Cognitive Decline in Early and Premature Menopause
Abstract
1. Introduction
2. Methodology
3. The Causes of Menopause
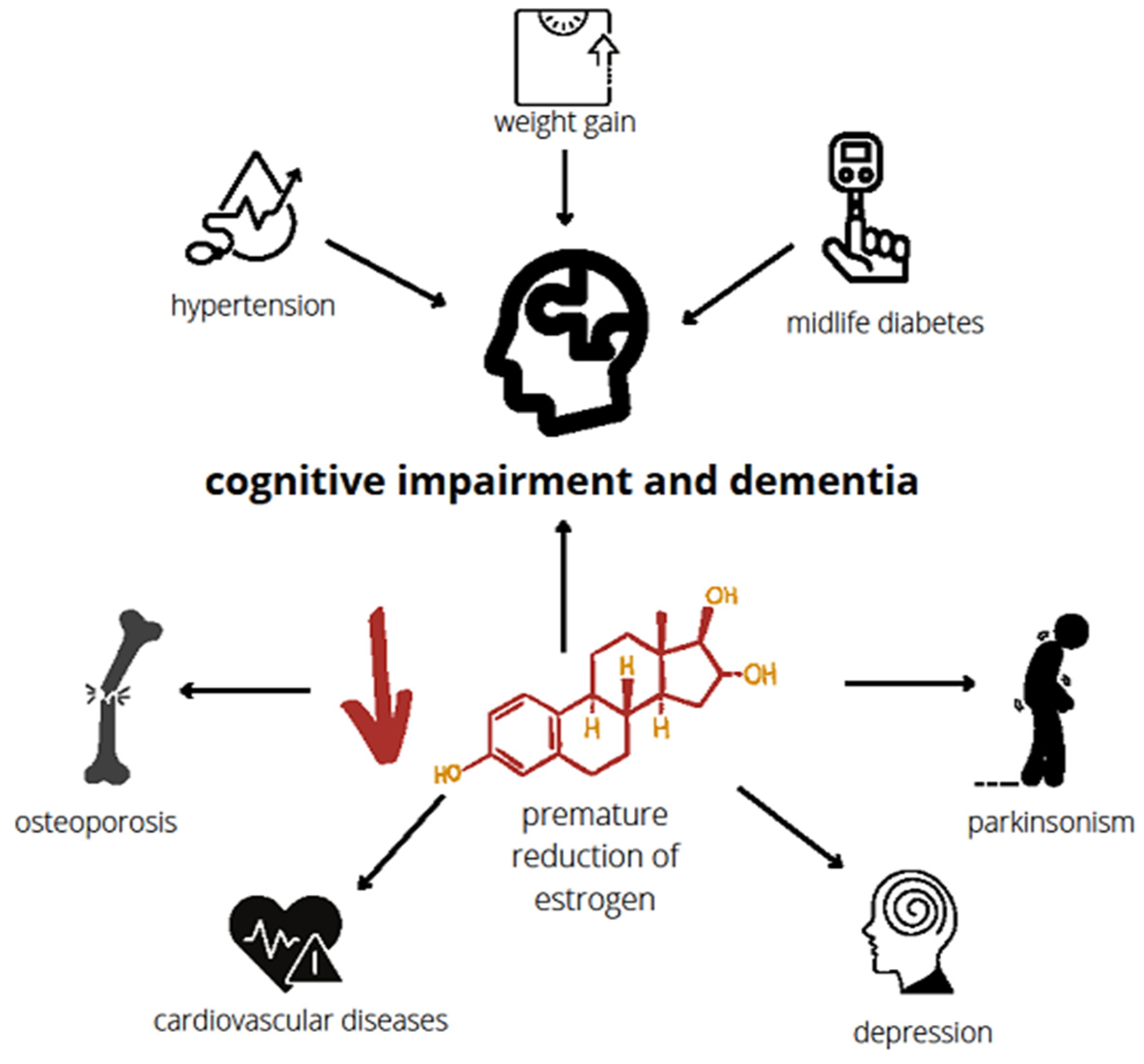
4. The Causes and Risk Factors of Premature and Early Menopause
5. Influence of Premature Menopause on Cognitive Functions
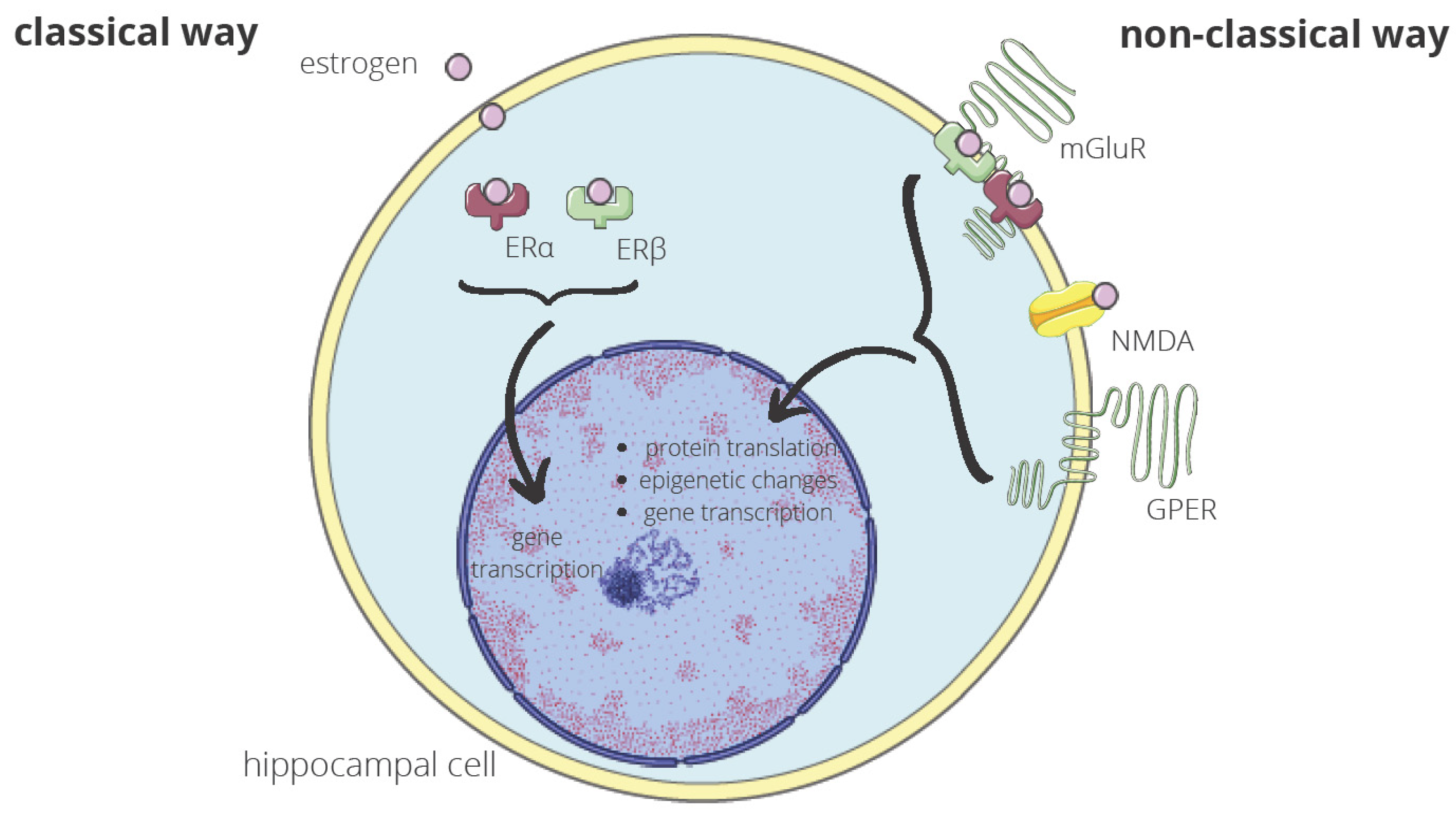
6. Molecular Mechanisms Underlying the Relation between Early Menopause and Cognition
7. Mild Cognitive Impairment (MCI) in the Perimenopausal Period
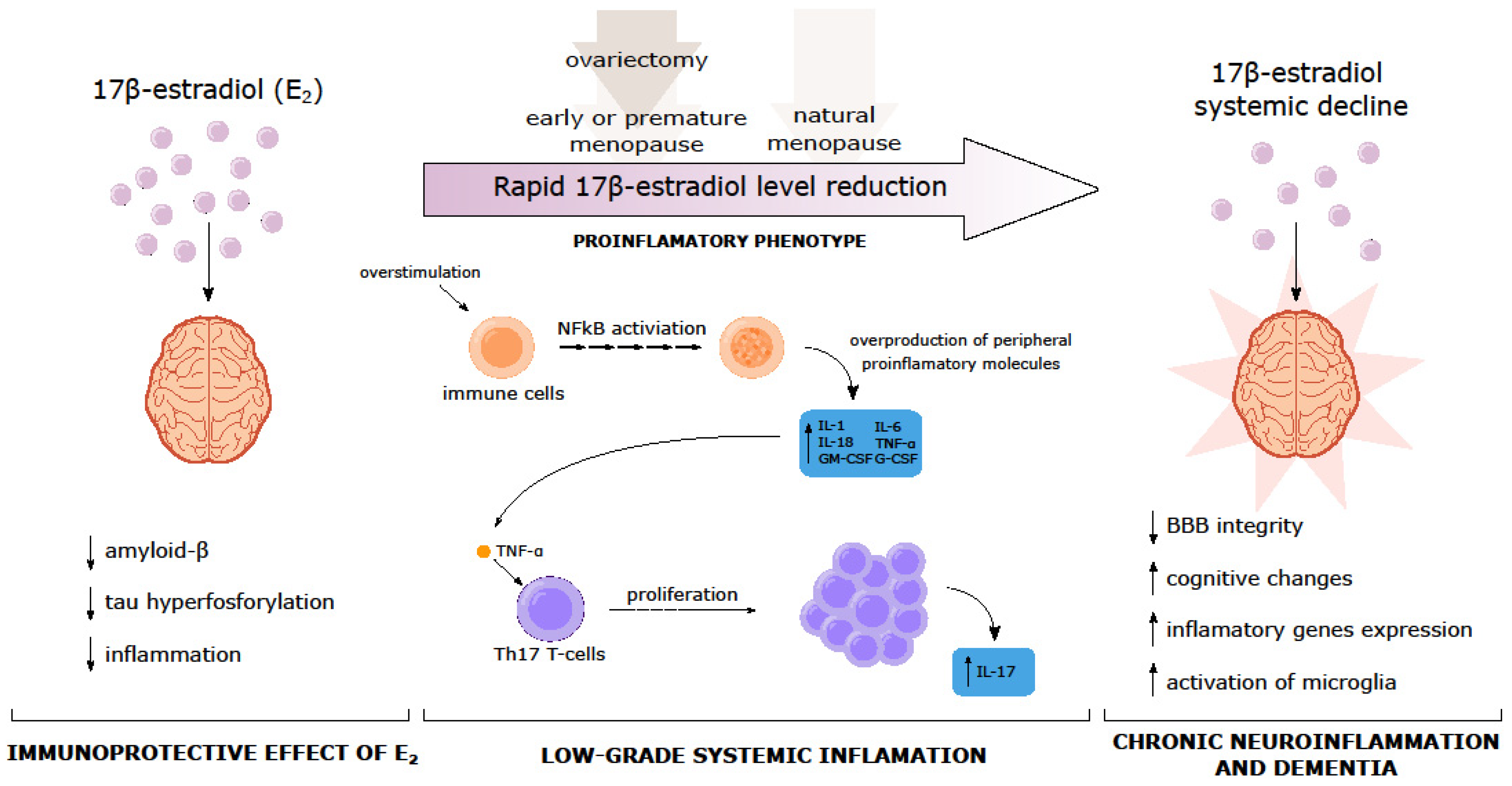
8. Early Menopause and Vascular Implications of Dementia
9. Surgical Menopause and Dementia
10. Early and Premature Menopause and Hormone Replacement Therapy (HRT)
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shuster, L.T.; Rhodes, D.J.; Gostout, B.S.; Grossardt, B.R.; Rocca, W.A. Premature menopause or early menopause: Long-term health consequences. Maturitas 2010, 65, 161–166. [Google Scholar] [CrossRef]
- Okeke, T.; Anyaehie, U.; Ezenyeaku, C. Premature Menopause. Ann. Med. Health Sci. Res. 2013, 3, 90–95. [Google Scholar] [CrossRef]
- Holesh, J.E.; Bass, A.N.; Lord, M. Physiology, Ovulation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK441996/ (accessed on 10 February 2023).
- Santoro, N.; Roeca, C.; Peters, B.A.; Neal-Perry, G. The Menopause Transition: Signs, Symptoms, and Management Options. J. Clin. Endocrinol. Metab. 2021, 106, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Luborsky, J.L.; Meyer, P.; Sowers, M.F.; Gold, E.B.; Santoro, N. Premature menopause in a multi-ethnic population study of the menopause transition*. Hum. Reprod. 2003, 18, 199–206. [Google Scholar] [CrossRef]
- de Souza Macêdo, P.R.; Rocha, T.N.; Fernandes, S.G.G.; Vieira, M.C.A.; Jerez-Roig, J.; da Câmara, S.M.A. Possible association of early menopause with worse physical function: A systematic review. Menopause 2021, 28, 467. [Google Scholar] [CrossRef]
- Seco, C.; Balint, O.; Pirtea, L.; Grigora, D.; Bălulescu, L.; Ilina, R. Surgically Induced Menopause—A Practical Review of Literature. Medicina 2019, 55, 482. [Google Scholar] [CrossRef]
- Boekhout, A.H.; Beijnen, J.H.; Schellens, J.H.M. Symptoms and Treatment in Cancer Therapy-Induced Early Menopause. Oncologist 2006, 11, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Torrealday, S.; Kodaman, P.; Pal, L. Premature Ovarian Insufficiency—An update on recent advances in understanding and management. F1000Research 2017, 6, 2069. [Google Scholar] [CrossRef]
- Becker, S.L.; Manson, J.E. Menopause, the gut microbiome, and weight gain: Correlation or causation? Menopause 2020, 28, 327–331. [Google Scholar] [CrossRef]
- Zhou, Z.; Bian, C.; Luo, Z.; Guille, C.; Ogunrinde, E.; Wu, J.; Zhao, M.; Fitting, S.; Kamen, D.L.; Oates, J.C.; et al. Pro-gesterone decreases gut permeability through upregulating occludin expression in primary human gut tissues and Ca-co-2 cells. Sci. Rep. 2019, 9, 8367. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.A.; Santoro, N.; Kaplan, R.C.; Qi, Q. Spotlight on the Gut Microbiome in Menopause: Current Insights. Int. J. Womens Health 2022, 14, 1059–1072. [Google Scholar] [CrossRef]
- Laven, J.S.E. Primary Ovarian Insufficiency. Semin. Reprod. Med. 2016, 34, 230–234. [Google Scholar] [CrossRef]
- Baronchelli, S.; Villa, N.; Redaelli, S.; Lissoni, S.; Saccheri, F.; Panzeri, E.; Conconi, D.; Bentivegna, A.; Crosti, F.; Sala, E.; et al. Investigating the role of X chromosome breakpoints in premature ovarian failure. Mol. Cytogenet. 2012, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Di-Battista, A.; Moysés-Oliveira, M.; Melaragno, M.I. Genetics of premature ovarian insufficiency and the association with X-autosome translocations. Reproduction 2020, 160, R55–R64. [Google Scholar] [CrossRef] [PubMed]
- Greendale, G.A.; Wight, R.G.; Huang, M.-H.; Avis, N.; Gold, E.B.; Joffe, H.; Seeman, T.; Vuge, M.; Karlamangla, A.S. Menopause-associated Symptoms and Cognitive Performance: Results from the Study of Women’s Health Across the Nation. Am. J. Epidemiol. 2010, 171, 1214–1224. [Google Scholar] [CrossRef]
- Sammaritano, L.R. Menopause in patients with autoimmune diseases. Autoimmun. Rev. 2012, 11, A430–A436. [Google Scholar] [CrossRef]
- Hernández-Angeles, C.; Castelo-Branco, C. Early menopause: A hazard to a woman’s health. Indian J. Med. Res. 2016, 143, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Laven, J.S.E. Genetics of Early and Normal Menopause. Semin. Reprod. Med. 2015, 33, 377–383. [Google Scholar] [CrossRef]
- Cox, L.; Liu, J.H. Primary ovarian insufficiency: An update. Int. J. Womens Health 2014, 6, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Vogt, E.C.; Breivik, L.; Røyrvik, E.C.; Grytaas, M.; Husebye, E.S.; Øksnes, M. Primary Ovarian Insufficiency in Women with Addison’s Disease. J. Clin. Endocrinol. Metab. 2021, 106, e2656–e2663. [Google Scholar] [CrossRef]
- Thakur, M.; Feldman, G.; Puscheck, E.E. Primary ovarian insufficiency in classic galactosemia: Current understanding and future research opportunities. J. Assist. Reprod. Genet. 2018, 35, 3–16. [Google Scholar] [CrossRef]
- Santoro, N. Mechanisms of premature ovarian failure. Ann. Endocrinol. 2003, 64, 87–92. [Google Scholar]
- Chen, Q.; Ke, H.; Luo, X.; Wang, L.; Wu, Y.; Tang, S.; Li, J.; Jin, L.; Zhang, F.; Qin, Y.; et al. Rare deleterious BUB1B variants induce premature ovarian insufficiency and early menopause. Hum. Mol. Genet. 2020, 29, 2698–2707. [Google Scholar] [CrossRef]
- Yasui, T.; Hayashi, K.; Mizunuma, H.; Kubota, T.; Aso, T.; Matsumura, Y.; Lee, J.-S.; Suzuki, S. Factors associated with premature ovarian failure, early menopause and earlier onset of menopause in Japanese women. Maturitas 2012, 72, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, B.W.; Purdue-Smithe, A.C.; Szegda, K.L.; Boutot, M.E.; Hankinson, S.E.; Manson, J.E.; Rosner, B.; Willett, W.C.; Eliassen, A.H.; Bertone-Johnson, E.R. Cigarette Smoking and Risk of Early Natural Menopause. Am. J. Epidemiol. 2018, 187, 696–704. [Google Scholar] [CrossRef]
- Sveinsson, O.; Tomson, T. Epilepsy and Menopause: Potential Implications for Pharmacotherapy. Drugs Aging 2014, 31, 671–675. [Google Scholar] [CrossRef]
- Mishra, G.D.; Chung, H.-F.; Cano, A.; Chedraui, P.; Goulis, D.G.; Lopes, P.; Mueck, A.; Rees, M.; Senturk, L.M.; Sim, T.; et al. EMAS position statement: Predictors of premature and early natural menopause. Maturitas 2019, 123, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.D.; Pandeya, N.; Dobson, A.J.; Chung, H.-F.; Anderson, D.; Kuh, D.; Sandin, S.; Giles, G.G.; Bruinsma, F.; Hayashi, K.; et al. Early menarche, nulliparity and the risk for premature and early natural menopause. Hum. Reprod. 2017, 32, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Szegda, K.L.; Whitcomb, B.W.; Purdue-Smithe, A.C.; Boutot, M.E.; Manson, J.E.; Hankinson, S.E.; Rosner, B.A.; Bertone-Johnson, E.R. Adult adiposity and risk of early menopause. Hum Reprod. 2017, 32, 2522–2531. [Google Scholar] [CrossRef]
- Whitcomb, B.W.; Purdue-Smithe, A.; Hankinson, S.E.; Manson, J.E.; Rosner, B.A.; Bertone-Johnson, E.R. Menstrual Cycle Characteristics in Adolescence and Early Adulthood Are Associated with Risk of Early Natural Menopause. J. Clin. Endocrinol. Metab. 2018, 103, 3909–3918. [Google Scholar] [CrossRef]
- Yasui, T.; Hayashi, K.; Mizunuma, H.; Kubota, T.; Aso, T.; Matsumura, Y.; Lee, J.-S.; Suzuki, S. Association of endometriosis-related infertility with age at menopause. Maturitas 2011, 69, 279–283. [Google Scholar] [CrossRef]
- Woods, N.F.; Mitchell, E.S.; Adams, C. Memory functioning among midlife women: Observations from the Seattle Midlife Women’s Health Study. Menopause 2000, 7, 257–265. [Google Scholar] [CrossRef]
- Betti, S.; Orsini, M.R.; Sciaky, R.; Cristini, C.; Cesa-Bianchi, G.; Zandonini, G.F. Attitudes towards menopause in a group of women followed in a public service for menopause counselling. Aging Milan Italy 2001, 13, 331–338. [Google Scholar] [CrossRef]
- Schaafsma, M.; Homewood, J.; Taylor, A. Subjective cognitive complaints at menopause associated with declines in performance of verbal memory and attentional processes. Climacteric 2010, 13, 84–98. [Google Scholar] [CrossRef]
- Georgakis, M.K.; Georgakis, M.K.; Kalogirou, E.I.; Diamantaras, A.-A.; Daskalopoulou, S.S.; Munro, C.A.; Lyketsos, C.G.; Skalkidou, A.; Petridou, E.T. Age at menopause and duration of reproductive period in association with dementia and cognitive function: A systematic review and meta-analysis. Psychoneuroendocrinology 2016, 73, 224–243. [Google Scholar] [CrossRef]
- Gilsanz; Lee, C.; Corrada, M.M.; Kawas, C.H.; Quesenberry, C.; Whitmer, R.A. Reproductive period and risk of dementia in a diverse cohort of health care members. Neurology 2019, 92, e2005–e2014. [Google Scholar] [CrossRef]
- Yoo, J.E.; Shin, D.W.; Han, K.; Kim, D.; Won, H.; Lee, J.; Kim, S.; Nam, G.E.; Park, H.S. Female reproductive factors and the risk of dementia: A nationwide cohort study. Eur. J. Neurol. 2020, 27, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Christou, K.; Artzouchaltzi, A.-M.; Gkekas, N.K.; Kosmidou, N.; Siolos, P.; Paschou, S.A.; Potoupnis, M.; Kenanidis, E.; Tsiridis, E.; et al. Early menopause and premature ovarian insufficiency are associated with increased risk of type 2 diabetes: A systematic review and meta-analysis. Eur. J. Endocrinol. 2019, 180, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Atsma, F.; Bartelink, M.L.E.L.; Grobbee, D.E.; Van Der Schouw, Y.T. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: A meta-analysis. Menopause 2006, 13, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, C. Effect of early menopause on bone mineral density and fractures. Menopause 2007, 14, 567–571. [Google Scholar] [CrossRef]
- Georgakis, M.K.; Thomopoulos, T.; Diamantaras, A.A.; Kalogirou, E.I.; Skalkidou, A.; Daskalopoulou, S.S.; Petridou, E.T. Association of Age at Menopause and Duration of Reproductive Period with Depression after Menopause: A Systematic Review and Meta-analysis. JAMA Psychiatry 2016, 73, 139–149. [Google Scholar] [CrossRef]
- Rocca, W.A.; Bower, J.H.; Maraganore, D.M.; Ahlskog, J.E.; Grossardt, B.R.; de Andrade, M.; Melton, L.J., III. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology 2007, 70, 200–209. [Google Scholar] [CrossRef]
- Zilberman, M.; Cerezo, G.H.; Del Sueldo, M.; Fernandez-Pérez, C.; Martell-Claros, N.; Vicario, A. Association between Hypertension, Menopause, and Cognition in Women. J. Clin. Hypertens. 2015, 17, 970–976. [Google Scholar] [CrossRef]
- Honigberg, M.C.; Zekavat, S.M.; Niroula, A.; Griffin, G.K.; Bick, A.G.; Pirruccello, J.P.; Nakao, T.; Whitsel, E.A.; Farland, L.V.; Laurie, C.; et al. Premature Menopause, Clonal Hematopoiesis, and Coronary Artery Disease in Postmenopausal Women. Circulation 2021, 143, 410–423. [Google Scholar] [CrossRef]
- Fitzpatrick, A.L.; Kuller, L.H.; Lopez, O.L.; Diehr, P.; O’Meara, E.S.; Longstreth, W.T.; Luchsinger, J. Midlife and Late-Life Obesity and the Risk of Dementia: Cardiovascular Health Study. Arch. Neurol. 2009, 66, 336–342. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Akama, K.T.; Spencer-Segal, J.L.; Milner, T.A.; Waters, E.M. Estrogen effects on the brain: Actions beyond the hypothalamus via novel mechanisms. Behav. Neurosci. 2012, 126, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Brinton, R.D.; Yao, J.; Yin, F.; Mack, W.J.; Cadenas, E. Perimenopause as a neurological transition state. Nat. Rev. Endocrinol. 2015, 11, 393–405. [Google Scholar] [CrossRef]
- Lund, T.D.; Rovis, T.; Chung, W.C.J.; Handa, R.J. Novel Actions of Estrogen Receptor-β on Anxiety-Related Behaviors. Endocrinology 2005, 146, 797–807. [Google Scholar] [CrossRef]
- Donner, N.C.; Handa, R.J. Estrogen receptor beta regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience 2009, 163, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.J.; Wu, T.-Y.; Handa, R.J. Estrogen Receptor-β Agonist Diarylpropionitrile: Biological Activities of R- and S-Enantiomers on Behavior and Hormonal Response to Stress. Endocrinology 2008, 150, 1817–1825. [Google Scholar] [CrossRef]
- Karlamangla, A.S.; Singer, B.H.; Chodosh, J.; McEwen, B.S.; Seeman, T.E. Urinary cortisol excretion as a predictor of incident cognitive impairment. Neurobiol. Aging 2005, 26, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Otte, C.; Hart, S.; Neylan, T.C.; Marmar, C.R.; Yaffe, K.; Mohr, D.C. A meta-analysis of cortisol response to challenge in human aging: Importance of gender. Psychoneuroendocrinology 2005, 30, 80–91. [Google Scholar] [CrossRef]
- Lu, Y.; Sareddy, G.R.; Wang, J.; Wang, R.; Li, Y.; Dong, Y.; Zhang, Q.; Liu, J.; O’Connor, J.C.; Xu, J.; et al. Neuron-Derived Estrogen Regulates Synaptic Plasticity and Memory. J. Neurosci. 2019, 39, 2792–2809. [Google Scholar] [CrossRef]
- Dumas, J.A.; Newhouse, A. The Cholinergic Hypothesis of Cognitive Aging Revisited Again: Cholinergic Functional Compensation. Pharmacol. Biochem. Behav. 2011, 99, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Simpkins, J.W.; Perez, E.; Wang, X.; Yang, S.; Wen, Y.; Singh, M. The Potential for Estrogens in Preventing Alzheimer’s Disease and Vascular Dementia. Pharmacol. Biochem. Behav. 2011, 99, 254–261. [Google Scholar] [CrossRef]
- Amin, Z.; Gueorguieva, R.; Cappiello, A.; Czarkowski, K.A.; Stiklus, S.; Anderson, G.M.; Naftolin, F.; Epperson, C.N. Estradiol and Tryptophan Depletion Interact to Modulate Cognition in Menopausal Women. Neuropsychopharmacology 2006, 31, 2489–2497. [Google Scholar] [CrossRef]
- Kugaya, A.; Epperson, C.N.; Zoghbi, S.; van Dyck, C.H.; Hou, Y.; Fujita, M.; Staley, J.K.; Garg, P.K.; Seibyl, J.P.; Innis, R.B. Increase in Prefrontal Cortex Serotonin2A Receptors Following Estrogen Treatment in Postmenopausal Women. Am. J. Psychiatry 2003, 160, 1522–1524. [Google Scholar] [CrossRef]
- Frick, K.M. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm. Behav. 2015, 74, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Prakapenka, A.V.; Korol, D.L. Estradiol selectively regulates metabolic substrates across memory systems in models of menopause. Climacteric 2021, 24, 366–372. [Google Scholar] [CrossRef]
- Fang, Y.-Y.; Zeng, P.; Qu, N.; Ning, L.-N.; Chu, J.; Zhang, T.; Zhou, X.-W.; Tian, Q. Evidence of altered depression and dementia-related proteins in the brains of young rats after ovariectomy. J. Neurochem. 2018, 146, 703–721. [Google Scholar] [CrossRef]
- Van Kempen, T.A.; Milner, T.A.; Waters, E.M. Accelerated Ovarian Failure: A novel, chemically induced animal model of menopause. Brain Res. 2011, 1379, 176–187. [Google Scholar] [CrossRef]
- McEwen, B. Estrogen actions throughout the brain. Recent Prog. Horm. Res. 2002, 57, 357–384. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Invited Review: Estrogens effects on the brain: Multiple sites and molecular mechanisms. J. Appl. Physiol. 2001, 91, 2785–2801. [Google Scholar] [CrossRef] [PubMed]
- Gleason, C.E.; Cholerton, B.; Carlsson, C.M.; Johnson, S.C.; Asthana, S. Alzheimer’s disease: The impact of age-related changes in reproductive hormones. Cell. Mol. Life Sci. 2005, 62, 299–312. [Google Scholar] [CrossRef]
- Morrison, J.H.; Brinton, R.D.; Schmidt, J.; Gore, A.C. Estrogen, Menopause, and the Aging Brain: How Basic Neuroscience Can Inform Hormone Therapy in Women. J. Neurosci. 2006, 26, 10332–10348. [Google Scholar] [CrossRef]
- Brann, D.W.; Dhandapani, K.; Wakade, C.; Mahesh, V.B.; Khan, M.M. Neurotrophic and Neuroprotective Actions of Estrogen: Basic Mechanisms and Clinical Implications. Steroids 2007, 72, 381–405. [Google Scholar] [CrossRef]
- Campos, G.V.; de Souza, A.M.A.; Ji, H.; West, C.A.; Wu, X.; Lee, D.L.; Aguilar, B.L.; Forcelli, P.A.; de Menezes, R.C.; Sandberg, K. The Angiotensin Type 1 Receptor Antagonist Losartan Prevents Ovariectomy-Induced Cognitive Dysfunction and Anxiety-Like Behavior in Long Evans Rats. Cell. Mol. Neurobiol. 2019, 40, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current Concepts in Mild Cognitive Impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef]
- Geerlings, M.I.; Bouter, L.; Schoevers, R.; Beekman, A.T.F.; Jonker, C.; Deeg, D.J.H.; Van Tilburg, W.; Adèr, H.J.; Schmand, B. Depression and risk of cognitive decline and Alzheimer’s disease: Results of two prospective community-based studies in the Netherlands. Br. J. Psychiatry 2000, 176, 568–575. [Google Scholar] [CrossRef]
- Henderson, V.W.; Guthrie, J.R.; Dudley, E.C.; Burger, H.G.; Dennerstein, L. Estrogen exposures and memory at midlife: A population-based study of women. Neurology 2003, 60, 1369–1371. [Google Scholar] [CrossRef]
- Vogel, B.; Acevedo, M.; Appelman, Y.; Merz, C.N.B.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.H.E.M.; et al. The Lancet women and cardiovascular disease Commission: Reducing the global burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef]
- Stampfer, M.J. Cardiovascular disease and Alzheimer’s disease: Common links. J. Intern. Med. 2006, 260, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Lloret, A.; Esteve, D.; Cervera-Ferri, A.; Lopez, B.; Nepomuceno, M.; Monllor, P. When Does Alzheimer′s Disease Really Start? The Role of Biomarkers. Int. J. Mol. Sci. 2019, 20, 5536. [Google Scholar] [CrossRef]
- Schneider, J.A.; Arvanitakis, Z.; Bang, W.; Bennett, D.A. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007, 69, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Launer, L.J.; Ross, G.; Petrovitch, H.; Masaki, K.; Foley, D.; White, L.R.; Havlik, R.J. Midlife blood pressure and dementia: The Honolulu–Asia aging study☆. Neurobiol. Aging 2000, 21, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Helkala, E.-L.; Hanninen, T.; Laakso, M.P.; Hallikainen, M.; Alhainen, K.; Soininen, H.; Tuomilehto, J.; Nissinen, A. Midlife vascular risk factors and late-life mild cognitive impairment: A population-based study. Neurology 2001, 56, 1683–1689. [Google Scholar] [CrossRef]
- Roberts, R.O.; Geda, Y.E.; Knopman, D.S.; Christianson, T.J.H.; Pankratz, V.S.; Boeve, B.F.; Vella, A.; Rocca, W.A.; Petersen, R.C. Association of Duration and Severity of Diabetes Mellitus with Mild Cognitive Impairment. Arch. Neurol. 2008, 65, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Qiu, C.; Gatz, M.; Pedersen, N.L.; Johansson, B.; Fratiglioni, L. Mid- and Late-Life Diabetes in Relation to the Risk of Dementia. Diabetes 2009, 58, 71–77. [Google Scholar] [CrossRef]
- Albanese, E.; Launer, L.J.; Egger, M.; Prince, M.J.; Giannakopoulos, P.; Wolters, F.J.; Egan, K. Body mass index in midlife and dementia: Systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimer’s Dementia: Diagn. Assess. Dis. Monit. 2017, 8, 165–178. [Google Scholar] [CrossRef]
- Au, R.; Massaro, J.; Wolf, P.A.; Young, M.; Beiser, A.; Seshadri, S.; D’Agostino, R.B.; DeCarli, C. Association of White Matter Hyperintensity Volume with Decreased Cognitive Functioning: The Framingham Heart Study. Arch. Neurol. 2006, 63, 246–250. [Google Scholar] [CrossRef]
- Anagnostis, P.; Paschou, S.A.; Katsiki, N.; Krikidis, D.; Lambrinoudaki, I.; Goulis, D.G. Menopausal Hormone Therapy and Cardiovascular Risk: Where are we Now? Curr. Vasc. Pharmacol. 2019, 17, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Rocca, W.A.; Mielke, M.M.; Rocca, L.G.; Stewart, E.A. Premature or early bilateral oophorectomy: A 2021 update. Climacteric 2021, 24, 466–473. [Google Scholar] [CrossRef]
- Farrag, A.F.; Khedr, E.M.; Abdel-Aleem, H.; Rageh, T.A. Effect of Surgical Menopause on Cognitive Functions. Dement. Geriatr. Cogn. Disord. 2002, 13, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Rocca, W.A.; Bower, J.H.; Maraganore, D.M.; Ahlskog, J.E.; Grossardt, B.R.; de Andrade, M.; Melton, L.J. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 2007, 69, 1074–1083. [Google Scholar] [CrossRef]
- Henderson, V.W. Cognitive changes after menopause: Influence of estrogen. Clin. Obstet. Gynecol. 2008, 51, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Rocca, W.A.; Shuster, L.T.; Grossardt, B.R.; Maraganore, D.M.; Gostout, B.S.; Geda, Y.E.; Melton, L.J.; Melton, I.L.J. Long-Term Effects of Bilateral Oophorectomy on Brain Aging: Unanswered Questions from the Mayo Clinic Cohort Study of Oophorectomy and Aging. Women’s Health 2009, 5, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Sohrabji, F. Premenopausal Oophorectomy and the Risk for Dementia. Women’s Health 2008, 4, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Phung, T.K.T.; Waltoft, B.L.; Laursen, T.M.; Settnes, A.; Kessing, L.V.; Mortensen, P.B.; Waldemar, G. Hysterectomy, Oophorectomy and Risk of Dementia: A Nationwide Historical Cohort Study. Dement. Geriatr. Cogn. Disord. 2010, 30, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Maki, M.; Henderson, V.W. Hormone therapy, dementia, and cognition: The Women’s Health Initiative 10 years on. Climacteric 2012, 15, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Gervais, N.J.; Au, A.; Almey, A.; Duchesne, A.; Gravelsins, L.; Brown, A.; Reuben, R.; Baker-Sullivan, E.; Schwartz, D.H.; Evans, K.; et al. Cognitive markers of dementia risk in middle-aged women with bilateral salpingo-oophorectomy prior to menopause. Neurobiol. Aging 2020, 94, 1–6. [Google Scholar] [CrossRef]
- Chang, H.; Kamara, D.; Bresee, C.; Lester, J.; Cass, I. Short-term impact of surgically induced menopause on cognitive function and wellbeing in women at high risk for ovarian cancer following risk-reducing bilateral salpingo-oophorectomy. Menopause 2020, 28, 354–359. [Google Scholar] [CrossRef]
- Kantarci, K.; Tosakulwong, N.; Lesnick, T.G.; Zuk, S.M.; Lowe, V.J.; Fields, J.A.; Gunter, J.L.; Senjem, M.L.; Settell, M.L.; Gleason, C.E.; et al. Brain structure and cognition 3 years after the end of an early menopausal hormone therapy trial. Neurology 2018, 90, e1404–e1412. [Google Scholar] [CrossRef]
- Georgakis, M.K.; Beskou-Kontou, T.; Theodoridis, I.; Skalkidou, A.; Petridou, E.T. Surgical menopause in association with cognitive function and risk of dementia: A systematic review and meta-analysis. Psychoneuroendocrinology 2019, 106, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-G.; Wang, R.-M.; Scott, E.; Han, N.; Dong, Y.; Tu, J.-Y.; Yang, F.; Sareddy, G.R.; Vadlamudi, R.K.; Brann, D.W. Female Sex and Alzheimer’s Risk: The Menopause Connection. J. Prev. Alzheimers Dis. 2018, 5, 225–230. [Google Scholar] [CrossRef]
- Scheyer, O.; Rahman, A.; Hristov, H.; Berkowitz, C.; Isaacson, R.S.; Diaz Brinton, R.; Mosconi, L. Hypersensitivity of the hippocampal CA3 region to stress-induced neurodegeneration and amyloidogenesis in a rat model of surgical menopause. J. Prev. Alzheimers Dis. 2018, 5, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Irwin, R.; Chen, S.; Hamilton, R.; Cadenas, E.; Brinton, R.D. Ovarian hormone loss induces bioenergetic deficits and mitochondrial β-amyloid. Neurobiol. Aging 2012, 33, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.; Magnus, T. Ageing and neuronal vulnerability. Nat. Rev. Neurosci. 2006, 7, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Lu, A.T.; Chen, B.H.; Hernandez, D.G.; Singleton, A.B.; Ferrucci, L.; Bandinelli, S.; Salfati, E.; Manson, J.E.; Quach, A.; et al. Menopause accelerates biological aging. Proc. Natl. Acad. Sci. USA 2016, 113, 9327–9332. [Google Scholar] [CrossRef]
- Mytton, J.; Evison, F.; Chilton, P.J.; Lilford, R.J. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: Study using routine data and data linkage. BMJ 2017, 356, j372. [Google Scholar] [CrossRef]
- Au, A.; Feher, A.; McPhee, L.; Jessa, A.; Oh, S.; Einstein, G. Estrogens, inflammation and cognition. Front. Neuroendocrinol. 2016, 40, 87–100. [Google Scholar] [CrossRef]
- Szoeke, C.; Downie, S.J.; Parker, A.F.; Phillips, S. Sex hormones, vascular factors and cognition. Front. Neuroendocr. 2021, 62, 100927. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Hsin, I.-L.; Chen, D.-R.; Chang, C.-C.; Kor, C.-T.; Chen, T.-Y.; Wu, H.-M. Circulating interleukin-8 and tumor necrosis factor-α are associated with hot flashes in healthy postmenopausal women. PLoS ONE 2017, 12, e0184011. [Google Scholar] [CrossRef] [PubMed]
- Najar, J.; Östling, S.; Waern, M.; Zettergren, A.; Kern, S.; Wetterberg, H.; Hällström, T.; Skoog, I. Reproductive period and dementia: A 44-year longitudinal population study of Swedish women. Alzheimer’s Dement. 2020, 16, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, Y.; Dening, T.; Hippisley-Cox, J.; Taylor, L.; Moore, M.; Coupland, C. Use of menopausal hormone therapy and risk of dementia: Nested case-control studies using QResearch and CPRD databases. BMJ 2021, 374, n2182. [Google Scholar] [CrossRef]
- Rocca, W.A.; Faubion, S.S. Estrogen and dementia. Maturitas 2022, 165, 120–121. [Google Scholar] [CrossRef]
- Ryan, J.; Scali, J.; Carrière, I.; Amieva, H.; Rouaud, O.; Berr, C.; Ritchie, K.; Ancelin, M.-L. Impact of a premature menopause on cognitive function in later life. BJOG: Int. J. Obstet. Gynaecol. 2014, 121, 1729–1739. [Google Scholar] [CrossRef]
- Koire, A.; Joffe, H.; Buckley, R. Menopausal Hormone Therapy and the Mind: The Role of Hormone Replacement in the Prevention and Treatment of Cognitive Decline, Dementia, and Cognitive Dysfunction of Depression. Harv. Rev. Psychiatry 2022, 30, 215–225. [Google Scholar] [CrossRef]
- Barha, C.K.; Liu-Ambrose, T. Sex differences in exercise efficacy: Is midlife a critical window for promoting healthy cognitive aging? FASEB J. 2020, 34, 11329–11336. [Google Scholar] [CrossRef] [PubMed]
- Girard, R.; Météreau, E.; Thomas, J.; Pugeat, M.; Qu, C.; Dreher, J.-C. Hormone therapy at early post-menopause increases cognitive control-related prefrontal activity. Sci. Rep. 2017, 7, srep44917. [Google Scholar] [CrossRef]
- Davey, D.A. Alzheimer’s Disease, Dementia, Mild Cognitive Impairment and the Menopause: A “Window of Opportunity”? Womens Health 2013, 9, 279–290. [Google Scholar] [CrossRef] [PubMed]

| Etiology | Effects | Ref. |
|---|---|---|
| Inheritance | Both numerical and structural changes in the X chromosome are the most common imbalances affecting about 13% of the POI cases | [15,16] |
| Autoimmune diseases | Antibodies produced in these diseases may also attack the ovaries:
| [17] |
| Primary ovarian insufficiency (POI) | The inhibition of the ovaries’ function before the age of 40 remains in close relationship with premature menopause. The causes of POI include the following:
| [18] |
| Etiology | Effects | Ref. |
|---|---|---|
| Genetic abnormality |
| [19,20] |
| Autoimmune disorders |
| [17,21] |
| Metabolic disorders |
| [20,22] |
| Infection |
| [20] |
| Idiopathic | Individual cases of women whose periods stop with no known cause | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sochocka, M.; Karska, J.; Pszczołowska, M.; Ochnik, M.; Fułek, M.; Fułek, K.; Kurpas, D.; Chojdak-Łukasiewicz, J.; Rosner-Tenerowicz, A.; Leszek, J. Cognitive Decline in Early and Premature Menopause. Int. J. Mol. Sci. 2023, 24, 6566. https://doi.org/10.3390/ijms24076566
Sochocka M, Karska J, Pszczołowska M, Ochnik M, Fułek M, Fułek K, Kurpas D, Chojdak-Łukasiewicz J, Rosner-Tenerowicz A, Leszek J. Cognitive Decline in Early and Premature Menopause. International Journal of Molecular Sciences. 2023; 24(7):6566. https://doi.org/10.3390/ijms24076566
Chicago/Turabian StyleSochocka, Marta, Julia Karska, Magdalena Pszczołowska, Michał Ochnik, Michał Fułek, Katarzyna Fułek, Donata Kurpas, Justyna Chojdak-Łukasiewicz, Anna Rosner-Tenerowicz, and Jerzy Leszek. 2023. "Cognitive Decline in Early and Premature Menopause" International Journal of Molecular Sciences 24, no. 7: 6566. https://doi.org/10.3390/ijms24076566
APA StyleSochocka, M., Karska, J., Pszczołowska, M., Ochnik, M., Fułek, M., Fułek, K., Kurpas, D., Chojdak-Łukasiewicz, J., Rosner-Tenerowicz, A., & Leszek, J. (2023). Cognitive Decline in Early and Premature Menopause. International Journal of Molecular Sciences, 24(7), 6566. https://doi.org/10.3390/ijms24076566








