Crosslinked Chitosan Nanoparticles with Muco-Adhesive Potential for Intranasal Delivery Applications
Abstract
:1. Introduction
2. Results
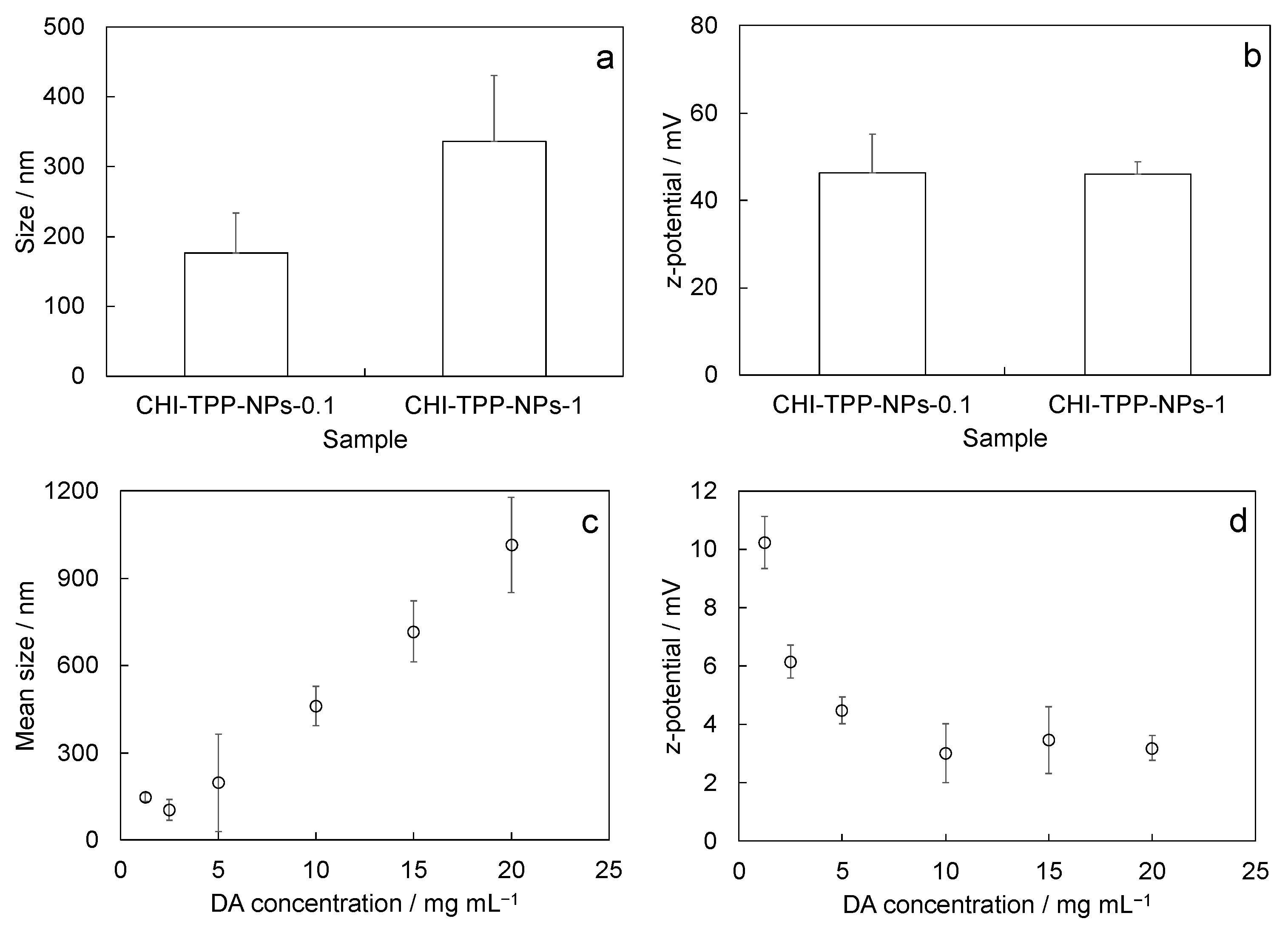
2.1. Physicochemical Characterization
2.2. In Vitro Cell Tests
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Nanoparticle Preparation via Ionotropic Gelation (CHI-TPP-NPs)
4.3. Crosslinked Nanoparticle Synthesis with 2,6-Pyridinedicarboxylic Acid (CHI-DA-NPs)
4.4. Physicochemical Characterization
4.4.1. Size and Surface Charge
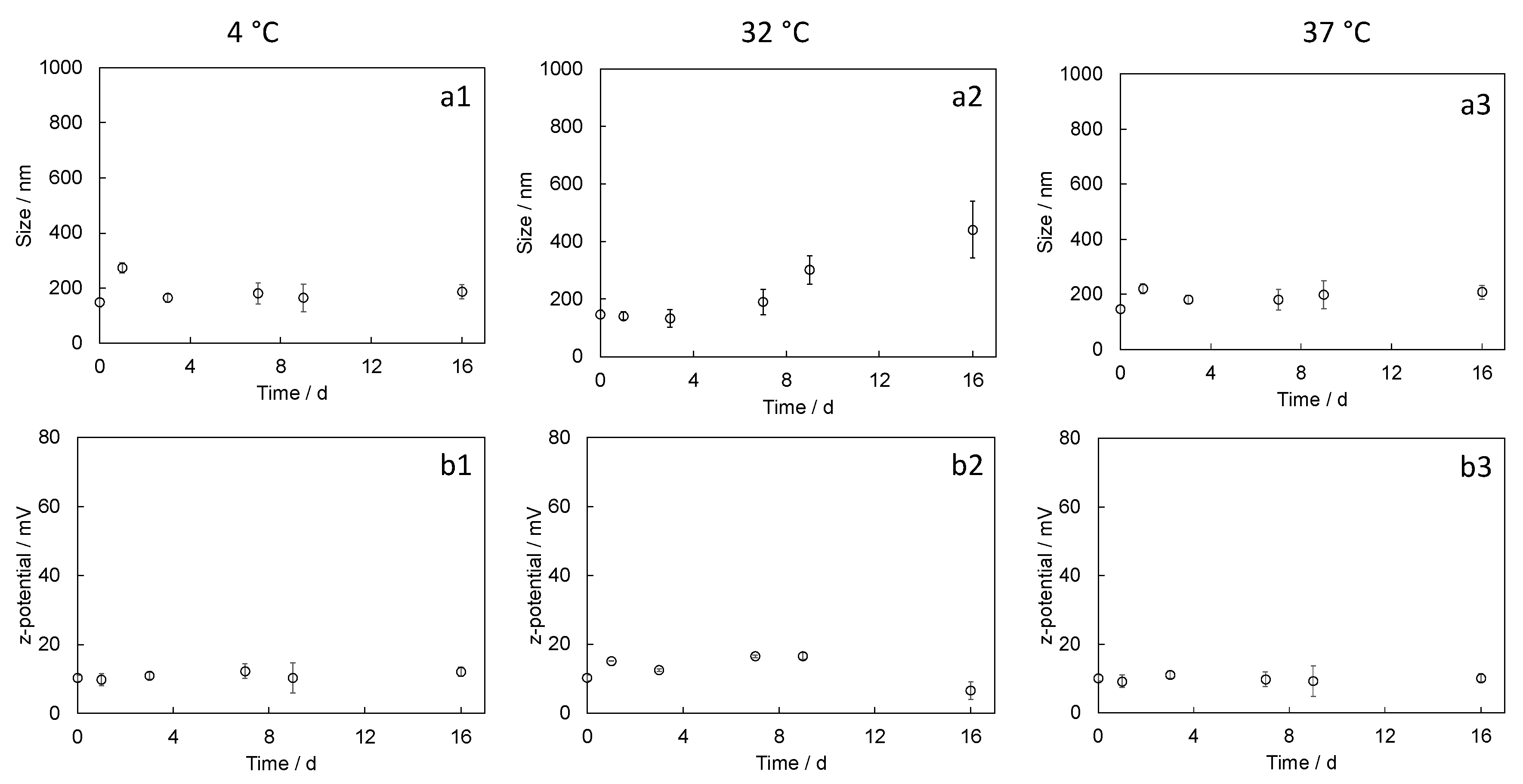
4.4.2. Nanoparticle Stability
4.5. Functional Characterization
4.5.1. In Vitro Biocompatibility Tests on Neuronal Cells
4.5.2. Statistics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Montegiove, N.; Calzoni, E.; Emiliani, C.; Cesaretti, A. Biopolymer nanoparticles for nose-to-brain drug delivery: A new promising approach for the treatment of neurological diseases. J. Funct. Biomater. 2022, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Mura, P.; Maestrelli, F.; Cirri, M.; Mennini, N. Multiple roles of chitosan in mucosal drug delivery: An updated review. Mar. Drugs 2022, 20, 335. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, L.D.; Duarte, J.; Fonseca-Santos, B.; Tavares Júnior, A.G.; Araújo, V.; Roque Borda, C.A.; Chorilli, M. Mucoadhesive nanosystems for nose-to-brain drug delivery in the treatment of central nervous system diseases. Curr. Med. Chem. 2022, 29, 3079–3110. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. Recent advances in mucoadhesive interface materials, mucoadhesion characterization, and technologies. Adv. Mater. Interf. 2022, 9, 2200211. [Google Scholar] [CrossRef]
- Gholizadeh, H.; Cheng, S.; Pozzoli, M.; Messerotti, E.; Traini, D.; Young, P.; Ong, H.X. Smart thermosensitive chitosan hydrogel for nasal delivery of ibuprofen to treat neurological disorders. Expert Opin. Drug Deliv. 2019, 16, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Syeda, J.T.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trenkel, M.; Scherließ, R. Optimising nasal powder drug delivery—Characterisation of the effect of excipients on drug absorption. Int. J. Pharm. 2023, 633, 122630. [Google Scholar] [CrossRef]
- Watchorn, J.; Clasky, A.J.; Prakash, G.; Johnston, I.A.; Chen, P.Z.; Gu, F.X. Untangling mucosal drug delivery: Engineering, designing, and testing nanoparticles to overcome the mucus barrier. ACS Biomater. Sci. Eng. 2022, 8, 1396–1426. [Google Scholar] [CrossRef]
- Viswanadhan Vasantha, P.; Sherafudeen, S.P.; Rahamathulla, M.; Mathew, S.T.; Murali, S.; Alshehri, S.; Narayana Iyer, B.A. Combination of Cellulose Derivatives and Chitosan-Based Polymers to Investigate the Effect of Permeation Enhancers Added to In Situ Nasal Gels for the Controlled Release of Loratadine and Chlorpheniramine. Polymers 2023, 15, 1206. [Google Scholar] [CrossRef]
- Teaima, M.H.; El-Nadi, M.T.; Hamed, R.R.; El-Nabarawi, M.A.; Abdelmonem, R. Lyophilized Nasal Inserts of Atomoxetine HCl Solid Lipid Nanoparticles for Brain Targeting as a Treatment of Attention-Deficit/Hyperactivity Disorder (ADHD): A Pharmacokinetics Study on Rats. Pharmaceuticals 2023, 16, 326. [Google Scholar] [CrossRef]
- Martins, P.P.; Smyth, H.D.; Cui, Z. Strategies to facilitate or block nose-to-brain drug delivery. Int. J. Pharm. 2019, 570, 118635. [Google Scholar] [CrossRef]
- Matias, M.; Santos, A.O.; Silvestre, S.; Alves, G. Fighting Epilepsy with Nanomedicines—Is This the Right Weapon? Pharmaceutics 2023, 15, 306. [Google Scholar] [CrossRef] [PubMed]
- Formica, M.L.; Real, D.A.; Picchio, M.L.; Catlin, E.; Donnelly, R.F.; Paredes, A.J. On a highway to the brain: A review on nose-to-brain drug delivery using nanoparticles. Appl. Mater. Today 2022, 29, 101631. [Google Scholar] [CrossRef]
- Surendranath, M.; Ramesan, R.M.; Parameswaran, R. Recent advances in developing functionally modified polymers for mucoadhesive drug delivery. J. Mater. Chem. B 2022, 10, 5913–5924. [Google Scholar] [CrossRef]
- Boyuklieva, R.; Pilicheva, B. Micro-and nanosized carriers for nose-to-brain drug delivery in neurodegenerative disorders. Biomedicines 2022, 10, 1706. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Qin, L.; Song, R.; Su, J.; Yuan, Y.; Zhang, X.; Mao, S. Elucidating inhaled liposome surface charge on its interaction with biological barriers in the lung. Eur. J. Pharm. Biopharm. 2022, 172, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.; Kaur, V.P.; Singh, A.; Singh, C. Recent advances on drug delivery applications of mucopenetrative/mucoadhesive particles: A review. J. Drug Deliv. Sci. Technol. 2022, 75, 103712. [Google Scholar] [CrossRef]
- Henning, A.; Schneider, M.; Nafee, N.; Muijs, L.; Rytting, E.; Wang, X.; Lehr, C.M. Influence of particle size and material properties on mucociliary clearance from the airways. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Butnarasu, C.; Petrini, P.; Bracotti, F.; Visai, L.; Guagliano, G.; Fiorio Pla, A.; Visentin, S. Mucosomes: Intrinsically Mucoadhesive Glycosylated Mucin Nanoparticles as Multi-Drug Delivery Platform. Adv. Healthc. Mater. 2022, 11, 2200340. [Google Scholar] [CrossRef]
- Wang, T.; Fleming, E.; Luo, Y. An overview of the biochemistry, synthesis, modification, and evaluation of mucoadhesive polymeric nanoparticles for oral delivery of bioactive compounds. Adv. Compos. Hybrid Mater. 2023, 6, 6. [Google Scholar] [CrossRef]
- Darwish, W.M.; Bayoumi, N.A.; Ebeid, N.H. Biocompatible mucoadhesive nanoparticles for brain targeting of ropinirole hydrochloride: Formulations, radiolabeling and biodistribution. Biopolymers 2022, 113, e23489. [Google Scholar] [CrossRef] [PubMed]
- Elkomy, M.H.; Ali, A.A.; Eid, H.M. Chitosan on the surface of nanoparticles for enhanced drug delivery: A comprehensive review. J. Control. Release 2022, 351, 923–940. [Google Scholar] [CrossRef] [PubMed]
- Mitsou, E.; Pletsa, V.; Sotiroudis, G.T.; Panine, P.; Zoumpanioti, M.; Xenakis, A. Development of a microemulsion for encapsulation and delivery of gallic acid. The role of chitosan. Colloids Surf. B 2020, 190, 110974. [Google Scholar] [CrossRef] [PubMed]
- Toy, R.; Pradhan, P.; Ramesh, V.; Di Paolo, N.C.; Lash, B.; Liu, J.; Roy, K. Modification of primary amines to higher order amines reduces in vivo hematological and immunotoxicity of cationic nanocarriers through TLR4 and complement pathways. Biomaterials 2019, 225, 119512. [Google Scholar] [CrossRef]
- Yermak, I.M.; Davydova, V.N.; Volod’Ko, A.V. Mucoadhesive Marine Polysaccharides. Mar. Drugs 2022, 20, 522. [Google Scholar] [CrossRef]
- Picos-Corrales, L.A.; Morales-Burgos, A.M.; Ruelas-Leyva, J.P.; Crini, G.; García-Armenta, E.; Jimenez-Lam, S.A.; Inzunza-Camacho, L.N. Chitosan as an Outstanding Polysaccharide Improving Health-Commodities of Humans and Environmental Protection. Polymers 2023, 15, 526. [Google Scholar] [CrossRef]
- Ways, T.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Abere, D.V.; Ojo, S.A.; Paredes-Epinosa, M.B.; Hakami, A. Derivation of composites of chitosan-nanoparticles from crustaceans source for nanomedicine: A mini review. Biomed. Eng. Adv. 2022, 4, 100058. [Google Scholar] [CrossRef]
- Naseer, F.; Ahmad, T.; Kousar, K.; Kakar, S.; Gul, R.; Anjum, S. Formulation of surface-functionalized hyaluronic acid-coated thiolated chitosan nano-formulation for the delivery of vincristine in prostate cancer: A multifunctional targeted drug delivery approach. J. Drug Deliv. Sci. Technol. 2022, 74, 103545. [Google Scholar] [CrossRef]
- Melamed, J.R.; Kreuzberger, N.L.; Goyal, R.; Day, E.S. Spherical nucleic acid architecture can improve the efficacy of polycation-mediated siRNA delivery. Mol. Ther. Nucleic Acids 2018, 12, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Pichla, M.; Bartosz, G.; Stefaniuk, I.; Sadowska-Bartosz, I. pH-Responsive Redox Nanoparticles Protect SH-SY5Y Cells at Lowered pH in a Cellular Model of Parkinson’s Disease. Molecules 2021, 26, 543. [Google Scholar] [CrossRef]
- Tonazzini, I.; Cecchini, A.; Elgersma, Y.; Cecchini, M. Interaction of SH-SY5Y cells with Nanogratings during neuronal differentiation: Comparison with primary neurons. Adv. Healthc. Mater. 2014, 3, 581–587. [Google Scholar] [CrossRef]
- Mezzena, R.; Masciullo, C.; Antonini, S.; Cremisi, F.; Scheffner, M.; Cecchini, M.; Tonazzini, I. Study of adhesion and migration dynamics in ubiquitin E3A ligase (UBE3A)-silenced SYSH5Y neuroblastoma cells by micro-structured surfaces. Nanotechnology 2020, 32, 025708. [Google Scholar] [CrossRef] [PubMed]
- Xicoy, H.; Wieringa, B.; Martens, G.J. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibinovska, N.; Žakelj, S.; Kristan, K. Suitability of RPMI 2650 cell models for nasal drug permeability prediction. Eur. J. Pharm. Biopharm. 2019, 145, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Clementino, A.; Batger, M.; Garrastazu, G.; Pozzoli, M.; Del Favero, E.; Rondelli, V.; Sonvico, F. The nasal delivery of nanoencapsulated statins–an approach for brain delivery. Int. J. Nanomed. 2016, 11, 6575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackenzie, C.G.; Mackenzie, J.B.; Beck, P. The effect of pH on growth, protein synthesis, and lipid-rich particles of cultured mammalian cells. J. Cell Biol. 1961, 9, 141–156. [Google Scholar] [CrossRef] [Green Version]
- Eagle, H. Buffer combinations for mammalian cell culture. Science 1971, 174, 500–503. [Google Scholar] [CrossRef]
- Washington, N.; Steele, R.J.C.; Jackson, S.J.; Bush, D.; Mason, J.; Gill, D.A.; Rawlins, D.A. Determination of baseline human nasal pH and the effect of intranasally administered buffers. Int. J. Pharm. 2000, 198, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K. (Ed.) Chitin and Chitosan Derivatives: Advances in Drug Discovery and Developments, 1st ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Vaz, J.M.; Pezzoli, D.; Chevallier, P.; Campelo, C.S.; Candiani, G.; Mantovani, D. Antibacterial coatings based on chitosan for pharmaceutical and biomedical applications. Curr. Pharm. Des. 2018, 24, 866–885. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C. (Ed.) Biochemistry of Microbial Degradation; Springer Science and Business Media: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Sims, G.K.; Sommers, L.E. Biodegradation of pyridine derivatives in soil suspensions. Environ. Toxicol. Chem. Int. J. 1986, 5, 503–509. [Google Scholar] [CrossRef]
- McClintock, M.K.; Fahnhorst, G.W.; Hoye, T.R.; Zhang, K. Engineering the production of dipicolinic acid in E. coli. Metab. Eng. 2018, 48, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.S.; Casey, K.P.; Pawar, S.S.; Garcia, G.J. Correlation of nasal mucosal temperature with subjective nasal patency in healthy individuals. JAMA Facial Plast. Surg. 2017, 19, 46–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthu, M.S.; Feng, S. Pharmaceutical stability aspects of nanomedicines. Nanomedicine 2009, 8, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Meucci, S.; Tonazzini, I.; Beltram, F.; Cecchini, M. Biocompatible noisy nanotopographies with specific directionality for controlled anisotropic cell cultures. Soft Matter 2012, 8, 1109–1119. [Google Scholar] [CrossRef]


| Size/nm | |||
|---|---|---|---|
| at | 0 h | 24 h | 48 h |
| CHI-TPP-NPs-0.1 | 268 ± 159 | 499 ± 33 | 177 ± 92 |
| CHI-TPP-NPs-1 | 203 ± 74 | 329 ± 128 | 494 ± 89 |
| CHI-DA-NPs-1.25 | 123 ± 9 | 149 ± 3 | 169 ± 4 |
| pH (2.5 mg/mL) | pH (2 mg/mL) | pH (1 mg/mL) | pH (0.5 mg/mL) | pH (0.25 mg/mL) | |
|---|---|---|---|---|---|
| CHI-TPP-NPs-0.1 | 6.2 ± 0.3 | 6.6 ± 0.1 | 7.4 ± 0.3 | 7.8 ± 0.2 | 7.9 ± 0.2 |
| CHI-TPP-NPs-1 | 6.1 ± 0.1 | 6.5 ± 0.2 | 7.4 ± 0.2 | 7.8 ± 0.2 | 7.8 ± 0.1 |
| CHI-DA-NPs-1.25 | 7.2 ± 0.1 | 7.3 ± 0.1 | 7.7 ± 0.1 | 7.9 ± 0.1 | 8.1 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagliardi, M.; Chiarugi, S.; De Cesari, C.; Di Gregorio, G.; Diodati, A.; Baroncelli, L.; Cecchini, M.; Tonazzini, I. Crosslinked Chitosan Nanoparticles with Muco-Adhesive Potential for Intranasal Delivery Applications. Int. J. Mol. Sci. 2023, 24, 6590. https://doi.org/10.3390/ijms24076590
Gagliardi M, Chiarugi S, De Cesari C, Di Gregorio G, Diodati A, Baroncelli L, Cecchini M, Tonazzini I. Crosslinked Chitosan Nanoparticles with Muco-Adhesive Potential for Intranasal Delivery Applications. International Journal of Molecular Sciences. 2023; 24(7):6590. https://doi.org/10.3390/ijms24076590
Chicago/Turabian StyleGagliardi, Mariacristina, Sara Chiarugi, Chiara De Cesari, Giulia Di Gregorio, Alessandra Diodati, Laura Baroncelli, Marco Cecchini, and Ilaria Tonazzini. 2023. "Crosslinked Chitosan Nanoparticles with Muco-Adhesive Potential for Intranasal Delivery Applications" International Journal of Molecular Sciences 24, no. 7: 6590. https://doi.org/10.3390/ijms24076590







