Sitagliptin Mitigates Diabetic Nephropathy in a Rat Model of Streptozotocin-Induced Type 2 Diabetes: Possible Role of PTP1B/JAK-STAT Pathway
Abstract
:1. Introduction
2. Results
2.1. Sitagliptin Reverses Hyperglycemia and Weight Loss and Alleviates DN in Diabetic Rats
2.1.1. Effects on Blood Glucose Level
2.1.2. Effects on Kidney-Weight-to-Body-Weight Ratio
2.1.3. Effects on Serum Creatinine, Blood Urea Nitrogen (BUN), and Urea Levels
2.2. Sitagliptin Alleviates STZ-Induced Alterations in Inflammatory Biomarkers in Experimental Diabetic Rats
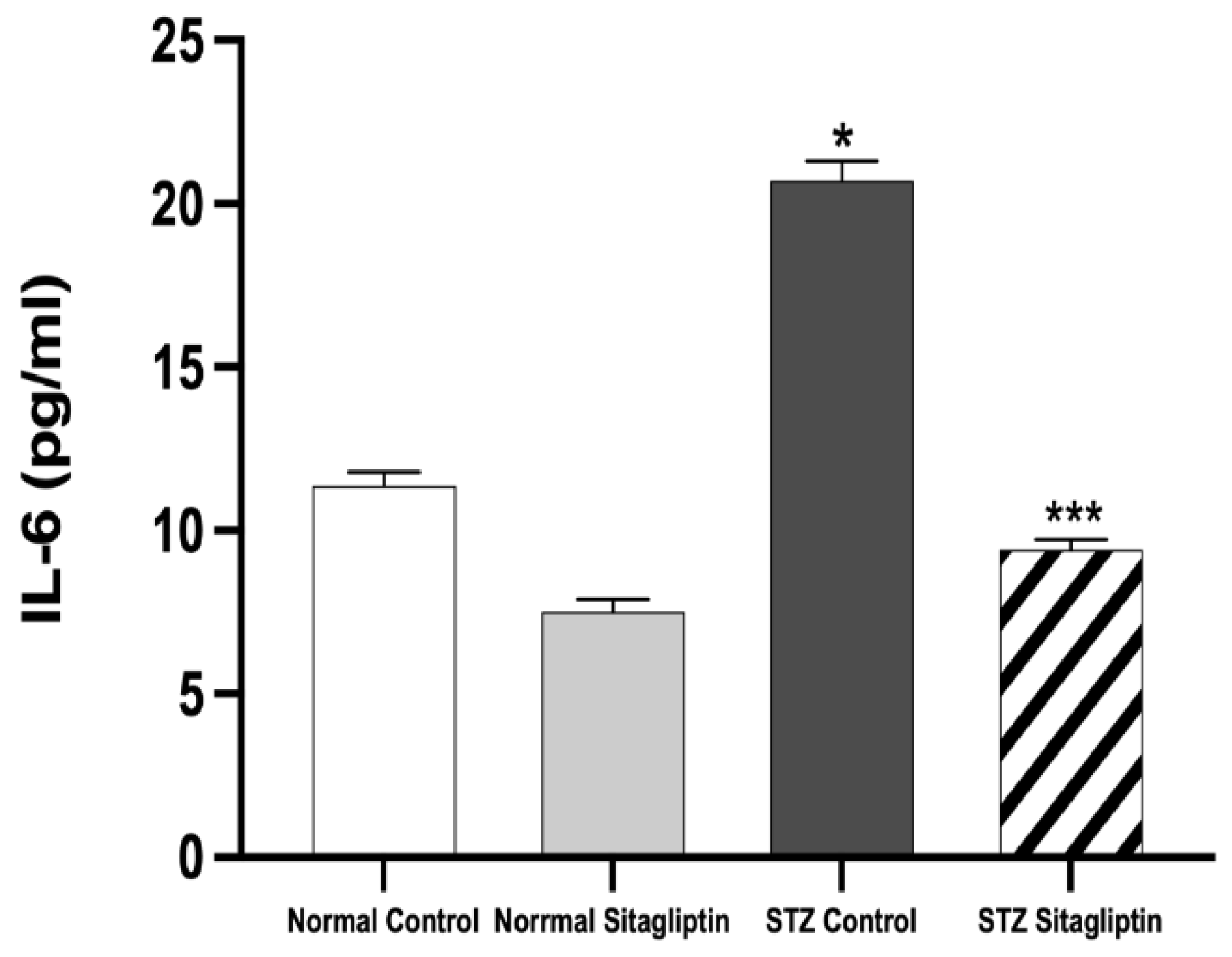
2.2.1. Effects on IL-6
2.2.2. Effects on TNF-α Levels
2.3. Effects of Sitagliptin on Protein Expression Levels in Experimental Diabetic Rats Using Western Blot Analysis
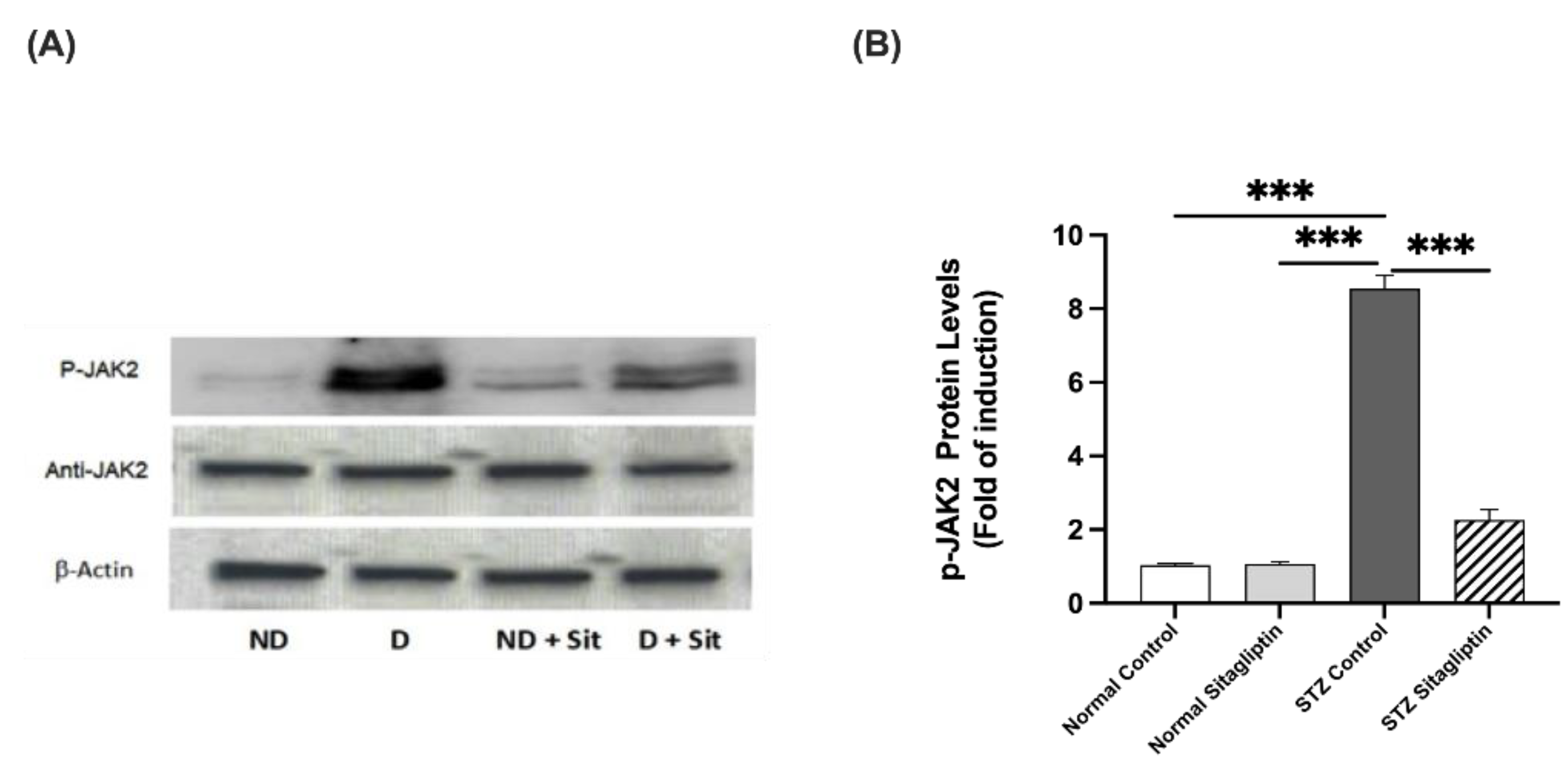
2.3.1. Effects on P-JAK2 Expression
2.3.2. Effects on P-STAT3 Expression
2.3.3. Effects on PTP1B Expression
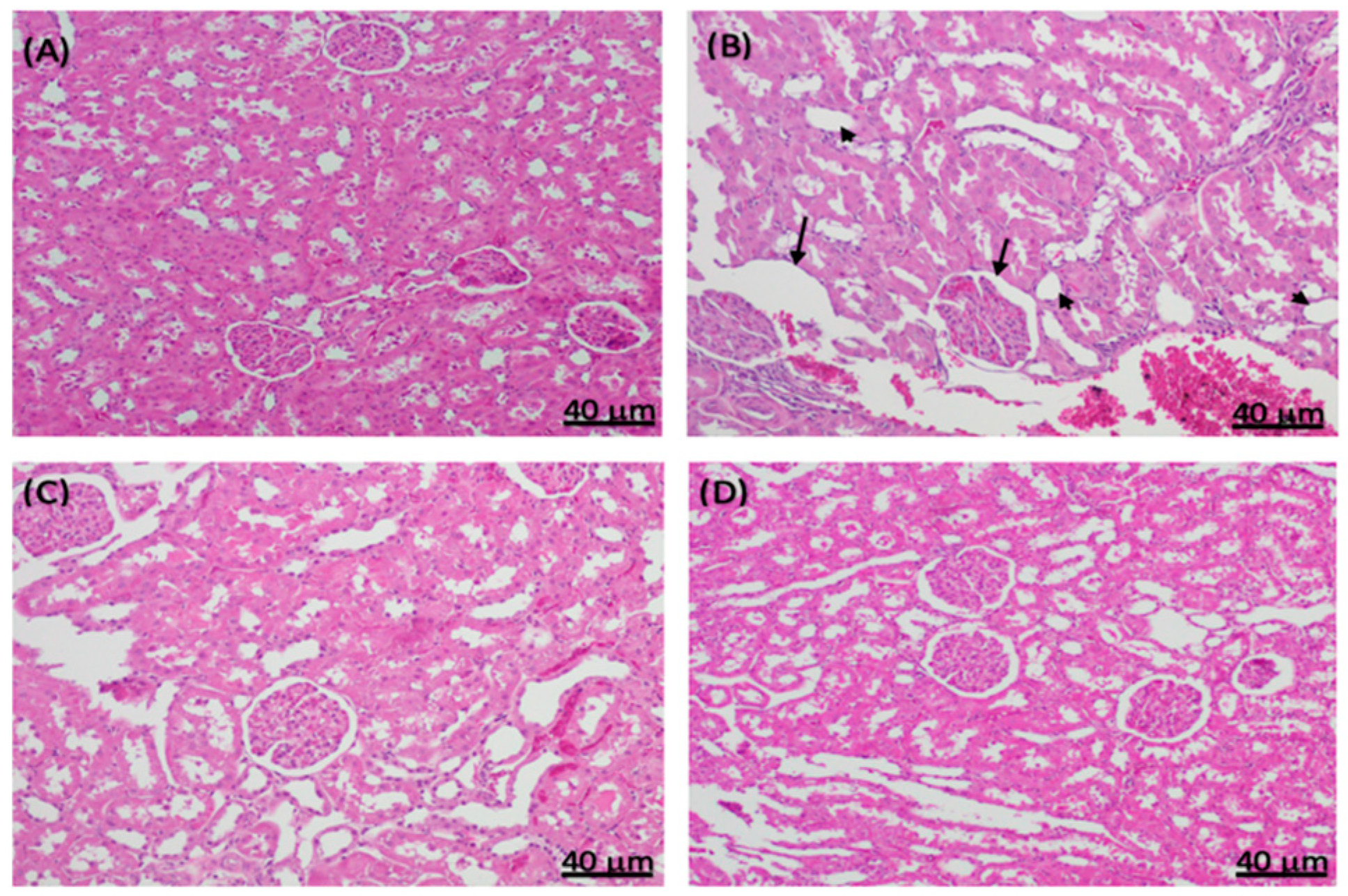
2.4. Histopathological Changes in Experimental Diabetic Rat Kidneys Treated with Sitagliptin
3. Discussion
4. Materials and Methods
4.1. Drugs, Chemicals, and Antibodies
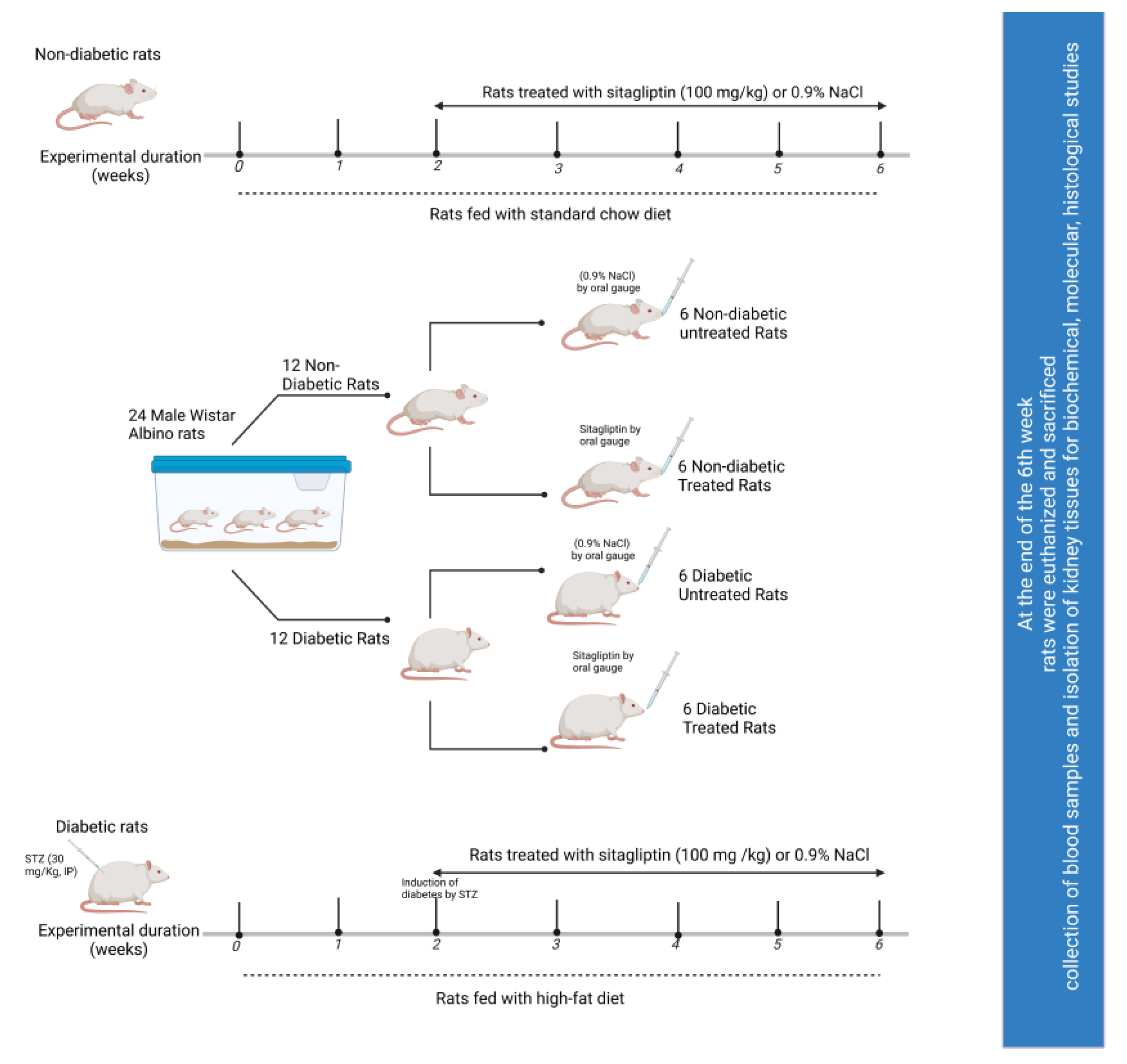
4.2. Experimental Animals
4.3. Induction of Diabetes
4.4. Experimental Design and Measurements
4.5. Measurement of Serum Glucose
4.6. Determination of DN Biomarkers
4.7. Assessment of Inflammatory Biomarkers
4.8. Western Blot Analysis
4.9. Histological Examination
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Boer, I.H.; Sibley, S.D.; Kestenbaum, B.; Sampson, J.N.; Young, B.; Cleary, P.A.; Steffes, M.W.; Weiss, N.S.; Brunzell, J.D. Central obesity, incident microalbuminuria, and change in creatinine clearance in the epidemiology of diabetes interventions and complications study. J. Am. Soc. Nephrol. 2007, 18, 235–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santilli, F.; D’Ardes, D.; Davì, G. Oxidative stress in chronic vascular disease: From prediction to prevention. Vascul. Pharmacol. 2015, 74, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Sahakyan, G.; Vejux, A.; Sahakyan, N. The Role of Oxidative Stress-Mediated Inflammation in the Development of T2DM-Induced Diabetic Nephropathy: Possible Preventive Action of Tannins and Other Oligomeric Polyphenols. Molecules 2022, 27, 9035. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Luis-Rodríguez, D.; Martín-Núñez, E.; Tagua, V.G.; Hernández-Carballo, C.; Ferri, C.; Rodríguez-Rodríguez, A.E.; Mora-Fernández, C.; Navarro-González, J.F. Inflammatory Targets in Diabetic Nephropathy. J. Clin. Med. 2020, 9, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przezak, A.; Bielka, W.; Pawlik, A. Incretins in the Therapy of Diabetic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 12312. [Google Scholar] [CrossRef] [PubMed]
- Mima, A. Incretin-Based Therapy for Prevention of Diabetic Vascular Complications. J. Diabetes Res. 2016, 2016, 1379274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matteucci, E.; Giampietro, O. Dipeptidyl peptidase-4 (CD26): Knowing the function before inhibiting the enzyme. Curr. Med. Chem. 2009, 16, 2943–2951. [Google Scholar] [CrossRef]
- Jo, C.H.; Kim, S.; Park, J.S.; Kim, G.H. Anti-Inflammatory Action of Sitagliptin and Linagliptin in Doxorubicin Nephropathy. Kidney Blood Press. Res. 2018, 43, 987–999. [Google Scholar] [CrossRef]
- Panchapakesan, U.; Pollock, C. The Role of Dipeptidyl Peptidase—4 Inhibitors in Diabetic Kidney Disease. Front. Immunol. 2015, 6, 443. [Google Scholar] [CrossRef] [Green Version]
- Kanasaki, K. The role of renal dipeptidyl peptidase-4 in kidney disease: Renal effects of dipeptidyl peptidase-4 inhibitors with a focus on linagliptin. Clin. Sci. 2018, 132, 489–507. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.H.; Kim, S.; Park, E.G.; Kim, S.G.; Hahn, S.; Kim, N.H. Effects of Dipeptidyl Peptidase-4 Inhibitors on Renal Outcomes in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Endocrinol. Metab. 2019, 34, 80–92. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, G.; Chen, X.; Wei, T.; Liu, C.; Chen, C.; Gong, Y.; Wei, Q. Sitagliptin ameliorates diabetic nephropathy by blocking TGF-β1/Smad signaling pathway. Int. J. Mol. Med. 2018, 41, 2784–2792. [Google Scholar] [CrossRef] [Green Version]
- Cho, E.H.; Kim, S.W. Soluble Dipeptidyl Peptidase-4 Levels Are Associated with Decreased Renal Function in Patients with Type 2 Diabetes Mellitus. Diabetes Metab. J. 2019, 43, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Feldhammer, M.; Uetani, N.; Miranda-Saavedra, D.; Tremblay, M.L. PTP1B: A simple enzyme for a complex world. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Hsu, M.F.; Bettaieb, A.; Koike, S.; Mello, A.; Calvo-Rubio, M.; Villalba, J.M.; Haj, F.G. Protein tyrosine phosphatase 1B deficiency in podocytes mitigates hyperglycemia-induced renal injury. Metabolism 2017, 76, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Pike, K.A.; Tremblay, M.L. TC-PTP and PTP1B: Regulating JAK-STAT signaling, controlling lymphoid malignancies. Cytokine 2016, 82, 52–57. [Google Scholar] [CrossRef]
- Rico-Fontalvo, J.; Aroca, G.; Cabrales, J.; Daza-Arnedo, R.; Yánez-Rodríguez, T.; Martínez-Ávila, M.C.; Uparella-Gulfo, I.; Raad-Sarabia, M. Molecular Mechanisms of Diabetic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 8668. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The JAK/STAT signaling pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef] [Green Version]
- Malemud, J.C.; Pearlman, E. Targeting JAK/STAT Signaling Pathway in Inflammatory Diseases. Curr. Signal Transduct. Ther. 2009, 4, 201–221. [Google Scholar] [CrossRef]
- Marrero, M.B.; Banes-Berceli, A.K.; Stern, D.M.; Eaton, D.C. Role of the JAK/STAT signaling pathway in diabetic nephropathy. Am. J. Physiol. Renal. Physiol. 2006, 290, F762–F768. [Google Scholar] [CrossRef]
- Zheng, C.; Huang, L.; Luo, W.; Yu, W.; Hu, X.; Guan, X.; Cai, Y.; Zou, C.; Yin, H.; Xu, Z.; et al. Inhibition of STAT3 in tubular epithelial cells prevents kidney fibrosis and nephropathy in STZ-induced diabetic mice. Cell Death Dis. 2019, 10, 848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhou, H.; Li, Y.; Han, L.; Song, M.; Chen, F.; Shang, G.; Wang, D.; Wang, Z.; Zhang, W.; et al. PTPN2 improved renal injury and fibrosis by suppressing STAT-induced inflammation in early diabetic nephropathy. J. Cell. Mol. Med. 2019, 23, 4179–4195. [Google Scholar] [CrossRef] [PubMed]
- Lone, A.H.; Behl, T.; Kumar, A.; Makkar, R.; Nijhawan, P.; Redhu, S.; Sharma, H.; Jaglan, D.; Goyal, A. Renoprotective potential of dimethyl fumarate in streptozotocin induced diabetic nephropathy in Wistar rats. Obes. Med. 2020, 18, 100237. [Google Scholar] [CrossRef]
- McGrath, K.; Edi, R. Diabetic Kidney Disease: Diagnosis, Treatment, and Prevention. Am. Fam. Physician 2019, 99, 751–759. [Google Scholar]
- Selby, N.M.; Taal, M.W. An updated overview of diabetic nephropathy: Diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes. Metab. 2020, 22 (Suppl. 1), 3–15. [Google Scholar] [CrossRef]
- Sawaf, H.; Thomas, G.; Taliercio, J.J.; Nakhoul, G.; Vachharajani, T.J.; Mehdi, A. Therapeutic Advances in Diabetic Nephropathy. J. Clin. Med. 2022, 11, 378. [Google Scholar] [CrossRef]
- Matoba, K.; Sekiguchi, K.; Nagai, Y.; Takeda, Y.; Takahashi, H.; Yokota, T.; Utsunomiya, K.; Nishimura, R. Renal ROCK Activation and Its Pharmacological Inhibition in Patients with Diabetes. Front. Pharmacol. 2021, 12, 738121. [Google Scholar] [CrossRef]
- Gojo, A.; Utsunomiya, K.; Taniguchi, K.; Yokota, T.; Ishizawa, S.; Kanazawa, Y.; Kurata, H.; Tajima, N. The Rho-kinase inhibitor, fasudil, attenuates diabetic nephropathy in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2007, 568, 242–247. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Norouzian, D.; Mehrabi, M.R.; Jamshidi, S.; Farhangi, A.; Verdi, A.A.; Mofidian, S.M.; Rad, B.L. Induction of diabetes by Streptozotocin in rats. Indian J. Clin. Biochem. 2007, 22, 60–64. [Google Scholar] [CrossRef] [Green Version]
- Kraynak, A.R.; Storer, R.D.; Jensen, R.D.; Kloss, M.W.; Soper, K.A.; Clair, J.H.; Deluca, J.G.; Nichols, W.W.; Eydelloth, R.S. Extent and Persistence of Streptozotocin-Induced DNA Damage and Cell Proliferation in Rat Kidney as Determined by in Vivo Alkaline Elution and BrdUrd Labeling Assays. Toxicol. Appl. Pharmacol. 1995, 135, 279–286. [Google Scholar] [CrossRef]
- King, G.L.; Loeken, M.R. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem. Cell Biol. 2004, 122, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, H.; Heidari, R.; Nejati, V.; Ilkhanipoor, M. Aqueous extract of Berberis integerrima root improves renal dysfunction in streptozotocin induced diabetic rats. Avicenna J. Phytomed. 2013, 3, 82–90. [Google Scholar] [PubMed]
- Indu, R.; Adhikari, A.; Basak, P.; Sur, T. Effect of concomitant therapy of anti-diabetics and hypolipidemics on biochemical and histological parameters in animal models. Asian J. Pharm. Pharmacol. 2019, 5, 771–778. [Google Scholar] [CrossRef]
- Zafar, M.; Naqvi, S.N.-U. Effects of STZ-Induced Diabetes on the Relative Weights of Kidney, Liver and Pancreas in Albino Rats: A Comparative Study. Int. J. Morphol. 2010, 28, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Jia, Q.; Yang, R.; Liu, X.F.; Ma, S.F.; Wang, L. Genistein attenuates renal fibrosis in streptozotocin-induced diabetic rats. Mol. Med. Rep. 2019, 19, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Avogaro, A.; Fadini, G.P. The effects of dipeptidyl peptidase-4 inhibition on microvascular diabetes complications. Diabetes Care 2014, 37, 2884–2894. [Google Scholar] [CrossRef] [Green Version]
- Scherberich, J.E.; Wiemer, J.; Schoeppe, W. Biochemical and immunological properties of urinary angiotensinase A and dipeptidylaminopeptidase IV. Their use as markers in patients with renal cell injury. Eur. J. Clin. Chem. Clin. Biochem. 1992, 30, 663–668. [Google Scholar]
- Sun, A.L.; Deng, J.T.; Guan, G.J.; Chen, S.H.; Liu, Y.T.; Cheng, J.; Li, Z.W.; Zhuang, X.H.; Sun, F.D.; Deng, H.P. Dipeptidyl peptidase-IV is a potential molecular biomarker in diabetic kidney disease. Diab. Vasc. Dis. Res. 2012, 9, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Haluzik, M.; Frolík, J.; Rychlík, I. Renal Effects of DPP-4 Inhibitors: A Focus on Microalbuminuria. Int. J. Endocrinol. 2013, 2013, 895102. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.T.; Wallace, C.G.; Yang, C.C.; Chen, C.H.; Chen, K.H.; Sung, P.H.; Chen, Y.L.; Chai, H.T.; Chung, S.Y.; Chua, S.; et al. DPP-4 enzyme deficiency protects kidney from acute ischemia-reperfusion injury: Role for remote intermittent bowel ischemia-reperfusion preconditioning. Oncotarget 2017, 8, 54821–54837. [Google Scholar] [CrossRef] [Green Version]
- Qiu, D.D.; Liu, J.; Shi, J.S.; An, Y.; Ge, Y.C.; Zhou, M.L.; Jiang, S. Renoprotection Provided by Dipeptidyl Peptidase-4 Inhibitors in Combination with Angiotensin Receptor Blockers in Patients with Type 2 Diabetic Nephropathy. Chin. Med. J. 2018, 131, 2658–2665. [Google Scholar] [CrossRef] [PubMed]
- Hattori, S. Sitagliptin reduces albuminuria in patients with type 2 diabetes. Endocr. J. 2011, 58, 69–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, E.V.; Russo, G.; Giandalia, A.; Viazzi, F.; Pontremoli, R.; De Cosmo, S. GLP-1 Receptor Agonists and Kidney Protection. Medicina (Kaunas) 2019, 55, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arakawa, M.; Mita, T.; Azuma, K.; Ebato, C.; Goto, H.; Nomiyama, T.; Fujitani, Y.; Hirose, T.; Kawamori, R.; Watada, H. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes 2010, 59, 1030–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, C.; Mega, C.; Gonçalves, A.; Rodrigues-Santos, P.; Teixeira-Lemos, E.; Teixeira, F.; Fontes-Ribeiro, C.; Reis, F.; Fernandes, R. Sitagliptin prevents inflammation and apoptotic cell death in the kidney of type 2 diabetic animals. Mediat. Inflamm. 2014, 2014, 538737. [Google Scholar] [CrossRef] [Green Version]
- Jensen, E.P.; Poulsen, S.S.; Kissow, H.; Holstein-Rathlou, N.H.; Deacon, C.F.; Jensen, B.L.; Holst, J.J.; Sorensen, C.M. Activation of GLP-1 receptors on vascular smooth muscle cells reduces the autoregulatory response in afferent arterioles and increases renal blood flow. Am. J. Physiol. Renal Physiol. 2015, 308, F867–F877. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Hu, L.; Chen, Y.; Fu, T.; Jiang, T.; Jiang, A.; You, X. Sitagliptin improves renal function in diabetic nephropathy in male Sprague Dawley rats through upregulating heme oxygenase-1 expression. Endocrine 2019, 63, 70–78. [Google Scholar] [CrossRef]
- Makdissi, A.; Ghanim, H.; Vora, M.; Green, K.; Abuaysheh, S.; Chaudhuri, A.; Dhindsa, S.; Dandona, P. Sitagliptin exerts an antinflammatory action. J. Clin. Endocrinol. Metab. 2012, 97, 3333–3341. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Biehl, A.; Gadina, M.; Hasni, S.; Schwartz, D.M. JAK-STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Drugs 2017, 77, 521–546. [Google Scholar] [CrossRef]
- Brooks, A.J.; Putoczki, T. JAK-STAT Signalling Pathway in Cancer. Cancers 2020, 12, 1971. [Google Scholar] [CrossRef]
- Alunno, A.; Padjen, I.; Fanouriakis, A.; Boumpas, D.T. Pathogenic and Therapeutic Relevance of JAK/STAT Signaling in Systemic Lupus Erythematosus: Integration of Distinct Inflammatory Pathways and the Prospect of Their Inhibition with an Oral Agent. Cells 2019, 8, 898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recio, C.; Lazaro, I.; Oguiza, A.; Lopez-Sanz, L.; Bernal, S.; Blanco, J.; Egido, J.; Gomez-Guerrero, C. Suppressor of Cytokine Signaling-1 Peptidomimetic Limits Progression of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2017, 28, 575–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, S.S.A.; Salama, M.A.; Selima, E.; Shehata, R.R. Sitagliptin and tofacitinib ameliorate adjuvant induced arthritis via modulating the cross talk between JAK/STAT and TLR-4/NF-κB signaling pathways. Life Sci. 2020, 260, 118261. [Google Scholar] [CrossRef] [PubMed]
- Zabolotny, J.M.; Kim, Y.B.; Welsh, L.A.; Kershaw, E.E.; Neel, B.G.; Kahn, B.B. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J. Biol. Chem. 2008, 283, 14230–14241. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Sun, Y.; Berthelet, J.; Bui, L.C.; Xu, X.; Viguier, M.; Dupret, J.M.; Deshayes, F.; Rodrigues Lima, F. Biochemical, Enzymatic, and Computational Characterization of Recurrent Somatic Mutations of the Human Protein Tyrosine Phosphatase PTP1B in Primary Mediastinal B Cell Lymphoma. Int. J. Mol. Sci. 2022, 23, 7060. [Google Scholar] [CrossRef] [PubMed]
- Teimouri, M.; Hosseini, H.; ArabSadeghabadi, Z.; Babaei-Khorzoughi, R.; Gorgani-Firuzjaee, S.; Meshkani, R. The role of protein tyrosine phosphatase 1B (PTP1B) in the pathogenesis of type 2 diabetes mellitus and its complications. J. Physiol. Biochem. 2022, 78, 307–322. [Google Scholar] [CrossRef]
- Huang, T.; Li, X.; Wang, F.; Lu, L.; Hou, W.; Zhu, M.; Miao, C. The CREB/KMT5A complex regulates PTP1B to modulate high glucose-induced endothelial inflammatory factor levels in diabetic nephropathy. Cell Death Dis. 2021, 12, 333. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, C.; Wang, M.; Fei, X.; Zhao, N. TRIM18-Regulated STAT3 Signaling Pathway via PTP1B Promotes Renal Epithelial-Mesenchymal Transition, Inflammation, and Fibrosis in Diabetic Kidney Disease. Front. Physiol. 2021, 12, 709506. [Google Scholar] [CrossRef]
- Tsunekawa, T.; Banno, R.; Mizoguchi, A.; Sugiyama, M.; Tominaga, T.; Onoue, T.; Hagiwara, D.; Ito, Y.; Iwama, S.; Goto, M.; et al. Deficiency of PTP1B Attenuates Hypothalamic Inflammation via Activation of the JAK2-STAT3 Pathway in Microglia. EBioMedicine 2017, 16, 172–183. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhu, Q.; Ye, Q.; Wang, J.; Dong, Q.; Chen, Y.; Wang, M.; Fu, Y.; Wu, R.; Wu, T. STAT3 Suppresses Cardiomyocytes Apoptosis in CVB3-Induced Myocarditis Via Survivin. Front. Pharmacol. 2020, 11, 613883. [Google Scholar] [CrossRef]
- Shawky, L.M.; Morsi, A.A.; El Bana, E.; Hanafy, S.M. The Biological Impacts of Sitagliptin on the Pancreas of a Rat Model of Type 2 Diabetes Mellitus: Drug Interactions with Metformin. Biology 2019, 9, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.; Choi, S.H.; Shin, H.; Cho, B.J.; Park, H.S.; Ahn, B.Y.; Kang, S.M.; Yoon, J.W.; Jang, H.C.; Kim, Y.B.; et al. Correction: Effect of a Dipeptidyl Peptidase-IV Inhibitor. PLoS ONE 2012, 7, e35007. [Google Scholar] [CrossRef]
- Forest, T.; Holder, D.; Smith, A.; Cunningham, C.; Yao, X.; Dey, M.; Frederick, C.; Prahalada, S. Characterization of the exocrine pancreas in the male Zucker diabetic fatty rat model of type 2 diabetes mellitus following 3 months of treatment with sitagliptin. Endocrinology 2014, 155, 783–792. [Google Scholar] [CrossRef] [Green Version]
- Pingree, S.D.; Simmonds, P.L.; Woods, J.S. Effects of 2,3-dimercapto-1-propanesulfonic acid (DMPS) on tissue and urine mercury levels following prolonged methylmercury exposure in rats. Toxicol. Sci. 2001, 61, 224–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, E.A.; Shubha, M.C.; D’Souza, C.J. Blood glucose determination: Plasma or serum? J. Clin. Lab. Anal. 2012, 26, 317–320. [Google Scholar] [CrossRef]



| Glucose (mg/dL) | Kidney Weight (g) | Kidney/Body Weight Ratio (%) (mg/g) | Urea (mg/dL) | BUN (mg/dL) | Creatinine (IU/L) | |
|---|---|---|---|---|---|---|
| Normal control | 3.72 ± 0.58 | 1.83 ± 0.16 | 0.57 ± 0.05 | 11.92 ± 2.85 ** | 5.56 ± 1.33 $$ | 0.28 ± 0.03 |
| Normal sitagliptin | 3.98 ± 0.25 | 2.00 ± 0.06 | 0.74 ± 0.03 | 9.65 ± 1.13 ** | 4.50 ± 0.53 $$ | 0.25 ± 0.44 |
| STZ control | 9.21 ± 1.41 *** | 2.80 ± 0.25 ** | 1.04 ± 0.08 *** | 49.70 ± 4.02 *** | 23.19 ± 1.87 *** | 0.64 ± 0.10 ** |
| STZ sitagliptin | 4.26 ± 1.06 ### | 1.87 ± 0.05 ## | 0.68 ± 0.02 ### | 35.31 ± 10.67 ### | 16.47 ± 4.98 ### | 0.31 ± 0.08 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AL-Qabbaa, S.M.; Qaboli, S.I.; Alshammari, T.K.; Alamin, M.A.; Alrajeh, H.M.; Almuthnabi, L.A.; Alotaibi, R.R.; Alonazi, A.S.; Bin Dayel, A.F.; Alrasheed, N.M.; et al. Sitagliptin Mitigates Diabetic Nephropathy in a Rat Model of Streptozotocin-Induced Type 2 Diabetes: Possible Role of PTP1B/JAK-STAT Pathway. Int. J. Mol. Sci. 2023, 24, 6532. https://doi.org/10.3390/ijms24076532
AL-Qabbaa SM, Qaboli SI, Alshammari TK, Alamin MA, Alrajeh HM, Almuthnabi LA, Alotaibi RR, Alonazi AS, Bin Dayel AF, Alrasheed NM, et al. Sitagliptin Mitigates Diabetic Nephropathy in a Rat Model of Streptozotocin-Induced Type 2 Diabetes: Possible Role of PTP1B/JAK-STAT Pathway. International Journal of Molecular Sciences. 2023; 24(7):6532. https://doi.org/10.3390/ijms24076532
Chicago/Turabian StyleAL-Qabbaa, Sarah M., Samaher I. Qaboli, Tahani K. Alshammari, Maha A. Alamin, Haya M. Alrajeh, Lama A. Almuthnabi, Rana R. Alotaibi, Asma S. Alonazi, Anfal F. Bin Dayel, Nawal M. Alrasheed, and et al. 2023. "Sitagliptin Mitigates Diabetic Nephropathy in a Rat Model of Streptozotocin-Induced Type 2 Diabetes: Possible Role of PTP1B/JAK-STAT Pathway" International Journal of Molecular Sciences 24, no. 7: 6532. https://doi.org/10.3390/ijms24076532
APA StyleAL-Qabbaa, S. M., Qaboli, S. I., Alshammari, T. K., Alamin, M. A., Alrajeh, H. M., Almuthnabi, L. A., Alotaibi, R. R., Alonazi, A. S., Bin Dayel, A. F., Alrasheed, N. M., & Alrasheed, N. M. (2023). Sitagliptin Mitigates Diabetic Nephropathy in a Rat Model of Streptozotocin-Induced Type 2 Diabetes: Possible Role of PTP1B/JAK-STAT Pathway. International Journal of Molecular Sciences, 24(7), 6532. https://doi.org/10.3390/ijms24076532







