Development of Antifouling Coatings Based on Quaternary Ammonium Compounds through a Multilayer Approach
Abstract
:1. Introduction
2. Results and Discussion
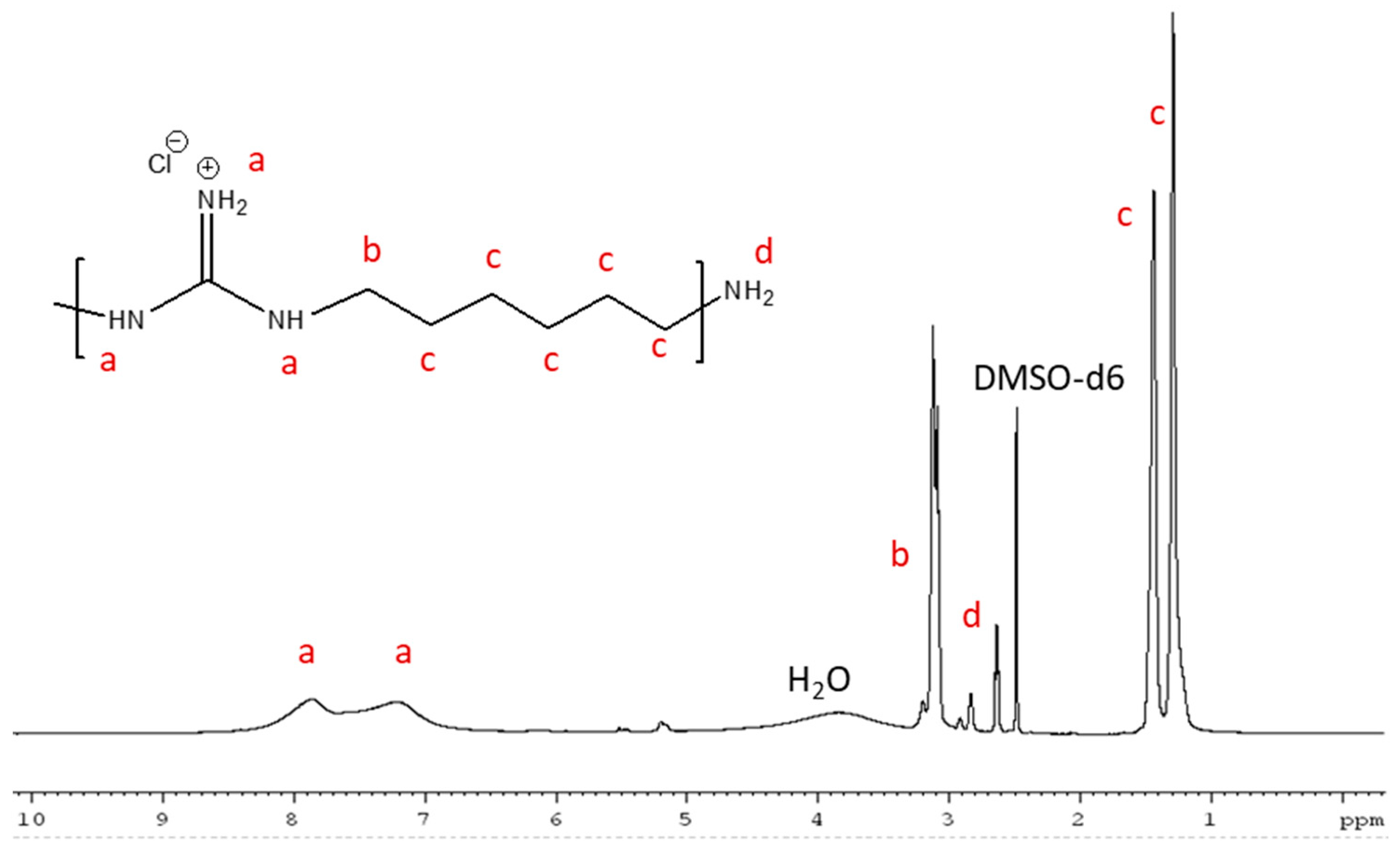
2.1. Characterization of Polymers
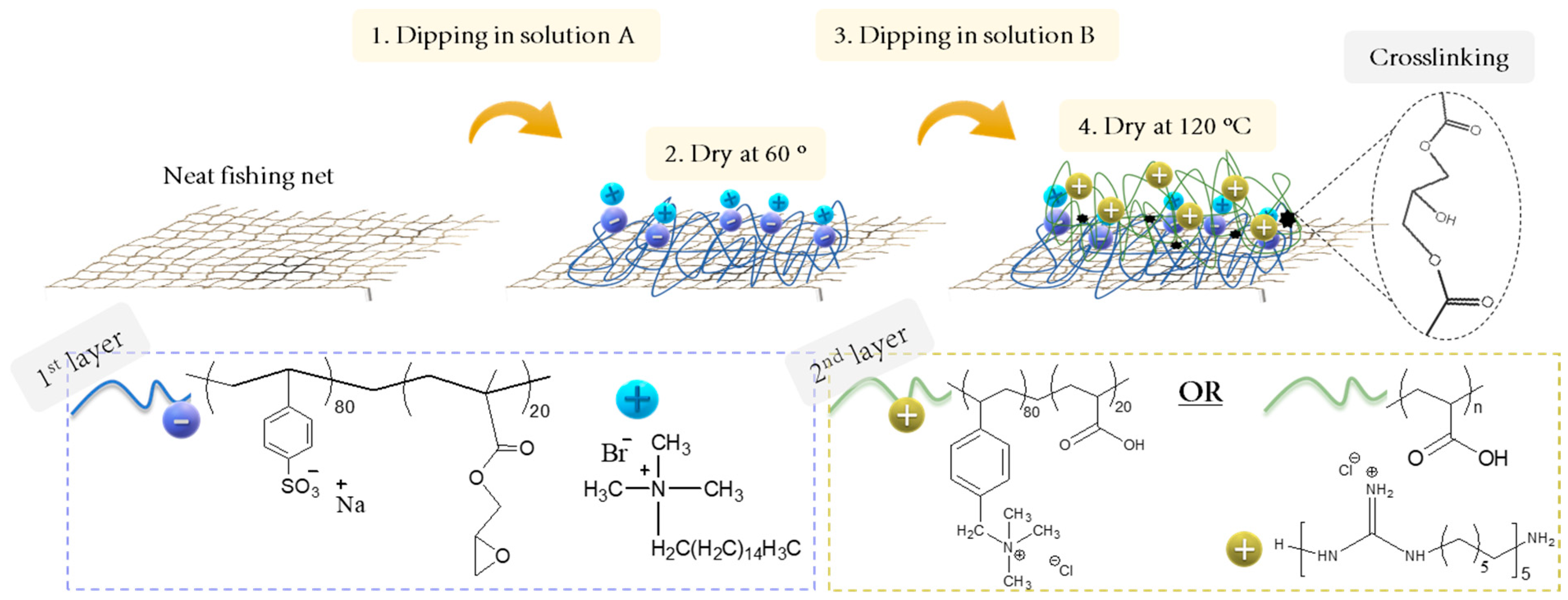
2.2. Preparation of Coated Nets
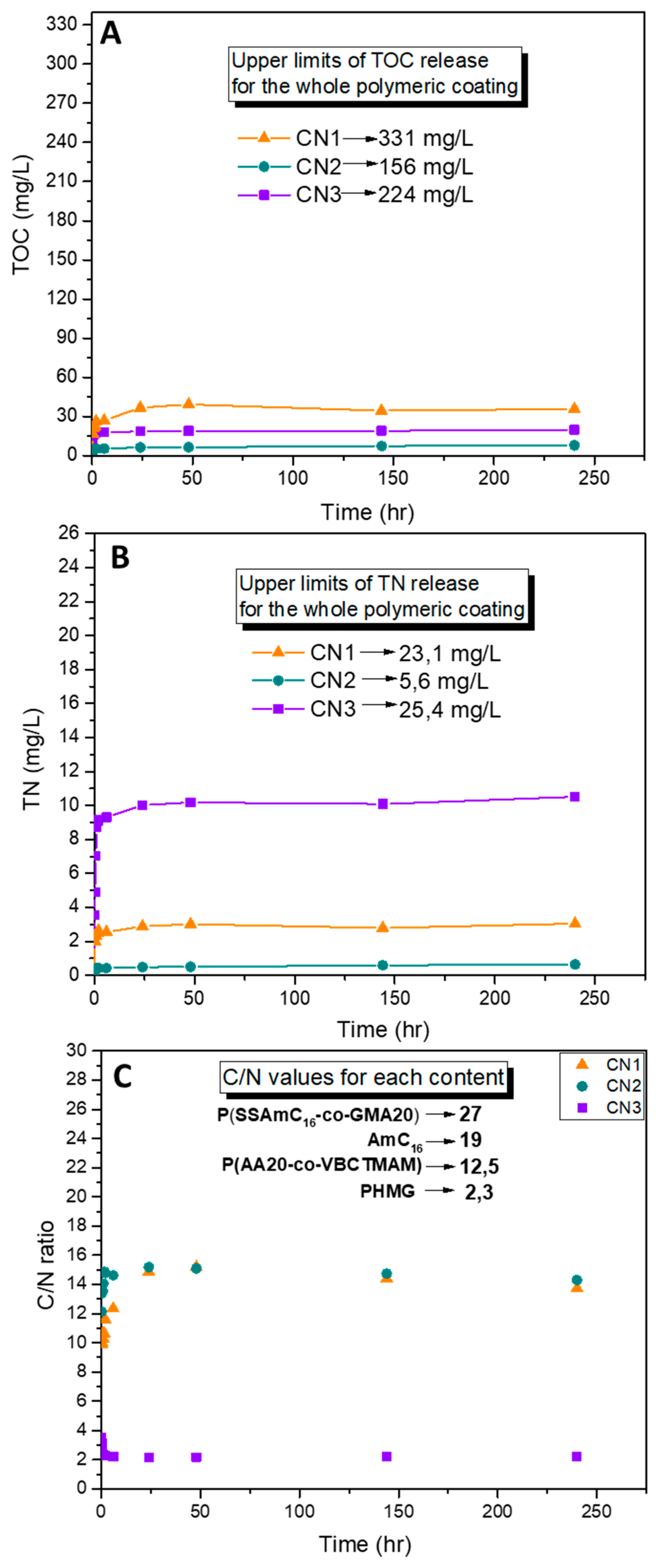
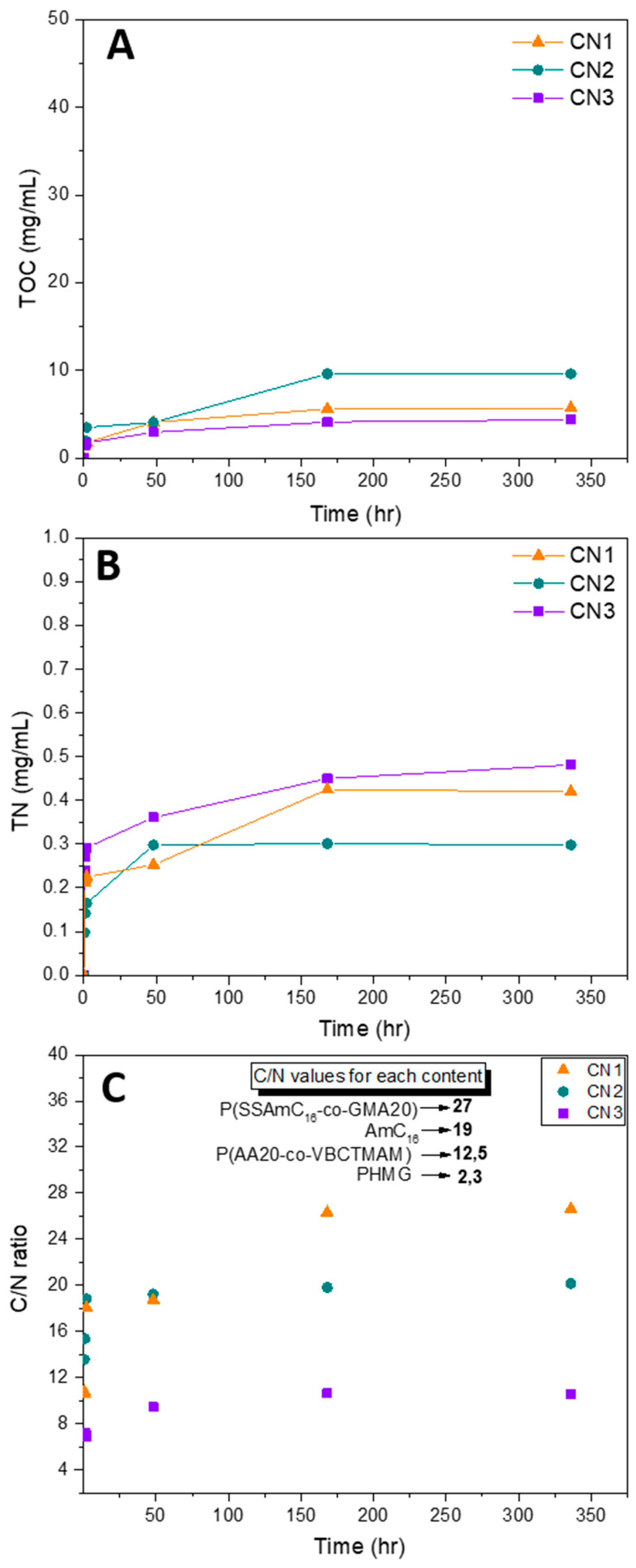
2.3. Release Study of Polymer Coatings
2.4. Action of New Polymeric Coatings against Biofouling
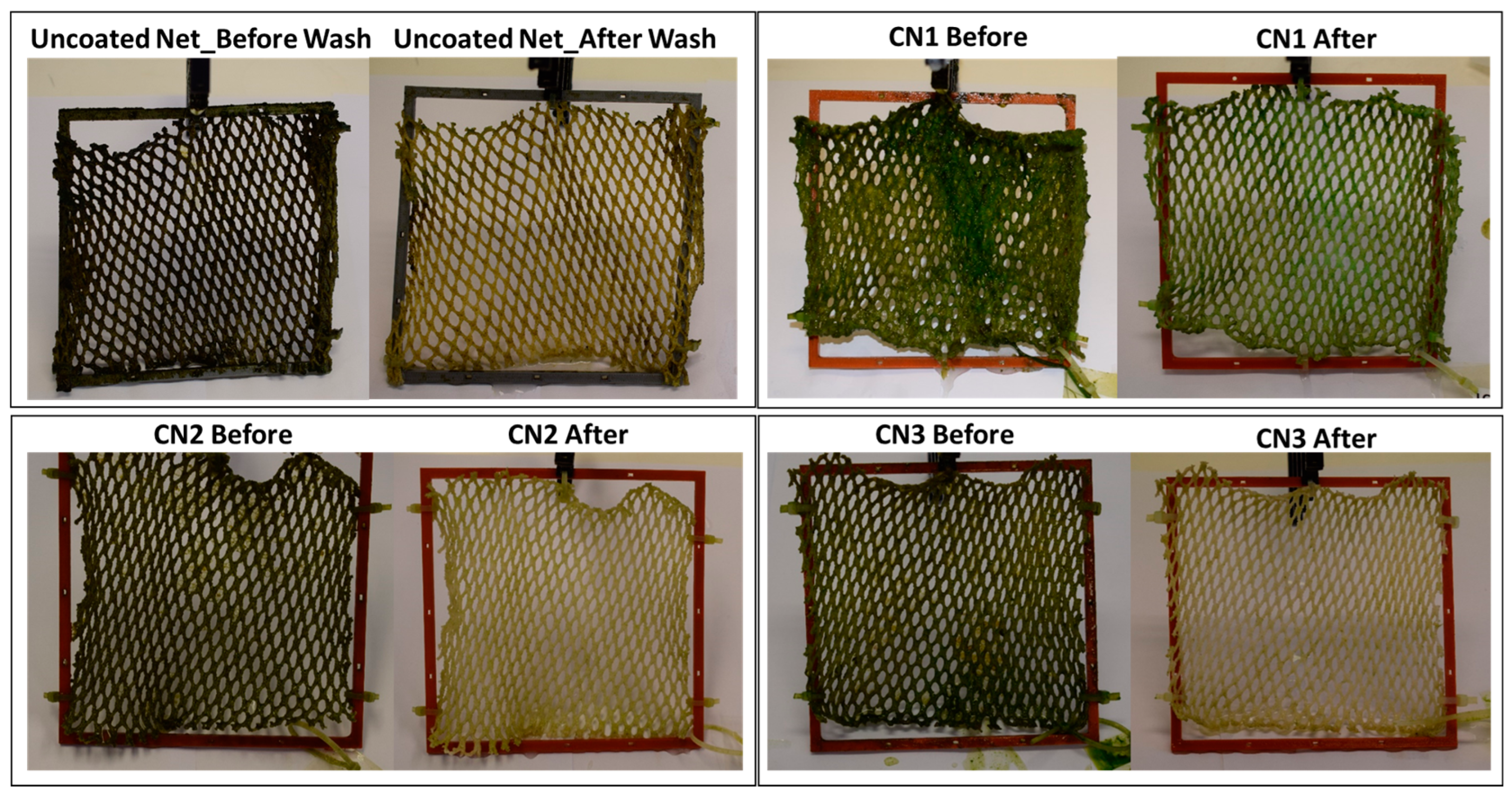
2.4.1. Testing under Accelerated Biofouling Conditions
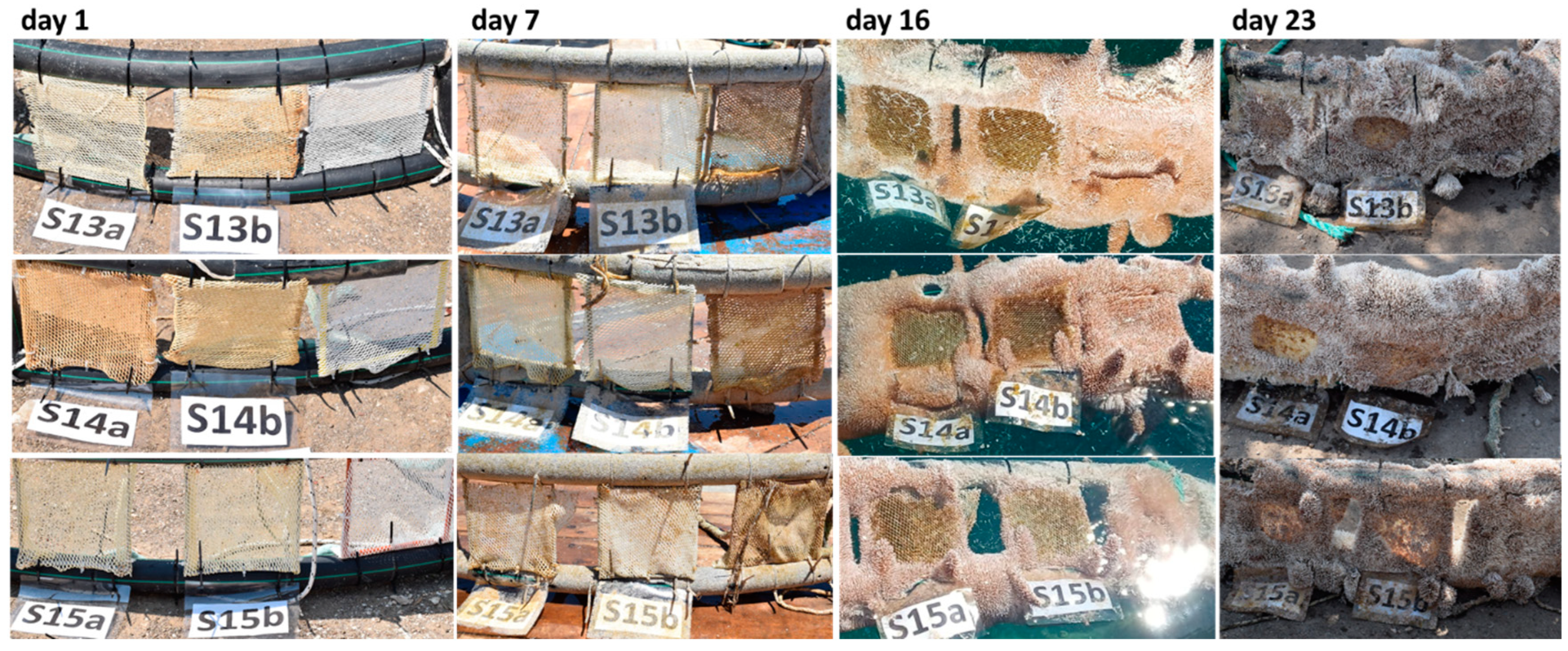
2.4.2. Testing under Real Field Conditions
3. Materials and Methods
3.1. Materials
3.2. Synthetic Procedures
3.2.1. P(SSAmC16-co-GMA20) and P(VBCTMAM-co-AA20)
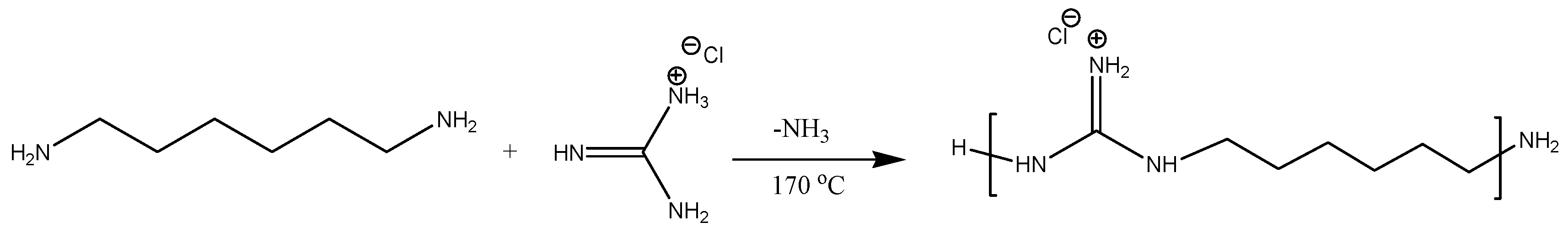
3.2.2. PHMG
3.3. Preparation of the Coated Aquaculture Nets
3.4. Characterization Techniques
3.4.1. Nuclear Magnetic Resonance Hydrogen Spectroscopy (1H-NMR)
3.4.2. Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
3.4.3. Scanning Electron Microscopy-Energy Dispersive Spectroscopy (SEM-EDS)
3.5. Release Studies
3.5.1. Soluble Fraction and Solvent Uptake Studies of Coated Nets
3.5.2. Total Organic Carbon (TOC) and Total Nitrogen (TN) Measurements
3.6. Antifouling Performance of Coated Nets
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amini, S.; Kolle, S.; Petrone, L.; Ahanotu, O.; Sunny, S.; Sutanto, C.N.; Hoon, S.; Cohen, L.; Weaver, J.C.; Aizenberg, J.; et al. Preventing mussel adhesion using lubricant-infused materials. Science 2017, 357, 668–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dürr, S.; Watson, D.I. Biofouling and antifouling in aquaculture. In Biofouling, 1st ed.; Dürr, S., Thomason, J.C., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 267–287. [Google Scholar]
- Hopkins, G.; Davidson, I.; Georgiades, E.; Floer, O.; Morrise, D.; Cahil, P. Managing Biofouling on Submerged Static Artificial Structures in the Marine Environment—Assessment of Current and Emerging Approaches. Front. Mar. Sci. 2021, 8, 759194. [Google Scholar] [CrossRef]
- Bloecher, N.; Floerl, O. Efficacy testing of novel antifouling coatings for pen nets in aquaculture: How good are alternatives to traditional copper coatings? Aquaculture 2020, 519, 734936. [Google Scholar] [CrossRef]
- Railkin, A.I. Marine Biofouling: Colonization Processes and Defenses; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Dobretsov, S. Marine Biofilms. In Biofouling, 1st ed.; Dürr, S., Thomason, J.C., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 123–153. [Google Scholar]
- Maki, J.S. Biofouling in the marine environment. In Encyclopedia of Environmental Micro-Biology; Bitton, G., Ed.; John Wiley & Sons: New York, NY, USA, 2002; pp. 610–619. [Google Scholar]
- Leonardi, A.K.; Ober, C.K. Polymer-Based Marine Antifouling and Fouling Release Surfaces: Strategies for Synthesis and Modification. Annu. Rev. Chem. Biomol. Eng. 2019, 10, 241–264. [Google Scholar] [CrossRef]
- Wu, X.; Fua, Q.; Kumar, D.; Ho, J.W.C.; Kanhere, P.; Zhou, H.; Chen, Z. Mechanically robust superhydrophobic and superoleophobic coatings derived by sol–gel method. Mater. Des. 2016, 89, 1302–1309. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Song, X.F.; Cui, L.Y.; Qi, Y.H. Synthesis of Polydimethylsiloxane-Modified Polyurethane and the Structure and Properties of its Antifouling Coatings. Coatings 2018, 8, 157. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Chen, Z.; Guo, Y.; Ju, Y.; Liu, Y.; Feng, R.; Xiong, C.; Ober, C.K.; Dong, L. Flexible hydrophobic antifouling coating with oriented nanotopography and non-leaking capsaicin. ACS Appl. Mater. Interfaces 2018, 10, 9718–9726. [Google Scholar] [CrossRef]
- Martinelli, E.; Gunes, D.; Wenning, B.M.; Ober, C.K.; Finlay, J.A.; Callow, M.E.; Callow, J.A.; Di Fino, A.; Clare, A.S.; Galli, G. Effects of surface-active block copolymers with oxyethylene and fluoroalkyl side chains on the antifouling performance of silicone-based films. Biofouling 2016, 32, 81–93. [Google Scholar] [CrossRef]
- Omae, I. General Aspects of Tin-Free Antifouling Paints. Chem. Rev. 2003, 103, 3431–3448. [Google Scholar] [CrossRef]
- Thomas, K.V.; Brooks, S. The environmental fate and effects of antifouling paint biocides. Biofouling 2010, 26, 73–88. [Google Scholar] [CrossRef]
- Kartal, G.E.; Sarıışık, A.M. Providing antifouling properties to fishing nets with encapsulated econea. J. Ind. Text 2022, 51 (Suppl. S5), 7569S–7586S. [Google Scholar] [CrossRef]
- Kartal, G.E.; Sarıışık, A.M. Modifying of UHMWPE fishing nets with layer-by-layer deposition method for antifouling properties. J. Coat. Technol. Res. 2021, 18, 163–171. [Google Scholar] [CrossRef]
- Frydenberg, T.; Weinell, C.E.; Dam-Johansen, K.; Wallström, E.; Kiil, S. Characterization and Release Mechanisms of Aerogel-Encapsulated Biocide Crystals for Low-Loading and High-Utilization Antifouling Coatings. ACS Omega 2022, 7, 34824–34838. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Liu, D.; Liu, Y.; Wang, H.; Zhang, C.; Yuan, C.; Liu, X. Marine antifouling behavior of the surfaces modified by dopamine and antibacterial peptide. J. Oceanol. Limnol. 2023, 41, 174–188. [Google Scholar] [CrossRef]
- Schönemann, E.; Koc, J.; Aldred, N.; Clare, A.S.; Laschewsky, A.; Rosenhahn, A.; Wischerhoff, E. Synthesis of Novel Sulfobetaine Polymers with Differing Dipole Orientations in Their Side Chains, and Their Effects on the Antifouling Properties. Macromol. Rapid Commun. 2019, 41, 1900447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, A.; Ashraf, P.M. Biofouling Control Using Nano Silicon Dioxide Reinforced Mixed-Charged Zwitterionic Hydrogel in Aquaculture Cage Nets. Langmuir 2019, 35, 4328–4335. [Google Scholar] [CrossRef]
- Koc, J.; Schönemann, E.; Amuthalingam, A.; Clarke, J.; Finlay, J.A.; Clare, A.S.; Laschewsky, A.; Rosenhahn, A. Low-Fouling Thin Hydrogel Coatings Made of Photo-cross-linked Polyzwitterions. Langmuir 2019, 35, 1552–1562. [Google Scholar] [CrossRef]
- Pranantyo, D.; Xu, L.Q.; Neoh, K.-G.; Kang, E.-T.; Ng, Y.X.; Teo, S.L.-M. Tea Stains-Inspired Initiator Primer for Surface Grafting of Antifouling and Antimicrobial Polymer Brush Coatings. Biomacromolecules 2015, 16, 723–732. [Google Scholar] [CrossRef]
- Gao, K.; Su, Y.; Zhou, L.; He, M.; Zhang, R.; Liu, Y.; Jiang, Z. Creation of active-passive integrated mechanisms on membrane surfaces for superior antifouling and antibacterial properties. J. Membr. Sci. 2018, 548, 621–631. [Google Scholar] [CrossRef]
- Ping, M.; Zhang, X.; Liu, M.; Wu, Z.; Wang, Z. Surface Modification of Polyvinylidene Fluoride Membrane by Atom-Transfer Radical-Polymerization of Quaternary Ammonium Compound for Mitigating Biofouling. J. Membr. Sci. 2019, 570–571, 286–293. [Google Scholar] [CrossRef]
- Yang, W.; Lin, P.; Cheng, D.; Zhang, L.; Wu, Y.; Liu, Y.; Pei, X.; Zhou, F. Contribution of Charges in Polyvinyl Alcohol Networks to Marine Antifouling. ACS Appl. Mater. Interfaces 2017, 9, 18295–18304. [Google Scholar] [CrossRef]
- Yandi, W.; Mieszkin, S.; Callow, M.E.; Callow, J.A.; Finlay, J.A.; Liedberg, B.; Ederth, T. Antialgal activity of poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA) brushes against the marine alga Ulva. Biofouling 2017, 33, 169–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Druvari, D.; Koromilas, N.D.; Lainioti, G.C.; Bokias, G.; Vasilopoulos, G.; Vandarakis, A.; Baras, I.; Dourala, N.; Kallitsis, J.K. Polymeric Quaternary Ammonium-Containing Coatings with Potential Dual Contact-Based and Release-Based Antimicrobial Activity. ACS Appl. Mater. Interfaces 2016, 8, 35593–35605. [Google Scholar] [CrossRef]
- Lainioti, G.C.; Tsapikouni, A.; Druvari, D.; Avramidis, P.; Prevedouros, I.; Glaropoulos, A.; Kallitsis, J.K. Environmentally Friendly Cross-Linked Antifouling Coatings Based on Dual Antimicrobial Action. Int. J. Mol. Sci. 2021, 22, 4658. [Google Scholar] [CrossRef] [PubMed]
- Lainioti, G.C.; Savva, P.; Druvari, D.; Avramidis, P.; Panagiotaras, D.; Karellou, E.I.E.; Kallitsis, J.K. Cross-linking of antimicrobial polymers with hexamethylene diamine to prevent biofouling in marine applications. Prog. Org. Coat. 2021, 157, 106336. [Google Scholar] [CrossRef]
- Tsagdi, A.; Druvari, D.; Panagiotaras, D.; Avramidis, P.; Bekiari, V.; Kallitsis, J.K. Polymeric Coatings Based on Water-Soluble Trimethylammonium Copolymers for Antifouling Applications. Molecules 2020, 25, 1678. [Google Scholar] [CrossRef] [Green Version]
- Marino, T.; Galiano, F.; Simone, S.; Figoli, A. DMSO EVOL™ as novel non-toxic solvent for polyethersulfone membrane preparation. Environ. Sci. Pollut. Res. 2019, 26, 14774–14785. [Google Scholar] [CrossRef]
- Protasov, A.; Bardeau, J.-F.; Morozovskaya, I.; Boretska, M.; Cherniavska, T.; Petrus, L.; Tarasyuk, O.; Metelytsia, L.; Kopernyk, I.; Kalashnikova, L.; et al. New promising antifouling agent based on polymeric biocide polyhexamethylene guanidine molybdate. J. Environ. Toxicol. 2016, 36, 2543–2551. [Google Scholar] [CrossRef]
- Han, J.-S.; Lim, K.-M.; Park, S.-J.; Song, W.-S. Polyhexamethyleneguanidine Phosphate Powder, Method of Making the Same and Antimicrobial Resin Containing the Same. European Patent No. EP1110948A2, 17 May 2001. [Google Scholar]
- Huang, G.; Shen, H.; Chen, X.; Wu, T.; Chen, Z.; Chen, Y.; Song, J.; Cai, Q.; Bai, Y.; Pu, H.; et al. A Degradable, Broad-Spectrum and Resistance-Resistant Antimicrobial Oligoguanidine as Disinfecting and Therapeutic Agent in Aquaculture. Polym. Chem. 2022, 13, 3539–3551. [Google Scholar] [CrossRef]
- Bazaron, L.U.; Stel’makh, S.A. Molecular-weight characteristics of polyhexamethyleneguanidine hydrochloride. Russ. J. Appl. Chem. 2008, 81, 2021–2025. [Google Scholar] [CrossRef]
- Druvari, D.; Kyriakopoulou, F.; Lainioti, G.C.; Vlamis-Gardikas, A.; Kallitsis, J.K. Humidity-Responsive Antimicrobial Membranes Based on Cross-Linked Copolymers Functionalized with Ionic Liquid Moieties. ACS Appl. Mater. Interfaces 2023, 15, 11193–11207. [Google Scholar] [CrossRef] [PubMed]
- Ba, C.; Ladner, D.A.; Economy, J. Using polyelectrolyte coatings to improve fouling resistance of a positively charged nanofiltration membrane. J. Membr. Sci. 2010, 347, 250–259. [Google Scholar] [CrossRef]
- Del Hoyo-Gallego, S.; Pérez-Álvarez, L.; Gómez-Galván, F.; Lizundia, E.; Kuritka, I.; Sedlarik, V.; Vila-Vilela, J.L. Construction of antibacterial poly(ethylene terephthalate) films via layer by layer assembly of chitosan and hyaluronic acid. Carbohydr. Polym. 2016, 143, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Jańczewski, D.; Guo, S.; Lee, S.S.C.; Parra Velandia, F.J.; Teo, S.L.-M.; Vancso, G.J. Polyion Multilayers with Precise Surface Charge Control for Antifouling. ACS Appl. Mater. Interfaces 2014, 7, 852–861. [Google Scholar] [CrossRef]
- Zhu, X.; Jańczewski, D.; Lee, S.S.C.; Teo, S.L.-M.; Vancso, G.J. Cross-Linked Polyelectrolyte Multilayers for Marine Antifouling Applications. ACS Appl. Mater. Interfaces 2013, 5, 5961–5968. [Google Scholar] [CrossRef]
- Wei, D.; Ma, Q.; Guan, Y.; Hu, F.; Zheng, A.; Zhang, X.; Jiang, H. Structural characterization and antibacterial activity of oligoguanidine (polyhexamethylene guanidine hydrochloride). Mater. Sci. Eng. C 2009, 29, 1776–1780. [Google Scholar] [CrossRef]
- Bekiari, V.; Avramidis, P. Data quality in water analysis: Validation of combustion-infrared and combustion-chemiluminescence methods for the simultaneous determination of Total Organic Carbon (TOC) Total Nitrogen (TN). Int. J. Environ. Anal. Chem. 2014, 94, 65–76. [Google Scholar] [CrossRef]



| Concentration (g/dL) | [η] (dL/g) in 0.3 N NaCl | Mη |
|---|---|---|
| 8.00 | 0.02095 | 646 |
| Net Code | 1st Layer (in DMSO) | 2nd Layer (in H2O) | Layers’ Ratio % wt. | Coatings’ Loading % wt. |
|---|---|---|---|---|
| CN1 | P(SSAmC16-co-GMA20) | P(VBCTMAM-co-AA20) | 55/45 | 34 |
| CN2 | P(SSAmC16-co-GMA20) | PAA | 70/30 | 30 |
| CN3 | P(SSAmC16-co-GMA20) | PAA/PHMG | 70/30 | 35 |
| Net Code | Immersion Time | Soluble Fraction % wt. | Solvent Uptake % wt. | ||
|---|---|---|---|---|---|
| H2O | ΝaCl 0.6 M | H2O | ΝaCl 0.6 M | ||
| CN1 | 1st cycle (10 d) | 32 | 6 | 167 | 159 |
| 2nd cycle (25 d) | 35 | 9 | 154 | 170 | |
| CN2 | 1st cycle (10 d) | 5 | 4 | 30 | 190 |
| 2nd cycle (25 d) | 13 | 2 | 108 | 89 | |
| CN3 | 1st cycle (10 d) | 14 | 6 | 80 | 130 |
| 2nd cycle (25 d) | 5 | 8 | 14 | 74 | |
| Net Code | 1st (and 3rd) Layer (in DMSO) | 2nd (and 4th) Layer (in H2O) | Number of Layers | Layers’ Ratio % wt. | Coatings’ Loading % wt. |
|---|---|---|---|---|---|
| S13a | P(SSAmC16-co-GMA20) | PAA | 2 | 70/30 | 30 |
| S13b | P(SSAmC16-co-GMA20) | PAA | 4 | 70/30 | 50 |
| S14a | P(SSAmC16-co-GMA20) | PAA/PHMG | 4 | 70/30 | 60 |
| S14b | P(SSAmC16-co-GMA20) | PAA/PHMG | 2 | 70/30 | 29 |
| S15a | P(SSAmC16-co-GMA20) | P(VBCTMAM-co-AA20) | 2 | 55/45 | 30 |
| S15b | P(SSAmC16-co-GMA20) | P(VBCTMAM-co-AA20) | 4 | 55/45 | 43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Druvari, D.; Lainioti, G.C.; Bekiari, V.; Avramidis, P.; Kallitsis, J.K.; Bokias, G. Development of Antifouling Coatings Based on Quaternary Ammonium Compounds through a Multilayer Approach. Int. J. Mol. Sci. 2023, 24, 6594. https://doi.org/10.3390/ijms24076594
Druvari D, Lainioti GC, Bekiari V, Avramidis P, Kallitsis JK, Bokias G. Development of Antifouling Coatings Based on Quaternary Ammonium Compounds through a Multilayer Approach. International Journal of Molecular Sciences. 2023; 24(7):6594. https://doi.org/10.3390/ijms24076594
Chicago/Turabian StyleDruvari, Denisa, Georgia C. Lainioti, Vlasoula Bekiari, Pavlos Avramidis, Joannis K. Kallitsis, and Georgios Bokias. 2023. "Development of Antifouling Coatings Based on Quaternary Ammonium Compounds through a Multilayer Approach" International Journal of Molecular Sciences 24, no. 7: 6594. https://doi.org/10.3390/ijms24076594





