The Reduction of Uromodulin, Complement Factor H, and Their Interaction Is Associated with Acute Kidney Injury to Chronic Kidney Disease Transition in a Four-Time Cisplatin-Injected Rat Model
Abstract
1. Introduction
2. Results
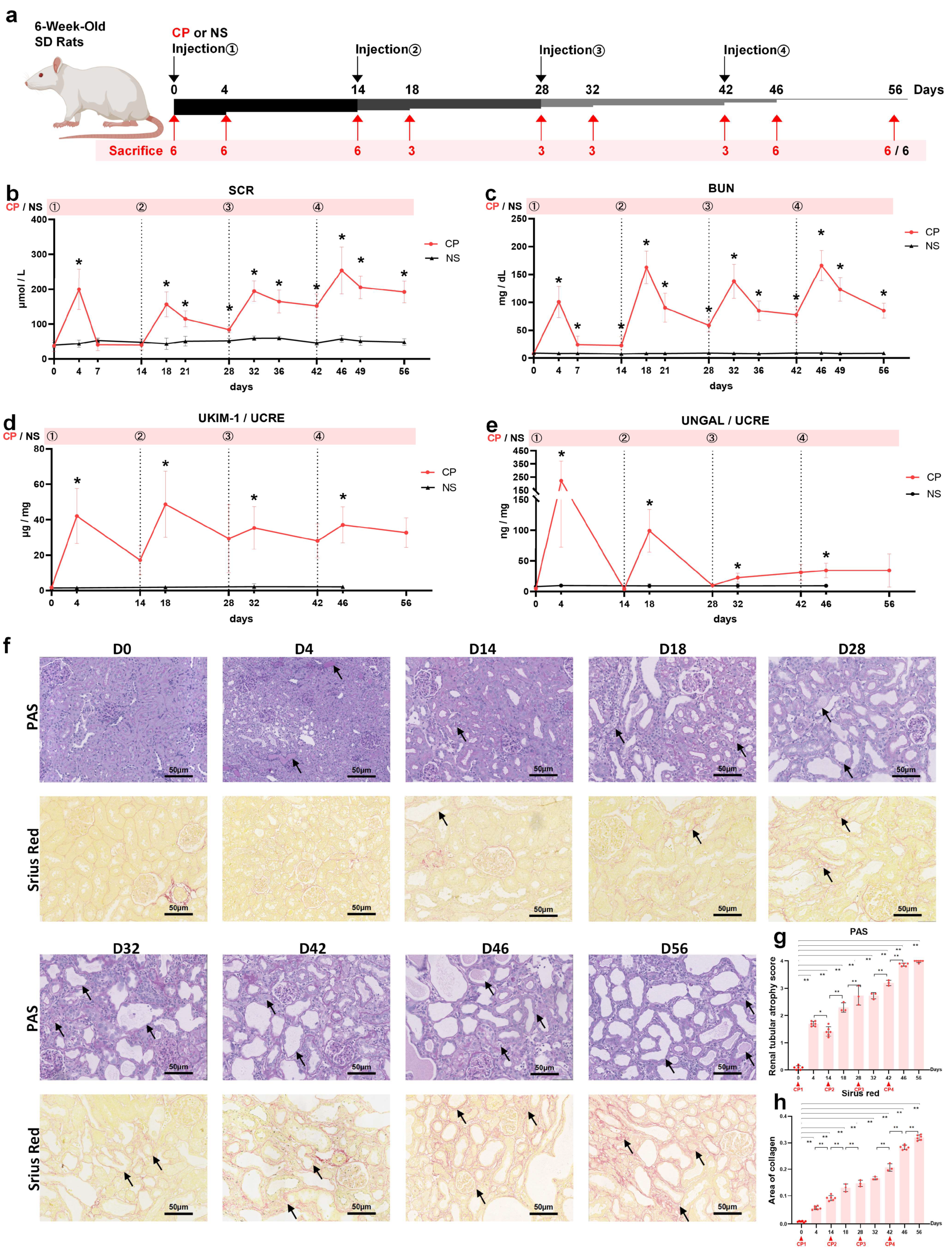
2.1. Setting Up a Rat Model of AKI-to-CKD Transition Induced by Repeated Injections with Low-Dose Cisplatin
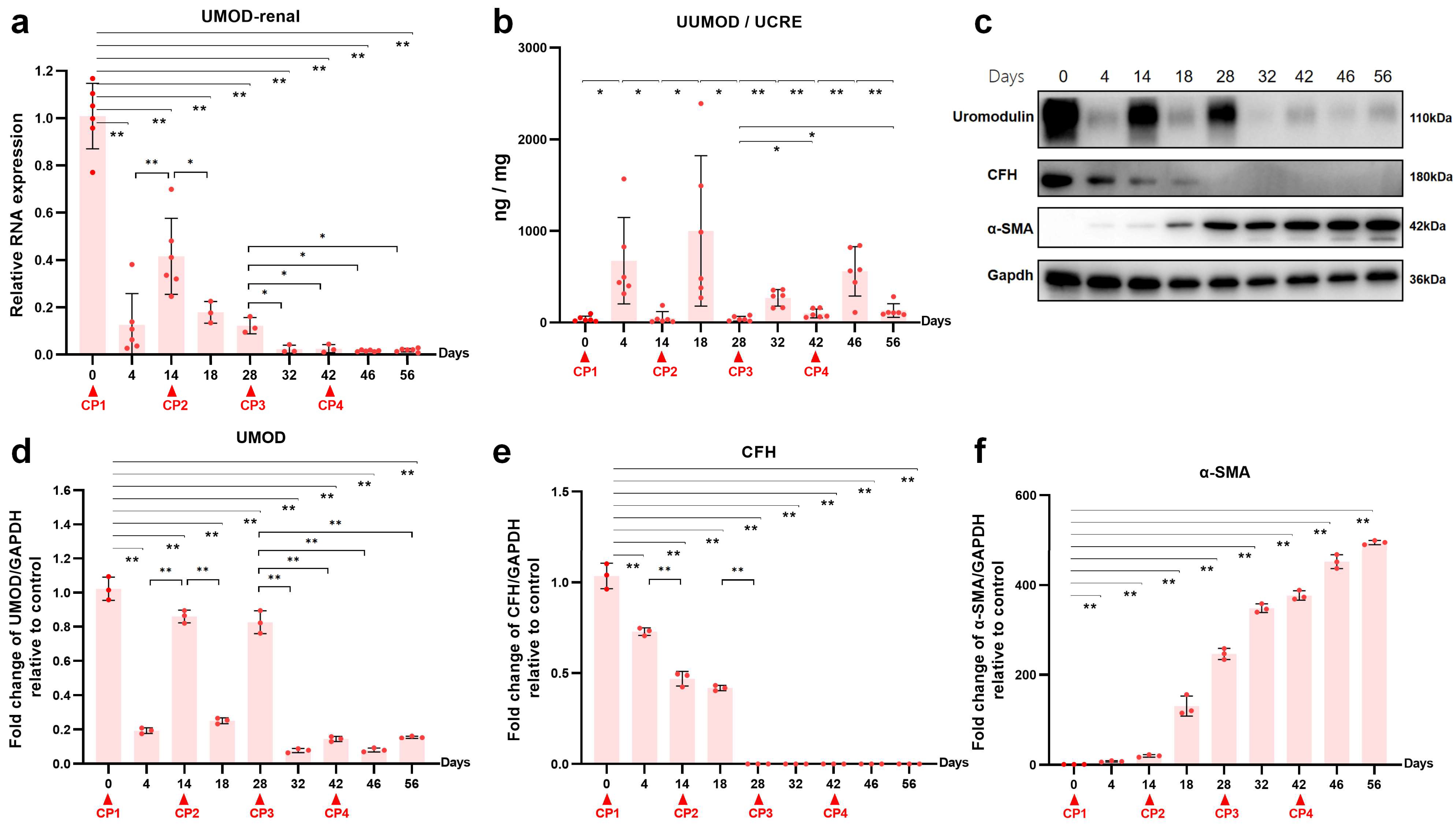
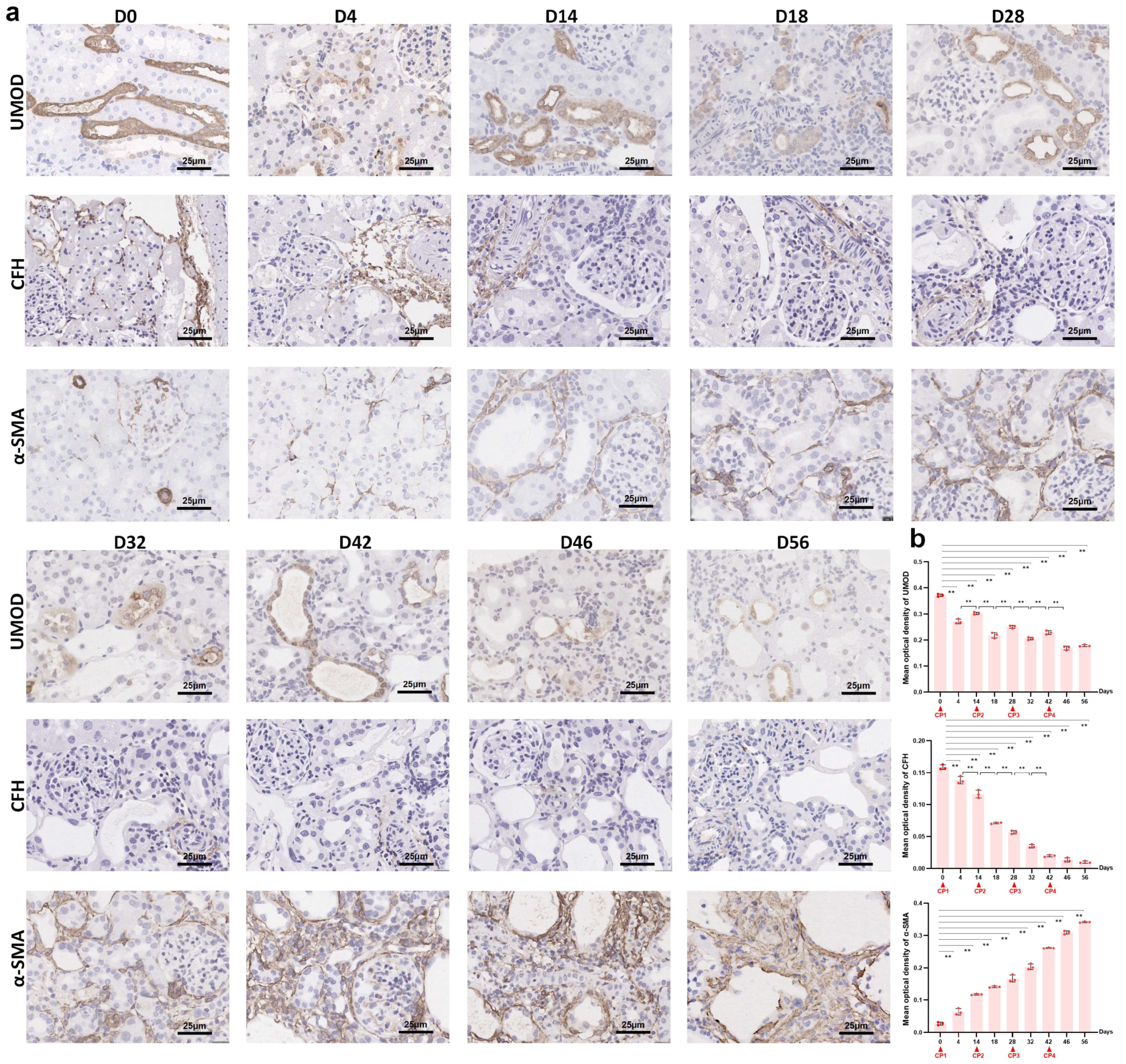
2.2. Uromodulin as a Biomarker of Kidney Injury Episodes during AKI-to-CKD Transition
2.3. Transcriptomic Analysis of the Kidneys in the Acute Phase after the First Cisplatin Injection
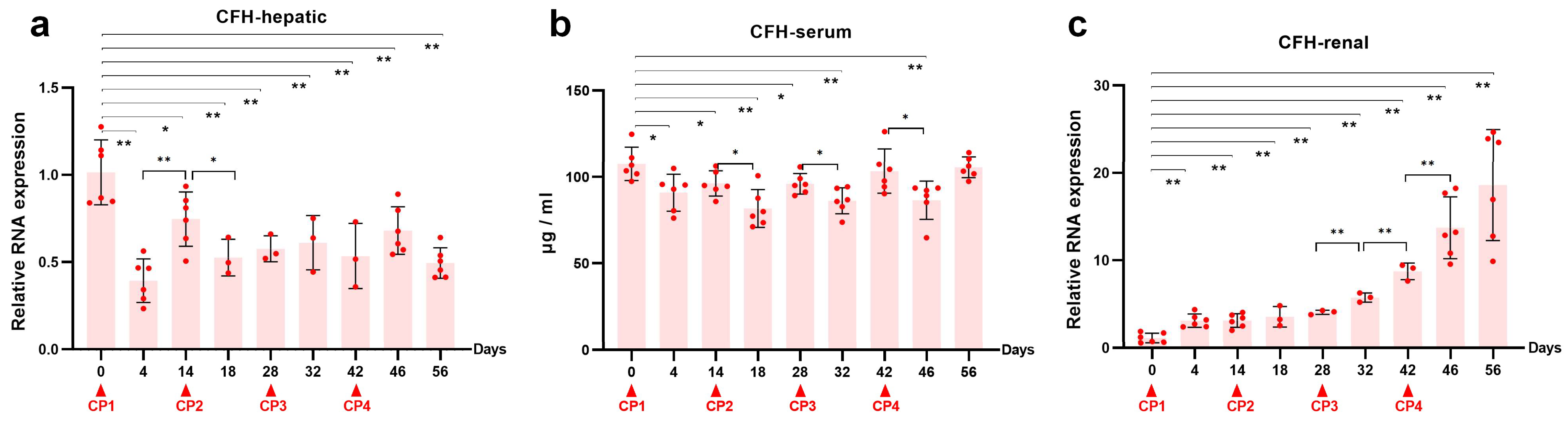
2.4. Reduced Renal CFH Is Associated with the Progression of CKD
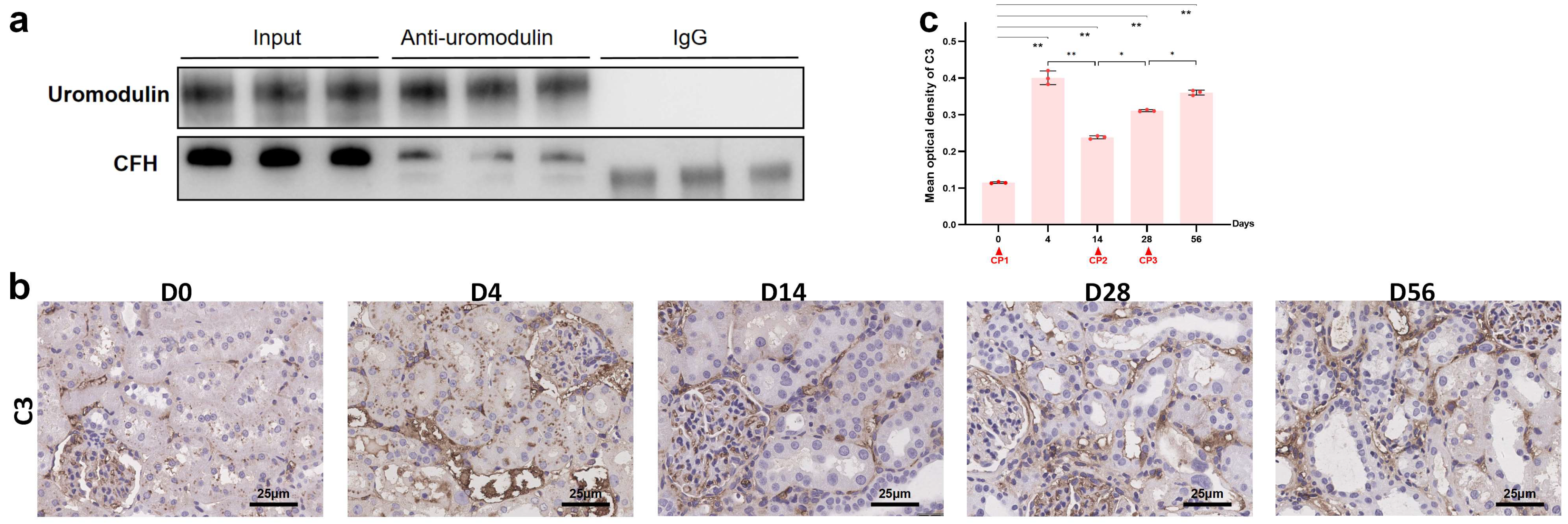
2.5. The Interaction between Uromodulin and CFH May Be Decreased with the Development of AKI to CKD
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Protocol
4.3. Blood and Urine Examination
4.4. Renal Histology
4.5. RNA Sequencing
4.6. Real-Time PCR (RT-PCR)
4.7. Antibodies
4.8. Western Bolts and Co-Immunoprecipitation (Co-IP)
4.9. Immunohistochemistry (IHC)
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- McSweeney, K.; Gadanec, L.; Qaradakhi, T.; Ali, B.; Zulli, A.; Apostolopoulos, V. Mechanisms of Cisplatin-Induced Acute Kidney Injury: Pathological Mechanisms, Pharmacological Interventions, and Genetic Mitigations. Cancers 2021, 13, 1572. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, M.K.; Burkhardt, G.; Kohler, H. Insights into potential cellular mechanisms of cisplatin nephrotoxicity and their clinical application. Nephrol. Dial. Transplant. 1997, 12, 2478–2480. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Z.Y.; Cadnapaphornchai, P.; Ginsburg, K.; Sivagnanam, M.; Chopra, S.; Treadway, C.K.; Lin, H.-S.; Yoo, G.; Sukari, A.; Doshi, M.D. Understanding the Risk Factors and Long-Term Consequences of Cisplatin-Associated Acute Kidney Injury: An Observational Cohort Study. PLoS ONE 2015, 10, e0142225. [Google Scholar] [CrossRef]
- Lam, A.Q.; Humphreys, B.D. Onco-nephrology: AKI in the cancer patient. Clin. J. Am. Soc. Nephrol. 2012, 7, 1692–1700. [Google Scholar] [CrossRef]
- Chawla, L.S.; Amdur, R.L.; Amodeo, S.; Kimmel, P.L.; Palant, C.E. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011, 79, 1361–1369. [Google Scholar] [CrossRef]
- Jiang, M.; Bai, M.; Lei, J.; Xie, Y.; Xu, S.; Jia, Z.; Zhang, A. Mitochondrial dysfunction and the AKI-to-CKD transition. Am. J. Physiol. Physiol. 2020, 319, F1105–F1116. [Google Scholar] [CrossRef]
- Sears, S.M.; Siskind, L.J. Potential Therapeutic Targets for Cisplatin-Induced Kidney Injury: Lessons from Other Models of AKI and Fibrosis. J. Am. Soc. Nephrol. 2021, 32, 1559–1567. [Google Scholar] [CrossRef]
- Devuyst, O.; Olinger, E.; Rampoldi, L. Uromodulin: From physiology to rare and complex kidney disorders. Nat. Rev. Nephrol. 2017, 13, 525–544. [Google Scholar] [CrossRef]
- El-Achkar, T.M.; McCracken, R.; Liu, Y.; Heitmeier, M.R.; Bourgeois, S.; Ryerse, J.; Wu, X.-R. Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am. J. Physiol. Physiol. 2013, 304, F1066–F1075. [Google Scholar] [CrossRef] [PubMed]
- Micanovic, R.; Khan, S.; Janosevic, D.; Lee, M.E.; Hato, T.; Srour, E.F.; Winfree, S.; Ghosh, J.; Tong, Y.; Rice, S.E.; et al. Tamm-Horsfall Protein Regulates Mononuclear Phagocytes in the Kidney. J. Am. Soc. Nephrol. 2018, 29, 841–856. [Google Scholar] [CrossRef] [PubMed]
- El-Achkar, T.M.; Wu, X.-R.; Rauchman, M.; McCracken, R.; Kiefer, S.; Dagher, P.C. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am. J. Physiol. Physiol. 2008, 295, F534–F544. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Pei, F.; Sun, Q.; Shen, F.; Yang, X.; Hu, Z.; Liu, M. Uromodulin aggravates renal tubulointerstitial injury through activation of the complement pathway in rats. J. Cell. Physiol. 2021, 236, 5012–5021. [Google Scholar] [CrossRef]
- Wen, Y.; Parikh, C.R. Current concepts and advances in biomarkers of acute kidney injury. Crit. Rev. Clin. Lab. Sci. 2021, 58, 354–368. [Google Scholar] [CrossRef]
- Skowron, B.; Baranowska, A.; Dobrek, L.; Ciesielczyk, K.; Kaszuba-Zwoinska, J.; Wiecek, G.; Malska-Wozniak, A.; Strus, M.; Gil, K. Urinary neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, uromodulin, and cystatin C concentrations in an experimental rat model of ascending acute kidney injury induced by pyelonephritis. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2018, 69, 625–637. [Google Scholar]
- McCullough, J.W.; Renner, B.; Thurman, J.M. The Role of the Complement System in Acute Kidney Injury. Semin. Nephrol. 2013, 33, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Farrar, C.A.; Abe, K.; Pratt, J.R.; Marsh, J.E.; Wang, Y.; Stahl, G.L.; Sacks, S.H. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J. Clin. Investig. 2000, 105, 1363–1371. [Google Scholar] [CrossRef]
- Rodríguez, E.; Gimeno, J.; Arias-Cabrales, C.; Barrios, C.; Redondo-Pachón, D.; Soler, M.J.; Crespo, M.; Sierra-Ochoa, A.; Riera, M.; Pascual, J. Membrane Attack Complex and Factor H in Humans with Acute Kidney Injury. Kidney Blood Press. Res. 2018, 43, 1655–1665. [Google Scholar] [CrossRef]
- Thurman, J.M.; Ljubanovic, D.; Edelstein, C.L.; Gilkeson, G.S.; Holers, V.M. Lack of a Functional Alternative Complement Pathway Ameliorates Ischemic Acute Renal Failure in Mice. J. Immunol. 2003, 170, 1517–1523. [Google Scholar] [CrossRef]
- Rhodes, D.C.J. Human Tamm-Horsfall protein, a renal specific protein, serves as a cofactor in complement 3b degradation. PLoS ONE 2017, 12, e0181857. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Y.; Wang, F.; Xia, M.; Liu, Y.; Chen, Y.; Zhao, M. Interaction of uromodulin and complement factor H enhances C3b inactivation. J. Cell. Mol. Med. 2016, 20, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Xie, Q.; Xia, M.; Gong, K.; Wang, N.; Chen, Y.; Zhao, M. The importance of sialic acid, pH and ion concentration on the interaction of uromodulin and complement factor H. J. Cell. Mol. Med. 2021, 25, 4316–4325. [Google Scholar] [CrossRef]
- Landau, S.I.; Guo, X.; Velazquez, H.; Torres, R.; Olson, E.; Garcia-Milian, R.; Moeckel, G.W.; Desir, G.V.; Safirstein, R. Regulated necrosis and failed repair in cisplatin-induced chronic kidney disease. Kidney Int. 2019, 95, 797–814. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, Q.; Wen, J.; Chen, T.; He, L.; Wang, Y.; Yin, J.; Wu, R.; Xue, R.; Li, S.; et al. Ischemic Duration and Frequency Determines AKI-to-CKD Progression Monitored by Dynamic Changes of Tubular Biomarkers in IRI Mice. Front. Physiol. 2019, 10, 153. [Google Scholar] [CrossRef]
- Wasung, M.E.; Chawla, L.S.; Madero, M. Biomarkers of renal function, which and when? Clin. Chim. Acta 2015, 438, 350–357. [Google Scholar] [CrossRef]
- Yoshida, T.; Kurella, M.; Beato, F.; Min, H.; Ingelfinger, J.R.; Stears, R.L.; Swinford, R.D.; Gullans, S.R.; Tang, S.-S. Monitoring changes in gene expression in renal ischemia-reperfusion in the rat. Kidney Int. 2002, 61, 1646–1654. [Google Scholar] [CrossRef]
- Pruijm, M.; Ponte, B.; Ackermann, D.; Paccaud, F.; Guessous, I.; Ehret, G.; Pechère-Bertschi, A.; Vogt, B.; Mohaupt, M.G.; Martin, P.-Y.; et al. Associations of Urinary Uromodulin with Clinical Characteristics and Markers of Tubular Function in the General Population. Clin. J. Am. Soc. Nephrol. 2016, 11, 70–80. [Google Scholar] [CrossRef]
- Vyletal, P.; Bleyer, A.J.; Kmoch, S. Uromodulin biology and pathophysiology—An update. Kidney Blood Press. Res. 2010, 33, 456–475. [Google Scholar] [CrossRef]
- Thurman, J.M.; Holers, V.M. The Central Role of the Alternative Complement Pathway in Human Disease. J. Immunol. 2006, 176, 1305–1310. [Google Scholar] [CrossRef]
- Lemaire, M.; Noone, D.; Lapeyraque, A.-L.; Licht, C.; Frémeaux-Bacchi, V. Inherited Kidney Complement Diseases. Clin. J. Am. Soc. Nephrol. 2021, 16, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Renner, B.; Coleman, K.; Goldberg, R.; Amura, C.; Holland-Neidermyer, A.; Pierce, K.; Orth, H.N.; Molina, H.; Ferreira, V.P.; Cortes, C.; et al. The Complement Inhibitors Crry and Factor H Are Critical for Preventing Autologous Complement Activation on Renal Tubular Epithelial Cells. J. Immunol. 2010, 185, 3086–3094. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.C. Importance of carbohydrate in the interaction of Tamm-Horsfall protein with complement 1q and inhibition of classical complement activation. Immunol. Cell Biol. 2006, 84, 357–365. [Google Scholar] [CrossRef]
- Rhodes, D.C. Binding of Tamm-Horsfall protein to complement 1q measured by ELISA and resonant mirror biosensor techniques under various ionic-strength conditions. Immunol. Cell Biol. 2000, 78, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Xia, M.; Wang, Y.; Bai, L.; Ying, W.; Zhu, F.; Chen, Y. Importance of glycosylation in the interaction of Tamm-Horsfall protein with collectin-11 and acute kidney injury. J. Cell. Mol. Med. 2020, 24, 3572–3581. [Google Scholar] [CrossRef]
- Mathew, M.; Bolton, W.K. Linear C3 Deposits on the Tubular Basement Membrane in Renal Allograft Biopsies. Am. J. Kidney Dis. 1988, 12, 121–125. [Google Scholar] [CrossRef]

| Gene | Primer Sequences | |
|---|---|---|
| UMOD | Forward | 5′-TGCTGGAAACTATGACCTAG-3′ |
| Reverse | 5′-GATGGGACCCAAGTTCAGGA-3′ | |
| CFH | Forward | 5′-CCCTAATTTCCCAACGTGTG-3′ |
| Reverse | 5′-TCATATTCCACCACGTCACC-3′ | |
| GAPDH | Forward | 5′-TGGCCAAGGTCATCCATGA-3′ |
| Reverse | 5′-GCAGTGATGGCATGGACTGT-3′ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Z.; Gong, K.; Hu, N.; Chen, Y. The Reduction of Uromodulin, Complement Factor H, and Their Interaction Is Associated with Acute Kidney Injury to Chronic Kidney Disease Transition in a Four-Time Cisplatin-Injected Rat Model. Int. J. Mol. Sci. 2023, 24, 6636. https://doi.org/10.3390/ijms24076636
Xing Z, Gong K, Hu N, Chen Y. The Reduction of Uromodulin, Complement Factor H, and Their Interaction Is Associated with Acute Kidney Injury to Chronic Kidney Disease Transition in a Four-Time Cisplatin-Injected Rat Model. International Journal of Molecular Sciences. 2023; 24(7):6636. https://doi.org/10.3390/ijms24076636
Chicago/Turabian StyleXing, Zheyu, Kunjing Gong, Nan Hu, and Yuqing Chen. 2023. "The Reduction of Uromodulin, Complement Factor H, and Their Interaction Is Associated with Acute Kidney Injury to Chronic Kidney Disease Transition in a Four-Time Cisplatin-Injected Rat Model" International Journal of Molecular Sciences 24, no. 7: 6636. https://doi.org/10.3390/ijms24076636
APA StyleXing, Z., Gong, K., Hu, N., & Chen, Y. (2023). The Reduction of Uromodulin, Complement Factor H, and Their Interaction Is Associated with Acute Kidney Injury to Chronic Kidney Disease Transition in a Four-Time Cisplatin-Injected Rat Model. International Journal of Molecular Sciences, 24(7), 6636. https://doi.org/10.3390/ijms24076636






