M. Conopeptides [V11L;V16D]ArIB and RgIA4: Powerful Tools for the Identification of Novel Nicotinic Acetylcholine Receptors in Monocytes. Front. Pharmacol. 2018, 9, 1499. [Google Scholar] [CrossRef]
- Tan, Y.; Chu, Z.; Shan, H.; Zhangsun, D.; Zhu, X.; Luo, S. Inflammation Regulation via an Agonist and Antagonists of α7 Nicotinic Acetylcholine Receptors in RAW264.7 Macrophages. Mar. Drugs 2022, 20, 200. [Google Scholar] [CrossRef]
- Clark, R.J.; Fischer, H.; Nevin, S.T.; Adams, D.J.; Craik, D.J. The synthesis, structural characterization, and receptor specificity of the α-conotoxin Vc1.1. J. Biol. Chem. 2006, 281, 23254–23263. [Google Scholar] [CrossRef] [PubMed]
- Vincler, M.; Wittenauer, S.; Parker, R.; Ellison, M.; Olivera, B.M.; McIntosh, J.M. Molecular mechanism for analgesia involving specific antagonism of α9α10 nicotinic acetylcholine receptors. Proc. Natl. Acad. Sci. USA 2006, 103, 17880–17884. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, J.M.; Plazas, P.V.; Watkins, M.; Gomez-Casati, M.E.; Olivera, B.M.; Elgoyhen, A.B. A novel α-conotoxin, PeIA, cloned from Conus pergrandis, discriminates between rat α9α10 and α7 nicotinic cholinergic receptors. J. Biol. Chem. 2005, 280, 30107–30112. [Google Scholar] [CrossRef] [PubMed]
- Ellison, M.; Haberlandt, C.; Gomez-Casati, M.E.; Watkins, M.; Elgoyhen, A.B.; McIntosh, J.M.; Olivera, B.M. α-RgIA: A novel conotoxin that specifically and potently blocks the α9α10 nAChR. Biochemistry 2006, 45, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, C.; Liu, Z.; Wang, X.; Liu, N.; Du, W.; Dai, Q. Structural and Functional Characterization of a Novel α-Conotoxin Mr1.7 from Conus marmoreus Targeting Neuronal nAChR α3β2, α9α10 and α6/α3β2β3 Subtypes. Mar. Drugs 2015, 13, 3259–3275. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Tae, H.S.; Zhao, Z.; Li, X.; Zhang, J.; Chen, S.; Jiang, T.; Adams, D.J.; Yu, R. Mechanism of Action and Structure-Activity Relationship of α-Conotoxin Mr1.1 at the Human α9α10 Nicotinic Acetylcholine Receptor. J. Med. Chem. 2022, 65, 16204–16217. [Google Scholar] [CrossRef]
- Zouridakis, M.; Papakyriakou, A.; Ivanov, I.A.; Kasheverov, I.E.; Tsetlin, V.; Tzartos, S.; Giastas, P. Crystal Structure of the Monomeric Extracellular Domain of α9 Nicotinic Receptor Subunit in Complex With α-Conotoxin RgIA: Molecular Dynamics Insights Into RgIA Binding to α9α10 Nicotinic Receptors. Front. Pharmacol. 2019, 10, 474. [Google Scholar] [CrossRef]
- Satkunanathan, N.; Livett, B.; Gayler, K.; Sandall, D.; Down, J.; Khalil, Z. α-conotoxin Vc1.1 alleviates neuropathic pain and accelerates functional recovery of injured neurones. Brain Res. 2005, 1059, 149–158. [Google Scholar] [CrossRef]
- Nevin, S.T.; Clark, R.J.; Klimis, H.; Christie, M.J.; Craik, D.J.; Adams, D.J. Are α9α10 nicotinic acetylcholine receptors a pain target for α-conotoxins? Mol. Pharmacol. 2007, 72, 1406–1410. [Google Scholar] [CrossRef]
- Mohammadi, S.A.; Christie, M.J. Conotoxin Interactions with α9α10-nAChRs: Is the α9α10-Nicotinic Acetylcholine Receptor an Important Therapeutic Target for Pain Management? Toxins 2015, 7, 3916–3932. [Google Scholar] [CrossRef] [PubMed]
- Carstens, B.B.; Berecki, G.; Daniel, J.T.; Lee, H.S.; Jackson, K.A.; Tae, H.S.; Sadeghi, M.; Castro, J.; O’Donnell, T.; Deiteren, A.; et al. Structure-Activity Studies of Cysteine-Rich α-Conotoxins that Inhibit High-Voltage-Activated Calcium Channels via GABA(B) Receptor Activation Reveal a Minimal Functional Motif. Angew. Chem. Int. Ed. Engl. 2016, 55, 4692–4696. [Google Scholar] [CrossRef] [PubMed]
- Azam, L.; McIntosh, J.M. Molecular basis for the differential sensitivity of rat and human α9α10 nAChRs to α-conotoxin RgIA. J. Neurochem. 2012, 122, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Kompella, S.N.; Adams, D.J.; Craik, D.J.; Kaas, Q. Determination of the α-conotoxin Vc1.1 binding site on the α9α10 nicotinic acetylcholine receptor. J. Med. Chem. 2013, 56, 3557–3567. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Tae, H.S.; Xu, Q.; Jiang, T.; Adams, D.J.; Yu, R. α-Conotoxin Vc1.1 Structure-Activity Relationship at the Human α9α10 Nicotinic Acetylcholine Receptor Investigated by Minimal Side Chain Replacement. ACS Chem. Neurosci. 2019, 10, 4328–4336. [Google Scholar] [CrossRef]
- Romero, H.K.; Christensen, S.B.; Di Cesare Mannelli, L.; Gajewiak, J.; Ramachandra, R.; Elmslie, K.S.; Vetter, D.E.; Ghelardini, C.; Iadonato, S.P.; Mercado, J.L.; et al. Inhibition of α9α10 nicotinic acetylcholine receptors prevents chemotherapy-induced neuropathic pain. Proc. Natl. Acad. Sci. USA 2017, 114, E1825–E1832. [Google Scholar] [CrossRef]
- Christensen, S.B.; Hone, A.J.; Roux, I.; Kniazeff, J.; Pin, J.P.; Upert, G.; Servent, D.; Glowatzki, E.; McIntosh, J.M. RgIA4 Potently Blocks Mouse α9α10 nAChRs and Provides Long Lasting Protection against Oxaliplatin-Induced Cold Allodynia. Front. Cell. Neurosci. 2017, 11, 219. [Google Scholar] [CrossRef]
- Gajewiak, J.; Christensen, S.B.; Dowell, C.; Hararah, F.; Fisher, F.; Huynh, P.N.; Olivera, B.M.; McIntosh, J.M. Selective Penicillamine Substitution Enables Development of a Potent Analgesic Peptide that Acts through a Non-Opioid-Based Mechanism. J. Med. Chem. 2021, 64, 9271–9278. [Google Scholar] [CrossRef]
- Huynh, P.N.; Giuvelis, D.; Christensen, S.; Tucker, K.L.; McIntosh, J.M. RgIA4 Accelerates Recovery from Paclitaxel-Induced Neuropathic Pain in Rats. Mar. Drugs 2019, 18, 12. [Google Scholar] [CrossRef]
- Luo, S.; Zhangsun, D.; Harvey, P.J.; Kaas, Q.; Wu, Y.; Zhu, X.; Hu, Y.; Li, X.; Tsetlin, V.I.; Christensen, S.; et al. Cloning, synthesis, and characterization of αO-conotoxin GeXIVA, a potent α9α10 nicotinic acetylcholine receptor antagonist. Proc. Natl. Acad. Sci. USA 2015, 112, E4026–E4035. [Google Scholar] [CrossRef]
- Jiang, S.; Tae, H.S.; Xu, S.; Shao, X.; Adams, D.J.; Wang, C. Identification of a Novel O-Conotoxin Reveals an Unusual and Potent Inhibitor of the Human α9α10 Nicotinic Acetylcholine Receptor. Mar. Drugs 2017, 15, 170. [Google Scholar] [CrossRef]
- Christensen, S.B.; Bandyopadhyay, P.K.; Olivera, B.M.; McIntosh, J.M. αS-conotoxin GVIIIB potently and selectively blocks α9α10 nicotinic acetylcholine receptors. Biochem. Pharmacol. 2015, 96, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, K.; Wang, S.; Sun, T.; Yu, S.; Dai, Q.; Liu, Z. Cloning, expression and functional characterization of a D-superfamily conotoxin Lt28.1 with previously undescribed cysteine pattern. Peptides 2017, 94, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Zhangsun, D.; Yu, G.; Su, R.; Luo, S. The α9α10 Nicotinic Acetylcholine Receptor Antagonist αO-Conotoxin GeXIVA[1,2] Alleviates and Reverses Chemotherapy-Induced Neuropathic Pain. Mar. Drugs 2019, 17, 265. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, Y.; Wu, Y.; Huang, Y.; Yu, S.; Ding, Q.; Zhangsun, D.; Luo, S. Anti-hypersensitive effect of intramuscular administration of αO-conotoxin GeXIVA[1,2] and GeXIVA[1,4] in rats of neuropathic pain. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 66, 112–119. [Google Scholar] [CrossRef]
- Lebedev, D.S.; Kryukova, E.V.; Ivanov, I.A.; Egorova, N.S.; Timofeev, N.D.; Spirova, E.N.; Tufanova, E.Y.; Siniavin, A.E.; Kudryavtsev, D.S.; Kasheverov, I.E.; et al. Oligoarginine Peptides, a New Family of Nicotinic Acetylcholine Receptor Inhibitors. Mol. Pharmacol. 2019, 96, 664–673. [Google Scholar] [CrossRef]
- Zhang, B.; Ren, M.; Yang, F.; Li, R.; Yu, L.; Luo, A.; Zhangsun, D.; Luo, S.; Dong, S. Oligo-basic amino acids, potential nicotinic acetylcholine receptor inhibitors. Biomed. Pharmacother. 2022, 152, 113215. [Google Scholar] [CrossRef]
- MacDougall, G.; Anderton, R.S.; Edwards, A.B.; Knuckey, N.W.; Meloni, B.P. The Neuroprotective Peptide Poly-Arginine-12 (R12) Reduces Cell Surface Levels of NMDA NR2B Receptor Subunit in Cortical Neurons; Investigation into the Involvement of Endocytic Mechanisms. J. Mol. Neurosci. 2017, 61, 235–246. [Google Scholar] [CrossRef]
- Kelly, M.J.; Breathnach, C.; Tracey, K.J.; Donnelly, S.C. Manipulation of the inflammatory reflex as a therapeutic strategy. Cell Rep. Med. 2022, 3, 100696. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef]
- Sansonetti, P.J. The innate signaling of dangers and the dangers of innate signaling. Nat. Immunol. 2006, 7, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Luyer, M.D.; Greve, J.W.; Hadfoune, M.; Jacobs, J.A.; Dejong, C.H.; Buurman, W.A. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J. Exp. Med. 2005, 202, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, W.J.; van der Zanden, E.P.; The, F.O.; Bijlsma, M.F.; van Westerloo, D.J.; Bennink, R.J.; Berthoud, H.R.; Uematsu, S.; Akira, S.; van den Wijngaard, R.M.; et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 2005, 6, 844–851. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Ballina, M.; Ochani, M.; Parrish, W.R.; Ochani, K.; Harris, Y.T.; Huston, J.M.; Chavan, S.; Tracey, K.J. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl. Acad. Sci. USA 2008, 105, 11008–11013. [Google Scholar] [CrossRef]
- Vida, G.; Pena, G.; Deitch, E.A.; Ulloa, L. α7-cholinergic receptor mediates vagal induction of splenic norepinephrine. J. Immunol. 2011, 186, 4340–4346. [Google Scholar] [CrossRef]
- Huston, J.M.; Ochani, M.; Rosas-Ballina, M.; Liao, H.; Ochani, K.; Pavlov, V.A.; Gallowitsch-Puerta, M.; Ashok, M.; Czura, C.J.; Foxwell, B.; et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med. 2006, 203, 1623–1628. [Google Scholar] [CrossRef]
- Vida, G.; Pena, G.; Kanashiro, A.; Thompson-Bonilla Mdel, R.; Palange, D.; Deitch, E.A.; Ulloa, L. β2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 4476–4485. [Google Scholar] [CrossRef]
- Rosas-Ballina, M.; Olofsson, P.S.; Ochani, M.; Valdes-Ferrer, S.I.; Levine, Y.A.; Reardon, C.; Tusche, M.W.; Pavlov, V.A.; Andersson, U.; Chavan, S.; et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 2011, 334, 98–101. [Google Scholar] [CrossRef]
- Vieira-Alves, I.; Coimbra-Campos, L.M.C.; Sancho, M.; da Silva, R.F.; Cortes, S.F.; Lemos, V.S. Role of the α7 Nicotinic Acetylcholine Receptor in the Pathophysiology of Atherosclerosis. Front. Physiol. 2020, 11, 621769. [Google Scholar] [CrossRef] [PubMed]
- Zoheir, N.; Lappin, D.F.; Nile, C.J. Acetylcholine and the α 7 nicotinic receptor: A potential therapeutic target for the treatment of periodontal disease? Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2012, 61, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Physiological functions of the cholinergic system in immune cells. J. Pharmacol. Sci. 2017, 134, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Tsuchiya, T.; Yamada, S.; Fujimoto, K.; Suzuki, T.; Kasahara, T.; Kawashima, K. Localization and synthesis of acetylcholine in human leukemic T cell lines. J. Neurosci. Res. 1996, 44, 66–72. [Google Scholar] [CrossRef]
- Fujii, T.; Yamada, S.; Watanabe, Y.; Misawa, H.; Tajima, S.; Fujimoto, K.; Kasahara, T.; Kawashima, K. Induction of choline acetyltransferase mRNA in human mononuclear leukocytes stimulated by phytohemagglutinin, a T-cell activator. J. Neuroimmunol. 1998, 82, 101–107. [Google Scholar] [CrossRef]
- Peng, H.; Ferris, R.L.; Matthews, T.; Hiel, H.; Lopez-Albaitero, A.; Lustig, L.R. Characterization of the human nicotinic acetylcholine receptor subunit α (α) 9 (CHRNA9) and α (α) 10 (CHRNA10) in lymphocytes. Life Sci. 2004, 76, 263–280. [Google Scholar] [CrossRef]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Expression and Function of the Cholinergic System in Immune Cells. Front. Immunol. 2017, 8, 1085. [Google Scholar] [CrossRef]
- Willemze, R.A.; Brinkman, D.J.; Welting, O.; van Hamersveld, P.H.P.; Verseijden, C.; Luyer, M.D.; Wildenberg, M.E.; Seppen, J.; de Jonge, W.J. Acetylcholine-producing T cells augment innate immune-driven colitis but are redundant in T cell-driven colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G557–G568. [Google Scholar] [CrossRef]
- Fujii, Y.X.; Fujigaya, H.; Moriwaki, Y.; Misawa, H.; Kasahara, T.; Grando, S.A.; Kawashima, K. Enhanced serum antigen-specific IgG1 and proinflammatory cytokine production in nicotinic acetylcholine receptor α7 subunit gene knockout mice. J. Neuroimmunol. 2007, 189, 69–74. [Google Scholar] [CrossRef]
- Oloris, S.C.; Frazer-Abel, A.A.; Jubala, C.M.; Fosmire, S.P.; Helm, K.M.; Robinson, S.R.; Korpela, D.M.; Duckett, M.M.; Baksh, S.; Modiano, J.F. Nicotine-mediated signals modulate cell death and survival of T lymphocytes. Toxicol. Appl. Pharmacol. 2010, 242, 299–309. [Google Scholar] [CrossRef]
- Nouri-Shirazi, M.; Guinet, E. Evidence for the immunosuppressive role of nicotine on human dendritic cell functions. Immunology 2003, 109, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Mashimo, M.; Komori, M.; Matsui, Y.Y.; Murase, M.X.; Fujii, T.; Takeshima, S.; Okuyama, H.; Ono, S.; Moriwaki, Y.; Misawa, H.; et al. Distinct Roles of α7 nAChRs in Antigen-Presenting Cells and CD4(+) T Cells in the Regulation of T Cell Differentiation. Front. Immunol. 2019, 10, 1102. [Google Scholar] [CrossRef]
- Nizri, E.; Irony-Tur-Sinai, M.; Lory, O.; Orr-Urtreger, A.; Lavi, E.; Brenner, T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J. Immunol. 2009, 183, 6681–6688. [Google Scholar] [CrossRef]
- Wang, D.W.; Zhou, R.B.; Yao, Y.M.; Zhu, X.M.; Yin, Y.M.; Zhao, G.J.; Dong, N.; Sheng, Z.Y. Stimulation of α7 nicotinic acetylcholine receptor by nicotine increases suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro. J. Pharm. Exp. Ther. 2010, 335, 553–561. [Google Scholar] [CrossRef]
- Nakata, Y.; Miura, K.; Yamasaki, N.; Ogata, S.; Miura, S.; Hosomi, N.; Kaminuma, O. Expression and Function of Nicotinic Acetylcholine Receptors in Induced Regulatory T Cells. Int. J. Mol. Sci. 2022, 23, 1779. [Google Scholar] [CrossRef]
- Zhao, X.; Wilson, K.; Uteshev, V.; He, J.J. Activation of α7 nicotinic acetylcholine receptor ameliorates HIV-associated neurology and neuropathology. Brain 2021, 144, 3355–3370. [Google Scholar] [CrossRef] [PubMed]
- Reardon, C.; Duncan, G.S.; Brustle, A.; Brenner, D.; Tusche, M.W.; Olofsson, P.S.; Rosas-Ballina, M.; Tracey, K.J.; Mak, T.W. Lymphocyte-derived ACh regulates local innate but not adaptive immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Skok, M.V.; Grailhe, R.; Agenes, F.; Changeux, J.P. The role of nicotinic receptors in B-lymphocyte development and activation. Life Sci. 2007, 80, 2334–2336. [Google Scholar] [CrossRef]
- Skok, M.; Grailhe, R.; Agenes, F.; Changeux, J.P. The role of nicotinic acetylcholine receptors in lymphocyte development. J. Neuroimmunol. 2006, 171, 86–98. [Google Scholar] [CrossRef]
- Skok, M.; Grailhe, R.; Changeux, J.P. Nicotinic receptors regulate B lymphocyte activation and immune response. Eur. J. Pharm. 2005, 517, 246–251. [Google Scholar] [CrossRef]
- Koval, L.; Kalashnyk, O.; Lykhmus, O.; Skok, M. α7 nicotinic acetylcholine receptors are involved in suppression of the antibody immune response. J. Neuroimmunol. 2018, 318, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ness, S.; Lin, S.; Gordon, J.R. Regulatory Dendritic Cells, T Cell Tolerance, and Dendritic Cell Therapy for Immunologic Disease. Front. Immunol. 2021, 12, 633436. [Google Scholar] [CrossRef] [PubMed]
- Hogg, N. Nicotine has suppressive effects on dendritic cell function. Immunology 2003, 109, 329–330. [Google Scholar] [CrossRef]
- Fujii, T.; Horiguchi, K.; Sunaga, H.; Moriwaki, Y.; Misawa, H.; Kasahara, T.; Tsuji, S.; Kawashima, K. SLURP-1, an endogenous α7 nicotinic acetylcholine receptor allosteric ligand, is expressed in CD205(+) dendritic cells in human tonsils and potentiates lymphocytic cholinergic activity. J. Neuroimmunol. 2014, 267, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, T.; Luo, H.; Zuo, X.; Liu, S.; Wu, S. The effect of the cholinergic anti-inflammatory pathway on collagen-induced arthritis involves the modulation of dendritic cell differentiation. Arthritis Res. Ther. 2018, 20, 263. [Google Scholar] [CrossRef]
- Mashimo, M.; Takeshima, S.; Okuyama, H.; Matsurida, A.; Murase, M.; Ono, S.; Kawashima, K.; Fujii, T. α7 nAChRs expressed on antigen presenting cells are insensitive to the conventional antagonists α-bungarotoxin and methyllycaconitine. Int. Immunopharmacol. 2020, 81, 106276. [Google Scholar] [CrossRef]
- Gori, S.; Vermeulen, M.; Remes-Lenicov, F.; Jancic, C.; Scordo, W.; Ceballos, A.; Towstyka, N.; Bestach, Y.; Belli, C.; Sabbione, F.; et al. Acetylcholine polarizes dendritic cells toward a Th2-promoting profile. Allergy 2017, 72, 221–231. [Google Scholar] [CrossRef]
- Nouri-Shirazi, M.; Kahlden, C.; Nishino, P.; Guinet, E. Nicotine exposure alters the mRNA expression of Notch ligands in dendritic cells and their response to Th1-/Th2-promoting stimuli. Scand. J. Immunol. 2015, 81, 110–120. [Google Scholar] [CrossRef]
- Li, R.; Hu, X.; Chen, H.; Zhao, Y.; Gao, X.; Yuan, Y.; Guo, H.; Huang, H.; Zou, X.; Qi, H.; et al. Role of Cholinergic Anti-Inflammatory Pathway in Protecting Sepsis-Induced Acute Lung Injury through Regulation of the Conventional Dendritic Cells. Mediat. Inflamm. 2022, 2022, 1474891. [Google Scholar] [CrossRef]
- Chernyavsky, A.I.; Arredondo, J.; Skok, M.; Grando, S.A. Auto/paracrine control of inflammatory cytokines by acetylcholine in macrophage-like U937 cells through nicotinic receptors. Int. Immunopharmacol. 2010, 10, 308–315. [Google Scholar] [CrossRef]
- van der Zanden, E.P.; Hilbers, F.W.; Verseijden, C.; van den Wijngaard, R.M.; Skynner, M.; Lee, K.; Ulloa, L.; Boeckxstaens, G.E.; de Jonge, W.J. Nicotinic acetylcholine receptor expression and susceptibility to cholinergic immunomodulation in human monocytes of smoking individuals. Neuroimmunomodulation 2012, 19, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.K.; Iwagaki, H.; Hamano, R.; Yoshino, T.; Tanaka, N.; Nishibori, M. Effect of nicotine on IL-18-initiated immune response in human monocytes. J. Leukoc. Biol. 2006, 80, 1388–1394. [Google Scholar] [CrossRef]
- Takahashi, H.K.; Iwagaki, H.; Hamano, R.; Yoshino, T.; Tanaka, N.; Nishibori, M. α7 Nicotinic acetylcholine receptor stimulation inhibits lipopolysaccharide-induced interleukin-18 and -12 production in monocytes. J. Pharmacol. Sci. 2006, 102, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, C.; Chen, X.; Jiang, L.; Su, X. Monocytes primed with GTS-21/α7 nAChR (nicotinic acetylcholine receptor) agonist develop anti-inflammatory memory. QJM Mon. J. Assoc. Physicians 2017, 110, 437–445. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, S.; Jiang, W.; Roy, P.; Champigny, C.; LeBlanc, E.; Morley, B.J.; Hao, J.; Simard, A.R. Nicotinic Acetylcholine Receptors Modulate Bone Marrow-Derived Pro-Inflammatory Monocyte Production and Survival. PLoS ONE 2016, 11, e0150230. [Google Scholar] [CrossRef]
- Jiang, W.; St-Pierre, S.; Roy, P.; Morley, B.J.; Hao, J.; Simard, A.R. Infiltration of CCR2+Ly6Chigh Proinflammatory Monocytes and Neutrophils into the Central Nervous System Is Modulated by Nicotinic Acetylcholine Receptors in a Model of Multiple Sclerosis. J. Immunol. 2016, 196, 2095–2108. [Google Scholar] [CrossRef]
- Hu, J.N.; Liu, Y.; Liu, S.C.; Zhang, T.; Chen, G.B.; Zhao, J.; Ma, T. The α7 Nicotinic Acetylcholine Receptor Agonist GTS-21 Improves Bacterial Clearance via Regulation of Monocyte Recruitment and Activity in Polymicrobial Septic Peritonitis. Front. Immunol. 2022, 13, 839290. [Google Scholar] [CrossRef]
- Richter, K.; Mathes, V.; Fronius, M.; Althaus, M.; Hecker, A.; Krasteva-Christ, G.; Padberg, W.; Hone, A.J.; McIntosh, J.M.; Zakrzewicz, A.; et al. Phosphocholine—An agonist of metabotropic but not of ionotropic functions of α9-containing nicotinic acetylcholine receptors. Sci. Rep. 2016, 6, 28660. [Google Scholar] [CrossRef]
- Zakrzewicz, A.; Richter, K.; Agne, A.; Wilker, S.; Siebers, K.; Fink, B.; Krasteva-Christ, G.; Althaus, M.; Padberg, W.; Hone, A.J.; et al. Canonical and Novel Non-Canonical Cholinergic Agonists Inhibit ATP-Induced Release of Monocytic Interleukin-1β via Different Combinations of Nicotinic Acetylcholine Receptor Subunits α7, α9 and α10. Front. Cell. Neurosci. 2017, 11, 189. [Google Scholar] [CrossRef]
- Backhaus, S.; Zakrzewicz, A.; Richter, K.; Damm, J.; Wilker, S.; Fuchs-Moll, G.; Kullmar, M.; Hecker, A.; Manzini, I.; Ruppert, C.; et al. Surfactant inhibits ATP-induced release of interleukin-1β via nicotinic acetylcholine receptors. J. Lipid Res. 2017, 58, 1055–1066. [Google Scholar] [CrossRef]
- Hecker, A.; Kullmar, M.; Wilker, S.; Richter, K.; Zakrzewicz, A.; Atanasova, S.; Mathes, V.; Timm, T.; Lerner, S.; Klein, J.; et al. Phosphocholine-Modified Macromolecules and Canonical Nicotinic Agonists Inhibit ATP-Induced IL-1β Release. J. Immunol. 2015, 195, 2325–2334. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Sagawe, S.; Hecker, A.; Kullmar, M.; Askevold, I.; Damm, J.; Heldmann, S.; Pohlmann, M.; Ruhrmann, S.; Sander, M.; et al. C-Reactive Protein Stimulates Nicotinic Acetylcholine Receptors to Control ATP-Mediated Monocytic Inflammasome Activation. Front. Immunol. 2018, 9, 1604. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Koch, C.; Perniss, A.; Wolf, P.M.; Schweda, E.K.H.; Wichmann, S.; Wilker, S.; Magel, I.; Sander, M.; McIntosh, J.M.; et al. Phosphocholine-Modified Lipooligosaccharides of Haemophilus influenzae Inhibit ATP-Induced IL-1β Release by Pulmonary Epithelial Cells. Molecules 2018, 23, 1979. [Google Scholar] [CrossRef] [PubMed]
- Mikulski, Z.; Hartmann, P.; Jositsch, G.; Zaslona, Z.; Lips, K.S.; Pfeil, U.; Kurzen, H.; Lohmeyer, J.; Clauss, W.G.; Grau, V.; et al. Nicotinic receptors on rat alveolar macrophages dampen ATP-induced increase in cytosolic calcium concentration. Respir. Res. 2010, 11, 133. [Google Scholar] [CrossRef]
- Tarnawski, L.; Reardon, C.; Caravaca, A.S.; Rosas-Ballina, M.; Tusche, M.W.; Drake, A.R.; Hudson, L.K.; Hanes, W.M.; Li, J.H.; Parrish, W.R.; et al. Adenylyl Cyclase 6 Mediates Inhibition of TNF in the Inflammatory Reflex. Front. Immunol. 2018, 9, 2648. [Google Scholar] [CrossRef]
- Siniavin, A.E.; Streltsova, M.A.; Kudryavtsev, D.S.; Shelukhina, I.V.; Utkin, Y.N.; Tsetlin, V.I. Activation of α7 Nicotinic Acetylcholine Receptor Upregulates HLA-DR and Macrophage Receptors: Potential Role in Adaptive Immunity and in Preventing Immunosuppression. Biomolecules 2020, 10, 507. [Google Scholar] [CrossRef]
- Yang, Y.H.; Li, D.L.; Bi, X.Y.; Sun, L.; Yu, X.J.; Fang, H.L.; Miao, Y.; Zhao, M.; He, X.; Liu, J.J.; et al. Acetylcholine Inhibits LPS-Induced MMP-9 Production and Cell Migration via the α7 nAChR-JAK2/STAT3 Pathway in RAW264.7 Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 36, 2025–2038. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, W.; Zhang, R.; Yuan, Q.; Wu, R.; Liu, X.; Wuri, J.; Li, R.; Yan, T. Activation of Cholinergic Anti-Inflammatory Pathway Ameliorates Cerebral and Cardiac Dysfunction After Intracerebral Hemorrhage Through Autophagy. Front. Immunol. 2022, 13, 870174. [Google Scholar] [CrossRef]
- Su, X.; Lee, J.W.; Matthay, Z.A.; Mednick, G.; Uchida, T.; Fang, X.; Gupta, N.; Matthay, M.A. Activation of the α7 nAChR reduces acid-induced acute lung injury in mice and rats. Am. J. Respir. Cell Mol. Biol. 2007, 37, 186–192. [Google Scholar] [CrossRef]
- Giebelen, I.A.; van Westerloo, D.J.; LaRosa, G.J.; de Vos, A.F.; van der Poll, T. Local stimulation of α7 cholinergic receptors inhibits LPS-induced TNF-α release in the mouse lung. Shock 2007, 28, 700–703. [Google Scholar] [CrossRef]
- Delgado-Velez, M.; Baez-Pagan, C.A.; Gerena, Y.; Quesada, O.; Santiago-Perez, L.I.; Capo-Velez, C.M.; Wojna, V.; Melendez, L.; Leon-Rivera, R.; Silva, W.; et al. The α7-nicotinic receptor is upregulated in immune cells from HIV-seropositive women: Consequences to the cholinergic anti-inflammatory response. Clin. Transl. Immunol. 2015, 4, e53. [Google Scholar] [CrossRef]
- Rios, S.C.; Colon Saez, J.O.; Quesada, O.; Figueroa, K.Q.; Lasalde Dominicci, J.A. Disruption of the cholinergic anti-inflammatory response by R5-tropic HIV-1 protein gp120(JRFL). J. Biol. Chem. 2021, 296, 100618. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.C.; Rir-sima-ah, J.; Boyd, R.T.; Singh, S.P.; Gundavarapu, S.; Langley, R.J.; Razani-Boroujerdi, S.; Sopori, M.L. Nicotine inhibits Fc epsilon RI-induced cysteinyl leukotrienes and cytokine production without affecting mast cell degranulation through α 7/α 9/α 10-nicotinic receptors. J. Immunol. 2010, 185, 588–596. [Google Scholar] [CrossRef]
- Fantozzi, R.; Masini, E.; Blandina, P.; Mannaioni, P.F.; Bani-Sacchi, T. Release of histamine from rat mast cells by acetylcholine. Nature 1978, 273, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Sudheer, P.S.; Hall, J.E.; Donev, R.; Read, G.; Rowbottom, A.; Williams, P.E. Nicotinic acetylcholine receptors on basophils and mast cells. Anaesthesia 2006, 61, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Radosa, J.; Dyck, W.; Goerdt, S.; Kurzen, H. The cholinergic system in guttate psoriasis with special reference to mast cells. Exp. Dermatol. 2011, 20, 677–679. [Google Scholar] [CrossRef]
- Kageyama-Yahara, N.; Suehiro, Y.; Yamamoto, T.; Kadowaki, M. IgE-induced degranulation of mucosal mast cells is negatively regulated via nicotinic acetylcholine receptors. Biochem. Biophys. Res. Commun. 2008, 377, 321–325. [Google Scholar] [CrossRef]
- Safronova, V.G.; Vulfius, C.A.; Shelukhina, I.V.; Mal’tseva, V.N.; Berezhnov, A.V.; Fedotova, E.I.; Miftahova, R.G.; Kryukova, E.V.; Grinevich, A.A.; Tsetlin, V.I. Nicotinic receptor involvement in regulation of functions of mouse neutrophils from inflammatory site. Immunobiology 2016, 221, 761–772. [Google Scholar] [CrossRef]
- Safronova, V.G.; Vulfius, C.A.; Astashev, M.E.; Tikhonova, I.V.; Serov, D.A.; Jirova, E.A.; Pershina, E.V.; Senko, D.A.; Zhmak, M.N.; Kasheverov, I.E.; et al. α9α10 nicotinic acetylcholine receptors regulate murine bone marrow granulocyte functions. Immunobiology 2021, 226, 152047. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Thompson, P.R.; Segal, B.H.; Urban, C.F. Nicotine induces neutrophil extracellular traps. J. Leukoc. Biol. 2016, 100, 1105–1112. [Google Scholar] [CrossRef]
- Su, X.; Matthay, M.A.; Malik, A.B. Requisite role of the cholinergic α7 nicotinic acetylcholine receptor pathway in suppressing Gram-negative sepsis-induced acute lung inflammatory injury. J. Immunol. 2010, 184, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, S.R.; Ziblat, A.; Torres, N.I.; Zwirner, N.W.; Bouzat, C. Expression and Functional Role of α7 Nicotinic Receptor in Human Cytokine-stimulated Natural Killer (NK) Cells. J. Biol. Chem. 2016, 291, 16541–16552. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, D.; Han, R.; Zhang, C.; Jin, W.N.; Wood, K.; Liu, Q.; Shi, F.D.; Hao, J. Acetylcholine-producing NK cells attenuate CNS inflammation via modulation of infiltrating monocytes/macrophages. Proc. Natl. Acad. Sci. USA 2017, 114, E6202–E6211. [Google Scholar] [CrossRef]
- Hao, J.; Shi, F.D.; Abdelwahab, M.; Shi, S.X.; Simard, A.; Whiteaker, P.; Lukas, R.; Zhou, Q. Nicotinic receptor β2 determines NK cell-dependent metastasis in a murine model of metastatic lung cancer. PLoS ONE 2013, 8, e57495. [Google Scholar] [CrossRef]
- Jiang, J.L.; Qiu, Y.H.; Peng, Y.P. Effect of acetylcholine on the cytotoxicity of natural killer cells. Chin. J. Appl. Physiol. 2005, 21, 330–333. [Google Scholar]
- Toma, W.; Ulker, E.; Alqasem, M.; AlSharari, S.D.; McIntosh, J.M.; Damaj, M.I. Behavioral and Molecular Basis of Cholinergic Modulation of Pain: Focus on Nicotinic Acetylcholine Receptors. Curr. Top. Behav. Neurosci. 2020, 45, 153–166. [Google Scholar]
- Zhou, Y.Q.; Liu, D.Q.; Liu, C.; Xu, A.J.; Tian, Y.K.; Mei, W.; Tian, X.B. Targeting α7 nicotinic acetylcholine receptors for chronic pain. Front. Mol. Neurosci. 2022, 15, 970040. [Google Scholar] [CrossRef]
- Alfonso-Rodriguez, J.; Wang, S.; Zeng, X.; Candiotti, K.A.; Zhang, Y. Mechanism of Electroacupuncture Analgesia on Nicotine Withdrawal-Induced Hyperalgesia in a Rat Model. Evid. Based Complement. Altern. Med. 2022, 2022, 7975803. [Google Scholar] [CrossRef]
- Papke, R.L.; Horenstein, N.A. Therapeutic Targeting of α7 Nicotinic Acetylcholine Receptors. Pharm. Rev. 2021, 73, 1118–1149. [Google Scholar] [CrossRef]
- Quadri, M.; Bagdas, D.; Toma, W.; Stokes, C.; Horenstein, N.A.; Damaj, M.I.; Papke, R.L. The Antinociceptive and Anti-Inflammatory Properties of the α7 nAChR Weak Partial Agonist p-CF(3) N,N-diethyl-N’-phenylpiperazine. J. Pharm. Exp. Ther. 2018, 367, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Papke, R.L.; Andleeb, H.; Stokes, C.; Quadri, M.; Horenstein, N.A. Selective Agonists and Antagonists of α9 Versus α7 Nicotinic Acetylcholine Receptors. ACS Chem. Neurosci. 2022, 13, 624–637. [Google Scholar] [CrossRef]
- Richter, K.; Papke, R.L.; Stokes, C.; Roy, D.C.; Espinosa, E.S.; Wolf, P.M.K.; Hecker, A.; Liese, J.; Singh, V.K.; Padberg, W.; et al. Comparison of the Anti-inflammatory Properties of Two Nicotinic Acetylcholine Receptor Ligands, Phosphocholine and pCF3-diEPP. Front. Cell. Neurosci. 2022, 16, 779081. [Google Scholar] [CrossRef] [PubMed]
- Di Lascio, S.; Fornasari, D.; Benfante, R. The Human-Restricted Isoform of the α7 nAChR, CHRFAM7A: A Double-Edged Sword in Neurological and Inflammatory Disorders. Int. J. Mol. Sci. 2022, 23, 3463. [Google Scholar] [CrossRef]
- Maroli, A.; Di Lascio, S.; Drufuca, L.; Cardani, S.; Setten, E.; Locati, M.; Fornasari, D.; Benfante, R. Effect of donepezil on the expression and responsiveness to LPS of CHRNA7 and CHRFAM7A in macrophages: A possible link to the cholinergic anti-inflammatory pathway. J. Neuroimmunol. 2019, 332, 155–166. [Google Scholar] [CrossRef]
- de Lucas-Cerrillo, A.M.; Maldifassi, M.C.; Arnalich, F.; Renart, J.; Atienza, G.; Serantes, R.; Cruces, J.; Sanchez-Pacheco, A.; Andres-Mateos, E.; Montiel, C. Function of partially duplicated human α77 nicotinic receptor subunit CHRFAM7A gene: Potential implications for the cholinergic anti-inflammatory response. J. Biol. Chem. 2011, 286, 594–606. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, C.; Indersmitten, T.; Freedman, R.; Leonard, S.; Lester, H.A. The duplicated α7 subunits assemble and form functional nicotinic receptors with the full-length α7. J. Biol. Chem. 2014, 289, 26451–26463. [Google Scholar] [CrossRef]
- Araud, T.; Graw, S.; Berger, R.; Lee, M.; Neveu, E.; Bertrand, D.; Leonard, S. The chimeric gene CHRFAM7A, a partial duplication of the CHRNA7 gene, is a dominant negative regulator of α7*nAChR function. Biochem. Pharm. 2011, 82, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Martin-Sanchez, C.; Ales, E.; Balseiro-Gomez, S.; Atienza, G.; Arnalich, F.; Bordas, A.; Cedillo, J.L.; Extremera, M.; Chavez-Reyes, A.; Montiel, C. The human-specific duplicated α7 gene inhibits the ancestral α7, negatively regulating nicotinic acetylcholine receptor-mediated transmitter release. J. Biol. Chem. 2021, 296, 100341. [Google Scholar] [CrossRef]
- Peng, W.; Mao, L.; Dang, X. The emergence of the uniquely human α7 nicotinic acetylcholine receptor gene and its roles in inflammation. Gene 2022, 842, 146777. [Google Scholar] [CrossRef]
- Courties, A.; Petit, J.; Do, A.; Legris, M.; Kouki, I.; Pigenet, A.; Sacitharan, P.K.; Ehkirch, F.P.; Berenbaum, F.; Sellam, J. α-7 Nicotinic Receptor Dampens Murine Osteoblastic Response to Inflammation and Age-Related Osteoarthritis. Front. Immunol. 2022, 13, 842538. [Google Scholar] [CrossRef] [PubMed]
- Courties, A.; Olmer, M.; Myers, K.; Ordoukhanian, P.; Head, S.R.; Natarajan, P.; Berenbaum, F.; Sellam, J.; Lotz, M.K. Human-specific duplicate CHRFAM7A gene is associated with more severe osteoarthritis and amplifies pain behaviours. Ann. Rheum. Dis. 2023. [Google Scholar] [CrossRef] [PubMed]
- Baird, A.; Coimbra, R.; Dang, X.; Eliceiri, B.P.; Costantini, T.W. Up-regulation of the human-specific CHRFAM7A gene in inflammatory bowel disease. BBA Clin. 2016, 5, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhang, Y.; Dang, X.; Li, B.; Wang, H.; Gong, S.; Li, S.; Meng, F.; Xing, J.; Li, T.; et al. Up-regulation of the human-specific CHRFAM7A gene protects against renal fibrosis in mice with obstructive nephropathy. J. Cell. Mol. Med. 2023, 27, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Kabbani, N.; Brannan, T.K.; Lin, M.K.; Theiss, M.M.; Hamilton, J.F.; Ecklund, J.M.; Conley, Y.P.; Vodovotz, Y.; Brienza, D.; et al. Association of a Functional Polymorphism in the CHRFAM7A Gene with Inflammatory Response Mediators and Neuropathic Pain after Spinal Cord Injury. J. Neurotrauma 2019, 36, 3026–3033. [Google Scholar] [CrossRef]
- Hone, A.J.; Servent, D.; McIntosh, J.M. α9-containing nicotinic acetylcholine receptors and the modulation of pain. Br. J. Pharm. 2018, 175, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Margiotta, F.; Micheli, L.; Ciampi, C.; Ghelardini, C.; McIntosh, J.M.; Di Cesare Mannelli, L. Conus regius-Derived Conotoxins: Novel Therapeutic Opportunities from a Marine Organism. Mar. Drugs 2022, 20, 773. [Google Scholar] [CrossRef]
- Li, X.; Tae, H.S.; Chu, Y.; Jiang, T.; Adams, D.J.; Yu, R. Medicinal chemistry, pharmacology, and therapeutic potential of α-conotoxins antagonizing the α9α10 nicotinic acetylcholine receptor. Pharmacol. Ther. 2021, 222, 107792. [Google Scholar] [CrossRef]
- Pacini, A.; Micheli, L.; Maresca, M.; Branca, J.J.; McIntosh, J.M.; Ghelardini, C.; Di Cesare Mannelli, L. The α9α10 nicotinic receptor antagonist α-conotoxin RgIA prevents neuropathic pain induced by oxaliplatin treatment. Exp. Neurol. 2016, 282, 37–48. [Google Scholar] [CrossRef]
- Rabik, C.A.; Dolan, M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2007, 33, 9–23. [Google Scholar] [CrossRef]
- Cragg, G.M. Paclitaxel (Taxol): A success story with valuable lessons for natural product drug discovery and development. Med. Res. Rev. 1998, 18, 315–331. [Google Scholar] [CrossRef]
- Song, S.J.; Min, J.; Suh, S.Y.; Jung, S.H.; Hahn, H.J.; Im, S.A.; Lee, J.Y. Incidence of taxane-induced peripheral neuropathy receiving treatment and prescription patterns in patients with breast cancer. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2017, 25, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Staff, N.P.; Cavaletti, G.; Islam, B.; Lustberg, M.; Psimaras, D.; Tamburin, S. Platinum-induced peripheral neurotoxicity: From pathogenesis to treatment. J. Peripher. Nerv. Syst. JPNS 2019, 24, S26–S39. [Google Scholar] [CrossRef] [PubMed]
- Salat, K. Chemotherapy-induced peripheral neuropathy-part 2: Focus on the prevention of oxaliplatin-induced neurotoxicity. Pharmacol. Rep. PR 2020, 72, 508–527. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Kavelaars, A.; Dougherty, P.M.; Heijnen, C.J. Beyond symptomatic relief for chemotherapy-induced peripheral neuropathy: Targeting the source. Cancer 2018, 124, 2289–2298. [Google Scholar] [CrossRef]
- Dyachenko, I.A.; Palikova, Y.A.; Palikov, V.A.; Korolkova, Y.V.; Kazakov, V.A.; Egorova, N.S.; Garifulina, A.I.; Utkin, Y.N.; Tsetlin, V.I.; Kryukova, E.V. α-Conotoxin RgIA and oligoarginine R8 in the mice model alleviate long-term oxaliplatin induced neuropathy. Biochimie 2022, 194, 127–136. [Google Scholar] [CrossRef]
- Li, Z.; Han, X.; Hong, X.; Li, X.; Gao, J.; Zhang, H.; Zheng, A. Lyophilization Serves as an Effective Strategy for Drug Development of the α9α10 Nicotinic Acetylcholine Receptor Antagonist α-Conotoxin GeXIVA[1,2]. Mar. Drugs 2021, 19, 121. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Cinci, L.; Micheli, L.; Zanardelli, M.; Pacini, A.; McIntosh, J.M.; Ghelardini, C. α-conotoxin RgIA protects against the development of nerve injury-induced chronic pain and prevents both neuronal and glial derangement. Pain 2014, 155, 1986–1995. [Google Scholar] [CrossRef]
- Holtman, J.R.; Dwoskin, L.P.; Dowell, C.; Wala, E.P.; Zhang, Z.; Crooks, P.A.; McIntosh, J.M. The novel small molecule α9α10 nicotinic acetylcholine receptor antagonist ZZ-204G is analgesic. Eur. J. Pharm. 2011, 670, 500–508. [Google Scholar] [CrossRef]
- Westlake, S.; Jones, M.; Sharma, K.D.; Xie, J.Y. Letters to the editor: Nicotinic acetylcholine receptor ligands as potential targets for managing neuropathic pain induced by diabetic peripheral neuropathy. eNeurologicalSci 2022, 28, 100416. [Google Scholar] [CrossRef]
- Lips, K.S.; Pfeil, U.; Kummer, W. Coexpression of α 9 and α 10 nicotinic acetylcholine receptors in rat dorsal root ganglion neurons. Neuroscience 2002, 115, 1–5. [Google Scholar] [CrossRef]
- Shelukhina, I.; Paddenberg, R.; Kummer, W.; Tsetlin, V. Functional expression and axonal transport of α7 nAChRs by peptidergic nociceptors of rat dorsal root ganglion. Brain Struct. Funct. 2015, 220, 1885–1899. [Google Scholar] [CrossRef]
- McIntosh, J.M.; Absalom, N.; Chebib, M.; Elgoyhen, A.B.; Vincler, M. α9 nicotinic acetylcholine receptors and the treatment of pain. Biochem. Pharm. 2009, 78, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Vincler, M.; McIntosh, J.M. Targeting the α9α10 nicotinic acetylcholine receptor to treat severe pain. Expert Opin. Ther. Targets 2007, 11, 891–897. [Google Scholar] [CrossRef]
- Moalem, G.; Tracey, D.J. Immune and inflammatory mechanisms in neuropathic pain. Brain Res. Rev. 2006, 51, 240–264. [Google Scholar] [CrossRef]
- Huynh, P.N.; Christensen, S.B.; McIntosh, J.M. RgIA4 Prevention of Acute Oxaliplatin-Induced Cold Allodynia Requires α9-Containing Nicotinic Acetylcholine Receptors and CD3+ T-Cells. Cells 2022, 11, 3561. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.; Haythornthwaite, A.; Berecki, G.; Clark, R.J.; Craik, D.J.; Adams, D.J. Analgesic α-conotoxins Vc1.1 and Rg1A inhibit N-type calcium channels in rat sensory neurons via GABAB receptor activation. J. Neurosci. 2008, 28, 10943–10951. [Google Scholar] [CrossRef] [PubMed]
- Cuny, H.; de Faoite, A.; Huynh, T.G.; Yasuda, T.; Berecki, G.; Adams, D.J. γ-Aminobutyric acid type B (GABAB) receptor expression is needed for inhibition of N-type (Cav2.2) calcium channels by analgesic α-conotoxins. J. Biol. Chem. 2012, 287, 23948–23957. [Google Scholar] [CrossRef]
- Daly, N.L.; Callaghan, B.; Clark, R.J.; Nevin, S.T.; Adams, D.J.; Craik, D.J. Structure and activity of α-conotoxin PeIA at nicotinic acetylcholine receptor subtypes and GABA(B) receptor-coupled N-type calcium channels. J. Biol. Chem. 2011, 286, 10233–10237. [Google Scholar] [CrossRef]
- Huynh, T.G.; Cuny, H.; Slesinger, P.A.; Adams, D.J. Novel mechanism of voltage-gated N-type (Cav2.2) calcium channel inhibition revealed through α-conotoxin Vc1.1 activation of the GABA(B) receptor. Mol. Pharm. 2015, 87, 240–250. [Google Scholar] [CrossRef]
- van Lierop, B.J.; Robinson, S.D.; Kompella, S.N.; Belgi, A.; McArthur, J.R.; Hung, A.; MacRaild, C.A.; Adams, D.J.; Norton, R.S.; Robinson, A.J. Dicarba α-conotoxin Vc1.1 analogues with differential selectivity for nicotinic acetylcholine and GABAB receptors. ACS Chem. Biol. 2013, 8, 1815–1821. [Google Scholar] [CrossRef]
- Callaghan, B.; Adams, D.J. Analgesic α-conotoxins Vc1.1 and RgIA inhibit N-type calcium channels in sensory neurons of α9 nicotinic receptor knockout mice. Channels 2010, 4, 51–54. [Google Scholar] [CrossRef]
- Adams, D.J.; Callaghan, B.; Berecki, G. Analgesic conotoxins: Block and G protein-coupled receptor modulation of N-type (Ca(V) 2.2) calcium channels. Br. J. Pharm. 2012, 166, 486–500. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, A.; Wu, X.; Bony, A.R.; Sadeghi, M.; Huang, Y.; Craik, D.J.; Adams, D.J. ɑO-Conotoxin GeXIVA isomers modulate N-type calcium (CaV 2.2) channels and inwardly-rectifying potassium (GIRK) channels via GABAB receptor activation. J. Neurochem. 2022, 160, 154–171. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Pope, J.E.; Hanes, M.C.; McDowell, G.C. Intrathecal Therapy for Chronic Pain: A Review of Morphine and Ziconotide as Firstline Options. Pain Med. 2019, 20, 784–798. [Google Scholar] [CrossRef]
- Wright, A.B.; Norimatsu, Y.; McIntosh, J.M.; Elmslie, K.S. Limited efficacy of α-conopeptides, Vc1.1 and RgIA, to inhibit sensory neuron Ca(V) current. eNeuro 2015, 2, e0057-14.2015. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Christie, M.J. α9-nicotinic acetylcholine receptors contribute to the maintenance of chronic mechanical hyperalgesia, but not thermal or mechanical allodynia. Mol. Pain 2014, 10, 64. [Google Scholar] [CrossRef]
- Napier, I.A.; Klimis, H.; Rycroft, B.K.; Jin, A.H.; Alewood, P.F.; Motin, L.; Adams, D.J.; Christie, M.J. Intrathecal α-conotoxins Vc1.1, AuIB and MII acting on distinct nicotinic receptor subtypes reverse signs of neuropathic pain. Neuropharmacology 2012, 62, 2202–2207. [Google Scholar] [CrossRef]
- Zheng, N.; Christensen, S.B.; Dowell, C.; Purushottam, L.; Skalicky, J.J.; McIntosh, J.M.; Chou, D.H. Discovery of Methylene Thioacetal-Incorporated α-RgIA Analogues as Potent and Stable Antagonists of the Human α9α10 Nicotinic Acetylcholine Receptor for the Treatment of Neuropathic Pain. J. Med. Chem. 2021, 64, 9513–9524. [Google Scholar] [CrossRef]
- Zhu, X.; Yuan, M.; Wang, H.; Zhangsun, D.; Yu, G.; Che, J.; Luo, S. Novel αO-conotoxin GeXIVA[1,2] Nonaddictive Analgesic with Pharmacokinetic Modelling-Based Mechanistic Assessment. Pharmaceutics 2022, 14, 1789. [Google Scholar] [CrossRef]
- Jin, A.H.; Muttenthaler, M.; Dutertre, S.; Himaya, S.W.A.; Kaas, Q.; Craik, D.J.; Lewis, R.J.; Alewood, P.F. Conotoxins: Chemistry and Biology. Chem. Rev. 2019, 119, 11510–11549. [Google Scholar] [CrossRef] [PubMed]
- Akondi, K.B.; Muttenthaler, M.; Dutertre, S.; Kaas, Q.; Craik, D.J.; Lewis, R.J.; Alewood, P.F. Discovery, synthesis, and structure-activity relationships of conotoxins. Chem. Rev. 2014, 114, 5815–5847. [Google Scholar] [CrossRef]
- Li, L.; Li, Z.; Guo, Y.; Zhang, K.; Mi, W.; Liu, J. Preparation of uniform-sized GeXIVA[1,2]-loaded PLGA microspheres as long-effective release system with high encapsulation efficiency. Drug Deliv. 2022, 29, 2283–2295. [Google Scholar] [CrossRef]
- Bavo, F.; Pallavicini, M.; Pucci, S.; Appiani, R.; Giraudo, A.; Eaton, B.; Lucero, L.; Gotti, C.; Moretti, M.; Whiteaker, P.; et al. From 2-Triethylammonium Ethyl Ether of 4-Stilbenol (MG624) to Selective Small-Molecule Antagonists of Human α9α10 Nicotinic Receptor by Modifications at the Ammonium Ethyl Residue. J. Med. Chem. 2022, 65, 10079–10097. [Google Scholar] [CrossRef]
- Huang, Q.; Chu, X.; Zhang, H.; Yu, S.; Zhang, L.; Zhang, X.; Yu, R.; Guo, C.; Dai, Q. Discovery and Structural and Functional Characterization of a Novel A-Superfamily Conotoxin Targeting α9α10 Nicotinic Acetylcholine Receptor. ACS Chem. Biol. 2022, 17, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
- Hueffer, K.; Khatri, S.; Rideout, S.; Harris, M.B.; Papke, R.L.; Stokes, C.; Schulte, M.K. Rabies virus modifies host behaviour through a snake-toxin like region of its glycoprotein that inhibits neurotransmitter receptors in the CNS. Sci. Rep. 2017, 7, 12818. [Google Scholar] [CrossRef] [PubMed]
- Embregts, C.W.E.; Begeman, L.; Voesenek, C.J.; Martina, B.E.E.; Koopmans, M.P.G.; Kuiken, T.; GeurtsvanKessel, C.H. Street RABV Induces the Cholinergic Anti-inflammatory Pathway in Human Monocyte-Derived Macrophages by Binding to nAChr α7. Front. Immunol. 2021, 12, 622516. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Zhang, A.; Chen, Z.; Zhang, X.; Zhang, R.; Yin, C.; Zhang, J.; Zhang, Y.; Yan, Y. Migration of gastric cancer is suppressed by recombinant Newcastle disease virus (rL-RVG) via regulating α7-nicotinic acetylcholine receptors/ERK- EMT. BMC Cancer 2019, 19, 976. [Google Scholar] [CrossRef]
- Callaway, H.M.; Zyla, D.; Larrous, F.; de Melo, G.D.; Hastie, K.M.; Avalos, R.D.; Agarwal, A.; Corti, D.; Bourhy, H.; Saphire, E.O. Structure of the rabies virus glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Sci. Adv. 2022, 8, eabp9151. [Google Scholar] [CrossRef]
- Neri, P.; Bracci, L.; Rustici, M.; Santucci, A. Sequence homology between HIV gp120, rabies virus glycoprotein, and snake venom neurotoxins. Is the nicotinic acetylcholine receptor an HIV receptor? Arch. Virol. 1990, 114, 265–269. [Google Scholar] [CrossRef]
- Bracci, L.; Lozzi, L.; Rustici, M.; Neri, P. Binding of HIV-1 gp120 to the nicotinic receptor. FEBS Lett. 1992, 311, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Vélez, M.; Lasalde-Dominicci, J.A. The Cholinergic Anti-Inflammatory Response and the Role of Macrophages in HIV-Induced Inflammation. Int. J. Mol. Sci. 2018, 19, 1473. [Google Scholar] [CrossRef] [PubMed]
- Capó-Vélez, C.M.; Delgado-Vélez, M.; Báez-Pagán, C.A.; Lasalde-Dominicci, J.A. Nicotinic Acetylcholine Receptors in HIV: Possible Roles During HAND and Inflammation. Cell. Mol. Neurobiol. 2018, 38, 1335–1348. [Google Scholar] [CrossRef]
- Farsalinos, K.; Barbouni, A.; Niaura, R. Systematic review of the prevalence of current smoking among hospitalized COVID-19 patients in China: Could nicotine be a therapeutic option? Intern. Emerg. Med. 2020, 15, 845–852. [Google Scholar] [CrossRef]
- Fudim, M.; Qadri, Y.J.; Ghadimi, K.; MacLeod, D.B.; Molinger, J.; Piccini, J.P.; Whittle, J.; Wischmeyer, P.E.; Patel, M.R.; Ulloa, L. Implications for Neuromodulation Therapy to Control Inflammation and Related Organ Dysfunction in COVID-19. J. Cardiovasc. Transl. Res. 2020, 13, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Kloc, M.; Ghobrial, R.M.; Kubiak, J.Z. How nicotine can inhibit cytokine storm in the lungs and prevent or lessen the severity of COVID-19 infection? Immunol. Lett. 2020, 224, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Lupacchini, L.; Maggi, F.; Tomino, C.; De Dominicis, C.; Mollinari, C.; Fini, M.; Bonassi, S.; Merlo, D.; Russo, P. Nicotine Changes Airway Epithelial Phenotype and May Increase the SARS-CoV-2 Infection Severity. Molecules 2020, 26, 101. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Goniewicz, M.L.; Halpern-Felsher, B.; Krishnan-Sarin, S.; Ling, P.M.; O’Connor, R.J.; Pentz, M.A.; Robertson, R.M.; Bhatnagar, A. Tobacco product use and the risks of SARS-CoV-2 infection and COVID-19: Current understanding and recommendations for future research. Lancet Respir. Med. 2022, 10, 900–915. [Google Scholar] [CrossRef]
- Clift, A.K.; von Ende, A.; Tan, P.S.; Sallis, H.M.; Lindson, N.; Coupland, C.A.C.; Munafò, M.R.; Aveyard, P.; Hippisley-Cox, J.; Hopewell, J.C. Smoking and COVID-19 outcomes: An observational and Mendelian randomisation study using the UK Biobank cohort. Thorax 2022, 77, 65–73. [Google Scholar] [CrossRef]
- Maggi, F.; Rosellini, A.; Spezia, P.G.; Focosi, D.; Macera, L.; Lai, M.; Pistello, M.; de Iure, A.; Tomino, C.; Bonassi, S.; et al. Nicotine upregulates ACE2 expression and increases competence for SARS-CoV-2 in human pneumocytes. ERJ Open Res. 2021, 7, 00713–02020. [Google Scholar] [CrossRef]
- Russo, P.; Bonassi, S.; Giacconi, R.; Malavolta, M.; Tomino, C.; Maggi, F. COVID-19 and smoking: Is nicotine the hidden link? Eur. Respir. J. 2020, 55, 2001116. [Google Scholar] [CrossRef] [PubMed]
- Allahverdi Khani, M.; SalehiRad, M.; Darbeheshti, S.; Motaghinejad, M. Survival of COVID-19 patients requires precise immune regulation: The hypothetical immunoprotective role of nicotinic agonists. Med. Hypotheses 2020, 143, 109871. [Google Scholar] [CrossRef] [PubMed]
- Ballestero, J.A.; Plazas, P.V.; Kracun, S.; Gomez-Casati, M.E.; Taranda, J.; Rothlin, C.V.; Katz, E.; Millar, N.S.; Elgoyhen, A.B. Effects of quinine, quinidine, and chloroquine on α9α10 nicotinic cholinergic receptors. Mol. Pharm. 2005, 68, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Changeux, J.P.; Amoura, Z.; Rey, F.A.; Miyara, M. A nicotinic hypothesis for COVID-19 with preventive and therapeutic implications. Comptes Rendus Biol. 2020, 343, 33–39. [Google Scholar] [CrossRef]
- Farsalinos, K.; Eliopoulos, E.; Leonidas, D.D.; Papadopoulos, G.E.; Tzartos, S.; Poulas, K. Nicotinic Cholinergic System and COVID-19: In Silico Identification of an Interaction between SARS-CoV-2 and Nicotinic Receptors with Potential Therapeutic Targeting Implications. Int. J. Mol. Sci. 2020, 21, 5807. [Google Scholar] [CrossRef]
- Lagoumintzis, G.; Chasapis, C.T.; Alexandris, N.; Kouretas, D.; Tzartos, S.; Eliopoulos, E.; Farsalinos, K.; Poulas, K. Nicotinic cholinergic system and COVID-19: In silico identification of interactions between α7 nicotinic acetylcholine receptor and the cryptic epitopes of SARS-Co-V and SARS-CoV-2 Spike glycoproteins. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2021, 149, 112009. [Google Scholar] [CrossRef]
- Oliveira, A.S.F.; Ibarra, A.A.; Bermudez, I.; Casalino, L.; Gaieb, Z.; Shoemark, D.K.; Gallagher, T.; Sessions, R.B.; Amaro, R.E.; Mulholland, A.J. A potential interaction between the SARS-CoV-2 spike protein and nicotinic acetylcholine receptors. Biophys. J. 2021, 120, 983–993. [Google Scholar] [CrossRef]
- Lykhmus, O.; Kalashnyk, O.; Koval, L.; Krynina, O.; Komisarenko, S.; Skok, M. Immunization with 674-685 fragment of SARS-CoV-2 spike protein induces neuroinflammation and impairs episodic memory of mice. Biochem. Biophys. Res. Commun. 2022, 622, 57–63. [Google Scholar] [CrossRef]
- Chrestia, J.F.; Oliveira, A.S.; Mulholland, A.J.; Gallagher, T.; Bermudez, I.; Bouzat, C. A Functional Interaction Between Y674-R685 Region of the SARS-CoV-2 Spike Protein and the Human α7 Nicotinic Receptor. Mol. Neurobiol. 2022, 59, 6076–6090. [Google Scholar] [CrossRef]
- Godellas, N.E.; Cymes, G.D.; Grosman, C. An experimental test of the nicotinic hypothesis of COVID-19. Proc. Natl. Acad. Sci. USA 2022, 119, e2204242119. [Google Scholar] [CrossRef]
| Immune Cell Type | nAChR Subtype | Agonists and Antagonists | Biological Effect | References |
|---|---|---|---|---|
| T-cells | α7 | Nicotine | Increased FasL expression and suppressed the development of CD4+ T-cells | [120] |
| α7 | GTS-21 | Enhanced the differentiation and proliferation of Tregs and effector T-cells | [122] | |
| α7 | Nicotine | Reduced T-cell proliferation and Th1 cytokine production | [123] | |
| α7 | Nicotine | Increased expression of CTLA-4 and Foxp3 | [124] | |
| α9 | Nicotine | Suppressed TGF-β1 | [125] | |
| α7 | GTS-21 | Promoted transcription of HIV-1 proviral DNA, increased reactive oxygen species, decreased DUSP1 and DUSP6; increased p38 MAPK phosphorylation | [126] | |
| B cells | α7 | MLA | Enhanced proliferation | [131] |
| Dendritic cells | α7 | Nicotine | Reduced production of IL-1β, IL-10, TNF-α and IL-12 | [121] |
| α7 | SLURP-1 | Attenuated cell proliferation | [134] | |
| α7 | GTS-21 | Reduced secretion of pro-inflammatory cytokines and downregulation of the CD80 and MHC II expression | [135] | |
| α7 | GTS-21 | Suppressed APC-dependent differentiation of CD4+ T-cells | [136] | |
| n.d. * | Acetylcholine | Stimulated OX40L expression, induced Th2 profile, increased production of IL-4, IL-5, and IL-13 by CD4+ T-cells | [137] | |
| n.d. | Nicotine | Increased expression of CD86 and production of less IL-12, modulation of the Th1/Th2 balance towards Th2 | [138] | |
| Monocytes | α9α10 | Epibatidine | Inhibition of pro-inflammatory cytokines | [140] |
| α7 | Nicotine and GSK1345038 | Inhibition of TNF production | [141] | |
| α7 | Nicotine | Inhibition of IL-18-enhanced expression of ICAM-1, B7.2 and CD40 and production of IL-12, IFN-γ and TNF-α | [142] | |
| α7 | GTS-21 | Suppressed TNF-α production | [144] | |
| n.d. | Nicotine | Inhibition of the production of TNF-α, IL-1β and IL-12 and stimulation of the IL-10 secretion | [145] | |
| α9α10 and α7 | Nicotine, acetylcholine, phosphocholine | Inhibition of ATP-induced release of IL-1β | [148,149] | |
| Macrophages | n.d. | Acetylcholine | Attenuation of the release of TNF, IL-1β, IL-6 and IL-18, but not IL-10 | [99] |
| α7 | PNU 282987 | Increased expression of HLA-DR, CD11b and CD54; decreased expression of CD14 and of IL-10 production | [156] | |
| α7 | Acetylcholine and PNU 282987 | Inhibition of MMP-9 production and cell migration | [157] | |
| GTS-21 | Inhibition of TNF-α production | [160] | ||
| Neutrophils and granulocytes | α7 and α9 | Nicotine and acetylcholine | Modified respiratory burst and affected neutrophil adhesion | [168] |
| α9 | Nicotine and choline | Increased cell adhesion and decreased reactive oxygen species production | [169] | |
| n.d. | Nicotine | Release of NET, activation of Akt and PAD4 | [170] | |
| Mast cells | α7 and α9 | Nicotine | Inhibition of C4 leukotriene (LTC4), TNF-α, and IL-1β | [163] |
| Acetylcholine | Induced release of histamine | [164] | ||
| Natural killer cells | α7 | PNU 282987 | Suppressed NKG2D expression, reduced cytotoxic activity and IFN-γ production | [173] |
| n.d. | Nicotine | Impairment of the ability of NK cells to kill cancer cells and release cytokines; decreased the expression of NKG2D, Ly49I and cell proliferation | [175,176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shelukhina, I.; Siniavin, A.; Kasheverov, I.; Ojomoko, L.; Tsetlin, V.; Utkin, Y. α7- and α9-Containing Nicotinic Acetylcholine Receptors in the Functioning of Immune System and in Pain. Int. J. Mol. Sci. 2023, 24, 6524. https://doi.org/10.3390/ijms24076524
Shelukhina I, Siniavin A, Kasheverov I, Ojomoko L, Tsetlin V, Utkin Y. α7- and α9-Containing Nicotinic Acetylcholine Receptors in the Functioning of Immune System and in Pain. International Journal of Molecular Sciences. 2023; 24(7):6524. https://doi.org/10.3390/ijms24076524
Chicago/Turabian StyleShelukhina, Irina, Andrei Siniavin, Igor Kasheverov, Lucy Ojomoko, Victor Tsetlin, and Yuri Utkin. 2023. "α7- and α9-Containing Nicotinic Acetylcholine Receptors in the Functioning of Immune System and in Pain" International Journal of Molecular Sciences 24, no. 7: 6524. https://doi.org/10.3390/ijms24076524
APA StyleShelukhina, I., Siniavin, A., Kasheverov, I., Ojomoko, L., Tsetlin, V., & Utkin, Y. (2023). α7- and α9-Containing Nicotinic Acetylcholine Receptors in the Functioning of Immune System and in Pain. International Journal of Molecular Sciences, 24(7), 6524. https://doi.org/10.3390/ijms24076524






