Electrophysiological Properties of Tetraploid Cardiomyocytes Derived from Murine Pluripotent Stem Cells Generated by Fusion of Adult Somatic Cells with Embryonic Stem Cells
Abstract
1. Introduction
2. Results
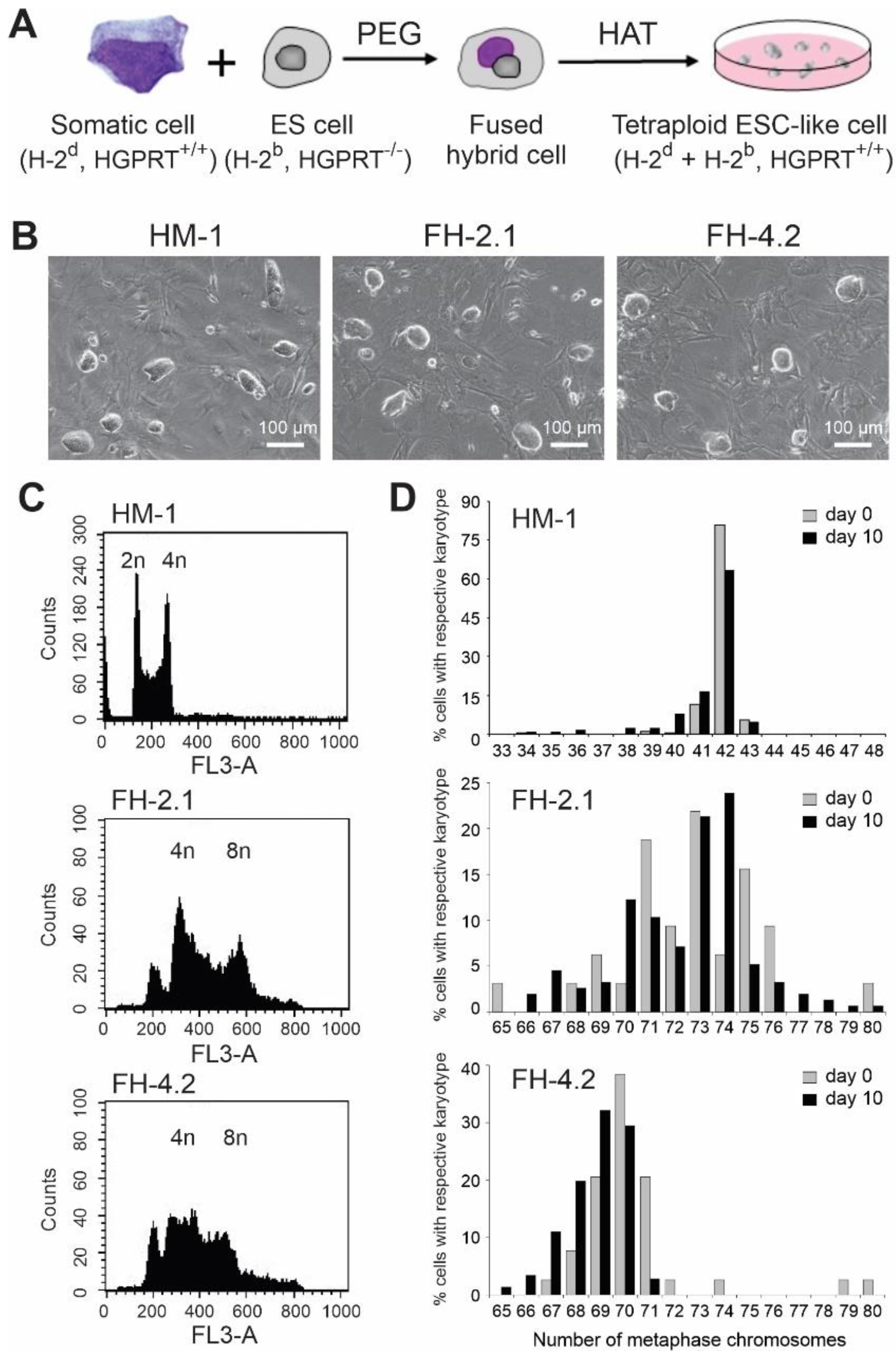
2.1. Generation of Hybrid Cells by Fusion of ESCs with Adult Somatic Cells
2.2. DNA Content and Cytogenetic Analysis
2.3. Multicolour Fluorescence In Situ Hybridization (mFISH)
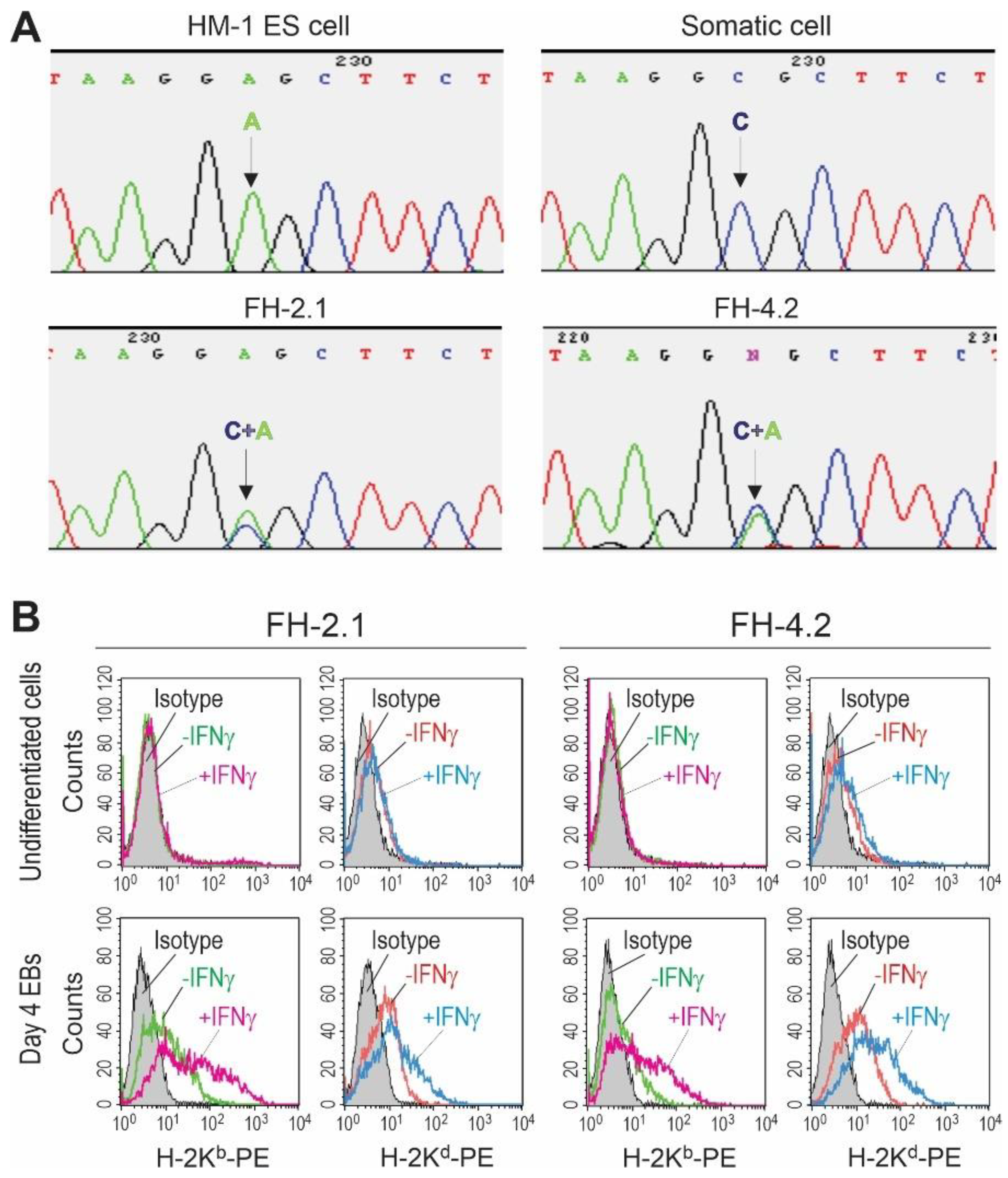
2.4. SNP Genotyping of Fusion-Derived Cells
2.5. MHC Class I Haplotyping of Fusion-Derived Cells
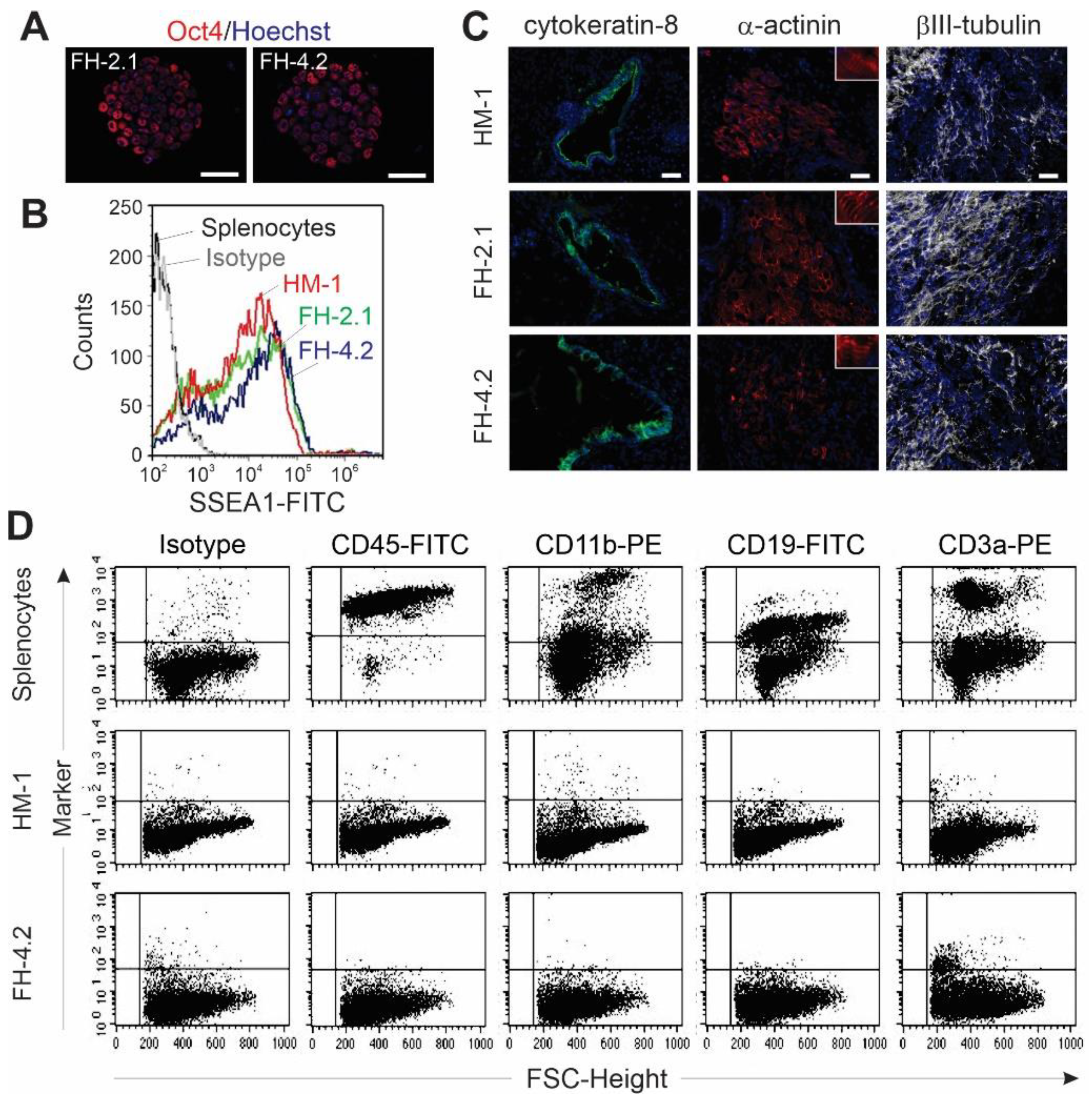
2.6. Pluripotency of FH Cells
2.7. Cardiac Differentiation of FH Cells
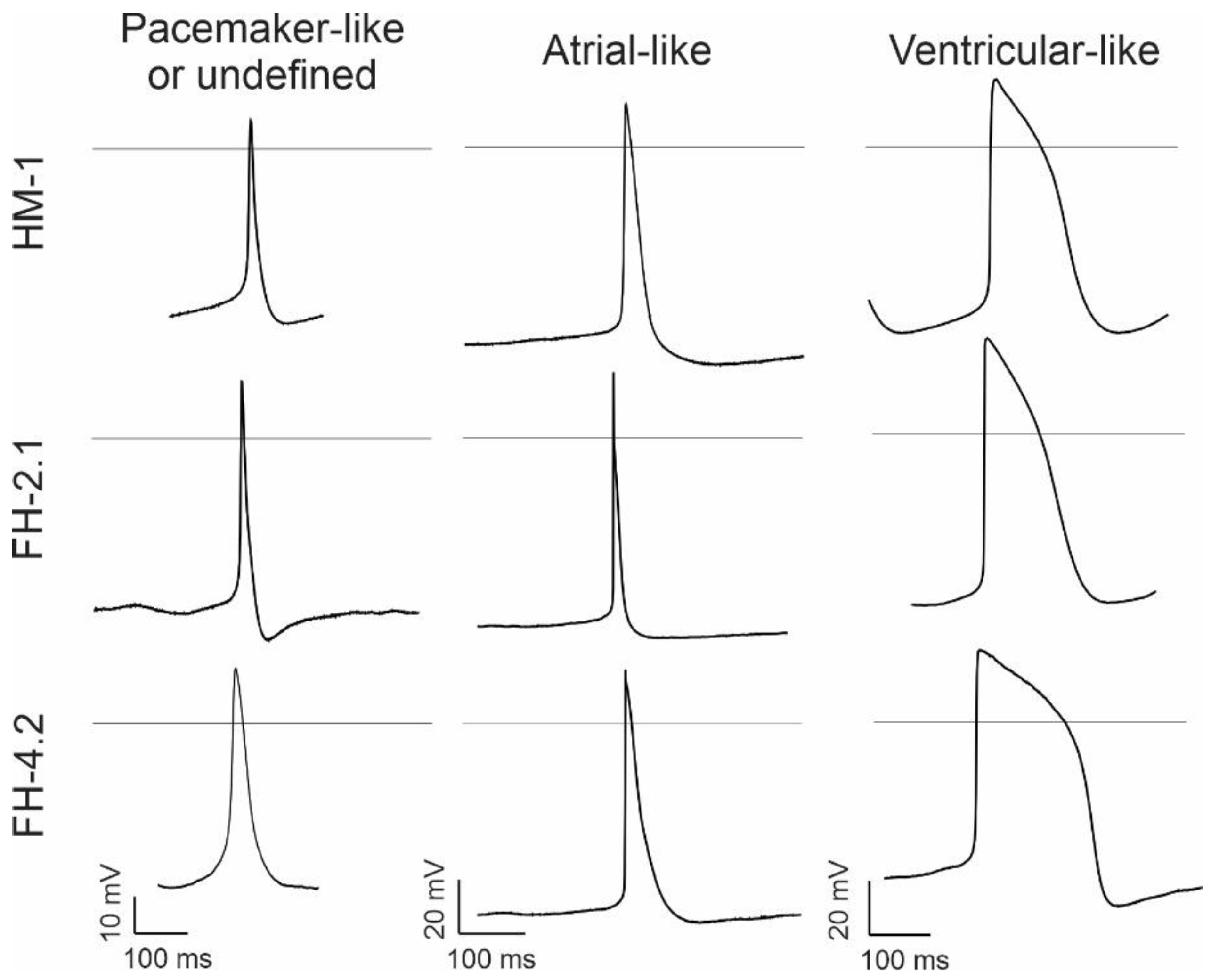
2.8. Characteristics of Spontaneous Action Potentials (APs) in Fusion-Derived CMs
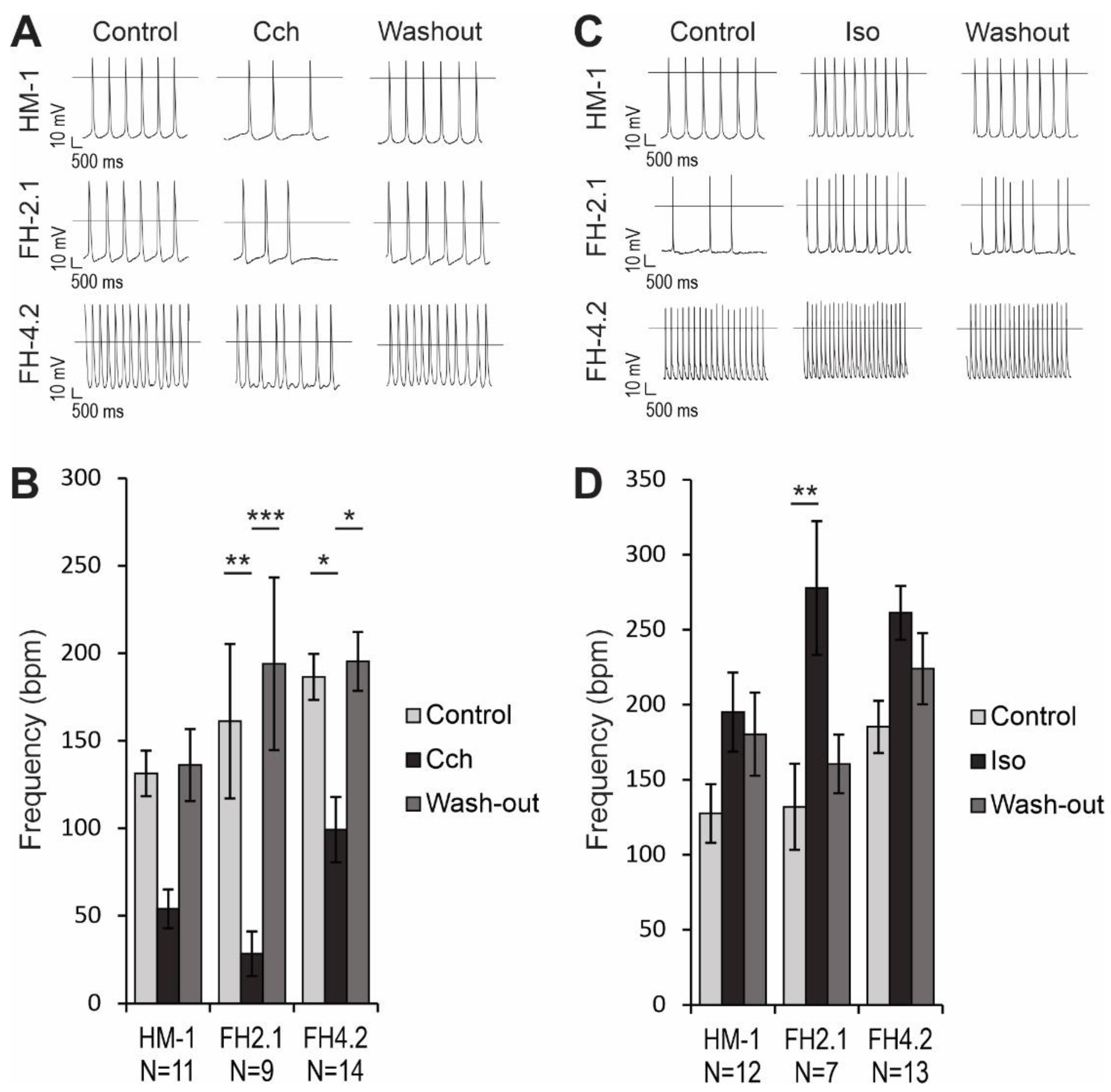
2.9. Effects of Muscarinic and β-Adrenergic Receptor Agonists on AP Frequency
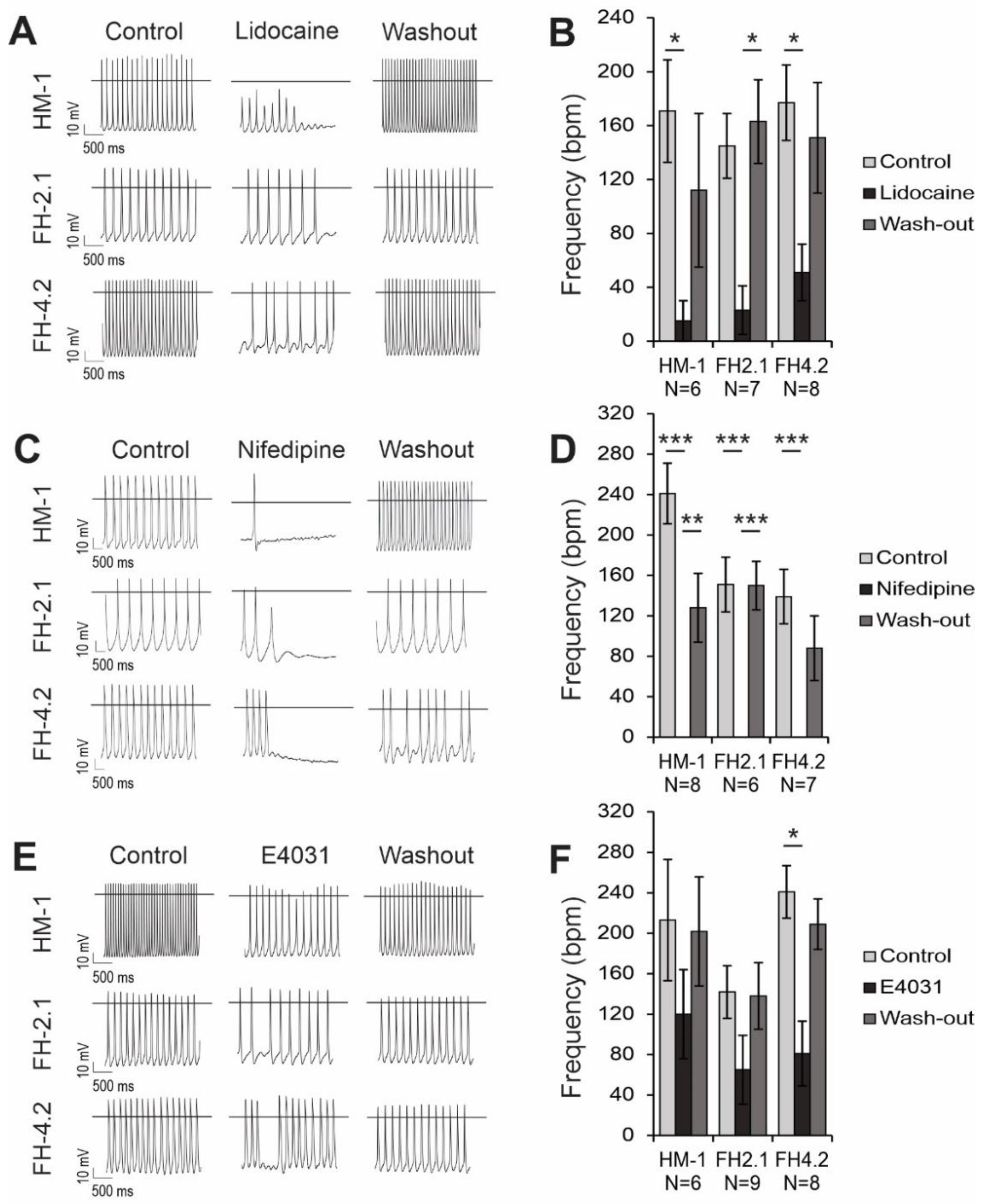
2.10. Effects of Ion Channel Blockers on Spontaneous APs
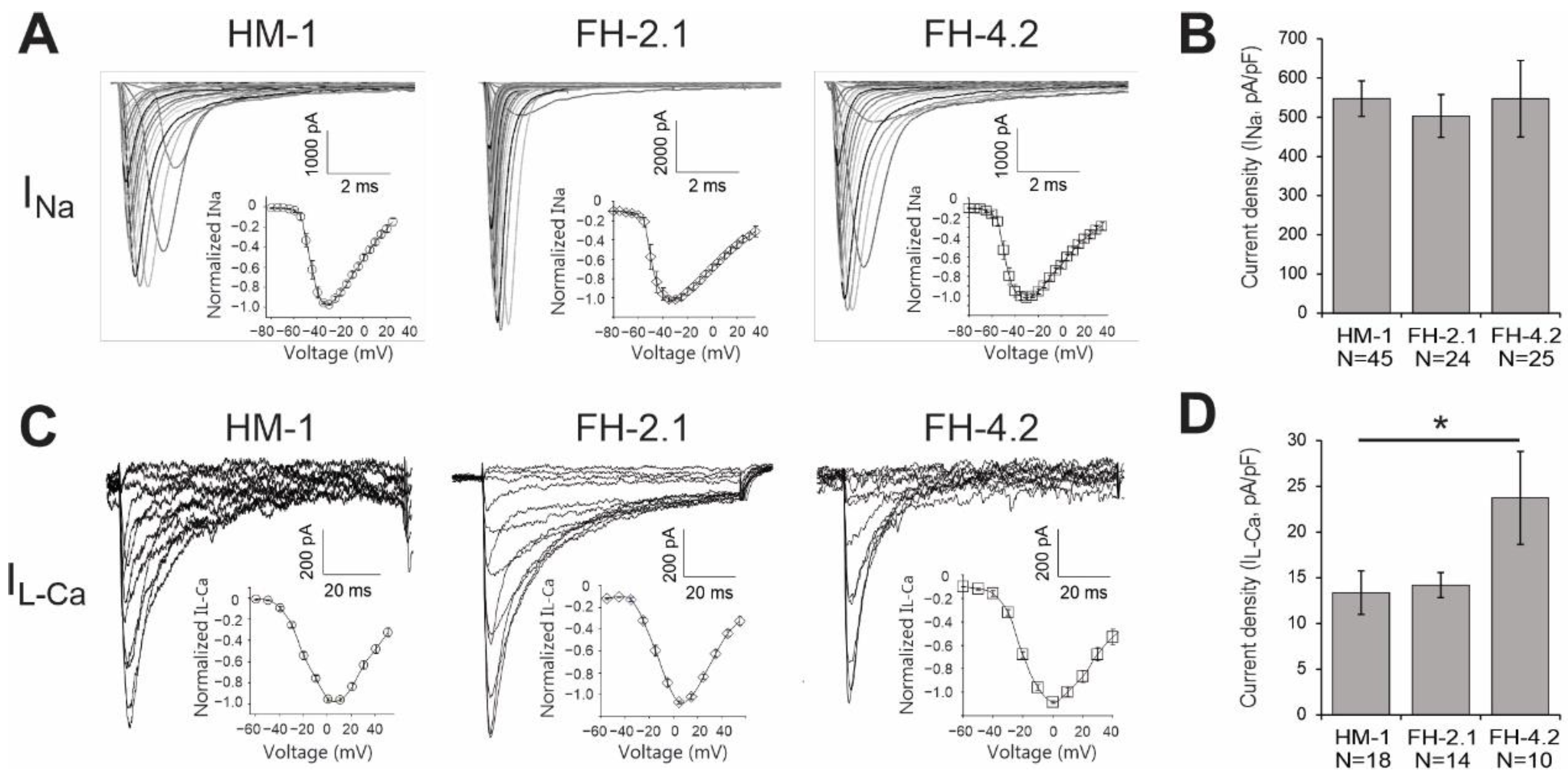
2.11. Expression of Cardiac-Specific Voltage-Gated Na+ and L-Type Ca2+ Channels
3. Discussion
4. Materials and Methods
4.1. ES Cells
4.2. Somatic Cells
4.3. Cell Fusion
4.4. Cardiac Differentiation
4.5. Karyotype Analysis
4.6. Flow Cytometry
4.7. Immunocytochemistry
4.8. Teratoma Assay
4.9. SNP Genotyping
4.10. RT-PCR Analysis
4.11. Patch-Clamp
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BM | bone marrow |
| αMHC | alpha myosin heavy chain |
| βME | β-mercaptoethanol |
| CCh | carbachol |
| CMs | cardiomyocytes |
| cTnT | cardiac troponin T |
| DMEM | Dulbecco’s modified Eagle’s medium |
| EBs | embryoid bodies |
| ESCs | embryonic stem cells |
| FBS | fetal bovine serum |
| FH cells | fusion-derived hybrid (cells) |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| HAT | hypoxanthine-aminopterin-thymidine |
| HGPRT | hypoxanthine-guanine phosphoribosyl transferase |
| IFNγ | interferon γ |
| iPSCs | induced pluripotent stem cells |
| Iso | isoproterenol |
| MEFs | murine embryonic fibroblasts |
| MI | myocardial infarction |
| MLC2v | myosin light chain 2 ventricular |
| MHC | major histocompatibility complex |
| NEAA | non-essential amino acids |
| PBS | phosphate-buffered saline |
| SEM | standard error of the mean |
| SNP | single nucleotide polymorphism |
References
- Orr-Weaver, T.L. When bigger is better: The role of polyploidy in organogenesis. Trends Genet. 2015, 31, 307–315. [Google Scholar] [CrossRef]
- Machlus, K.R.; Italiano, J.E., Jr. The incredible journey: From megakaryocyte development to platelet formation. J. Cell Biol. 2013, 201, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, J.-E.; Brégerie, O.; Robert, A.; Debey, P.; Brechot, C.; Desdouets, C. Liver Cell Polyploidization: A Pivotal Role for Binuclear Hepatocytes. J. Biol. Chem. 2003, 278, 19095–19101. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtsev, B.; Kudryavtseva, M.V.; Sakuta, G.A.; Stein, G. Human hepatocyte polyploidization kinetics in the course of life cycle. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1993, 64, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Donne, R.; Saroul-Aïnama, M.; Cordier, P.; Celton-Morizur, S.; Desdouets, C. Polyploidy in liver development, homeostasis and disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Wakefield, L.; Tarlow, B.D.; Grompe, M. In Vivo Lineage Tracing of Polyploid Hepatocytes Reveals Extensive Proliferation during Liver Regeneration. Cell Stem Cell 2020, 26, 34–47.e3. [Google Scholar] [CrossRef]
- Toyoda, H.; Bregerie, O.; Vallet, A.; Nalpas, B.; Pivert, G.; Brechot, C.; Desdouets, C. Changes to hepatocyte ploidy and binuclearity profiles during human chronic viral hepatitis. Gut 2005, 54, 297–302. [Google Scholar] [CrossRef]
- Gentric, G.; Maillet, V.; Paradis, V.; Couton, D.; L’Hermitte, A.; Panasyuk, G.; Fromenty, B.; Celton-Morizur, S.; Desdouets, C. Oxidative stress promotes pathologic polyploidization in nonalcoholic fatty liver disease. J. Clin. Investig. 2015, 125, 981–992. [Google Scholar] [CrossRef]
- Soonpaa, M.H.; Kim, K.K.; Pajak, L.; Franklin, M.; Field, L.J. Cardiomyocyte DNA synthesis and binucleation during murine development. Am. J. Physiol. Circ. Physiol. 1996, 271, H2183–H2189. [Google Scholar] [CrossRef]
- Engel, F.B.; Schebesta, M.; Keating, M.T. Anillin localization defect in cardiomyocyte binucleation. J. Mol. Cell. Cardiol. 2006, 41, 601–612. [Google Scholar] [CrossRef]
- Alkass, K.; Panula, J.; Westman, M.; Wu, T.-D.; Guerquin-Kern, J.-L.; Bergmann, O. No Evidence for Cardiomyocyte Number Expansion in Preadolescent Mice. Cell 2015, 163, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.; Barske, L.; Van Handel, B.; Rau, C.D.; Gan, P.; Sharma, A.; Parikh, S.; Denholtz, M.; Huang, Y.; Yamaguchi, Y.; et al. Frequency of mononuclear diploid cardiomyocytes underlies natural variation in heart regeneration. Nat. Genet. 2017, 49, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.P.; Zhou, Y.; Nakada, Y.; Zhang, J. Changes in Cardiomyocyte Cell Cycle and Hypertrophic Growth During Fetal to Adult in Mammals. J. Am. Heart Assoc. 2021, 10, e017839. [Google Scholar] [CrossRef] [PubMed]
- Mollova, M.; Bersell, K.; Walsh, S.; Savla, J.; Das, L.T.; Park, S.-Y.; Silberstein, L.E.; Dos Remedios, C.G.; Graham, D.; Colan, S.; et al. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc. Natl. Acad. Sci. USA 2013, 110, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; dos Remedios, C.; et al. Dynamics of Cell Generation and Turnover in the Human Heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef]
- Bischof, C.; Mirtschink, P.; Yuan, T.; Wu, M.; Zhu, C.; Kaur, J.; Pham, M.D.; Gonzalez-Gonoggia, S.; Hammer, M.; Rogg, E.-M.; et al. Mitochondrial–cell cycle cross-talk drives endoreplication in heart disease. Sci. Transl. Med. 2021, 13, eabi7964. [Google Scholar] [CrossRef]
- Meckert, P.C.; Rivello, H.G.; Vigliano, C.; González, P.; Favaloro, R.; Laguens, R. Endomitosis and polyploidization of myocardial cells in the periphery of human acute myocardial infarction. Cardiovasc. Res. 2005, 67, 116–123. [Google Scholar] [CrossRef]
- Herget, G.W.; Neuburger, M.; Plagwitz, R.; Adler, C.P. DNA content, ploidy level and number of nuclei in the human heart after myocardial infarction. Cardiovasc. Res. 1997, 36, 45–51. [Google Scholar] [CrossRef]
- Sukhacheva, T.V.; Serov, R.A.; Nizyaeva, N.V.; Burov, A.A.; Pavlovich, S.V.; Podurovskaya, Y.L.; Samsonova, M.V.; Chernyaev, A.L.; Shchegolev, A.I.; Kim, A.I.; et al. Accelerated Growth, Differentiation, and Ploidy with Reduced Proliferation of Right Ventricular Cardiomyocytes in Children with Congenital Heart Defect Tetralogy of Fallot. Cells 2022, 11, 175. [Google Scholar] [CrossRef]
- Hesse, M.; Bednarz, R.; Carls, E.; Becker, C.; Bondareva, O.; Lother, A.; Geisen, C.; Dreßen, M.; Krane, M.; Roell, W.; et al. Proximity to injury, but neither number of nuclei nor ploidy define pathological adaptation and plasticity in cardiomyocytes. J. Mol. Cell. Cardiol. 2021, 152, 95–104. [Google Scholar] [CrossRef]
- Paradis, A.N.; Gay, M.S.; Zhang, L. Binucleation of cardiomyocytes: The transition from a proliferative to a terminally differentiated state. Drug Discov. Today 2014, 19, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.; Han, L.; Liu, H.; Kühn, B. Polyploid cardiomyocytes: Implications for heart regeneration. Development 2021, 148, dev199401. [Google Scholar] [CrossRef] [PubMed]
- Gan, P.; Patterson, M.; Sucov, H.M. Cardiomyocyte Polyploidy and Implications for Heart Regeneration. Annu. Rev. Physiol. 2020, 82, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Derks, W.; Bergmann, O. Polyploidy in Cardiomyocytes: Roadblock to Heart Regeneration? Circ. Res. 2020, 126, 552–565. [Google Scholar] [CrossRef]
- Senyo, S.E.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.-D.; Guerquin-Kern, J.-L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2013, 493, 433–436. [Google Scholar] [CrossRef]
- González-Rosa, J.M.; Sharpe, M.; Field, D.; Soonpaa, M.H.; Field, L.J.; Burns, C.E.; Burns, C.G. Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish. Dev. Cell 2018, 44, 433–446.e437. [Google Scholar] [CrossRef]
- Bageghni, S.A.; Frentzou, G.A.; Drinkhill, M.J.; Mansfield, W.; Coverley, D.; Ainscough, J.F.X. Cardiomyocyte specific expression of the nuclear matrix protein, CIZ1, stimulates production of mononucleated cells with an extended window of proliferation in the postnatal mouse heart. Biol. Open 2017, 6, 92–99. [Google Scholar] [CrossRef]
- Shen, H.; Gan, P.; Wang, K.; Darehzereshki, A.; Wang, K.; Kumar, S.R.; Lien, C.-L.; Patterson, M.; Tao, G.; Sucov, H.M.; et al. Mononuclear diploid cardiomyocytes support neonatal mouse heart regeneration in response to paracrine IGF2 signaling. Elife 2020, 9, e53071. [Google Scholar] [CrossRef]
- Becker, C.; Hesse, M. Role of Mononuclear Cardiomyocytes in Cardiac Turnover and Regeneration. Curr. Cardiol. Rep. 2020, 22, 39. [Google Scholar] [CrossRef]
- Han, L.; Choudhury, S.; Mich-Basso, J.D.; Ammanamanchi, N.; Ganapathy, B.; Suresh, S.; Khaladkar, M.; Singh, J.; Maehr, R.; Zuppo, D.A.; et al. Lamin B2 Levels Regulate Polyploidization of Cardiomyocyte Nuclei and Myocardial Regeneration. Dev. Cell 2020, 53, 42–59.e11. [Google Scholar] [CrossRef]
- Alvarez-Dolado, M.; Pardal, R.; García-Verdugo, J.M.; Fike, J.R.; Lee, H.O.; Pfeffer, K.; Lois, C.; Morrison, S.; Alvarez-Buylla, A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nat. Cell Biol. 2003, 425, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Wada, H.; Nagai, T.; Iijima, Y.; Minamino, T.; Sano, M.; Akazawa, H.; Molkentin, J.; Kasanuki, H.; Komuro, I. Cardiomyocytes fuse with surrounding noncardiomyocytes and reenter the cell cycle. J. Cell Biol. 2004, 167, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Nygren, J.M.; Jovinge, S.; Breitbach, M.; Sawen, P.; Roll, W.; Hescheler, J.; Taneera, J.; Fleischmann, B.K.; Jacobsen, S.E. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentia-tion. Nat. Med. 2004, 10, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Yellamilli, A.; Ren, Y.; McElmurry, R.T.; Lambert, J.P.; Gross, P.; Mohsin, S.; Houser, S.R.; Elrod, J.W.; Tolar, J.; Garry, D.J.; et al. Abcg2 -expressing side population cells contribute to cardiomyocyte renewal through fusion. FASEB J. 2020, 34, 5642–5657. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.T.; Ogle, B.M. Viral-mediated fusion of mesenchymal stem cells with cells of the infarcted heart hinders healing via decreased vascularization and immune modulation. Sci. Rep. 2016, 6, 20283. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Cusella, G.; Angelis, D.; Coletta, M.; Paolucci, E.; Stornaiuolo, A.; Cossu, G.; Mavilio, F. Muscle Regeneration by Bone Marrow-Derived Myogenic Progenitors. Science 1998, 279, 1528–1530. [Google Scholar] [CrossRef] [PubMed]
- Ferrand, J.; Noël, D.; Lehours, P.; Prochazkova-Carlotti, M.; Chambonnier, L.; Ménard, A.; Mégraud, F.; Varon, C. Human Bone Marrow-Derived Stem Cells Acquire Epithelial Characteristics through Fusion with Gastrointestinal Epithelial Cells. PLoS ONE 2011, 6, e19569. [Google Scholar] [CrossRef]
- Vassilopoulos, G.; Wang, P.-R.; Russell, D.W. Transplanted bone marrow regenerates liver by cell fusion. Nat. Cell Biol. 2003, 422, 901–904. [Google Scholar] [CrossRef]
- Grompe, M. The Role of Bone Marrow Stem Cells in Liver Regeneration. Semin. Liver Dis. 2003, 23, 363–372. [Google Scholar] [CrossRef]
- Weimann, J.M.; Charlton, C.A.; Brazelton, T.R.; Hackman, R.C.; Blau, H.M. Contribution of transplanted bone marrow cells to Purkinje neurons in human adult brains. Proc. Natl. Acad. Sci. USA 2003, 100, 2088–2093. [Google Scholar] [CrossRef]
- Hokari, M.; Kuroda, S.; Shichinohe, H.; Yano, S.; Hida, K.; Iwasaki, Y. Bone marrow stromal cells protect and repair damaged neurons through multiple mechanisms. J. Neurosci. Res. 2008, 86, 1024–1035. [Google Scholar] [CrossRef]
- Nygren, J.; Liuba, K.; Breitbach, M.; Stott, S.; Thorén, L.; Roell, W.; Geisen, C.; Sasse, P.; Kirik, D.; Björklund, A.; et al. Myeloid and lymphoid contribution to non-haematopoietic lineages through irradiation-induced heterotypic cell fusion. Nature 2008, 10, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; Nygren, J.; Ponten, A.; Jovinge, S. Myogenic Reprogramming of Bone Marrow Derived Cells in a WDmd Deficient Mouse Model. PLoS ONE 2011, 6, e27500. [Google Scholar] [CrossRef]
- Quijada, P.; Salunga, H.T.; Hariharan, N.; Cubillo, J.D.; El-Sayed, F.G.; Moshref, M.; Bala, K.M.; Emathinger, J.M.; De La Torre, A.; Ormachea, L.; et al. Cardiac Stem Cell Hybrids Enhance Myocardial Repair. Circ. Res. 2015, 117, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Gaspar-Maia, A.; Ramalho-Santos, M.; Pera, R.A.R. High-Efficiency Stem Cell Fusion-Mediated Assay Reveals Sall4 as an Enhancer of Reprogramming. PLoS ONE 2008, 3, e1955. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.K.; Chiang, C.-H.J.; Ponnusamy, K.; Ming, G.-L.; Song, H. G9a and Jhdm2a Regulate Embryonic Stem Cell Fusion-Induced Reprogramming of Adult Neural Stem Cells. Stem Cells 2008, 26, 2131–2141. [Google Scholar] [CrossRef]
- Lluis, F.; Pedone, E.; Pepe, S.; Cosma, M.P. Periodic activation of Wnt/beta-catenin signaling enhances somatic cell repro-gramming mediated by cell fusion. Cell Stem Cell 2008, 3, 493–507. [Google Scholar] [CrossRef]
- Matveeva, N.M.; Shilov, A.G.; Kaftanovskaya, E.M.; Maximovsky, L.P.; Zhelezova, A.I.; Golubitsa, A.N.; Bayborodin, S.I.; Fokina, M.M.; Serov, O.L. In vitro and in vivo study of pluripotency in intraspecific hybrid cells obtained by fusion of murine embryonic stem cells with splenocytes. Mol. Reprod. Dev. 1998, 50, 128–138. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; He, P.; Slukvin, I.I.; Thomson, J.A. Human Embryonic Stem Cells Reprogram Myeloid Precursors Following Cell–Cell Fusion. Stem Cells 2006, 24, 168–176. [Google Scholar] [CrossRef]
- Sumer, H.; Jones, K.L.; Liu, J.; Heffernan, C.; Tat, P.A.; Upton, K.R.; Verma, P.J. Reprogramming of Somatic Cells After Fusion With Induced Pluripotent Stem Cells and Nuclear Transfer Embryonic Stem Cells. Stem Cells Dev. 2010, 19, 239–246. [Google Scholar] [CrossRef]
- Ying, Q.-L.; Nichols, J.; Evans, E.P.; Smith, A.G. Changing potency by spontaneous fusion. Nature 2002, 416, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Tada, M.; Takahama, Y.; Abe, K.; Nakatsuji, N.; Tada, T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr. Biol. 2001, 11, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, D.J.; Tanasijevic, B.; Kaur, A.; Obergfell, C.; O’Neill, R.J.; Krueger, W.; Rasmussen, T.P. Genome-Wide Reprogramming in Hybrids of Somatic Cells and Embryonic Stem Cells. Stem Cells 2007, 25, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Cowan, C.A.; Atienza, J.; Melton, D.A.; Eggan, K. Nuclear Reprogramming of Somatic Cells After Fusion with Human Embryonic Stem Cells. Science 2005, 309, 1369–1373. [Google Scholar] [CrossRef]
- Kruglova, A.A.; Kizilova, E.A.; Zhelezova, A.I.; Gridina, M.M.; Golubitsa, A.N.; Serov, O.L. Embryonic stem cell/fibroblast hybrid cells with near-tetraploid karyotype provide high yield of chimeras. Cell Tissue Res. 2008, 334, 371–380. [Google Scholar] [CrossRef]
- Matveeva, N.M.; Pristyazhnyuk, I.E.; Temirova, S.A.; Menzorov, A.G.; Vasilkova, A.; Shilov, A.G.; Smith, A.; Serov, O.L. Un-equal segregation of parental chromosomes in embryonic stem cell hybrids. Mol. Reprod. Dev. 2005, 71, 305–314. [Google Scholar] [CrossRef]
- Mittmann, J.; Kerkis, I.; Kawashima, C.; Sukoyan, M.; Santos, E.; Kerkis, A. Differentiation of mouse embryonic stem cells and their hybrids during embryoid body formation. Genet. Mol. Biol. 2002, 25, 103–111. [Google Scholar] [CrossRef]
- Gaztelumendi, N.; Nogués, C. Chromosome Instability in mouse Embryonic Stem Cells. Sci. Rep. 2014, 4, 5324. [Google Scholar] [CrossRef]
- Frenzel, L.P.; Abdullah, Z.; Kriegeskorte, A.K.; Dieterich, R.; Lange, N.; Busch, D.H.; Krönke, M.; Utermöhlen, O.; Hescheler, J.; Šarić, T. Role of Natural-Killer Group 2 Member D Ligands and Intercellular Adhesion Molecule 1 in Natural Killer Cell-Mediated Lysis of Murine Embryonic Stem Cells and Embryonic Stem Cell-Derived Cardiomyocytes. Stem Cells 2009, 27, 307–316. [Google Scholar] [CrossRef]
- Abdullah, Z.; Saric, T.; Kashkar, H.; Baschuk, N.; Yazdanpanah, B.; Fleischmann, B.K.; Hescheler, J.; Krönke, M.; Utermöhlen, O. Serpin-6 Expression Protects Embryonic Stem Cells from Lysis by Antigen-Specific CTL. J. Immunol. 2007, 178, 3390–3399. [Google Scholar] [CrossRef]
- Vasilkova, A.A.; Kizilova, H.A.; Puzakov, M.V.; Shilov, A.G.; Zhelezova, A.I.; Golubitsa, A.N.; Battulin, N.R.; Vedernikov, V.E.; Menzorov, A.G.; Matveeva, N.M.; et al. Dominant manifestation of pluripotency in embryonic stem cell hybrids with various numbers of somatic chromosomes. Mol. Reprod. Dev. 2007, 74, 941–951. [Google Scholar] [CrossRef]
- Goto, Y.; Matsui, J.; Takagi, N. Developmental potential of mouse tetraploid cells in diploid <--> tetraploid chimeric embryos. Int. J. Dev. Biol. 2002, 46, 741–745. [Google Scholar] [PubMed]
- Pandit, S.K.; Westendorp, B.; de Bruin, A. Physiological significance of polyploidization in mammalian cells. Trends Cell Biol. 2013, 23, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Mauritz, C.; Schwanke, K.; Reppel, M.; Neef, S.; Katsirntaki, K.; Maier, L.S.; Nguemo, F.; Menke, S.; Haustein, M.; Hescheler, J.; et al. Generation of Functional Murine Cardiac Myocytes From Induced Pluripotent Stem Cells. Circulation 2008, 118, 507–517. [Google Scholar] [CrossRef]
- Pfannkuche, K.; Liang, H.; Hannes, T.; Xi, J.; Fatima, A.; Nguemo, F.; Matzkies, M.; Wernig, M.; Jaenisch, R.; Pillekamp, F.; et al. Cardiac Myocytes Derived from Murine Reprogrammed Fibroblasts: Intact Hormonal Regulation, Cardiac Ion Channel Expression and Development of Contractility. Cell. Physiol. Biochem. 2009, 24, 73–86. [Google Scholar] [CrossRef]
- Insel, P.A.; Motulsky, H.J. Calcium-channel-blocking agents. N. Engl. J. Med. 1983, 308, 1361–1362. [Google Scholar]
- Nayler, W.G. Calcium antagonists. Med. J. Aust. 1983, 2, 506–512. [Google Scholar] [CrossRef]
- Spedding, M.; Kenny, B. Voltage-dependent calcium channels: Structures and drug-binding sites. Biochem. Soc. Trans. 1992, 20, 147–153. [Google Scholar] [CrossRef]
- Grant, A.O.; Dietz, M.A.; Gilliam, F.R., 3rd; Starmer, C.F. Blockade of cardiac sodium channels by lidocaine. Single-channel analysis. Circ. Res. 1989, 65, 1247–1262. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.V.; Courtney, K.R.; Clusin, W.T. Use-dependent block of single sodium channels by lidocaine in guinea pig ven-tricular myocytes. Biophys. J. 1989, 55, 1261–1266. [Google Scholar] [CrossRef]
- Sanguinetti, M.C.; Jurkiewicz, N.K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J. Gen. Physiol. 1990, 96, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Ito, H. Role of rapidly activating delayed rectifier K+ current in sinoatrial node pacemaker activity. Am. J. Physiol. Content 1995, 269, H453–H462. [Google Scholar] [CrossRef] [PubMed]
- Verheijck, E.E.; van Ginneken, A.C.; Bourier, J.; Bouman, L.N. Effects of Delayed Rectifier Current Blockade by E-4031 on Impulse Generation in Single Sinoatrial Nodal Myocytes of the Rabbit. Circ. Res. 1995, 76, 607–615. [Google Scholar] [CrossRef]
- Maltsev, V.A.; Rohwedel, J.; Hescheler, J.; Wobus, A.M. Embryonic stem cells differentiate in vitro into cardiomyocytes repre-senting sinusnodal, atrial and ventricular cell types. Mech. Dev. 1993, 44, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, V.A.; Wobus, A.M.; Rohwedel, J.; Bader, M.; Hescheler, J. Cardiomyocytes differentiated in vitro from embryonic stem cells developmentally express cardiac-specific genes and ionic currents. Circ. Res. 1994, 75, 233–244. [Google Scholar] [CrossRef]
- Ehrlich, J.R.; Cha, T.J.; Zhang, L.; Chartier, D.; Melnyk, P.; Hohnloser, S.H.; Nattel, S. Cellular electrophysiology of canine pulmonary vein cardiomyocytes: Action potential and ionic current properties. J. Physiol. 2003, 551, 801–813. [Google Scholar] [CrossRef]
- Lei, M.; Jones, S.A.; Liu, J.; Lancaster, M.K.; Fung, S.S.; Dobrzynski, H.; Camelliti, P.; Maier, S.K.; Noble, D.; Boyett, M.R. Re-quirement of neuronal- and cardiac-type sodium channels for murine sinoatrial node pacemaking. J. Physiol. 2004, 559, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Brown, H. Two components of the delayed rectifier potassium current, IK, in rabbit sino-atrial node cells. Exp. Physiol. 1996, 81, 725–741. [Google Scholar] [CrossRef]
- Kodama, I.; Boyett, M.R.; Nikmaram, M.R.; Yamamoto, M.; Honjo, H.; Niwa, R. Regional differences in effects of E-4031 within the sinoatrial node. Am. J. Physiol. Circ. Physiol. 1999, 276, H793–H802. [Google Scholar] [CrossRef]
- Pond, A.L.; Scheve, B.K.; Benedict, A.T.; Petrecca, K.; Van Wagoner, D.R.; Shrier, A.; Nerbonne, J.M. Expression of Distinct ERG Proteins in Rat, Mouse, and Human Heart. J. Biol. Chem. 2000, 275, 5997–6006. [Google Scholar] [CrossRef]
- Kuzmenkin, A.; Liang, H.; Xu, G.; Pfannkuche, K.; Eichhorn, H.; Fatima, A.; Luo, H.; Saric, T.; Wernig, M.; Jaenisch, R.; et al. Functional characterization of cardiomyocytes derived from murine induced pluripotent stem cells in vitro. FASEB J. 2009, 23, 4168–4180. [Google Scholar] [CrossRef] [PubMed]
- Carcamo-Orive, I.; Hoffman, G.E.; Cundiff, P.; Beckmann, N.D.; D’Souza, S.L.; Knowles, J.W.; Patel, A.; Hendry, C.; Papatsenko, D.; Abbasi, F.; et al. Analysis of Transcriptional Variability in a Large Human iPSC Library Reveals Genetic and Non-genetic Determinants of Heterogeneity. Cell Stem Cell 2017, 20, 518–532.e519. [Google Scholar] [CrossRef] [PubMed]
- Osafune, K.; Caron, L.; Borowiak, M.; Martinez, R.J.; Fitz-Gerald, C.S.; Sato, Y.; Cowan, C.A.; Chien, K.R.; Melton, D.A. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat. Biotechnol. 2008, 26, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Millrod, M.A.; Zambidis, E.T.; Tung, L. Variability of Action Potentials Within and Among Cardiac Cell Clusters Derived from Human Embryonic Stem Cells. Sci. Rep. 2016, 6, 18544. [Google Scholar] [CrossRef]
- Hannes, T.; Wolff, M.; Jesudoss, M.X.D.; Pfannkuche, K.P.; Haustein, M.; Müller-Ehmsen, J.; Sachinidis, A.; Hescheler, J.; Khalil, M.; Halbach, M. Electrophysiological Characteristics of Embryonic Stem Cell-Derived Cardiomyocytes are Cell Line-Dependent. Cell. Physiol. Biochem. 2015, 35, 305–314. [Google Scholar] [CrossRef]
- Sanchez-Freire, V.; Lee, A.S.; Hu, S.; Abilez, O.J.; Liang, P.; Lan, F.; Huber, B.C.; Ong, S.-G.; Hong, W.X.; Huang, M.; et al. Effect of Human Donor Cell Source on Differentiation and Function of Cardiac Induced Pluripotent Stem Cells. J. Am. Coll. Cardiol. 2014, 64, 436–448. [Google Scholar] [CrossRef]
- Ortmann, D.; Brown, S.; Czechanski, A.; Aydin, S.; Muraro, D.; Huang, Y.; Tomaz, R.A.; Osnato, A.; Canu, G.; Wesley, B.T.; et al. Naive Pluripotent Stem Cells Exhibit Phenotypic Variability that Is Driven by Genetic Variation. Cell Stem Cell 2020, 27, 470–481.e476. [Google Scholar] [CrossRef]
- Schmid, C.; Abi-Gerges, N.; Leitner, M.G.; Zellner, D.; Rast, G. Ion Channel Expression and Electrophysiology of Singular Human (Primary and Induced Pluripotent Stem Cell-Derived) Cardiomyocytes. Cells 2021, 10, 3370. [Google Scholar] [CrossRef]
- Yechikov, S.; Copaciu, R.; Gluck, J.M.; Deng, W.; Chiamvimonvat, N.; Chan, J.W.; Lieu, D.K. Same-Single-Cell Analysis of Pacemaker-Specific Markers in Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Subtypes Classified by Electrophysiology. Stem Cells 2016, 34, 2670–2680. [Google Scholar] [CrossRef]
- Biendarra-Tiegs, S.M.; Li, X.; Ye, D.; Brandt, E.B.; Ackerman, M.J.; Nelson, T.J. Single-Cell RNA-Sequencing and Optical Electrophysiology of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Reveal Discordance Between Cardiac Subtype-Associated Gene Expression Patterns and Electrophysiological Phenotypes. Stem Cells Dev. 2019, 28, 659–673. [Google Scholar] [CrossRef]
- Huang, C.-F.; Chen, Y.-C.; Yeh, H.-I.; Chen, S.-A. Mononucleated and binucleated cardiomyocytes in left atrium and pulmonary vein have different electrical activity and calcium dynamics. Prog. Biophys. Mol. Biol. 2012, 108, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.-W.; Chen, S.; Geng, L.; Shum, A.M.-Y.; Sun, D.; Li, R.A. Increasing the physical size and nucleation status of human pluripotent stem cell-derived ventricular cardiomyocytes by cell fusion. Stem Cell Res. 2017, 19, 76–81. [Google Scholar] [CrossRef]
- Yekelchyk, M.; Guenther, S.; Preussner, J.; Braun, T. Mono- and multi-nucleated ventricular cardiomyocytes constitute a transcriptionally homogenous cell population. Basic Res. Cardiol. 2019, 114, 36. [Google Scholar] [CrossRef] [PubMed]
- Windmueller, R.; Leach, J.P.; Babu, A.; Zhou, S.; Morley, M.P.; Wakabayashi, A.; Petrenko, N.B.; Viatour, P.; Morrisey, E.E. Direct Comparison of Mononucleated and Binucleated Cardiomyocytes Reveals Molecular Mechanisms Underlying Distinct Proliferative Competencies. Cell Rep. 2020, 30, 3105–3116.e3104. [Google Scholar] [CrossRef]
- Selfridge, J.; Pow, A.M.; McWhir, J.; Magin, T.M.; Melton, D.W. Gene targeting using a mouse HPRT minigene/HPRT-deficient embryonic stem cell system: Inactivation of the mouseERCC-1 gene. Somat. Cell Mol. Genet. 1992, 18, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Do, J.T.; Schöler, H.R. Nuclei of Embryonic Stem Cells Reprogram Somatic Cells. Stem Cells 2004, 22, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Hescheler, J.; Šaric, T. Chromosome Tracking in Fused Cells by Single Nucleotide Polymorphisms. Methods Mol. Biol. 2015, 1313, 95–106. [Google Scholar] [CrossRef]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981, 391, 85–100. [Google Scholar] [CrossRef]

| CM Origin | AP Type | Peak (mV) | MDP (mV) | Height (mV) | Vdd (mV/s) | Frequency (1/min) | Vmax (V/s) | APD90 (ms) | APD90/ APD50 | Cells (N) | Cells (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HM-1 | UD | 23 ± 9 | −49 ± 5 | 72 ± 8 | 0.06 ± 0.01 | 136 ± 27 | 13 ± 4 | 83 ± 14 | 2.7 ± 0.8 | 7 | 26 |
| A | 29 ± 4 | −53 ± 5 | 82 ± 7 | 0.06 ± 0.02 | 168 ± 39 | 21 ± 4 | 86 ± 15 | 2.5 ± 0.3 | 6 | 22 | |
| V | 36 ± 2 | −56 ± 2 | 90 ± 3 | 0.07 ± 0.01 | 163 ± 24 | 23 ± 2 | 136 ± 13 | 1.6 ± 0.1 | 14 | 52 | |
| FH-2.1 | UD | 22 ± 3 | −41 ± 4 | 63 ± 6 | 0.06 ± 0.01 | 125 ± 14 | 6 ± 1 | 100 ± 23 | 2.4 ± 0.2 | 8 | 33 |
| A | 38 ± 6 | −52 ± 2 | 90 ± 5 | 0.03 ± 0.01 | 167 ± 36 | 21 ± 2 | 86 ± 14 | 3.7 ± 0.8 | 11 | 46 | |
| V | 36 ± 1 | −54 ± 4 | 93 ± 3 | 0.03 ± 0.02 | 118 ± 45 | 21 ± 4 | 122 ± 14 | 1.5 ± 0.1 | 5 | 21 | |
| FH-4.2 | UD | 25 ± 2 | −49 ± 2 | 74 ± 2 | 0.11 ± 0.02 | 190 ± 42 | 8 ± 2 | 79 ± 10 | 3.8 ± 1.3 | 8 | 33 |
| A | 25 ± 4 | −57 ± 2 | 83 ± 4 | 0.07 ± 0.01 | 182 ± 16 | 21 ± 2 | 77 ± 8 | 3.4 ± 0.6 | 13 | 54 | |
| V | 29 ± 4 | −48 ± 14 | 78 ± 18 | 0.07 ± 0.01 | 119 ± 18 | 12 ± 3 | 194 ± 74 | 1.5 ± 0.1 | 3 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Fatima, A.; Breitbach, M.; Kuzmenkin, A.; Fügemann, C.J.; Ivanyuk, D.; Kim, K.P.; Cantz, T.; Pfannkuche, K.; Schöler, H.R.; et al. Electrophysiological Properties of Tetraploid Cardiomyocytes Derived from Murine Pluripotent Stem Cells Generated by Fusion of Adult Somatic Cells with Embryonic Stem Cells. Int. J. Mol. Sci. 2023, 24, 6546. https://doi.org/10.3390/ijms24076546
Xu G, Fatima A, Breitbach M, Kuzmenkin A, Fügemann CJ, Ivanyuk D, Kim KP, Cantz T, Pfannkuche K, Schöler HR, et al. Electrophysiological Properties of Tetraploid Cardiomyocytes Derived from Murine Pluripotent Stem Cells Generated by Fusion of Adult Somatic Cells with Embryonic Stem Cells. International Journal of Molecular Sciences. 2023; 24(7):6546. https://doi.org/10.3390/ijms24076546
Chicago/Turabian StyleXu, Guoxing, Azra Fatima, Martin Breitbach, Alexey Kuzmenkin, Christopher J. Fügemann, Dina Ivanyuk, Kee Pyo Kim, Tobias Cantz, Kurt Pfannkuche, Hans R. Schöler, and et al. 2023. "Electrophysiological Properties of Tetraploid Cardiomyocytes Derived from Murine Pluripotent Stem Cells Generated by Fusion of Adult Somatic Cells with Embryonic Stem Cells" International Journal of Molecular Sciences 24, no. 7: 6546. https://doi.org/10.3390/ijms24076546
APA StyleXu, G., Fatima, A., Breitbach, M., Kuzmenkin, A., Fügemann, C. J., Ivanyuk, D., Kim, K. P., Cantz, T., Pfannkuche, K., Schöler, H. R., Fleischmann, B. K., Hescheler, J., & Šarić, T. (2023). Electrophysiological Properties of Tetraploid Cardiomyocytes Derived from Murine Pluripotent Stem Cells Generated by Fusion of Adult Somatic Cells with Embryonic Stem Cells. International Journal of Molecular Sciences, 24(7), 6546. https://doi.org/10.3390/ijms24076546








