Those That Remain: Sorption/Desorption Behaviour and Kinetics of the Neonicotinoids Still in Use
Abstract
1. Introduction
2. Results
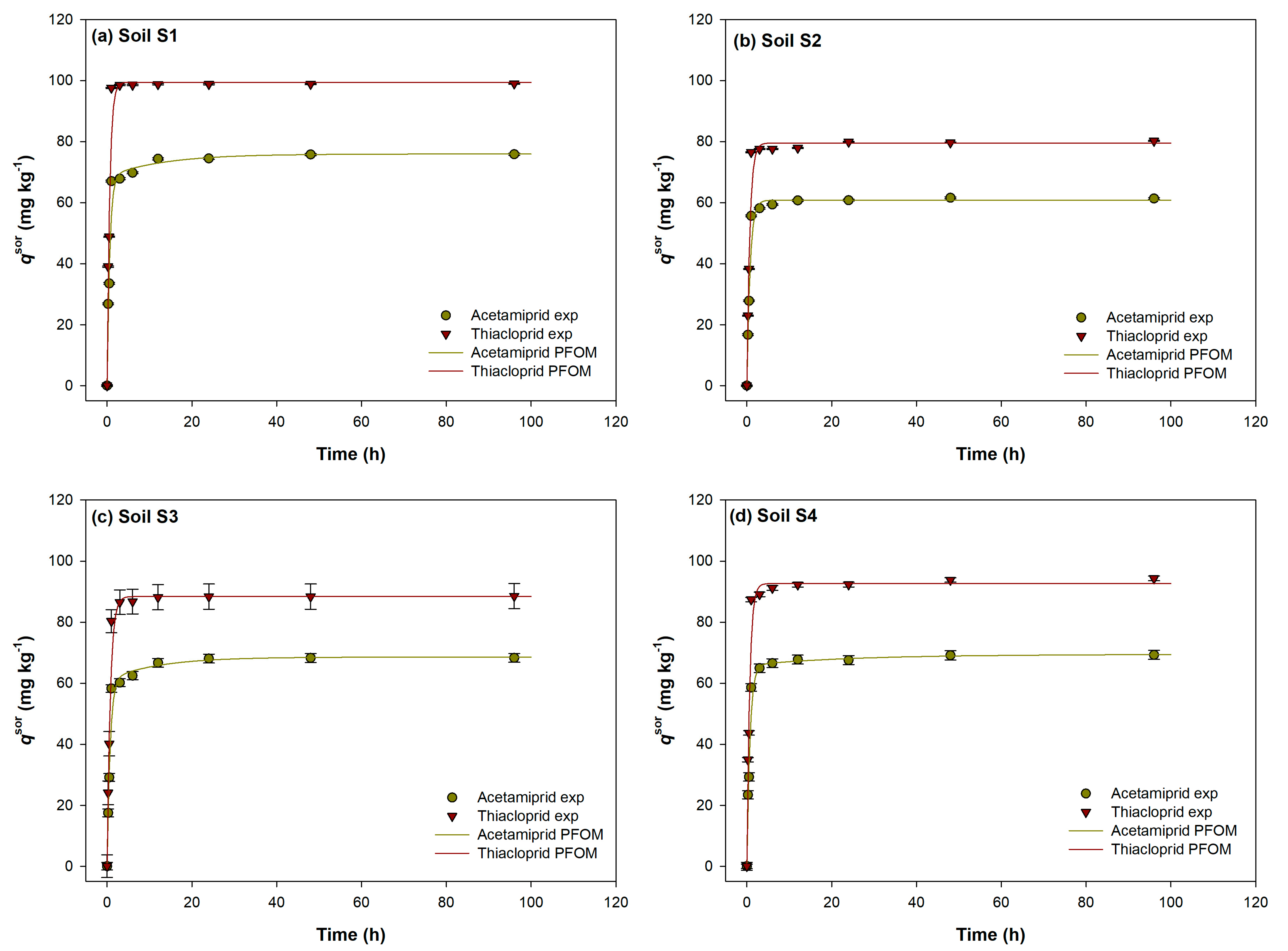
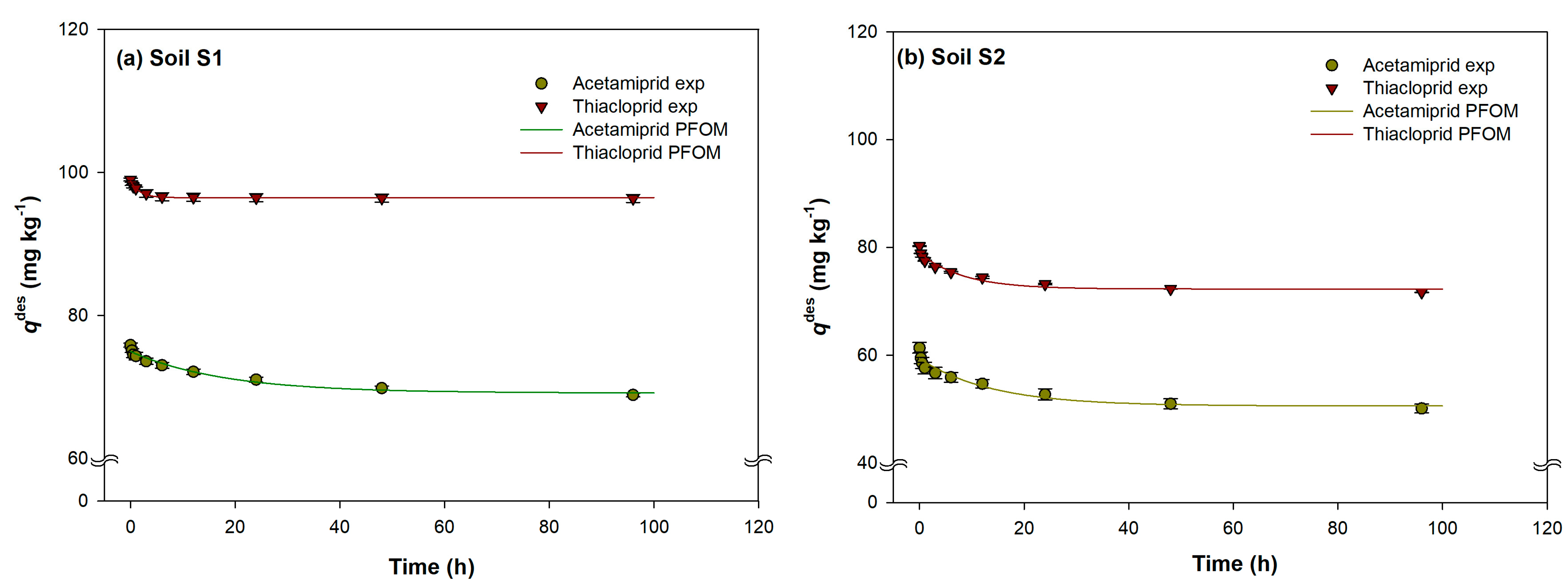
2.1. Evaluation of Acetamiprid and Thiacloprid Sorption and Desorption Equilibrium Time in Experimental Soils
2.2. Sorption/Desorption Kinetic Models
2.2.1. Pseudo-First Order Model (PFOM)
2.2.2. Elovich’s Model (EM)
2.2.3. Intraparticle Diffusion Model (WMM)
2.2.4. Two-Site Model (TSM)
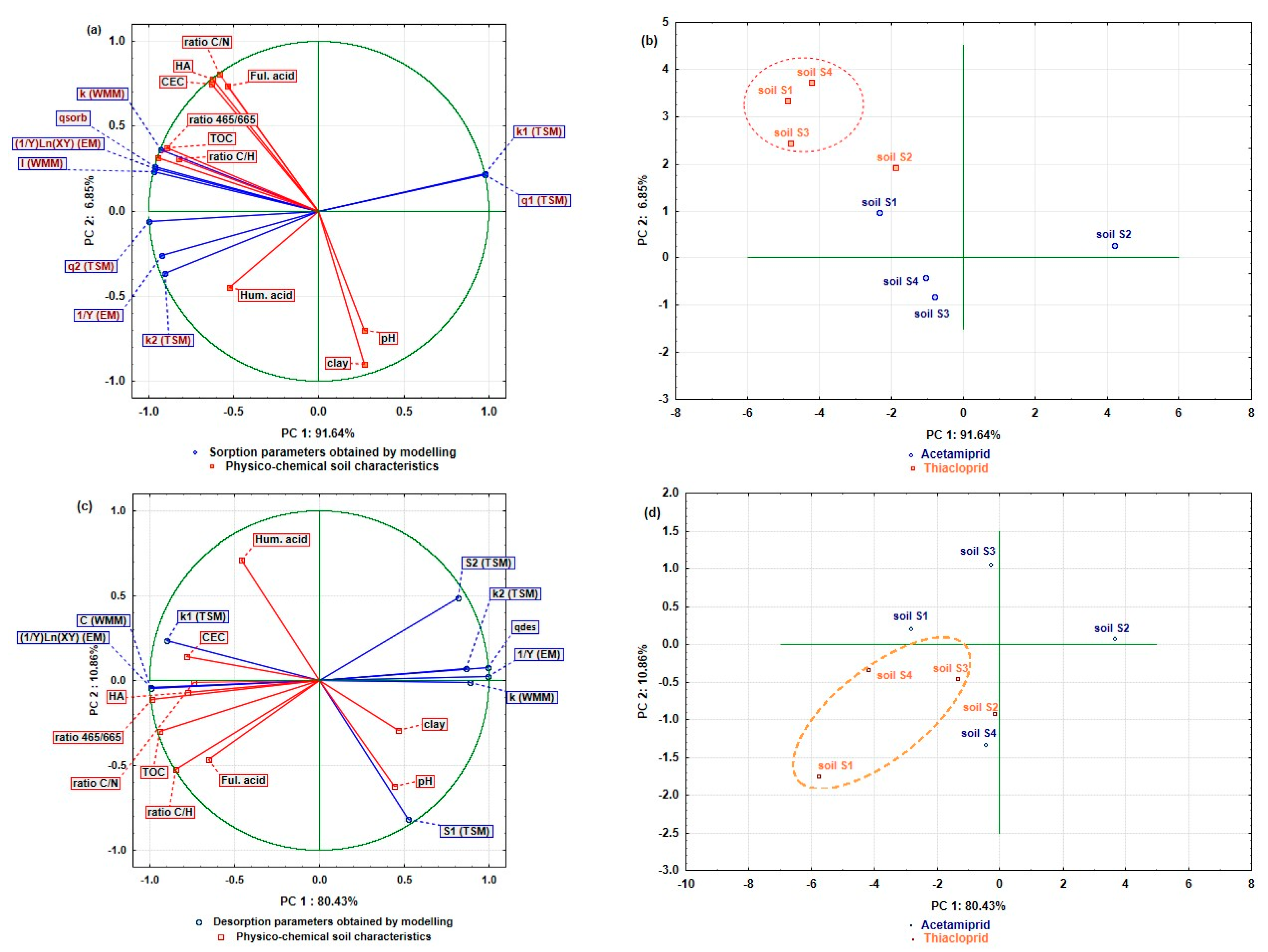
2.3. Effect of Physico-Chemical Soil Characteristics on Acetamiprid/Thiacloprid Sorption/Desorption Parameters
2.4. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals
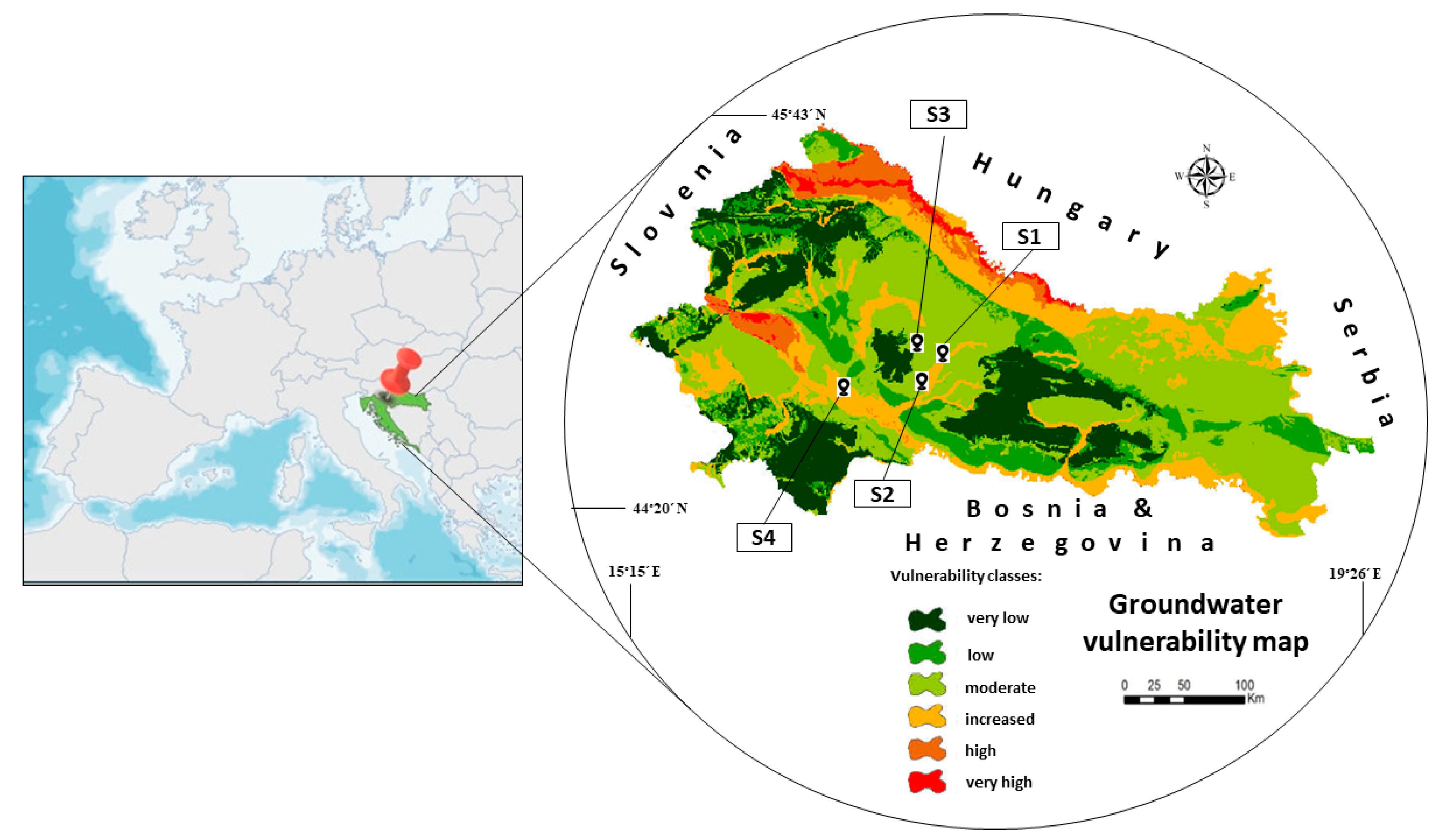
4.2. Experimental Soils and Physico-Chemical Characteristics Soil Determination
4.3. Sorption/Desorption Kinetic Experiments
4.4. Instrumentation and Operating Conditions
4.5. MS/MS (Detector AB SCIEX 4500 QTRAP) Conditions
4.6. Sorption/Desorption Kinetic Models
4.6.1. Lagergren’s Pseudo First-Order Model (PFOM)
4.6.2. Nonequlibrium Two-Site Model (TSM)
4.6.3. Weber–Morris Intraparticle Diffusion Model (WMM)
4.6.4. Elovich’s Model (EM)
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexandratos, N.; Bruinsma, J.; Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; FAO: Rome, Italy, 2012. [Google Scholar] [CrossRef]
- FAO. Proceedings of the Expert Meeting on How to Feed the World in 2050: 24–26 June 2009, FAO Headquarters, Rome; FAO: Rome, Italy, 2009; ISBN 978-92-5-106363-7. [Google Scholar]
- Rosegrant, M.W.; Ringler, C.; Zhu, T. Water for Agriculture: Maintaining Food Security under Growing Scarcity. Annu. Rev. Environ. Resour. 2009, 34, 205–222. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the Status and Global Strategy for Neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- Maienfisch, P.; Angst, M.; Brandl, F.; Fischer, W.; Hofer, D.; Kayser, H.; Kobel, W.; Rindlisbacher, A.; Senn, R.; Steinemann, A.; et al. Chemistry and Biology of Thiamethoxam: A Second Generation Neonicotinoid. Pest. Manag. Sci. 2001, 57, 906–913. [Google Scholar] [CrossRef]
- Mörtl, M.; Kereki, O.; Darvas, B.; Klátyik, S.; Vehovszky, Á.; Győri, J.; Székács, A. Study on Soil Mobility of Two Neonicotinoid Insecticides. J. Chem. 2016, 2016, 4546584. [Google Scholar] [CrossRef]
- Yamamuro, M.; Komuro, T.; Kamiya, H.; Kato, T.; Hasegawa, H.; Kameda, Y. Neonicotinoids Disrupt Aquatic Food Webs and Decrease Fishery Yields. Science 2019, 366, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Poliserpi, M.B.; Cristos, D.S.; Brodeur, J.C. Imidacloprid Seed Coating Poses a Risk of Acute Toxicity to Small Farmland Birds: A Weight-of-Evidence Analysis Using Data from the Grayish Baywing Agelaioides Badius. Sci. Total Environ. 2021, 763, 142957. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Foppen, R.P.B.; van Turnhout, C.A.M.; de Kroon, H.; Jongejans, E. Declines in Insectivorous Birds Are Associated with High Neonicotinoid Concentrations. Nature 2014, 511, 341–343. [Google Scholar] [CrossRef]
- Yan, S.; Meng, Z.; Tian, S.; Teng, M.; Yan, J.; Jia, M.; Li, R.; Zhou, Z.; Zhu, W. Neonicotinoid Insecticides Exposure Cause Amino Acid Metabolism Disorders, Lipid Accumulation and Oxidative Stress in ICR Mice. Chemosphere 2020, 246, 125661. [Google Scholar] [CrossRef]
- Goulson, D. 232 signatories Call to Restrict Neonicotinoids. Science 2018, 360, 973. [Google Scholar] [CrossRef]
- Goulson, D. REVIEW: An Overview of the Environmental Risks Posed by Neonicotinoid Insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Wood, T.J.; Goulson, D. The Environmental Risks of Neonicotinoid Pesticides: A Review of the Evidence Post 2013. Env. Sci. Pollut Res. 2017, 24, 17285–17325. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Evaluation of the Data on Clothianidin, Imidacloprid and Thiamethoxam for the Updated Risk Assessment to Bees for Seed Treatments and Granules in the EU. EFS3 2018, 31. [Google Scholar] [CrossRef]
- Andres, P. EU Court Puts End to Emergency Use of Bee-Toxic Pesticides. Euractiv 2023. Available online: https://www.euractiv.com/section/agriculture-food/news/eu-high-court-bans-use-of-bee-toxic-pesticides/ (accessed on 29 January 2023).
- EFSA Panel on Plant Protection Products and their Residues (PPR); Jerez, A.H.; Adriaanse, P.; Berny, P.; Coja, T.; Duquesne, S.; Focks, A.; Marinovich, M.; Millet, M.; Pelkonen, O.; et al. Statement on the Active Substance Acetamiprid. EFSA J. 2022, 20, 7031. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An International Database for Pesticide Risk Assessments and Management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Wang, W.; Chen, H.; Gao, D.; Long, J.; Long, H.; Zhang, Y. Dissipation and Dietary Risk Assessment of Thiacloprid and Tolfenpyrad in Tea in China. Agronomy 2022, 12, 3166. [Google Scholar] [CrossRef]
- Rodríguez-Liébana, J.A.; Mingorance, M.D.; Peña, A. Thiacloprid Adsorption and Leaching in Soil: Effect of the Composition of Irrigation Solutions. Sci. Total Environ. 2018, 610–611, 367–376. [Google Scholar] [CrossRef]
- Yi, X.; Zhang, C.; Liu, H.; Wu, R.; Tian, D.; Ruan, J.; Zhang, T.; Huang, M.; Ying, G. Occurrence and Distribution of Neonicotinoid Insecticides in Surface Water and Sediment of the Guangzhou Section of the Pearl River, South China. Environ. Pollut. 2019, 251, 892–900. [Google Scholar] [CrossRef]
- Dankyi, E.; Gordon, C.; Carboo, D.; Apalangya, V.A.; Fomsgaard, I.S. Sorption and Degradation of Neonicotinoid Insecticides in Tropical Soils. J. Environ. Sci. Health Part B 2018, 53, 587–594. [Google Scholar] [CrossRef]
- Xie, Z.; Lu, G.; Yu, Y. Early-Stage High-Concentration Thiacloprid Exposure Induced Persistent Behavioral Alterations in Zebrafish. IJERPH 2022, 19, 10920. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Bi, G.; Ward, T.; Li, L. Adsorption and Degradation of Neonicotinoid Insecticides in Agricultural Soils. Environ. Sci. Pollut. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Hladik, M.L.; Kolpin, D.W. First National-Scale Reconnaissance of Neonicotinoid Insecticides in Streams across the USA. Environ. Chem. 2016, 13, 12. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Hyne, R.V. Detection and Analysis of Neonicotinoids in River Waters—Development of a Passive Sampler for Three Commonly Used Insecticides. Chemosphere 2014, 99, 143–151. [Google Scholar] [CrossRef]
- Mitchell, E.A.D.; Mulhauser, B.; Mulot, M.; Mutabazi, A.; Glauser, G.; Aebi, A. A Worldwide Survey of Neonicotinoids in Honey. Science 2017, 358, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Republic of Croatia. Hrvatske Vode, Effect of Agriculture on Surface Waters and Groundwaters Pollution in Croatia 2014, Report in Croatian: Utjecaj Poljoprivrede Na Onečišćenje Površinskih I Podzemnih Voda u Republici Hrvatskoj. Available online: https://www.Voda.Hr/Sites/Default/Files/Dokumenti/Utjecaj_poljoprivrede_na_Onečišćenje_površinskih_i_podzemnih_voda_u_Republici_Hrvatskoj.Pdf (accessed on 19 December 2022).
- Gasparic, H.V.; Grubelic, M.; Uzelac, V.D.; Bazok, R.; Cacija, M.; Drmic, Z.; Lemic, D. Neonicotinoid Residues in Sugar Beet Plants and Soil under Different Agro-Climatic Conditions. Agriculture 2020, 10, 484. [Google Scholar] [CrossRef]
- Thompson, D.A.; Lehmler, H.-J.; Kolpin, D.W.; Hladik, M.L.; Vargo, J.D.; Schilling, K.E.; LeFevre, G.H.; Peeples, T.L.; Poch, M.C.; LaDuca, L.E.; et al. A Critical Review on the Potential Impacts of Neonicotinoid Insecticide Use: Current Knowledge of Environmental Fate, Toxicity, and Implications for Human Health. Environ. Sci. Process. Impacts 2020, 22, 1315–1346. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. USEPA Fact Sheet for Acetamiprid. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-099050_15-Mar-02.pdf (accessed on 3 January 2023).
- United States Environmental Protection Agency. USEPA Fact Sheet for Thiacloprid. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-014019_26-Sep-03.pdf (accessed on 3 January 2023).
- Petković Didović, M.; Kowalkowski, T.; Broznić, D. Emerging Contaminant Imidacloprid in Mediterranean Soils: The Risk of Accumulation Is Greater than the Risk of Leaching. Toxics 2022, 10, 358. [Google Scholar] [CrossRef]
- Aseperi, A.K.; Busquets, R.; Hooda, P.S.; Cheung, P.C.W.; Barker, J. Behaviour of Neonicotinoids in Contrasting Soils. J. Environ. Manag. 2020, 276, 111329. [Google Scholar] [CrossRef]
- Broznić, D.; Marinić, J.; Tota, M.; Jurešić, G.Č.; Petković, O.; Milin, Č. Hysteretic Behavior of Imidacloprid Sorption-Desorption in Soils of Croatian Coastal Regions. Soil Sediment Contam. Int. J. 2012, 21, 850–871. [Google Scholar] [CrossRef]
- Kah, M.; Walch, H.; Hofmann, T. Environmental Fate of Nanopesticides: Durability, Sorption and Photodegradation of Nanoformulated Clothianidin. Environ. Sci. Nano 2018, 5, 882–889. [Google Scholar] [CrossRef]
- Broznić, D.; Didović, M.P.; Rimac, V.; Marinić, J. Sorption and Leaching Potential of Organophosphorus Insecticide Dimethoate in Croatian Agricultural Soils. Chemosphere 2021, 273, 128563. [Google Scholar] [CrossRef] [PubMed]
- Calvet, R. Adsorption of Organic Chemicals in Soils. Environ. Health Perspect. 1989, 83, 145–177. [Google Scholar] [CrossRef] [PubMed]
- Oliver, D.P.; Kookana, R.S.; Quintana, B. Sorption of Pesticides in Tropical and Temperate Soils from Australia and the Philippines. J. Agric. Food Chem. 2005, 53, 6420–6425. [Google Scholar] [CrossRef]
- Zhang, P.; Ren, C.; Sun, H.; Min, L. Sorption, Desorption and Degradation of Neonicotinoids in Four Agricultural Soils and Their Effects on Soil Microorganisms. Sci. Total Environ. 2018, 615, 59–69. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA) Peer Review of the Pesticide Risk Assessment of the Active Substance Acetamiprid. EFSA Journal 2016, 14, 4610. [CrossRef]
- European Food Safety Authority (EFSA); Abdourahime, H.; Anastassiadou, M.; Arena, M.; Auteri, D.; Barmaz, S.; Brancato, A.; Brocca, D.; Bura, L.; Carrasco Cabrera, L.; et al. Peer Review of the Pesticide Risk Assessment of the Active Substance Thiacloprid. EFSA J. 2019, 17, 5595. [Google Scholar] [CrossRef]
- Bonmatin, J.-M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.D.; et al. Environmental Fate and Exposure; Neonicotinoids and Fipronil. Env. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef] [PubMed]
- Kurwadkar, S.T.; Dewinne, D.; Wheat, R.; McGahan, D.G.; Mitchell, F.L. Time Dependent Sorption Behavior of Dinotefuran, Imidacloprid and Thiamethoxam. J. Environ. Sci. Health Part B 2013, 48, 237–242. [Google Scholar] [CrossRef]
- Fernández-Bayo, J.D.; Nogales, R.; Romero, E. Evaluation of the Sorption Process for Imidacloprid and Diuron in Eight Agricultural Soils from Southern Europe Using Various Kinetic Models. J. Agric. Food Chem. 2008, 56, 5266–5272. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic Models of Sorption: A Theoretical Analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef]
- Haerifar, M.; Azizian, S. Mixed Surface Reaction and Diffusion-Controlled Kinetic Model for Adsorption at the Solid/Solution Interface. J. Phys. Chem. C 2013, 117, 8310–8317. [Google Scholar] [CrossRef]
- Nelson, P.N. A Density Functional Theoretical Study of the Hydrolysis Mechanism of Three Neonicotinoid Based Pesticides. J. Mol. Struct. 2021, 1230, 129909. [Google Scholar] [CrossRef]
- Rodríguez-Liébana, J.A.; Peña, A. Differences in the Sorption Kinetics of Various Non-Ionisable Pesticides in a Limited Number of Agricultural Soils from the Mediterranean Basin. J. Environ. Manag. 2020, 276, 111336. [Google Scholar] [CrossRef] [PubMed]
- Francisco, R.; Almeida, C.; Sousa, A.C.A.; Neves, M.C.; Freire, M.G. High Performance of Ionic-Liquid-Based Materials to Remove Insecticides. IJMS 2022, 23, 2989. [Google Scholar] [CrossRef]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical Models of Sorption Kinetics Including a Surface Reaction Mechanism: A Review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef]
- Salvestrini, S.; Canzano, S.; Iovino, P.; Leone, V.; Capasso, S. Modelling the Biphasic Sorption of Simazine, Imidacloprid, and Boscalid in Water/Soil Systems. J. Environ. Sci. Health Part B 2014, 49, 578–590. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations FAO. Specifications and Evaluations for Agricultural Pesticides: Thiacloprid; FAO: Washington, WA, USA, 1977. [Google Scholar]
- Abraham, M.H.; Le, J. The Correlation and Prediction of the Solubility of Compounds in Water Using an Amended Solvation Energy Relationship. J. Pharm. Sci. 1999, 88, 868–880. [Google Scholar] [CrossRef]
- Prabhakar, Y.S.; Raja Solomon, V.; Gupta, M.K.; Katti, S.B. QSAR Studies on Thiazolidines: A Biologically Privileged Scaffold. In QSAR and Molecular Modeling Studies in Heterocyclic Drugs II.; Gupta, S.P., Ed.; Topics in Heterocyclic Chemistry; Springer-Verlag: Berlin/Heidelberg, Germany, 2006; Volume 4, pp. 161–249. ISBN 978-3-540-33233-6. [Google Scholar]
- Kagabu, S. Studies on the Synthesis and Insecticidal Activity of Neonicotinoid Compounds. J. Pestic. Sci. 1996, 21, 231–239. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R. Neonicotinoids-from Zero to Hero in Insecticide Chemistry. Pest. Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef]
- Chandran, J.; Aravind, U.K.; Rajalakshmi, C.; Thomas, V.I.; Nguyen, P.T.; Aravindakumar, C.T. Solvent Dependent ESI-Collisionally Induced Dissociation of Protonated Nitenpyram. Int. J. Mass Spectrom. 2019, 445, 116207. [Google Scholar] [CrossRef]
- Fusetto, R.; White, J.M.; Hutton, C.A.; O’Hair, R.A.J. Structure of Olefin–Imidacloprid and Gas-Phase Fragmentation Chemistry of Its Protonated Form. Org. Biomol. Chem. 2016, 14, 1715–1726. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Standard Operating Procedures. Soil Sampling; U.S. EPA Contract 68-C4-0022; EPA: Washington, WA, USA, 2012. Available online: https://dem.ri.gov/sites/g/files/xkgbur861/files/pubs/sops/wmsr2012.pdf (accessed on 19 December 2022).
- Kroetsch, D.; Wang, C. Particle Size Distribution. In Soil Sampling and Methods of Analysis; Carter, M.R., Gregorich, E.H., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 713–727. [Google Scholar]
- Hendershot, W.H.; Lalande, H.; Duquette, M. Soil Reaction and Exchangeable Acidity. In Soil Sampling and Methods of Analysis; Carter, M.R., Gregorich, E.H., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 173–179. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation Exchange Capacity and Exchange Coefficients. In SSSA Book Series; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 2018; pp. 1201–1229. ISBN 978-0-89118-866-7. [Google Scholar]
- Skjemstad, J.O.; Baldock, J.A. Total and Organic Carbon. In Soil Sampling and Methods of Analysis; Carter, M.R., Gregorich, E.H., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 225–239. [Google Scholar]
- Kononova, M.M.; Belcikova, N.P. A Rapid Analysis of Humus Composition in Mineral Soil. Pochvovedenie 1961, 10, 75–87. [Google Scholar]
- OECD. OECD Guidelines for Testing of Chemicals, Proposal for Updating Guideline No 106:Adsorption/Desorption Using a Batch Equilibrium Method, pp. 1e45. Paris, France. 2000. Available online: www.oecd-ilibrary.org/test-no-106-adsorption-desorption-using-a-batch-equilibrium-method_5lmqcr2k7tkl.pdf?itemId=%2Fcontent%2Fpublication%2F9789264069602-en&mimeType=pdf (accessed on 19 December 2022).
- Willis, B.G.; Woodruff, W.H.; Frysinger, J.R.; Margerum, D.W.; Pardue, H.L. Simultaneous Kinetic Determination of Mixtures by On-Line Regression Analysis. Anal. Chem. 1970, 42, 1350–1355. [Google Scholar] [CrossRef]
- van Genuchten, M.T.; Wagenet, R.J. Two-Site/Two-Region Models for Pesticide Transport and Degradation: Theoretical Development and Analytical Solutions. Soil Sci. Soc. Am. J. 1989, 53, 1303–1310. [Google Scholar] [CrossRef]
- Gamerdinger, A.P.; Wagenet, R.J.; van Genuchten, M.T. Application of Two-Site/Two-Region Models for Studying Simultaneous Nonequilibrium Transport and Degradation of Pesticides. Soil Sci. Soc. Am. J. 1990, 54, 957–963. [Google Scholar] [CrossRef]
- Gaber, H.M.; Inskeep, W.P.; Comfort, S.D.; Wraith, J.M. Nonequilibrium Transport of Atrazine through Large Intact Soil Cores. Soil Sci. Soc. Am. J. 1995, 59, 60–67. [Google Scholar] [CrossRef]
- FOCUS. Guidance Document on Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration; Report of the FOCUS Work Group on Degradation Kinetics, EC Document Reference Sanco/10058/2005 Version 2.0; FOCUS: Golden, CO, USA, 2006; p. 434. [Google Scholar]

| Physico-Chemical Characteristics | Soil | |||
|---|---|---|---|---|
| S1 | S2 | S3 | S4 | |
| Textural classes | clay loam | clay loam | clay loam | clay loam |
| pH (a) | 4.94 (±0.11) | 5.29 (±0.06) | 5.25 (±0.04) | 5.55 (±0.04) |
| HA (b) (cmol/kg) | 13.39 (±1.02) | 4.62 (±0.46) | 4.59 (±0.44) | 6.59 (±0.26) |
| CEC (c) (cmol/kg) | 60.76 (±4.26) | 48.28 (±1.54) | 49.76 (±1.91) | 49.59 (±1.69) |
| Clay (%) | 30.75 (±1.25) | 35.26 (±0.86) | 36.62 (±0.67) | 37.60 (±1.07) |
| Ca2+ (mg/100 g) | 38.9 (±0.6) | 25.7 (±1.9) | 20.4 (±3.9) | 23.0 (±2.9) |
| Mg2+ (mg/100 g) | 450.8 (±33.8) | 401.1 (±21.6) | 447.0 (±34.81) | 352.4 (±24.4) |
| Na+ (mg/100 g) | 23.4 (±57.2) | 30.9 (±4.5) | 28.5 (±8.7) | 31.5 (±5.4) |
| K+ (mg/100 g) | 286.7 (±32.9) | 315.1 (±46.4) | 240.8 (±29.1) | 449.5 (±5.4) |
| humus (%) | 2.64 (±0.03) | 1.78 (±0.02) | 2.01 (±0.34) | 2.95 (±0.13) |
| TOC (d) (%) | 2.59 (±0.10) | 1.06 (±0.15) | 1.71 (±0.01) | 2.21 (±0.05) |
| CoxHa (e) (%) | 0.56 (±0.06) | 0.42 (±0.06) | 0.74 (±0.14) | 0.47 (±0.10) |
| CoxFa (f) (%) | 1.06 (±0.08) | 0.32 (±0.03) | 0.10 (±0.01) | 0.70 (±0.03) |
| N (%) | 0.22 (±0.009) | 0.13 (±0.002) | 0.18 (±0.002) | 0.22 (±0.011) |
| C (%) | 2.13 (±0.014) | 0.95 (±0.018) | 1.28 (±0.005) | 1.73 (±0.040) |
| H (%) | 0.59 (±0.005) | 0.37 (±0.005) | 0.46 (±0.009) | 0.49 (±0.014) |
| ratio C/H | 3.58 (±0.02) | 2.54 (±0.05) | 2.81 (±0.07) | 3.51 (±0.03) |
| ratio C/N | 9.63 (±0.44) | 7.39 (±0.05) | 7.32 (±0.13) | 7.71 (±0.23) |
| ratio 465/665 | 8.20 (±0.31) | 5.45 (±0.30) | 6.76 (±0.09) | 7.19 (±0.15) |
| Sorption | Desorption | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Statistical Parameter | S1 | S2 | S3 | S4 | Statistical Parameter | S1 | S2 | S3 | S4 |
| Pseudo First-Order (PFOM) | |||||||||
| R2 (a) | 0.9973 | 0.9981 | 0.9961 | 0.9976 | R2 (a) | 0.9999 | 0.9995 | 0.9999 | 0.9999 |
| SRMSE (b) | 0.0581 | 0.0463 | 0.0660 | 0.0518 | SRMSE (b) | 0.0069 | 0.0215 | 0.0115 | 0.0112 |
| err-% (c) | 4.67 | 3.71 | 5.30 | 4.16 | err% (c) | 0.58 | 1.81 | 0.97 | 0.99 |
| m (d) | 8 (χ2 tab = 15.507 at p = 0.05) | m (d) | 7 (χ2 tab = 14.067 at p = 0.05) | ||||||
| Nonequlibrium Two-Site (TSM) | |||||||||
| R2 (a) | 0.9999 | 0.9999 | 0.9999 | 0.9999 | R2 (a) | 0.9999 | 0.9999 | 0.9999 | 0.9999 |
| SRMSE (b) | 0.0107 | 0.0123 | 0.0058 | 0.0120 | SRMSE (b) | 0.0033 | 0.0077 | 0.0020 | 0.0019 |
| err-% (c) | 0.96 | 1.10 | 0.51 | 1.07 | err-% (c) | 0.31 | 0.73 | 0.19 | 0.18 |
| m (d) | 6 (χ2 tab = 12.592 at p = 0.05) | m (d) | 6 (χ2 tab = 12.592 at p = 0.05) | ||||||
| Elovich (EM) | |||||||||
| R2 (a) | 0.9880 | 0.9789 | 0.9534 | 0.9360 | R2 (a) | 0.9050 | 0.9503 | 0.9449 | 0.9537 |
| SRMSE (b) | 0.0437 | 0.0774 | 0.0967 | 0.1322 | SRMSE (b) | 0.1268 | 0.0803 | 0.0888 | 0.0841 |
| err-% (c) | 3.51 | 5.92 | 7.75 | 10.61 | err-% (c) | 10.18 | 6.45 | 7.13 | 6.76 |
| m (d) | 8 (χ2 tab = 15.507 at p = 0.05) | m (d) | 8 (χ2 tab = 15.507 at p = 0.05) | ||||||
| Weber–Morris (WMM) | |||||||||
| R2 (a) | 0.9172 | 0.7143 | 0.7370 | 0.8626 | R2 (a) | 0.6170 | 0.6819 | 0.9682 | 0.9576 |
| SRMSE (b) | 0.1864 | 0.2956 | 0.2903 | 0.2055 | SRMSE (b) | 0.3896 | 0.2986 | 0.0654 | 0.0765 |
| err-% (c) | 14.97 | 23.73 | 23.31 | 16.51 | err-% (c) | 32.68 | 24.03 | 5.96 | 7.88 |
| m (d) | 8 (χ2 tab = 15.507 at p = 0.05) | m (d) | 8 (χ2 tab = 15.507 at p = 0.05) | ||||||
| Sorption | Desorption | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Statistical Parameter | S1 | S2 | S3 | S4 | Statistical Parameter | S1 | S2 | S3 | S4 |
| Pseudo First-Order (PFOM) | |||||||||
| R2 (a) | 0.9997 | 0.9995 | 0.9989 | 0.9992 | R2 (a) | 0.9999 | 0.9999 | 0.9999 | 0.9999 |
| SRMSE (b) | 0.0191 | 0.0231 | 0.0348 | 0.0290 | SRMSE (b) | 0.0012 | 0.0083 | 0.0076 | 0.0018 |
| err-% (c) | 1.53 | 1.86 | 2.78 | 2.33 | err-% (c) | 0.10 | 0.70 | 0.64 | 0.15 |
| m (d) | 8 (χ2 tab = 15.507 at p = 0.05) | m (d) | 7 (χ2 tab = 14.067 at p = 0.05) | ||||||
| Nonequlibrium Two-Site (TSM) | |||||||||
| R2 (a) | 0.9999 | 0.9999 | 0.9999 | 0.9999 | R2 (a) | 0.9999 | 0.9999 | 0.9999 | 0.9999 |
| SRMSE (b) | 0.0011 | 0.0131 | 0.0070 | 0.0066 | SRMSE (b) | 0.0076 | 0.0017 | 0.0018 | 0.0007 |
| err-% (c) | 0.10 | 1.17 | 0.62 | 0.59 | err-% (c) | 0.72 | 0.16 | 0.17 | 0.06 |
| m (d) | 6 (χ2 tab = 12.592 at p = 0.05) | m (d) | 6 (χ2 tab = 12.592 at p = 0.05) | ||||||
| Elovich (EM) | |||||||||
| R2 (a) | 0.7225 | 0.6670 | 0.7156 | 0.8365 | R2 (a) | 0.9030 | 0.9924 | 0.9911 | 0.9748 |
| SRMSE (b) | 0.2572 | 0.2963 | 0.2668 | 0.2419 | SRMSE (b) | 0.1333 | 0.0126 | 0.0169 | 0.0452 |
| err-% (c) | 20.65 | 23.79 | 21.42 | 19.42 | err-% (c) | 10.71 | 1.01 | 1.36 | 3.63 |
| m (d) | 8 (χ2 tab = 15.507 at p = 0.05) | m (d) | 8 (χ2 tab = 15.507 at p = 0.05) | ||||||
| Weber–Morris (WMM) | |||||||||
| R2 (a) | 0.7325 | 0.4635 | 0.5851 | 0.5524 | R2 (a) | 0.5974 | 0.8860 | 0.7987 | 0.7477 |
| SRMSE (b) | 0.3061 | 0.4025 | 0.3204 | 0.3312 | SRMSE (b) | 0.3578 | 0.1682 | 0.2356 | 0.2576 |
| err-% (c) | 24.58 | 35.31 | 29.36 | 30.11 | err-% (c) | 30.12 | 14.78 | 18.56 | 20.46 |
| m (d) | 8 (χ2 tab = 15.507 at p = 0.05) | m (d) | 8 (χ2 tab = 15.507 at p = 0.05) | ||||||
| Sorption | Desorption | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fitted/Statistical Parameter | S1 | S2 | S3 | S4 | Fitted/Statistical Parameter | S1 | S2 | S3 | S4 |
| Acetamiprid | |||||||||
| k1sor (a) (1/h) | 0.0452 (±0.0117) | 14.1762 (±2.7099) | 0.1355 (±0.0130) | 0.3827 (±0.1377) | k1des (c) (1/h) | 1.6989 (±0.4831) | 0.0715 (±0.0138) | 1.7951 (±0.2964) | 1.2687 (±0.2002) |
| k2sor (b) (1/h) | 11.2921 (±1.1083) | 0.1135 (±0.0352) | 12.4820 (±0.8839) | 15.1937 (±0.4235) | k2des (d) (1/h) | 0.1326 (±0.0766) | 1.5064 (±0.3155) | 0.0259 (±0.0030) | 0.0194 (±0.0031) |
| q1sor (e) (mg/kg) | 11.06 (±0.98) | 48.96 (±0.64) | 12.12 (±0.42) | 11.56 (±1.27) | q1des (g) (mg/kg) | 2.34 (±0.78) | 5.83 (±0.80) | 3.07 (±0.23) | 7.45 (±0.46) |
| q2sor (f) (mg/kg) | 66.44 (±0.62) | 6.65 (±0.73) | 56.32 (±0.38) | 56.63 (±1.29) | q2des (h) (mg/kg) | 1.70 (±0.16) | 8.30 (±0.91) | 7.69 (±0.30) | 3.25 (±0.22) |
| R2 (i) | 0.9999 | 0.9999 | 0.9999 | 0.9999 | R2 (i) | 0.9999 | 0.9999 | 0.9999 | 0.9999 |
| SRMSE (j) | 0.0107 | 0.0123 | 0.0058 | 0.0120 | SRMSE (j) | 0.0033 | 0.0077 | 0.0020 | 0.0019 |
| err-% (k) | 0.96 | 1.10 | 0.51 | 1.07 | err-% (k) | 0.31 | 0.73 | 0.19 | 0.18 |
| Thiacloprid | |||||||||
| k1sor (a) (1/h) | 1.2472 (±0.0886) | 21.8250 (±0.0225) | 1.1266 (±0.2358) | 0.1877 (±0.0475) | k1des (c) (1/h) | 0.8057 (±0.0752) | 0.0567 (±0.0067) | 0.0888 (±0.0164) | 0.1433 (±0.0266) |
| k2sor (b) (1/h) | 15.9947 (±0.8151) | 2.4835 (±0.0235) | 7.7060 (±0.9418) | 8.9457 (±0.4233) | k2des (d) (1/h) | 0.0262 (±0.0041) | 1.7781 (±0.3213) | 2.2931 (±0.4723) | 2.1306 (±0.7705) |
| q1sor (e) (mg/kg) | 11.13 (±0.63) | 19.12 (±4.35) | 24.89 (±4.74) | 7.89 (±0.80) | q1des (g) (mg/kg) | 5.47 (±1.14) | 5.36 (±0.23) | 4.07 (±0.32) | 1.91 (±0.20) |
| q2sor (f) (mg/kg) | 87.67 (±0.64) | 55.57 (±4.30) | 63.09 (±4.80) | 85.61 (±0.77) | q2des (h) (mg/kg) | 0.27 (±0.01) | 3.04 (±0.27) | 3.58 (±0.37) | 1.02 (±0.21) |
| R2 (i) | 0.9999 | 0.9999 | 0.9999 | 0.9999 | R2 (i) | 0.9999 | 0.9999 | 0.9999 | 0.9999 |
| SRMSE (j) | 0.0011 | 0.0131 | 0.0070 | 0.0066 | SRMSE (j) | 0.0076 | 0.0017 | 0.0018 | 0.0007 |
| err-% (k) | 0.10 | 1.17 | 0.62 | 0.59 | err-% (k) | 0.72 | 0.16 | 0.17 | 0.06 |
| Variable | pH | HA (a) | CEC (b) | Clay | TOC (c) | CoxHa (d) | CoxFa (e) | Ratio 465/665 | Ratio C/H | Ratio C/N |
|---|---|---|---|---|---|---|---|---|---|---|
| Acetamiprid | ||||||||||
| qsor (f) | −0.472 | 0.809 | 0.816 | −0.511 | 0.950 (p = 0.049) | 0.419 | 0.691 | 0.994 (p = 0.006) | 0.856 | 0.780 |
| k1 (TSM) (g) | 0.106 | −0.438 | −0.450 | 0.061 | −0.789 | −0.613 | −0.356 | −0.853 | −0.735 | −0.391 |
| k2 (TSM) (h) | 0.154 | 0.288 | 0.259 | 0.158 | 0.729 | 0.482 | 0.307 | 0.756 | 0.738 | 0.228 |
| q1 (TSM) (i) | 0.098 | −0.448 | −0.455 | 0.063 | −0.801 | −0.591 | −0.376 | −0.860 | −0.752 | −0.400 |
| q2 (TSM) (j) | −0.234 | 0.576 | 0.587 | −0.216 | 0.865 | 0.565 | 0.479 | 0.924 | 0.796 | 0.534 |
| Thiacloprid | ||||||||||
| qsor (f) | −0.275 | 0.752 | 0.716 | −0.367 | 0.977 (p = 0.023) | 0.302 | 0.723 | 0.984 (p = 0.016) | 0.937 | 0.708 |
| k1 (TSM) (g) | 0.046 | −0.412 | −0.412 | 0.014 | −0.786 | −0.578 | −0.356 | −0.840 | −0.747 | −0.361 |
| k2 (TSM) (h) | −0.584 | 0.914 | 0.913 | −0.665 | 0.956 (p = 0.044) | 0.287 | 0.797 | 0.983 (p = 0.017) | 0.857 | 0.894 |
| q1 (TSM) (i) | −0.137 | −0.585 | −0.405 | 0.212 | −0.707 | 0.578 | −0.861 | −0.549 | −0.838 | −0.536 |
| q2 (TSM) (j) | −0.121 | 0.765 | 0.659 | −0.343 | 0.966 (p = 0.034) | −0.071 | 0.873 | 0.898 | 0.993 (p = 0.001) | 0.714 |
| Variable | pH | HA (a) | CEC (b) | Clay | TOC (c) | CoxHa (d) | CoxFa (e) | Ratio 465/665 | Ratio C/H | Ratio C/N |
|---|---|---|---|---|---|---|---|---|---|---|
| Acetamiprid | ||||||||||
| qdes (f) | 0.423 | −0.804 | −0.798 | 0.481 | −0.965 (p = 0.035) | −0.378 | −0.713 | −0.997 (p = 0.003) | −0.887 | −0.771 |
| k1 (TSM) (g) | −0.302 | 0.445 | 0.512 | −0.177 | 0.709 | 0.775 | 0.255 | 0.821 | 0.594 | 0.415 |
| k2 (TSM) (h) | 0.023 | −0.365 | −0.372 | −0.027 | −0.750 | −0.610 | −0.303 | −0.810 | −0.710 | −0.314 |
| q1 (TSM) (i) | 0.896 | −0.501 | −0.671 | 0.685 | −0.286 | −0.695 | −0.105 | −0.451 | −0.045 | −0.541 |
| q2 (TSM) (j) | 0.255 | −0.859 | −0.757 | 0.494 | −0.965 (p = 0.035) | 0.139 | −0.945 | −0.894 | −0.981 (p = 0.019) | −0.818 |
| Thiacloprid | ||||||||||
| qdes (f) | 0.114 | −0.776 | −0.656 | 0.366 | −0.946 (p = 0.048) | 0.171 | −0.908 | −0.861 | −0.992 (p = 0.008) | −0.726 |
| k1 (TSM) (g) | −0.796 | 0.990 (p = 0.010) | 0.997 (p = 0.003) | −0.913 | 0.805 | 0.0059 | 0.850 | 0.816 | 0.684 | 0.996 (p = 0.004) |
| k2 (TSM) (h) | 0.838 | −0.944 | −0.949 | 0.976 (p = 0.024) | −0.636 | 0.113 | −0.813 | −0.630 | −0.519 | −0.965 (p = 0.035) |
| q1 (TSM) (i) | −0.833 | 0.320 | 0.439 | −0.750 | −0.194 | 0.028 | 0.047 | −0.119 | −0.367 | 0.391 |
| q2 (TSM) (j) | 0.242 | −0.855 | −0.732 | 0.532 | −0.890 | 0.335 | −0.984 (p = 0.016) | −0.788 | −0.928 | −0.819 |
| Properties | Acetamiprid | Thiacloprid |
|---|---|---|
| Chemical structure | 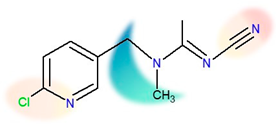 | 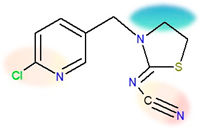 |
| IUPAC name | N-[(6-chloropyridin-3-yl)methyl]-N’-cyano-N-methylethanimidamide | [3-[(6-chloropyridin-3-yl)methyl]-1.3-thiazolidin-2-ylidene]cyanamide |
| Molecular formula | C10H11ClN4 | C10H9ClN4S |
| Molar mass/(g mol−1) | 222.67 | 255.72 |
| Melting point/°C | 98.9 | 136.0 |
| Vapor pressure/mPa | 1.73·10−4 (20 °C) | 3.00·10−7 (20 °C) |
| Water solubility/g L−1 | 2.95 (20 °C, pH 7) | 0.19 (20 °C) |
| KOW | 6.31 | 18.2 |
| pKa | 0.7 | no dissociation |
| hydrogen bond donor count | 0 | 0 |
| hydrogen bond acceptor count | 3 | 4 |
| topological polar surface area/Å2 | 52.3 | 77.6 |
| Compound | Q1 (m/z) | Q3 (m/z) | MRM Transition | CE (V) | DP (V) | EP (V) | CEP (V) |
|---|---|---|---|---|---|---|---|
| Acetamiprid | 223.2 | 126.1 | Quantitation | 39.0 | 50.0 | 10.0 | 10.0 |
| 223.2 | 99.1 | Confirmation | 67.0 | 50.0 | 10.0 | 10.0 | |
| Thiacloprid | 253.1 | 126.1 | Quantitation | 29.0 | 50.0 | 10.0 | 10.0 |
| 253.1 | 99.1 | Confirmation | 57.0 | 50.0 | 10.0 | 10.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinčić Modrić, G.; Petković Didović, M.; Dubrović, I.; Žurga, P.; Broznić, D. Those That Remain: Sorption/Desorption Behaviour and Kinetics of the Neonicotinoids Still in Use. Int. J. Mol. Sci. 2023, 24, 6548. https://doi.org/10.3390/ijms24076548
Sinčić Modrić G, Petković Didović M, Dubrović I, Žurga P, Broznić D. Those That Remain: Sorption/Desorption Behaviour and Kinetics of the Neonicotinoids Still in Use. International Journal of Molecular Sciences. 2023; 24(7):6548. https://doi.org/10.3390/ijms24076548
Chicago/Turabian StyleSinčić Modrić, Gordana, Mirna Petković Didović, Igor Dubrović, Paula Žurga, and Dalibor Broznić. 2023. "Those That Remain: Sorption/Desorption Behaviour and Kinetics of the Neonicotinoids Still in Use" International Journal of Molecular Sciences 24, no. 7: 6548. https://doi.org/10.3390/ijms24076548
APA StyleSinčić Modrić, G., Petković Didović, M., Dubrović, I., Žurga, P., & Broznić, D. (2023). Those That Remain: Sorption/Desorption Behaviour and Kinetics of the Neonicotinoids Still in Use. International Journal of Molecular Sciences, 24(7), 6548. https://doi.org/10.3390/ijms24076548







