Diet as a Source of Acrolein: Molecular Basis of Aldehyde Biological Activity in Diabetes and Digestive System Diseases
Abstract
:1. Introduction
2. Methodology
3. Watch What You Eat—Daily Diet as a Source of Acrolein
3.1. Carbohydrates and Acrolein
3.2. Alcohol and Acrolein
3.3. Lipids and Acrolein
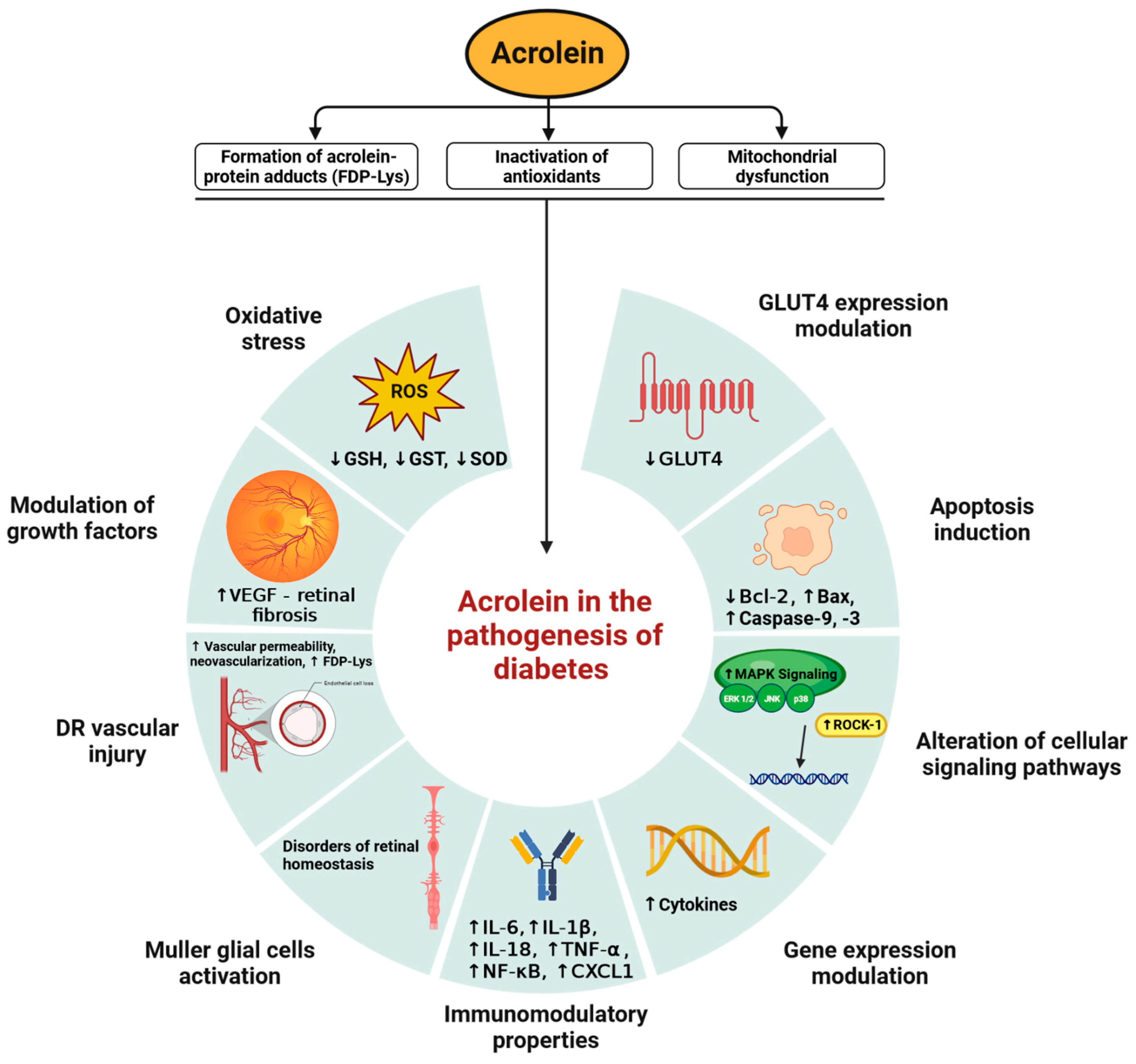
4. Acrolein in the Pathogenesis of Diabetes
4.1. Acrolein-Dependent Oxidative Stress Generation: Damage to Proteins and Mitochondria
4.2. Acrolein Cytotoxicity: Neurodegeneration, Vascular Damage and Nephrotoxicity
4.3. Immunomodulatory Properties of Acrolein and Diabetes
4.4. Acrolein-Dependent Activation of Müller Glial Cells
5. The Role of Acrolein in the Pathogenesis of Alcoholic Liver Disease
6. Acrolein as a Carcinogen in Colorectal Cancer
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burcham, P.C. Acrolein and Human Disease: Untangling the Knotty Exposure Scenarios Accompanying Several Diverse Disorders. Chem. Res. Toxicol. 2017, 30, 145–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghe, A.; Ghare, S.; Lamoreau, B.; Mohammad, M.; Barve, S.; McClain, C.; Joshi-Barve, S. Molecular mechanisms of acrolein toxicity: Relevance to human disease. Toxicol. Sci. 2015, 143, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Maier, C.S. Acrolein: Sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food Res. 2008, 52, 7–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muguruma, K.; Pradipta, A.R.; Ode, Y.; Terashima, K.; Michiba, H.; Fujii, M.; Tanaka, K. Disease-associated acrolein: A possible diagnostic and therapeutic substrate for in vivo synthetic chemistry. Bioorg. Med. Chem. 2020, 28, 115831. [Google Scholar] [CrossRef]
- Yasuo, M.; Droma, Y.; Kitaguchi, Y.; Ito, M.; Imamura, H.; Kawakubo, M.; Hanaoka, M. The relationship between acrolein and oxidative stress in COPD: In systemic plasma and in local lung tissue. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 1527–1537. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Song, Y.; Xie, J.; Xie, J.; Chen, Y.; Li, P.; Liu, D.; Hu, X.; Yu, Q. Acrolein Promotes Aging and Oxidative Stress via the Stress Response Factor DAF-16/FOXO in Caenorhabditis elegans. Foods 2022, 11, 1590. [Google Scholar] [CrossRef]
- Noerager, B.D.; Xu, X.; Davis, V.A.; Jones, C.W.; Okafor, S.; Whitehead, A.; Blalock, J.E.; Jackson, P.L. A Potential Role for Acrolein in Neutrophil-Mediated Chronic Inflammation. Inflammation 2015, 38, 2279–2287. [Google Scholar] [CrossRef]
- Sun, Y.; Ito, S.; Nishio, N.; Tanaka, Y.; Chen, N.; Isobe, K.-I. Acrolein induced both pulmonary inflammation and the death of lung epithelial cells. Toxicol. Lett. 2014, 229, 384–392. [Google Scholar] [CrossRef]
- Tsai, H.-C.; Tsou, H.-H.; Lin, C.-C.; Chen, S.-C.; Cheng, H.-W.; Liu, T.-Y.; Chen, W.-S.; Jiang, J.-K.; Yang, S.-H.; Chang, S.-C.; et al. Acrolein contributes to human colorectal tumorigenesis through the activation of RAS-MAPK pathway. Sci. Rep. 2021, 11, 12590. [Google Scholar] [CrossRef]
- Moretto, N.; Bertolini, S.; Iadicicco, C.; Marchini, G.; Kaur, M.; Volpi, G.; Patacchini, R.; Singh, D.; Facchinetti, F. Cigarette smoke and its component acrolein augment IL-8/CXCL8 mRNA stability via p38 MAPK/MK2 signaling in human pulmonary cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L929–L938. [Google Scholar] [CrossRef]
- Paiano, V.; Maertens, L.; Guidolin, V.; Yang, J.; Balbo, S.; Hecht, S.S. Quantitative Liquid Chromatography-Nanoelectrospray Ionization-High-Resolution Tandem Mass Spectrometry Analysis of Acrolein-DNA Adducts and Etheno-DNA Adducts in Oral Cells from Cigarette Smokers and Nonsmokers. Chem. Res. Toxicol. 2020, 33, 2197–2207. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-J.C. Mass Spectrometry Analysis of DNA and Protein Adducts as Biomarkers in Human Exposure to Cigarette Smoking: Acrolein as an Example. Chem. Res. Toxicol. 2023, 36, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-T.; Hu, Y.; Tong, D.; Huang, J.; Gu, L.; Wu, X.-R.; Chung, F.-L.; Li, G.-M.; Tang, M. Effect of Carcinogenic Acrolein on DNA Repair and Mutagenic Susceptibility. J. Biol. Chem. 2012, 287, 12379–12386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashiwagi, K.; Igarashi, K. Molecular Characteristics of Toxicity of Acrolein Produced from Spermine. Biomolecules 2023, 13, 298. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Huang, C.; Liu, F.; Zheng, J.; Ou, J.; Zhao, D.; Ou, S. Origin and Fate of Acrolein in Foods. Foods 2022, 11, 1976. [Google Scholar] [CrossRef] [PubMed]
- Lorkiewicz, P.; Keith, R.; Lynch, J.; Jin, L.; Theis, W.; Krivokhizhina, T.; Riggs, D.; Bhatnagar, A.; Srivastava, S.; Conklin, D.J. Electronic Cigarette Solvents, JUUL E-Liquids, and Biomarkers of Exposure: In Vivo Evidence for Acrolein and Glycidol in E-Cig-Derived Aerosols. Chem. Res. Toxicol. 2022, 35, 283–292. [Google Scholar] [CrossRef]
- Znyk, M.; Jurewicz, J.; Kaleta, D. Exposure to Heated Tobacco Products and Adverse Health Effects, a Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 6651. [Google Scholar] [CrossRef]
- Ding, Y.S.; Richter, P.; Hearn, B.; Zhang, L.; Bravo, R.; Yan, X.; Perez, J.J.; Chan, M.; Hughes, J.; Chen, P.; et al. Chemical Characterization of Mainstream Smoke from SPECTRUM Variable Nicotine Research Cigarettes. Tob. Regul. Sci. 2017, 3, 81–94. [Google Scholar] [CrossRef]
- Chen, M.; Carmella, S.G.; Lindgren, B.R.; Luo, X.; Ikuemonisan, J.; Niesen, B.; Thomson, N.M.; Murphy, S.E.; Hatsukami, D.K.; Hecht, S.S. Increased Levels of the Acrolein Metabolite 3-Hydroxypropyl Mercapturic Acid in the Urine of e-Cigarette Users. Chem. Res. Toxicol. 2022. [Google Scholar] [CrossRef]
- Cheng, G.; Guo, J.; Carmella, S.G.; Lindgren, B.; Ikuemonisan, J.; Niesen, B.; Jensen, J.; Hatsukami, D.K.; Balbo, S.; Hecht, S.S. Increased acrolein-DNA adducts in buccal brushings of e-cigarette users. Carcinogenesis 2022, 43, 437–444. [Google Scholar] [CrossRef]
- Alvarenga, G.F.; de Resende Machado, A.M.; Barbosa, R.B.; Ferreira, V.R.F.; Santiago, W.D.; Teixeira, M.L.; Nelson, D.L.; Cardoso, M.D.G. Correlation of the presence of acrolein with higher alcohols, glycerol, and acidity in cachaças. J. Food Sci. 2023; early view. [Google Scholar] [CrossRef]
- Uemura, T.; Uchida, M.; Nakamura, M.; Shimekake, M.; Sakamoto, A.; Terui, Y.; Higashi, K.; Ishii, I.; Kashiwagi, K.; Igarashi, K. A search for acrolein scavengers among food components. Amino Acids 2023, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abraham, K.; Andres, S.; Palavinskas, R.; Berg, K.; Appel, K.E.; Lampen, A. Toxicology and risk assessment of acrolein in food. Mol. Nutr. Food Res. 2011, 55, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Aizenbud, D.; Aizenbud, I.; Reznick, A.Z.; Avezov, K. Acrolein-an α,β-Unsaturated Aldehyde: A Review of Oral Cavity Exposure and Oral Pathology Effects. Rambam Maimonides Med. J. 2016, 7, e0024. [Google Scholar] [CrossRef] [PubMed]
- Ewert, A.; Granvogl, M.; Schieberle, P. Development of two stable isotope dilution assays for the quantitation of acrolein in heat-processed fats. J. Agric. Food Chem. 2011, 59, 3582–3589. [Google Scholar] [CrossRef]
- Ewert, A.; Granvogl, M.; Schieberle, P. Isotope-labeling studies on the formation pathway of acrolein during heat processing of oils. J. Agric. Food Chem. 2014, 62, 8524–8529. [Google Scholar] [CrossRef] [PubMed]
- Bachir, N.; Haddarah, A.; Sepulcre, F.; Pujola, M. Formation, Mitigation, and Detection of Acrylamide in Foods. Food Anal. Methods 2022, 15, 1736–1747. [Google Scholar] [CrossRef]
- Ho, S.S.H.; Yu, J.Z.; Chu, K.W.; Yeung, L.L. Carbonyl emissions from commercial cooking sources in Hong Kong. J. Air Waste Manag. Assoc. 2006, 56, 1091–1098. [Google Scholar] [CrossRef] [Green Version]
- Seaman, V.Y.; Bennett, D.H.; Cahill, T.M. Indoor acrolein emission and decay rates resulting from domestic cooking events. Atmos. Environ. 2009, 43, 6199–6204. [Google Scholar] [CrossRef]
- Hecht, S.S.; Koh, W.-P.; Wang, R.; Chen, M.; Carmella, S.G.; Murphy, S.E.; Yuan, J.-M. Elevated levels of mercapturic acids of acrolein and crotonaldehyde in the urine of Chinese women in Singapore who regularly cook at home. PLoS ONE 2015, 10, e0120023. [Google Scholar] [CrossRef] [Green Version]
- Yu, I.T.S.; Chiu, Y.-L.; Au, J.S.K.; Wong, T.-W.; Tang, J.-L. Dose-response relationship between cooking fumes exposures and lung cancer among Chinese nonsmoking women. Cancer Res. 2006, 66, 4961–4967. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.T.; Blot, W.J.; Zheng, W.; Ershow, A.G.; Hsu, C.W.; Levin, L.I.; Zhang, R.; Fraumeni, J.F. Lung cancer among Chinese women. Int. J. Cancer 1987, 40, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S.; Seow, A.; Wang, M.; Wang, R.; Meng, L.; Koh, W.-P.; Carmella, S.G.; Chen, M.; Han, S.; Yu, M.C.; et al. Elevated levels of volatile organic carcinogen and toxicant biomarkers in Chinese women who regularly cook at home. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1185–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.-W.; Liu, J.-H.; Tsou, H.-H.; Liu, T.-Y.; Wang, H.-T. Identification of acrolein metabolites in human buccal cells, blood, and urine after consumption of commercial fried food. Food Sci. Nutr. 2019, 7, 1668–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, G.A.; Gardiner, D.; Holmes, F.H. The pyrolysis of cellulose and the action of flame-retardants. J. Appl. Chem. 1966, 16, 81–88. [Google Scholar] [CrossRef]
- Paine, J.B.; Pithawalla, Y.B.; Naworal, J.D. Carbohydrate pyrolysis mechanisms from isotopic labeling. J. Anal. Appl. Pyrolysis 2008, 82, 42–69. [Google Scholar] [CrossRef]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef]
- Quan, W.; Li, Y.; Jiao, Y.; Xue, C.; Liu, G.; Wang, Z.; He, Z.; Qin, F.; Zeng, M.; Chen, J. Simultaneous generation of acrylamide, β-carboline heterocyclic amines and advanced glycation ends products in an aqueous Maillard reaction model system. Food Chem. 2020, 332, 127387. [Google Scholar] [CrossRef]
- Pfeifer, Y.V.; Kroh, L.W. Investigation of reactive alpha-dicarbonyl compounds generated from the Maillard reactions of L-methionine with reducing sugars via their stable quinoxaline derivatives. J. Agric. Food Chem. 2010, 58, 8293–8299. [Google Scholar] [CrossRef]
- Roemer, E.; Schorp, M.K.; Piadé, J.-J.; Seeman, J.I.; Leyden, D.E.; Haussmann, H.-J. Scientific assessment of the use of sugars as cigarette tobacco ingredients: A review of published and other publicly available studies. Crit. Rev. Toxicol. 2012, 42, 244–278. [Google Scholar] [CrossRef] [Green Version]
- Pennings, J.L.A.; Cremers, J.W.J.M.; Becker, M.J.A.; Klerx, W.N.M.; Talhout, R. Aldehyde and Volatile Organic Compound Yields in Commercial Cigarette Mainstream Smoke Are Mutually Related and Depend on the Sugar and Humectant Content in Tobacco. Nicotine Tob. Res. 2020, 22, 1748–1756. [Google Scholar] [CrossRef] [Green Version]
- Fagan, P.; Pokhrel, P.; Herzog, T.A.; Moolchan, E.T.; Cassel, K.D.; Franke, A.A.; Li, X.; Pagano, I.; Trinidad, D.R.; Sakuma, K.-L.K.; et al. Sugar and Aldehyde Content in Flavored Electronic Cigarette Liquids. Nicotine Tob. Res. 2018, 20, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Conklin, D.J.; Ogunwale, M.A.; Chen, Y.; Theis, W.S.; Nantz, M.H.; Fu, X.-A.; Chen, L.-C.; Riggs, D.W.; Lorkiewicz, P.; Bhatnagar, A.; et al. Electronic cigarette-generated aldehydes: The contribution of e-liquid components to their formation and the use of urinary aldehyde metabolites as biomarkers of exposure. Aerosol Sci. Technol. 2018, 52, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Burns, A.E.; Tran, L.N.; Abellar, K.A.; Poindexter, M.; Li, X.; Madl, A.K.; Pinkerton, K.E.; Nguyen, T.B. Impact of e-Liquid Composition, Coil Temperature, and Puff Topography on the Aerosol Chemistry of Electronic Cigarettes. Chem. Res. Toxicol. 2021, 34, 1640–1654. [Google Scholar] [CrossRef] [PubMed]
- Vreeke, S.; Korzun, T.; Luo, W.; Jensen, R.P.; Peyton, D.H.; Strongin, R.M. Dihydroxyacetone levels in electronic cigarettes: Wick temperature and toxin formation. Aerosol Sci. Technol. 2018, 52, 370–376. [Google Scholar] [CrossRef] [Green Version]
- Samburova, V.; Bhattarai, C.; Strickland, M.; Darrow, L.; Angermann, J.; Son, Y.; Khlystov, A. Aldehydes in Exhaled Breath during E-Cigarette Vaping: Pilot Study Results. Toxics 2018, 6, 46. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Kong, L.; Xue, R.; Wang, W.; Xia, X. Bitterness in alcoholic beverages: The profiles of perception, constituents, and contributors. Trends Food Sci. Technol. 2020, 96, 222–232. [Google Scholar] [CrossRef]
- Sung, H. A natural compound (reuterin) produced by Lactobacillus reuteri for biological-tissue fixation. Biomaterials 2003, 24, 1335–1347. [Google Scholar] [CrossRef]
- SERJAK, W.C.; DAY, W.H.; van Lanen, J.M.; BORUFF, C.S. Acrolein Production by Bacteria Found in Distillery Grain Mashes. Appl. Microbiol. 1954, 2, 14–20. [Google Scholar] [CrossRef]
- Smiley, K.L.; Sobolov, M. A cobamide-requiring glycerol dehydrase from an acrolein-forming lactobacillus. Arch. Biochem. Biophys. 1962, 97, 538–543. [Google Scholar] [CrossRef]
- Cespi, D.; Passarini, F.; Mastragostino, G.; Vassura, I.; Larocca, S.; Iaconi, A.; Chieregato, A.; Dubois, J.-L.; Cavani, F. Glycerol as feedstock in the synthesis of chemicals: A life cycle analysis for acrolein production. Green Chem. 2015, 17, 343–355. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry of deep-fat frying oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef] [PubMed]
- Spiteller, G. Peroxyl radicals: Inductors of neurodegenerative and other inflammatory diseases. Their origin and how they transform cholesterol, phospholipids, plasmalogens, polyunsaturated fatty acids, sugars, and proteins into deleterious products. Free Radic. Biol. Med. 2006, 41, 362–387. [Google Scholar] [CrossRef] [PubMed]
- Spiteller, P.; Kern, W.; Reiner, J.; Spiteller, G. Aldehydic lipid peroxidation products derived from linoleic acid. Biochim. Biophys. Acta 2001, 1531, 188–208. [Google Scholar] [CrossRef] [PubMed]
- Umano, K.; Shibamoto, T. Analysis of acrolein from heated cooking oils and beef fat. J. Agric. Food Chem. 1987, 35, 909–912. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Yin, H.; Porter, N.A. New insights regarding the autoxidation of polyunsaturated fatty acids. Antioxid. Redox Signal. 2005, 7, 170–184. [Google Scholar] [CrossRef]
- Endo, Y.; Hayashi, C.; Yamanaka, T.; Takayose, K.; Yamaoka, M.; Tsuno, T.; Nakajima, S. Linolenic Acid as the Main Source of Acrolein Formed During Heating of Vegetable Oils. J. Am. Oil. Chem. Soc. 2013, 90, 959–964. [Google Scholar] [CrossRef]
- Kato, S.; Shimizu, N.; Otoki, Y.; Ito, J.; Sakaino, M.; Sano, T.; Takeuchi, S.; Imagi, J.; Nakagawa, K. Determination of acrolein generation pathways from linoleic acid and linolenic acid: Increment by photo irradiation. NPJ Sci. Food 2022, 6, 21. [Google Scholar] [CrossRef]
- Pedersen, J.R.; Ingemarsson, Å.; Olsson, J.O. Oxidation of rapeseed oil, rapeseed methyl ester (RME) and diesel fuel studied with GC/MS. Chemosphere 1999, 38, 2467–2474. [Google Scholar] [CrossRef]
- Katragadda, H.R.; Fullana, A.; Sidhu, S.; Carbonell-Barrachina, Á.A. Emissions of volatile aldehydes from heated cooking oils. Food Chem. 2010, 120, 59–65. [Google Scholar] [CrossRef]
- Chan, W.R.; Sidheswaran, M.; Sullivan, D.P.; Cohn, S.; Fisk, W.J. Cooking-related PM2.5 and acrolein measured in grocery stores and comparison with other retail types. Indoor Air 2016, 26, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Shibata, A.; Uemura, M.; Hosokawa, M.; Miyashita, K. Formation of Acrolein in the Autoxidation of Triacylglycerols with Different Fatty Acid Compositions. J. Am. Oil. Chem. Soc. 2015, 92, 1661–1670. [Google Scholar] [CrossRef] [Green Version]
- Shibata, A.; Uemura, M.; Hosokawa, M.; Miyashita, K. Acrolein as a Major Volatile in the Early Stages of Fish Oil TAG Oxidation. J. Oleo Sci. 2018, 67, 515–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pop-Busui, R.; Januzzi, J.L.; Bruemmer, D.; Butalia, S.; Green, J.B.; Horton, W.B.; Knight, C.; Levi, M.; Rasouli, N.; Richardson, C.R. Heart Failure: An Underappreciated Complication of Diabetes. A Consensus Report of the American Diabetes Association. Diabetes Care 2022, 45, 1670–1690. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef]
- Ansari, P.; Tabasumma, N.; Snigdha, N.N.; Siam, N.H.; Panduru, R.V.N.R.S.; Azam, S.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Diabetic Retinopathy: An Overview on Mechanisms, Pathophysiology and Pharmacotherapy. Diabetology 2022, 3, 159–175. [Google Scholar] [CrossRef]
- Pelle, M.C.; Provenzano, M.; Busutti, M.; Porcu, C.V.; Zaffina, I.; Stanga, L.; Arturi, F. Up-Date on Diabetic Nephropathy. Life 2022, 12, 1202. [Google Scholar] [CrossRef]
- Henning, R.J.; Johnson, G.T.; Coyle, J.P.; Harbison, R.D. Acrolein Can Cause Cardiovascular Disease: A Review. Cardiovasc. Toxicol. 2017, 17, 227–236. [Google Scholar] [CrossRef]
- Chang, X.; Wang, Y.; Zheng, B.; Chen, Y.; Xie, J.; Song, Y.; Ding, X.; Hu, X.; Hu, X.; Yu, Q. The Role of Acrolein in Neurodegenerative Diseases and Its Protective Strategy. Foods 2022, 11, 3203. [Google Scholar] [CrossRef]
- Carcinogenicity of acrolein, crotonaldehyde, and arecoline. Lancet Oncol. 2021, 22, 19–20. [CrossRef] [PubMed]
- Feroe, A.G.; Attanasio, R.; Scinicariello, F. Acrolein metabolites, diabetes and insulin resistance. Environ. Res. 2016, 148, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Z.-J.; Kuo, C.-W.; Yen, P.-C.; Lin, C.-C.; Tsai, M.-T.; Lu, S.-H.; Chang, Y.-P.; Liu, W.-S.; Tsou, H.-H.; Cheng, H.-W.; et al. Acrolein plays a culprit role in the pathogenesis of diabetic nephropathy in vitro and in vivo. Eur. J. Endocrinol. 2022, 187, 579–592. [Google Scholar] [CrossRef]
- Daimon, M.; Sugiyama, K.; Kameda, W.; Saitoh, T.; Oizumi, T.; Hirata, A.; Yamaguchi, H.; Ohnuma, H.; Igarashi, M.; Kato, T. Increased urinary levels of pentosidine, pyrraline and acrolein adduct in type 2 diabetes. Endocr. J. 2003, 50, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.F.B.; Kishikawa, N.; Ohyama, K.; Mohamed, H.A.-M.; Abdel-Wadood, H.M.; Mahmoud, A.M.; Imazato, T.; Ueki, Y.; Wada, M.; Kuroda, N. Chromatographic determination of low-molecular mass unsaturated aliphatic aldehydes with peroxyoxalate chemiluminescence detection after fluorescence labeling with 4-(N,N-dimethylaminosulfonyl)-7-hydrazino-2,1,3-benzoxadiazole. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 953–954, 147–152. [Google Scholar] [CrossRef] [Green Version]
- McDowell, R.E.; Barabas, P.; Augustine, J.; Chevallier, O.; McCarron, P.; Chen, M.; McGeown, J.G.; Curtis, T.M. Müller glial dysfunction during diabetic retinopathy in rats is reduced by the acrolein-scavenging drug, 2-hydrazino-4,6-dimethylpyrimidine. Diabetologia 2018, 61, 2654–2667. [Google Scholar] [CrossRef] [Green Version]
- Tsukahara, H.; Sekine, K.; Uchiyama, M.; Kawakami, H.; Hata, I.; Todoroki, Y.; Hiraoka, M.; Kaji, M.; Yorifuji, T.; Momoi, T.; et al. Formation of advanced glycosylation end products and oxidative stress in young patients with type 1 diabetes. Pediatr. Res. 2003, 54, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Murata, M.; Noda, K.; Ishida, S. Pathological Role of Unsaturated Aldehyde Acrolein in Diabetic Retinopathy. Front. Immunol. 2020, 11, 589531. [Google Scholar] [CrossRef]
- Fukutsu, K.; Murata, M.; Kikuchi, K.; Yoshida, S.; Noda, K.; Ishida, S. ROCK1 Mediates Retinal Glial Cell Migration Promoted by Acrolein. Front. Med. 2021, 8, 717602. [Google Scholar] [CrossRef]
- Yao, L.; Wu, Y.-T.; Tian, G.-X.; Xia, C.-Q.; Zhang, F.; Zhang, W. Acrolein Scavenger Hydralazine Prevents Streptozotocin-Induced Painful Diabetic Neuropathy and Spinal Neuroinflammation in Rats. Anat. Rec. 2017, 300, 1858–1864. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Noda, K.; Murata, M.; Yoshida, S.; Saito, W.; Kanda, A.; Ishida, S. Localization of Acrolein-Lysine Adduct in Fibrovascular Tissues of Proliferative Diabetic Retinopathy. Curr. Eye Res. 2017, 42, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, J.; Betts, B.; Vidro-Kotchan, E.; Culbert, R.; Tsin, A. A possible role of acrolein in diabetic retinopathy: Involvement of a VEGF/TGFβ signaling pathway of the retinal pigment epithelium in hyperglycemia. Curr. Eye Res. 2012, 37, 1045–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masaki, H.; Sinomiya, D.; Okano, Y.; Yoshida, M.; Iwabuchi, T. Impact of protein carbonylation on the chemical characteristics of the hair surface. Int. J. Cosmet. Sci. 2021, 43, 764–771. [Google Scholar] [CrossRef]
- Kaminskas, L.M.; Pyke, S.M.; Burcham, P.C. Michael addition of acrolein to lysinyl and N-terminal residues of a model peptide: Targets for cytoprotective hydrazino drugs. Rapid Commun. Mass Spectrom. 2007, 21, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Bhatnagar, A.; Pierce, W.M. Protein modification by acrolein: Formation and stability of cysteine adducts. Chem. Res. Toxicol. 2009, 22, 708–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, P.H.; Zong, H.; Medina, R.J.; Limb, G.A.; Uchida, K.; Stitt, A.W.; Curtis, T.M. Evidence supporting a role for N-(3-formyl-3,4-dehydropiperidino)lysine accumulation in Müller glia dysfunction and death in diabetic retinopathy. Mol. Vis. 2010, 16, 2524–2538. [Google Scholar]
- Murata, M.; Noda, K.; Kawasaki, A.; Yoshida, S.; Dong, Y.; Saito, M.; Dong, Z.; Ando, R.; Mori, S.; Saito, W.; et al. Soluble Vascular Adhesion Protein-1 Mediates Spermine Oxidation as Semicarbazide-Sensitive Amine Oxidase: Possible Role in Proliferative Diabetic Retinopathy. Curr. Eye Res. 2017, 42, 1674–1683. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, A.; Zheng, J.; Bizzozero, O.A. Protein carbonylation and aggregation precede neuronal apoptosis induced by partial glutathione depletion. ASN Neuro 2012, 4, AN20110064. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, G.; Alam, K.; Nawaz, M.I.; Siddiquei, M.M.; Mousa, A.; Abu El-Asrar, A.M. Mutual enhancement between high-mobility group box-1 and NADPH oxidase-derived reactive oxygen species mediates diabetes-induced upregulation of retinal apoptotic markers. J. Physiol. Biochem. 2015, 71, 359–372. [Google Scholar] [CrossRef]
- Feng, Z.; Liu, Z.; Li, X.; Jia, H.; Sun, L.; Tian, C.; Jia, L.; Liu, J. α-Tocopherol is an effective Phase II enzyme inducer: Protective effects on acrolein-induced oxidative stress and mitochondrial dysfunction in human retinal pigment epithelial cells. J. Nutr. Biochem. 2010, 21, 1222–1231. [Google Scholar] [CrossRef]
- Murata, M.; Noda, K.; Yoshida, S.; Saito, M.; Fujiya, A.; Kanda, A.; Ishida, S. Unsaturated Aldehyde Acrolein Promotes Retinal Glial Cell Migration. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4425–4435. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tao, J.; Jiang, M.; Yao, Y. Apocynin ameliorates diabetic retinopathy in rats: Involvement of TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2019, 73, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Vodošek Hojs, N.; Bevc, S.; Ekart, R.; Hojs, R. Oxidative Stress Markers in Chronic Kidney Disease with Emphasis on Diabetic Nephropathy. Antioxidants 2020, 9, 925. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Liu, Z.; Sun, L.; Miller, S.S.; Ames, B.N.; Cotman, C.W.; Liu, J. Acrolein, a toxicant in cigarette smoke, causes oxidative damage and mitochondrial dysfunction in RPE cells: Protection by (R)-alpha-lipoic acid. Investig. Ophthalmol. Vis. Sci. 2007, 48, 339–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Noda, K.; Murata, M.; Liu, Y.; Kanda, A.; Ishida, S. Regulation of Spermine Oxidase through Hypoxia-Inducible Factor-1α Signaling in Retinal Glial Cells under Hypoxic Conditions. Investig. Ophthalmol. Vis. Sci. 2020, 61, 52. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Mishra, M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim. Biophys. Acta 2015, 1852, 2474–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, J.M.; Thorburn, D.R. Mitochondrial dysfunction in diabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 291–312. [Google Scholar] [CrossRef]
- Li, Y.; Zou, X.; Cao, K.; Xu, J.; Yue, T.; Dai, F.; Zhou, B.; Lu, W.; Feng, Z.; Liu, J. Curcumin analog 1, 5-bis (2-trifluoromethylphenyl)-1, 4-pentadien-3-one exhibits enhanced ability on Nrf2 activation and protection against acrolein-induced ARPE-19 cell toxicity. Toxicol. Appl. Pharmacol. 2013, 272, 726–735. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, L.; Zhu, L.; Jia, X.; Li, X.; Jia, H.; Wang, Y.; Weber, P.; Long, J.; Liu, J. Hydroxytyrosol protects retinal pigment epithelial cells from acrolein-induced oxidative stress and mitochondrial dysfunction. J. Neurochem. 2007, 103, 2690–2700. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-C.; Chen, H.-J.; Chan, D.-C.; Chiu, C.-Y.; Liu, S.-H.; Lan, K.-C. Low-Dose Acrolein, an Endogenous and Exogenous Toxic Molecule, Inhibits Glucose Transport via an Inhibition of Akt-Regulated GLUT4 Signaling in Skeletal Muscle Cells. Int. J. Mol. Sci. 2021, 22, 7228. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, R.; Bryant, N.J.; Boyle, J.G.; Petrie, J.R.; Gould, G.W. Diabetes is accompanied by changes in the levels of proteins involved in endosomal GLUT4 trafficking in obese human skeletal muscle. Endocrinol. Diabetes Metab. 2022, 5, e361. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Saul, A.B.; Pichavaram, P.; Xu, Z.; Rudraraju, M.; Somanath, P.R.; Smith, S.B.; Caldwell, R.B.; Narayanan, S.P. Pharmacological Inhibition of Spermine Oxidase Reduces Neurodegeneration and Improves Retinal Function in Diabetic Mice. J. Clin. Med. 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [Green Version]
- Vitalakumar, D.; Sharma, A.; Flora, S.J.S. Ferroptosis: A potential therapeutic target for neurodegenerative diseases. J. Biochem. Mol. Toxicol. 2021, 35, e22830. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; Huan, X.; Du, X.; Chen, X.; Bi, M.; Yan, C.; Jiao, Q.; Jiang, H. Correlation of Ferroptosis and Other Types of Cell Death in Neurodegenerative Diseases. Neurosci. Bull. 2022, 38, 938–952. [Google Scholar] [CrossRef]
- Qi, H.; Kan, K.; Sticht, C.; Bennewitz, K.; Li, S.; Qian, X.; Poschet, G.; Kroll, J. Acrolein-inducing ferroptosis contributes to impaired peripheral neurogenesis in zebrafish. Front. Neurosci. 2022, 16, 1044213. [Google Scholar] [CrossRef]
- Xiong, R.; Wu, Q.; Muskhelishvili, L.; Davis, K.; Shemansky, J.M.; Bryant, M.; Rosenfeldt, H.; Healy, S.M.; Cao, X. Evaluating Mode of Action of Acrolein Toxicity in an In Vitro Human Airway Tissue Model. Toxicol. Sci. 2018, 166, 451–464. [Google Scholar] [CrossRef]
- Comer, D.M.; Elborn, J.S.; Ennis, M. Inflammatory and cytotoxic effects of acrolein, nicotine, acetylaldehyde and cigarette smoke extract on human nasal epithelial cells. BMC Pulm. Med. 2014, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Sorrentino, F.S.; Allkabes, M.; Salsini, G.; Bonifazzi, C.; Perri, P. The importance of glial cells in the homeostasis of the retinal microenvironment and their pivotal role in the course of diabetic retinopathy. Life Sci. 2016, 162, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Zhang, J.; Chen, L. The cells involved in the pathological process of diabetic retinopathy. Biomed. Pharmacother. 2020, 132, 110818. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Micera, A.; Bini, S.; Berton, M.; Esposito, G.; Midena, E. Aqueous Humor Biomarkers of Müller Cell Activation in Diabetic Eyes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3913–3918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picconi, F.; Parravano, M.; Sciarretta, F.; Fulci, C.; Nali, M.; Frontoni, S.; Varano, M.; Caccuri, A.M. Activation of retinal Müller cells in response to glucose variability. Endocrine 2019, 65, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Amin, S.; Roy, S. Retinal fibrosis in diabetic retinopathy. Exp. Eye Res. 2016, 142, 71–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, M.; Xin, X.; Jee, K.; Babapoor-Farrokhran, S.; Kashiwabuchi, F.; Ma, T.; Bhutto, I.; Hassan, S.J.; Daoud, Y.; Baranano, D.; et al. VEGF secreted by hypoxic Müller cells induces MMP-2 expression and activity in endothelial cells to promote retinal neovascularization in proliferative diabetic retinopathy. Diabetes 2013, 62, 3863–3873. [Google Scholar] [CrossRef] [Green Version]
- Lange, C.A.K.; Stavrakas, P.; Luhmann, U.F.O.; de Silva, D.J.; Ali, R.R.; Gregor, Z.J.; Bainbridge, J.W.B. Intraocular oxygen distribution in advanced proliferative diabetic retinopathy. Am. J. Ophthalmol. 2011, 152, 406–412.e3. [Google Scholar] [CrossRef]
- Fukutsu, K.; Noda, K.; Murata, M.; Yoshida, S.; Kanda, A.; Ishida, S. Role of acrolein and ROCK1 in retinal glial cells. Investig. Ophthalmol. Vis. Sci. 2020, 61, 311. [Google Scholar]
- Rothschild, P.-R.; Salah, S.; Berdugo, M.; Gélizé, E.; Delaunay, K.; Naud, M.-C.; Klein, C.; Moulin, A.; Savoldelli, M.; Bergin, C.; et al. ROCK-1 mediates diabetes-induced retinal pigment epithelial and endothelial cell blebbing: Contribution to diabetic retinopathy. Sci. Rep. 2017, 7, 8834. [Google Scholar] [CrossRef]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Primers 2018, 4, 16. [Google Scholar] [CrossRef]
- Mitra, S.; De, A.; Chowdhury, A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl. Gastroenterol. Hepatol. 2020, 5, 16. [Google Scholar] [CrossRef]
- Stickel, F.; Datz, C.; Hampe, J.; Bataller, R. Pathophysiology and Management of Alcoholic Liver Disease: Update 2016. Gut Liver 2017, 11, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.; Shah, V.H. Pathogenesis of Alcoholic Liver Disease. Clin. Liver Dis. 2016, 20, 445–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, S.M. Alcoholic Liver Disease. Clin. Liver Dis. 2016, 20, xiii–xiv. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Zhang, J.; Ghare, S.; Barve, S.; McClain, C.; Joshi-Barve, S. Acrolein Is a Pathogenic Mediator of Alcoholic Liver Disease and the Scavenger Hydralazine Is Protective in Mice. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 685–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Cheng, Y.; Mei, X.; Xie, Y.; Tang, Z.; Liu, J.; Cao, X. Mechanisms of acrolein induces toxicity in human umbilical vein endothelial cells: Oxidative stress, DNA damage response, and apoptosis. Environ. Toxicol. 2022, 37, 708–719. [Google Scholar] [CrossRef]
- Liu, D.; Cheng, Y.; Tang, Z.; Mei, X.; Cao, X.; Liu, J. Toxicity mechanism of acrolein on DNA damage and apoptosis in BEAS-2B cells: Insights from cell biology and molecular docking analyses. Toxicology 2022, 466, 153083. [Google Scholar] [CrossRef]
- Rashad, W.A.; Sakr, S.; Domouky, A.M. Comparative study of oral versus parenteral crocin in mitigating acrolein-induced lung injury in albino rats. Sci. Rep. 2022, 12, 10233. [Google Scholar] [CrossRef]
- Mohammad, M.K.; Avila, D.; Zhang, J.; Barve, S.; Arteel, G.; McClain, C.; Joshi-Barve, S. Acrolein cytotoxicity in hepatocytes involves endoplasmic reticulum stress, mitochondrial dysfunction and oxidative stress. Toxicol. Appl. Pharmacol. 2012, 265, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Aydın, B.; Oğuz, A.; Şekeroğlu, V.; Atlı Şekeroğlu, Z. Whey protein protects liver mitochondrial function against oxidative stress in rats exposed to acrolein. Arh. Hig. Rada Toksikol. 2022, 73, 200–206. [Google Scholar] [CrossRef]
- Yin, Z.; Guo, H.; Jiang, K.; Ou, J.; Wang, M.; Huang, C.; Liu, F.; Bai, W.; Zheng, J.; Ou, S. Morin decreases acrolein-induced cell injury in normal human hepatocyte cell line LO2. J. Funct. Foods 2020, 75, 104234. [Google Scholar] [CrossRef]
- Vatsalya, V.; Kong, M.; Gobejishvili, L.; Chen, W.-Y.; Srivastava, S.; Barve, S.; McClain, C.; Joshi-Barve, S. Urinary acrolein metabolite levels in severe acute alcoholic hepatitis patients. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G115–G122. [Google Scholar] [CrossRef]
- Shah, H.; Speen, A.M.; Saunders, C.; Brooke, E.A.S.; Nallasamy, P.; Zhu, H.; Li, Y.R.; Jia, Z. Protection of HepG2 cells against acrolein toxicity by 2-cyano-3,12-dioxooleana-1,9-dien-28-imidazolide via glutathione-mediated mechanism. Exp. Biol. Med. 2015, 240, 1340–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafie, B.; Pourahmad, J.; Rezaei, M. N-acetylcysteine is more effective than ellagic acid in preventing acrolein induced dysfunction in mitochondria isolated from rat liver. J. Food Biochem. 2021, 45, e13775. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.R.; Myers, J.M. The effects of acrolein on peroxiredoxins, thioredoxins, and thioredoxin reductase in human bronchial epithelial cells. Toxicology 2009, 257, 95–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, C.R.; Myers, J.M.; Kufahl, T.D.; Forbes, R.; Szadkowski, A. The effects of acrolein on the thioredoxin system: Implications for redox-sensitive signaling. Mol. Nutr. Food Res. 2011, 55, 1361–1374. [Google Scholar] [CrossRef] [Green Version]
- Spiess, P.C.; Deng, B.; Hondal, R.J.; Matthews, D.E.; van der Vliet, A. Proteomic profiling of acrolein adducts in human lung epithelial cells. J. Proteom. 2011, 74, 2380–2394. [Google Scholar] [CrossRef] [Green Version]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [Green Version]
- Park, S.L.; Le Marchand, L.; Cheng, G.; Balbo, S.; Chen, M.; Carmella, S.G.; Thomson, N.M.; Lee, Y.; Patel, Y.M.; Stram, D.O.; et al. Quantitation of DNA Adducts Resulting from Acrolein Exposure and Lipid Peroxidation in Oral Cells of Cigarette Smokers from Three Racial/Ethnic Groups with Differing Risks for Lung Cancer. Chem. Res. Toxicol. 2022, 35, 1914–1922. [Google Scholar] [CrossRef]
- Tsou, H.-H.; Hu, C.-H.; Liu, J.-H.; Liu, C.-J.; Lee, C.-H.; Liu, T.-Y.; Wang, H.-T. Acrolein Is Involved in the Synergistic Potential of Cigarette Smoking- and Betel Quid Chewing-Related Human Oral Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 954–962. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, M.; Yamano, S.; Senoh, H.; Umeda, Y.; Hirai, S.; Saito, A.; Kasai, T.; Aiso, S. Carcinogenicity and chronic toxicity of acrolein in rats and mice by two-year inhalation study. Regul. Toxicol. Pharmacol. 2021, 121, 104863. [Google Scholar] [CrossRef]
- Peterson, L.A.; Seabloom, D.; Smith, W.E.; Vevang, K.R.; Seelig, D.M.; Zhang, L.; Wiedmann, T.S. Acrolein Increases the Pulmonary Tumorigenic Activity of the Tobacco-Specific Nitrosamine 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Chem. Res. Toxicol. 2022, 35, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-H.; Tong, Z.-J.; Wei, T.-E.; Lu, Y.-C.; Huang, C.-Y.; Huang, C.-Y.; Chiang, C.-H.; Jaw, F.-S.; Cheng, H.-W.; Wang, H.-T. Cigarette Smoke Containing Acrolein Contributes to Cisplatin Resistance in Human Bladder Cancers through the Regulation of HER2 Pathway or FGFR3 Pathway. Mol. Cancer Ther. 2022, 21, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Zarkovic, K.; Uchida, K.; Kolenc, D.; Hlupic, L.; Zarkovic, N. Tissue distribution of lipid peroxidation product acrolein in human colon carcinogenesis. Free Radic. Res. 2006, 40, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zou, X.; Yang, L.; Feng, Z.; Liu, J. Hydroxytyrosol protects against acrolein induced preosteoblast cell toxicity: Involvement of Nrf2/Keap1 pathway. J. Funct. Foods 2015, 19, 28–38. [Google Scholar] [CrossRef]
- Tirumalai, R.; Rajesh Kumar, T.; Mai, K.H.; Biswal, S. Acrolein causes transcriptional induction of phase II genes by activation of Nrf2 in human lung type II epithelial (A549) cells. Toxicol. Lett. 2002, 132, 27–36. [Google Scholar] [CrossRef]
- Feng, Z.; Hu, W.; Hu, Y.; Tang, M. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc. Natl. Acad. Sci. USA 2006, 103, 15404–15409. [Google Scholar] [CrossRef] [Green Version]
- Jain, V.; Alcheva, A.; Huang, D.; Caruso, R.; Jain, A.; Lay, M.; O’Connor, R.; Stepanov, I. Comprehensive Chemical Characterization of Natural American Spirit Cigarettes. Tob. Regul. Sci. 2019, 5, 381–399. [Google Scholar] [CrossRef]
- Wells, J.M.; O’Reilly, P.J.; Szul, T.; Sullivan, D.I.; Handley, G.; Garrett, C.; McNicholas, C.M.; Roda, M.A.; Miller, B.E.; Tal-Singer, R.; et al. An Aberrant Leukotriene A 4 Hydrolase–Proline-Glycine-Proline Pathway in the Pathogenesis of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2014, 190, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, S.; Dyba, M.; Pan, J.; Roy, R.; Chung, F.-L. Repair kinetics of acrolein- and (E)-4-hydroxy-2-nonenal-derived DNA adducts in human colon cell extracts. Mutat. Res. 2013, 751–752, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-W.; Wang, H.-T.; Weng, M.; Chin, C.; Huang, W.; Lepor, H.; Wu, X.-R.; Rom, W.N.; Chen, L.-C.; Tang, M. Cigarette side-stream smoke lung and bladder carcinogenesis: Inducing mutagenic acrolein-DNA adducts, inhibiting DNA repair and enhancing anchorage-independent-growth cell transformation. Oncotarget 2015, 6, 33226–33236. [Google Scholar] [CrossRef] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hikisz, P.; Jacenik, D. Diet as a Source of Acrolein: Molecular Basis of Aldehyde Biological Activity in Diabetes and Digestive System Diseases. Int. J. Mol. Sci. 2023, 24, 6579. https://doi.org/10.3390/ijms24076579
Hikisz P, Jacenik D. Diet as a Source of Acrolein: Molecular Basis of Aldehyde Biological Activity in Diabetes and Digestive System Diseases. International Journal of Molecular Sciences. 2023; 24(7):6579. https://doi.org/10.3390/ijms24076579
Chicago/Turabian StyleHikisz, Pawel, and Damian Jacenik. 2023. "Diet as a Source of Acrolein: Molecular Basis of Aldehyde Biological Activity in Diabetes and Digestive System Diseases" International Journal of Molecular Sciences 24, no. 7: 6579. https://doi.org/10.3390/ijms24076579
APA StyleHikisz, P., & Jacenik, D. (2023). Diet as a Source of Acrolein: Molecular Basis of Aldehyde Biological Activity in Diabetes and Digestive System Diseases. International Journal of Molecular Sciences, 24(7), 6579. https://doi.org/10.3390/ijms24076579







