The Roles of Innate Lymphoid Cells in the Gastric Mucosal Immunology and Oncogenesis of Gastric Cancer
Abstract
1. Introduction
2. ILCs in Maintaining Gastric Mucosal Homeostasis and Regulation of Mucosal Immunity
3. Crosstalk between ILCs and Gastric Microbiota and Its Impact on Oncogenesis
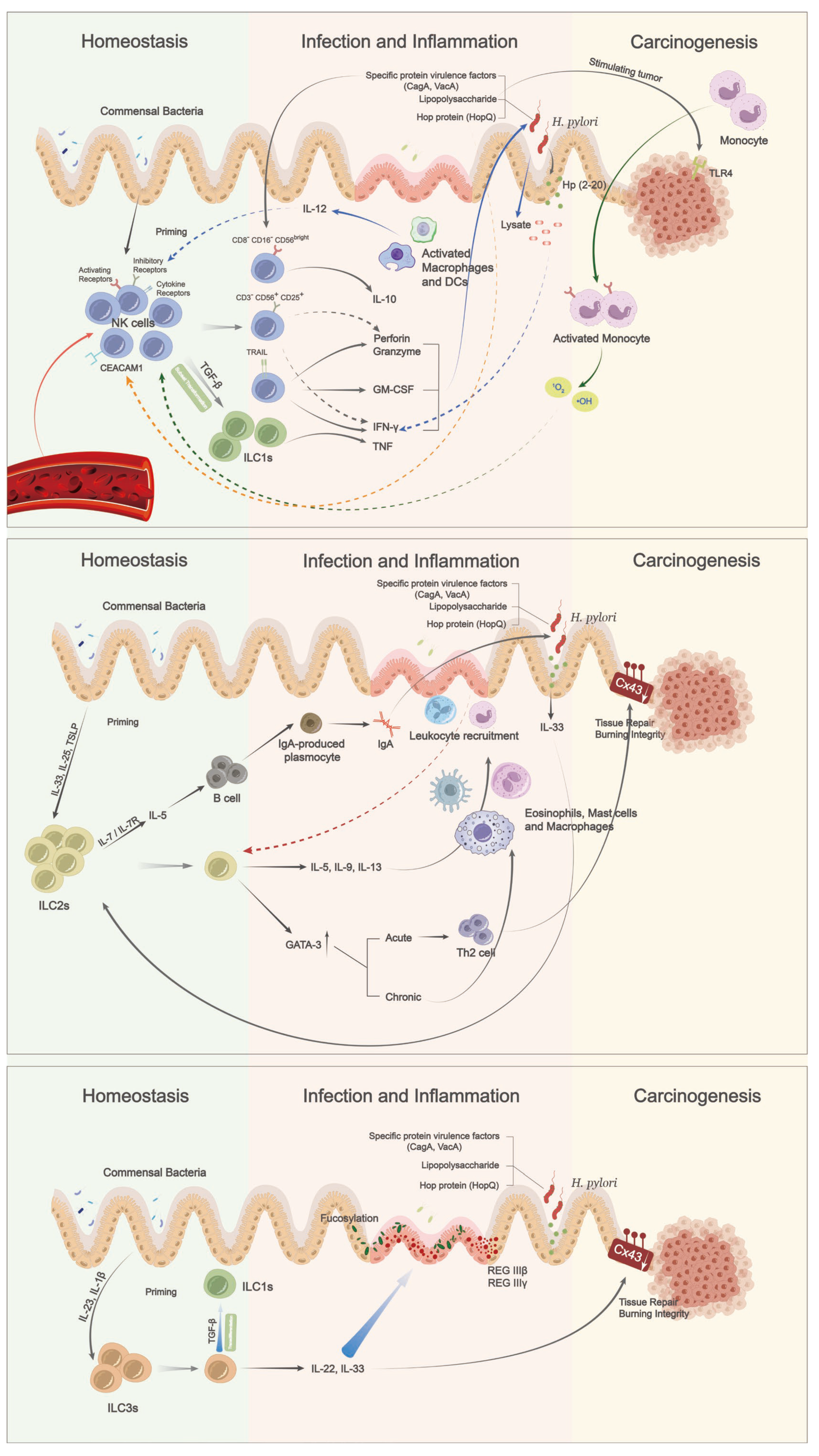
4. ILCs and Helicobacter pylori Infection
5. Roles of ILC Subsets in Gastric Oncogenesis
5.1. Group 1 ILCs: NK Cells and ILC1s
5.2. Group 2 ILCs
5.3. Group 3 ILCs
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.; Mebius, R.E. Innate lymphoid cells: 10 years on. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Finke, D. Fate and function of lymphoid tissue inducer cells. Curr. Opin. Immunol. 2005, 17, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Cupedo, T.; Crellin, N.K.; Papazian, N.; Rombouts, E.J.; Weijer, K.; Grogan, J.L.; Fibbe, W.E.; Cornelissen, J.J.; Spits, H. Human fetal lymphoid tissue–inducer cells are interleukin 17–producing precursors to RORC+ CD127+ natural killer–like cells. Nat. Immunol. 2009, 10, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Freud, A.G.; Caligiuri, M.A. Human natural killer cell development. Immunol. Rev. 2006, 214, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Boos, M.D.; Yokota, Y.; Eberl, G.; Kee, B.L. Mature natural killer cell and lymphoid tissue–inducing cell development requires Id2-mediated suppression of E protein activity. J. Exp. Med. 2007, 204, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Melo-Gonzalez, F.; Hepworth, M.R. Functional and phenotypic heterogeneity of group 3 innate lymphoid cells. Immunology 2017, 150, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wu, L.; Huntington, N.D.; Zhang, X. Crosstalk between gut microbiota and innate immunity and its implication in autoimmune diseases. Front. Immunol. 2020, 11, 282. [Google Scholar] [CrossRef]
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.; Mebius, R.E. Innate lymphoid cells—A proposal for uniform nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef]
- Bernink, J.H.; Krabbendam, L.; Germar, K.; de Jong, E.; Gronke, K.; Kofoed-Nielsen, M.; Munneke, J.M.; Hazenberg, M.D.; Villaudy, J.; Buskens, C.J. Interleukin-12 and-23 control plasticity of CD127+ group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 2015, 43, 146–160. [Google Scholar] [CrossRef]
- Fuchs, A.; Vermi, W.; Lee, J.S.; Lonardi, S.; Gilfillan, S.; Newberry, R.D.; Cella, M.; Colonna, M. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12-and IL-15-responsive IFN-γ-producing cells. Immunity 2013, 38, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Klose, C.S.; Flach, M.; Möhle, L.; Rogell, L.; Hoyler, T.; Ebert, K.; Fabiunke, C.; Pfeifer, D.; Sexl, V.; Fonseca-Pereira, D. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 2014, 157, 340–356. [Google Scholar] [CrossRef] [PubMed]
- Hoyler, T.; Klose, C.S.; Souabni, A.; Turqueti-Neves, A.; Pfeifer, D.; Rawlins, E.L.; Voehringer, D.; Busslinger, M.; Diefenbach, A. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity 2012, 37, 634–648. [Google Scholar] [CrossRef] [PubMed]
- Mjösberg, J.; Bernink, J.; Golebski, K.; Karrich, J.J.; Peters, C.P.; Blom, B.; te Velde, A.A.; Fokkens, W.J.; van Drunen, C.M.; Spits, H. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity 2012, 37, 649–659. [Google Scholar] [CrossRef]
- Peters, C.; Mjösberg, J.; Bernink, J.; Spits, H. Innate lymphoid cells in inflammatory bowel diseases. Immunol. Lett. 2016, 172, 124–131. [Google Scholar] [CrossRef]
- Panda, S.K.; Colonna, M. Innate lymphoid cells in mucosal immunity. Front. Immunol. 2019, 10, 861. [Google Scholar] [CrossRef]
- Jacquelot, N.; Seillet, C.; Vivier, E.; Belz, G.T. Innate lymphoid cells and cancer. Nat. Immunol. 2022, 23, 371–379. [Google Scholar] [CrossRef]
- Etemadi, A.; Safiri, S.; Sepanlou, S.G.; Ikuta, K.; Bisignano, C.; Shakeri, R.; Amani, M.; Fitzmaurice, C.; Nixon, M.; Abbasi, N. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 42–54. [Google Scholar] [CrossRef]
- World Health Organization. International Agency for Research on Cancer; WHO: Geneva, Switzerland, 2020.
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef]
- Food and Drug Adminitration. FDA Grants Accelerated Approval to Pembrolizumab for First Tissue/Site Agnostic Indication; FDA: Silver Spring, MD, USA, 2017.
- Takei, S.; Kawazoe, A.; Shitara, K. The new era of immunotherapy in gastric cancer. Cancers 2022, 14, 1054. [Google Scholar] [CrossRef]
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, G.; Fan, X.; Dikiy, S.; Lee, S.Y.; Rudensky, A.Y. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 2015, 350, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Kunz, S.; Witte, E.; Friedrich, M.; Asadullah, K.; Sabat, R. IL-22 increases the innate immunity of tissues. Immunity 2004, 21, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, G.F.; Hepworth, M.R. Functional interactions between innate lymphoid cells and adaptive immunity. Nat. Rev. Immunol. 2019, 19, 599–613. [Google Scholar] [CrossRef]
- Kärre, K. Natural killer cell recognition of missing self. Nat. Immunol. 2008, 9, 477–480. [Google Scholar] [CrossRef]
- Melsen, J.E.; Lugthart, G.; Lankester, A.C.; Schilham, M.W. Human circulating and tissue-resident CD56bright natural killer cell populations. Front. Immunol. 2016, 7, 262. [Google Scholar] [CrossRef]
- Sojka, D.K.; Tian, Z.; Yokoyama, W.M. Tissue-resident natural killer cells and their potential diversity. Semin. Immunol. 2014, 26, 127–131. [Google Scholar] [CrossRef]
- Pegram, H.J.; Andrews, D.M.; Smyth, M.J.; Darcy, P.K.; Kershaw, M.H. Activating and inhibitory receptors of natural killer cells. Immunol. Cell Biol. 2011, 89, 216–224. [Google Scholar] [CrossRef]
- Kumar, S. Natural killer cell cytotoxicity and its regulation by inhibitory receptors. Immunology 2018, 154, 383–393. [Google Scholar] [CrossRef]
- Caligiuri, M.A. Human natural killer cells. Blood J. Am. Soc. Hematol. 2008, 112, 461–469. [Google Scholar] [CrossRef]
- Jonsson, A.H.; Yokoyama, W.M. Natural killer cell tolerance: Licensing and other mechanisms. Adv. Immunol. 2009, 101, 27–79. [Google Scholar]
- Orr, M.T.; Lanier, L.L. Natural killer cell education and tolerance. Cell 2010, 142, 847–856. [Google Scholar] [CrossRef]
- Ganal, S.C.; Sanos, S.L.; Kallfass, C.; Oberle, K.; Johner, C.; Kirschning, C.; Lienenklaus, S.; Weiss, S.; Staeheli, P.; Aichele, P. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity 2012, 37, 171–186. [Google Scholar] [CrossRef]
- Rizzello, V.; Bonaccorsi, I.; Dongarra, M.L.; Fink, L.N.; Ferlazzo, G. Role of natural killer and dendritic cell crosstalk in immunomodulation by commensal bacteria probiotics. J. Biomed. Biotechnol. 2011, 2011, 473097. [Google Scholar] [CrossRef]
- Bartizal, K.F.; Salkowski, C.; Balish, E.; Pleasants, J.R. The effect of microbial flora, diet, and age on the tumoricidal activity of natural killer cells. J. Leukoc. Biol. 1984, 36, 739–750. [Google Scholar] [CrossRef]
- Souza-Fonseca-Guimaraes, F.; Parlato, M.; De Oliveira, R.B.; Golenbock, D.; Fitzgerald, K.; Shalova, I.N.; Biswas, S.K.; Cavaillon, J.-M.; Adib-Conquy, M. Interferon-γ and granulocyte/monocyte colony-stimulating factor production by natural killer cells involves different signaling pathways and the adaptor stimulator of interferon genes (STING). J. Biol. Chem. 2013, 288, 10715–10721. [Google Scholar] [CrossRef]
- Zaghi, E.; Calvi, M.; Marcenaro, E.; Mavilio, D.; Di Vito, C. Targeting NKG2A to elucidate natural killer cell ontogenesis and to develop novel immune-therapeutic strategies in cancer therapy. J. Leukoc. Biol. 2019, 105, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Souza-Fonseca-Guimaraes, F.; Bald, T.; Ng, S.S.; Young, A.; Ngiow, S.F.; Rautela, J.; Straube, J.; Waddell, N.; Blake, S.J. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat. Immunol. 2017, 18, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Cuff, A.O.; Sillito, F.; Dertschnig, S.; Hall, A.; Luong, T.V.; Chakraverty, R.; Male, V. The obese liver environment mediates conversion of NK cells to a less cytotoxic ILC1-like phenotype. Front. Immunol. 2019, 10, 2180. [Google Scholar] [CrossRef] [PubMed]
- Vosshenrich, C.A.; García-Ojeda, M.E.; Samson-Villéger, S.I.; Pasqualetto, V.; Enault, L.; Goff, O.R.-L.; Corcuff, E.; Guy-Grand, D.; Rocha, B.; Cumano, A. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat. Immunol. 2006, 7, 1217–1224. [Google Scholar] [CrossRef]
- Seillet, C.; Belz, G.T.; Huntington, N.D. Development, homeostasis, and heterogeneity of NK cells and ILC1. In Natural Killer Cells; Springer: Cham, Switzerland, 2016; pp. 37–61. [Google Scholar]
- Jiao, Y.; Huntington, N.D.; Belz, G.T.; Seillet, C. Type 1 innate lymphoid cell biology: Lessons learnt from natural killer cells. Front. Immunol. 2016, 7, 426. [Google Scholar] [CrossRef] [PubMed]
- Ducimetière, L.; Lucchiari, G.; Litscher, G.; Nater, M.; Heeb, L.; Nuñez, N.G.; Wyss, L.; Burri, D.; Vermeer, M.; Gschwend, J. Conventional NK cells and tissue-resident ILC1s join forces to control liver metastasis. Proc. Natl. Acad. Sci. USA 2021, 118, e2026271118. [Google Scholar] [CrossRef] [PubMed]
- Satoh-Takayama, N.; Kato, T.; Motomura, Y.; Kageyama, T.; Taguchi-Atarashi, N.; Kinoshita-Daitoku, R.; Kuroda, E.; Di Santo, J.P.; Mimuro, H.; Moro, K. Bacteria-induced group 2 innate lymphoid cells in the stomach provide immune protection through induction of IgA. Immunity 2020, 52, 635–649.e4. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jiang, X.-X.; Zhang, L.-F.; Liu, X.-M.; Hu, T.-Z.; Xia, X.-J.; Li, M.; Xu, C.-X. Group 2 innate lymphoid cells are involved in skewed type 2 immunity of gastric diseases induced by Helicobacter pylori infection. Mediat. Inflamm. 2017, 2017, 4927964. [Google Scholar] [CrossRef]
- Herbert, D.B.R.; Douglas, B.; Zullo, K. Group 2 innate lymphoid cells (ILC2): Type 2 immunity and helminth immunity. Int. J. Mol. Sci. 2019, 20, 2276. [Google Scholar] [CrossRef]
- Moro, K.; Yamada, T.; Tanabe, M.; Takeuchi, T.; Ikawa, T.; Kawamoto, H.; Furusawa, J.-i.; Ohtani, M.; Fujii, H.; Koyasu, S. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+ Sca-1+ lymphoid cells. Nature 2010, 463, 540–544. [Google Scholar] [CrossRef]
- Price, A.E.; Liang, H.-E.; Sullivan, B.M.; Reinhardt, R.L.; Eisley, C.J.; Erle, D.J.; Locksley, R.M. Systemically dispersed innate IL-13–expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. USA 2010, 107, 11489–11494. [Google Scholar] [CrossRef]
- Halim, T.Y.; Steer, C.A.; Mathä, L.; Gold, M.J.; Martinez-Gonzalez, I.; McNagny, K.M.; McKenzie, A.N.; Takei, F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 2014, 40, 425–435. [Google Scholar] [CrossRef]
- Pelly, V.; Kannan, Y.; Coomes, S.; Entwistle, L.; Rückerl, D.; Seddon, B.; MacDonald, A.; McKenzie, A.; Wilson, M. IL-4-producing ILC2s are required for the differentiation of TH2 cells following Heligmosomoides polygyrus infection. Mucosal Immunol. 2016, 9, 1407–1417. [Google Scholar] [CrossRef]
- Campbell, L.; Hepworth, M.R.; Whittingham-Dowd, J.; Thompson, S.; Bancroft, A.J.; Hayes, K.S.; Shaw, T.N.; Dickey, B.F.; Flamar, A.-L.; Artis, D. ILC2s mediate systemic innate protection by priming mucus production at distal mucosal sites. J. Exp. Med. 2019, 216, 2714–2723. [Google Scholar] [CrossRef]
- Moral, J.A.; Leung, J.; Rojas, L.A.; Ruan, J.; Zhao, J.; Sethna, Z.; Ramnarain, A.; Gasmi, B.; Gururajan, M.; Redmond, D. ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature 2020, 579, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Trabanelli, S.; Curti, A.; Lecciso, M.; Salomé, B.; Riether, C.; Ochsenbein, A.; Romero, P.; Jandus, C. CD127+ innate lymphoid cells are dysregulated in treatment naive acute myeloid leukemia patients at diagnosis. Haematologica 2015, 100, e257. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, M.F.; Trabanelli, S.; Racle, J.; Salomé, B.; Cesson, V.; Gharbi, D.; Bohner, P.; Domingos-Pereira, S.; Dartiguenave, F.; Fritschi, A.-S. ILC2-modulated T cell–to-MDSC balance is associated with bladder cancer recurrence. J. Clin. Investig. 2017, 127, 2916–2929. [Google Scholar] [CrossRef] [PubMed]
- Trabanelli, S.; Chevalier, M.F.; Martinez-Usatorre, A.; Gomez-Cadena, A.; Salomé, B.; Lecciso, M.; Salvestrini, V.; Verdeil, G.; Racle, J.; Papayannidis, C. Tumour-derived PGD2 and NKp30-B7H6 engagement drives an immunosuppressive ILC2-MDSC axis. Nat. Commun. 2017, 8, 593. [Google Scholar] [CrossRef]
- Zheng, Y.; Valdez, P.A.; Danilenko, D.M.; Hu, Y.; Sa, S.M.; Gong, Q.; Abbas, A.R.; Modrusan, Z.; Ghilardi, N.; De Sauvage, F.J. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008, 14, 282–289. [Google Scholar] [CrossRef]
- Goto, Y.; Obata, T.; Kunisawa, J.; Sato, S.; Ivanov, I.I.; Lamichhane, A.; Takeyama, N.; Kamioka, M.; Sakamoto, M.; Matsuki, T. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 2014, 345, 1254009. [Google Scholar] [CrossRef]
- Hepworth, M.R.; Monticelli, L.A.; Fung, T.C.; Ziegler, C.G.; Grunberg, S.; Sinha, R.; Mantegazza, A.R.; Ma, H.-L.; Crawford, A.; Angelosanto, J.M. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature 2013, 498, 113–117. [Google Scholar] [CrossRef]
- Hepworth, M.R.; Fung, T.C.; Masur, S.H.; Kelsen, J.R.; McConnell, F.M.; Dubrot, J.; Withers, D.R.; Hugues, S.; Farrar, M.A.; Reith, W. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria–specific CD4+ T cells. Science 2015, 348, 1031–1035. [Google Scholar] [CrossRef]
- Wang, S.; Qu, Y.; Xia, P.; Chen, Y.; Zhu, X.; Zhang, J.; Wang, G.; Tian, Y.; Ying, J.; Fan, Z. Transdifferentiation of tumor infiltrating innate lymphoid cells during progression of colorectal cancer. Cell Res. 2020, 30, 610–622. [Google Scholar] [CrossRef]
- Polk, D.B.; Peek Jr, R.M. Helicobacter pylori: Gastric cancer and beyond. Nat. Rev. Cancer 2010, 10, 403–414. [Google Scholar] [CrossRef]
- Wehkamp, J.; Schauber, J.; Stange, E.F. Defensins and cathelicidins in gastrointestinal infections. Curr. Opin. Gastroenterol. 2007, 23, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Ge, L.; Muthupalani, S.; Feng, Y.; Fox, J.G. Male-Dependent Promotion of Colitis in 129 Rag2(-/-) Mice Co-Infected with Helicobacter pylori and Helicobacter hepaticus. Int. J. Mol. Sci. 2020, 21, 8886. [Google Scholar] [CrossRef] [PubMed]
- Moyat, M.; Bouzourene, H.; Ouyang, W.; Iovanna, J.; Renauld, J.C.; Velin, D. IL-22-induced antimicrobial peptides are key determinants of mucosal vaccine-induced protection against H. pylori in mice. Mucosal Immunol. 2017, 10, 271–281. [Google Scholar] [CrossRef]
- Manta, C.; Heupel, E.; Radulovic, K.; Rossini, V.; Garbi, N.; Riedel, C.U.; Niess, J.H. CX3CR1+ macrophages support IL-22 production by innate lymphoid cells during infection with Citrobacter rodentium. Mucosal Immunol. 2013, 6, 177–188. [Google Scholar] [CrossRef]
- Bruce, D.W.; Stefanski, H.E.; Vincent, B.G.; Dant, T.A.; Reisdorf, S.; Bommiasamy, H.; Serody, D.A.; Wilson, J.E.; McKinnon, K.P.; Shlomchik, W.D. Type 2 innate lymphoid cells treat and prevent acute gastrointestinal graft-versus-host disease. J. Clin. Investig. 2017, 127, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.M.; Maurice, C.F.; Kinnebrew, M.A.; Abt, M.C.; Schenten, D.; Golovkina, T.V.; Bogatyrev, S.R.; Ismagilov, R.F.; Pamer, E.G.; Turnbaugh, P.J. Rapid fucosylation of intestinal epithelium sustains host–commensal symbiosis in sickness. Nature 2014, 514, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Jowett, G.M.; Norman, M.D.; Yu, T.T.; Rosell Arevalo, P.; Hoogland, D.; Lust, S.T.; Read, E.; Hamrud, E.; Walters, N.J.; Niazi, U. ILC1 drive intestinal epithelial and matrix remodelling. Nat. Mater. 2021, 20, 250–259. [Google Scholar] [CrossRef]
- Uhde, M.; Yu, X.; Bunin, A.; Brauner, C.; Lewis, S.; Lebwohl, B.; Krishnareddy, S.; Alaedini, A.; Reizis, B.; Ghosh, S. Phenotypic shift of small intestinal intra-epithelial type 1 innate lymphoid cells in celiac disease is associated with enhanced cytotoxic potential. Clin. Exp. Immunol. 2020, 200, 163–175. [Google Scholar] [CrossRef]
- Bik, E.M.; Eckburg, P.B.; Gill, S.R.; Nelson, K.E.; Purdom, E.A.; Francois, F.; Perez-Perez, G.; Blaser, M.J.; Relman, D.A. Molecular analysis of the bacterial microbiota in the human stomach. Proc. Natl. Acad. Sci. USA 2006, 103, 732–737. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Stewart, O.A.; Wu, F.; Chen, Y. The role of gastric microbiota in gastric cancer. Gut Microbes 2020, 11, 1220–1230. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Rajilic-Stojanovic, M.; Figueiredo, C.; Smet, A.; Hansen, R.; Kupcinskas, J.; Rokkas, T.; Andersen, L.; Machado, J.C.; Ianiro, G.; Gasbarrini, A. Systematic review: Gastric microbiota in health and disease. Aliment. Pharmacol. Ther. 2020, 51, 582–602. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Molina-Infante, J.; Gasbarrini, A. Gastric microbiota. Helicobacter 2015, 20, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Kronsteiner, B.; Bassaganya-Riera, J.; Philipson, C.; Viladomiu, M.; Carbo, A.; Abedi, V.; Hontecillas, R. Systems-wide analyses of mucosal immune responses to Helicobacter pylori at the interface between pathogenicity and symbiosis. Gut Microbes 2016, 7, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.M.; Pereira-Marques, J.; Pinto-Ribeiro, I.; Costa, J.L.; Carneiro, F.; Machado, J.C.; Figueiredo, C. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 2018, 67, 226–236. [Google Scholar] [CrossRef]
- Yun, C.H.; Lundgren, A.; Azem, J.; Sjöling, A.; Holmgren, J.; Svennerholm, A.-M.; Lundin, B.S. Natural killer cells and Helicobacter pylori infection: Bacterial antigens and interleukin-12 act synergistically to induce gamma interferon production. Infect. Immun. 2005, 73, 1482–1490. [Google Scholar] [CrossRef]
- Tarkkanen, J.; Kosunen, T.; Saksela, E. Contact of lymphocytes with Helicobacter pylori augments natural killer cell activity and induces production of gamma interferon. Infect. Immun. 1993, 61, 3012–3016. [Google Scholar] [CrossRef]
- Rudnicka, K.; Matusiak, A.; Miszczyk, E.; Rudnicka, W.; Tenderenda, M.; Chmiela, M. Immunophenotype of peripheral blood natural killer cells and IL-10 serum levels in relation to Helicobacter pylori status. Apmis 2013, 121, 806–813. [Google Scholar] [CrossRef]
- Ricardo-Gonzalez, R.R.; Van Dyken, S.J.; Schneider, C.; Lee, J.; Nussbaum, J.C.; Liang, H.-E.; Vaka, D.; Eckalbar, W.L.; Molofsky, A.B.; Erle, D.J. Tissue signals imprint ILC2 identity with anticipatory function. Nat. Immunol. 2018, 19, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, L.A.; Sonnenberg, G.F.; Abt, M.C.; Alenghat, T.; Ziegler, C.G.; Doering, T.A.; Angelosanto, J.M.; Laidlaw, B.J.; Yang, C.Y.; Sathaliyawala, T. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 2011, 12, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA A Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Hu, W.; Ouyang, Q.; Zhang, S.; He, L.; Chen, W.; Li, X.; Hu, C. Helicobacter pylori infection induces stem cell-like properties in Correa cascade of gastric cancer. Cancer Lett. 2022, 542, 215764. [Google Scholar] [CrossRef]
- Vakil, N.; Megraud, F. Eradication therapy for Helicobacter pylori. Gastroenterology 2007, 133, 985–1001. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Dore, M.P.; Graham, D.Y. Diagnosis and treatment of Helicobacter pylori infection. Annu. Rev. Med. 2022, 73, 183–195. [Google Scholar] [CrossRef]
- Wang, F.; Meng, W.; Wang, B.; Qiao, L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014, 345, 196–202. [Google Scholar] [CrossRef]
- Zavros, Y.; Merchant, J.L. The immune microenvironment in gastric adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 451–467. [Google Scholar] [CrossRef]
- Odenbreit, S.; Püls, J.; Sedlmaier, B.; Gerland, E.; Fischer, W.; Haas, R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 2000, 287, 1497–1500. [Google Scholar] [CrossRef]
- Fujii, Y.; Yoshihashi, K.; Suzuki, H.; Tsutsumi, S.; Mutoh, H.; Maeda, S.; Yamagata, Y.; Seto, Y.; Aburatani, H.; Hatakeyama, M. CDX1 confers intestinal phenotype on gastric epithelial cells via induction of stemness-associated reprogramming factors SALL4 and KLF5. Proc. Natl. Acad. Sci. USA 2012, 109, 20584–20589. [Google Scholar] [CrossRef]
- Ito, N.; Tsujimoto, H.; Ueno, H.; Xie, Q.; Shinomiya, N. Helicobacter pylori-mediated immunity and signaling transduction in gastric cancer. J. Clin. Med. 2020, 9, 3699. [Google Scholar] [CrossRef] [PubMed]
- Otani, K.; Tanigawa, T.; Watanabe, T.; Nadatani, Y.; Sogawa, M.; Yamagami, H.; Shiba, M.; Watanabe, K.; Tominaga, K.; Fujiwara, Y. Toll-like receptor 9 signaling has anti-inflammatory effects on the early phase of Helicobacter pylori-induced gastritis. Biochem. Biophys. Res. Commun. 2012, 426, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Chmiela, M.; Miszczyk, E.; Rudnicka, K. Structural modifications of Helicobacter pylori lipopolysaccharide: An idea for how to live in peace. World J. Gastroenterol. WJG 2014, 20, 9882. [Google Scholar] [CrossRef] [PubMed]
- Hafsi, N.; Voland, P.; Schwendy, S.; Rad, R.; Reindl, W.; Gerhard, M.; Prinz, C. Human dendritic cells respond to Helicobacter pylori, promoting NK cell and Th1-effector responses in vitro. J. Immunol. 2004, 173, 1249–1257. [Google Scholar] [CrossRef]
- Rudnicka, K.; Włodarczyk, M.; Moran, A.P.; Rechciński, T.; Miszczyk, E.; Matusiak, A.; Szczęsna, E.; Walencka, M.; Rudnicka, W.; Chmiela, M. Helicobacter pylori antigens as potential modulators of lymphocytes’ cytotoxic activity. Microbiol. Immunol. 2012, 56, 62–75. [Google Scholar] [CrossRef]
- Lindgren, Å.; Yun, C.-H.; Sjöling, Å.; Berggren, C.; Sun, J.-B.; Jonsson, E.; Holmgren, J.; Svennerholm, A.-M.; Lundin, S.B. Impaired IFN-γ production after stimulation with bacterial components by natural killer cells from gastric cancer patients. Exp. Cell Res. 2011, 317, 849–858. [Google Scholar] [CrossRef]
- Ma, H.-Y.; Liu, X.-Z.; Liang, C.-M. Inflammatory microenvironment contributes to epithelial-mesenchymal transition in gastric cancer. World J. Gastroenterol. 2016, 22, 6619. [Google Scholar] [CrossRef]
- Lindgren, Å.; Pavlovic, V.; Flach, C.-F.; Sjöling, Å.; Lundin, S. Interferon-gamma secretion is induced in IL-12 stimulated human NK cells by recognition of Helicobacter pylori or TLR2 ligands. Innate Immun. 2011, 17, 191–203. [Google Scholar] [CrossRef]
- Yang, C.A.; Scheibenbogen, C.; Bauer, S.; Kleinle, C.; Wex, T.; Bornschein, J.; Malfertheiner, P.; Hellmig, S.; Schumann, R.R.; Hamann, L. A frequent toll-like receptor 1 gene polymorphism affects NK-and T-cell IFN-γ production and is associated with helicobacter pylori-induced gastric disease. Helicobacter 2013, 18, 13–21. [Google Scholar] [CrossRef]
- Rudnicka, K.; Miszczyk, E.; Matusiak, A.; Walencka, M.; Moran, A.P.; Rudnicka, W.; Chmiela, M. Helicobacter pylori-driven modulation of NK cell expansion, intracellular cytokine expression and cytotoxic activity. Innate Immun. 2015, 21, 127–139. [Google Scholar] [CrossRef]
- Lindgren, Å.; Yun, C.H.; Lundgren, A.; Sjöling, Å.; Öhman, L.; Svennerholm, A.-M.; Holmgren, J.; Lundin, S.B. CD8–natural killer cells are greatly enriched in the human gastrointestinal tract and have the capacity to respond to bacteria. J. Innate Immun. 2010, 2, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, W.; Bai, J.; Li, J.; Li, H. Emerging role of Helicobacter pylori in the immune evasion mechanism of gastric cancer: An insight into tumor microenvironment-pathogen interaction. Front. Oncol. 2022, 12, 862462. [Google Scholar] [CrossRef] [PubMed]
- Chochi, K.; Ichikura, T.; Kinoshita, M.; Majima, T.; Shinomiya, N.; Tsujimoto, H.; Kawabata, T.; Sugasawa, H.; Ono, S.; Seki, S. Helicobacter pylori augments growth of gastric cancers via the lipopolysaccharide-toll-like receptor 4 pathway whereas its lipopolysaccharide attenuates antitumor activities of human mononuclear cells. Clin. Cancer Res. 2008, 14, 2909–2917. [Google Scholar] [CrossRef]
- Gur, C.; Maalouf, N.; Gerhard, M.; Singer, B.B.; Emgård, J.; Temper, V.; Neuman, T.; Mandelboim, O.; Bachrach, G. The Helicobacter pylori HopQ outermembrane protein inhibits immune cell activities. Oncoimmunology 2019, 8, e1553487. [Google Scholar] [CrossRef]
- Betten, Å.; Bylund, J.; Cristophe, T.; Boulay, F.; Romero, A.; Hellstrand, K.; Dahlgren, C. A proinflammatory peptide from Helicobacter pylori activates monocytes to induce lymphocyte dysfunction and apoptosis. J. Clin. Investig. 2001, 108, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, K.; Xu, C.; Hu, T.; Zhou, L.; Cao, D.; Xiao, J.; Luo, L.; Guo, Y.; Qi, Y. GATA-3 augmentation down-regulates Connexin43 in Helicobacter pylori associated gastric carcinogenesis. Cancer Biol. Ther. 2015, 16, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Buzzelli, J.N.; Chalinor, H.V.; Pavlic, D.I.; Sutton, P.; Menheniott, T.R.; Giraud, A.S.; Judd, L.M. IL33 is a stomach alarmin that initiates a skewed Th2 response to injury and infection. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 203–221.e3. [Google Scholar] [CrossRef]
- Kim, M.H.; Taparowsky, E.J.; Kim, C.H. Retinoic acid differentially regulates the migration of innate lymphoid cell subsets to the gut. Immunity 2015, 43, 107–119. [Google Scholar] [CrossRef]
- Huang, Q.; Cao, W.; Mielke, L.A.; Seillet, C.; Belz, G.T.; Jacquelot, N. Innate Lymphoid Cells in Colorectal Cancers: A Double-Edged Sword. Front. Immunol. 2019, 10, 3080. [Google Scholar] [CrossRef]
- Salimi, M.; Wang, R.; Yao, X.; Li, X.; Wang, X.; Hu, Y.; Chang, X.; Fan, P.; Dong, T.; Ogg, G. Activated innate lymphoid cell populations accumulate in human tumour tissues. BMC Cancer 2018, 18, 341. [Google Scholar] [CrossRef]
- Chen, L.-j.; Zheng, X.; Shen, Y.-p.; Zhu, Y.-b.; Li, Q.; Chen, J.; Xia, R.; Zhou, S.-m.; Wu, C.-p.; Zhang, X.-g. Higher numbers of T-bet+ intratumoral lymphoid cells correlate with better survival in gastric cancer. Cancer Immunol. Immunother. 2013, 62, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Maehara, Y.; Tokunaga, E.; Koga, T.; Kakeji, Y.; Sugimachi, K. Prognostic significance of natural killer cell activity in patients with gastric carcinoma: A multivariate analysis. Am. J. Gastroenterol. 2001, 96, 574–578. [Google Scholar] [CrossRef]
- González, S.; López-Soto, A.; Suarez-Alvarez, B.; López-Vázquez, A.; López-Larrea, C. NKG2D ligands: Key targets of the immune response. Trends Immunol. 2008, 29, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Konagai, A.; Yoshimura, K.; Hazama, S.; Yamamoto, N.; Aoki, K.; Ueno, T.; Fujioka, M.; Iijima, H.; Kato, M.; Uchida, M.; et al. Correlation between NKG2DL expression and antitumor effect of protein-bound polysaccharide-K in tumor-bearing mouse models. Anticancer Res. 2017, 37, 4093–4101. [Google Scholar] [PubMed]
- Yang, Y.; Lee, J.-H.; Kim, K.Y.; Song, H.K.; Kim, J.K.; Yoon, S.R.; Cho, D.; Song, K.S.; Lee, Y.H.; Choi, I. The interferon-inducible 9-27 gene modulates the susceptibility to natural killer cells and the invasiveness of gastric cancer cells. Cancer Lett. 2005, 221, 191–200. [Google Scholar] [CrossRef]
- Kursunel, M.A.; Esendagli, G. The untold story of IFN-γ in cancer biology. Cytokine Growth Factor Rev. 2016, 31, 73–81. [Google Scholar] [CrossRef]
- Martini, M.; Testi, M.G.; Pasetto, M.; Picchio, M.C.; Innamorati, G.; Mazzocco, M.; Ugel, S.; Cingarlini, S.; Bronte, V.; Zanovello, P. IFN-γ-mediated upmodulation of MHC class I expression activates tumor-specific immune response in a mouse model of prostate cancer. Vaccine 2010, 28, 3548–3557. [Google Scholar] [CrossRef]
- Mojic, M.; Takeda, K.; Hayakawa, Y. The dark side of IFN-γ: Its role in promoting cancer immunoevasion. Int. J. Mol. Sci. 2017, 19, 89. [Google Scholar] [CrossRef]
- Yoshida, T.; Ogata, H.; Kamio, M.; Joo, A.; Shiraishi, H.; Tokunaga, Y.; Sata, M.; Nagai, H.; Yoshimura, A. SOCS1 is a suppressor of liver fibrosis and hepatitis-induced carcinogenesis. J. Exp. Med. 2004, 199, 1701–1707. [Google Scholar] [CrossRef]
- Hanada, T.; Kobayashi, T.; Chinen, T.; Saeki, K.; Takaki, H.; Koga, K.; Minoda, Y.; Sanada, T.; Yoshioka, T.; Mimata, H. IFNγ-dependent, spontaneous development of colorectal carcinomas in SOCS1-deficient mice. J. Exp. Med. 2006, 203, 1391–1397. [Google Scholar] [CrossRef]
- Zou, Q.; Jin, J.; Xiao, Y.; Zhou, X.; Hu, H.; Cheng, X.; Kazimi, N.; Ullrich, S.E.; Sun, S.-C. T cell intrinsic USP15 deficiency promotes excessive IFN-γ production and an immunosuppressive tumor microenvironment in MCA-induced fibrosarcoma. Cell Rep. 2015, 13, 2470–2479. [Google Scholar] [CrossRef] [PubMed]
- Abiko, K.; Matsumura, N.; Hamanishi, J.; Horikawa, N.; Murakami, R.; Yamaguchi, K.; Yoshioka, Y.; Baba, T.; Konishi, I.; Mandai, M. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br. J. Cancer 2015, 112, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, R.; Martin, A.; Bommarito, D.; Wang, K.; Hansen, S.H.; Freeman, G.J.; Ritz, J. Interferon-γ-induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD-L1 expression. Oncoimmunology 2015, 4, e1008824. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.Y.; Nguyen, P.M.; Putoczki, T. The expanding role of innate lymphoid cells and their T-cell counterparts in gastrointestinal cancers. Mol. Immunol. 2019, 110, 48–56. [Google Scholar] [CrossRef]
- Mjösberg, J.M.; Trifari, S.; Crellin, N.K.; Peters, C.P.; Van Drunen, C.M.; Piet, B.; Fokkens, W.J.; Cupedo, T.; Spits, H. Human IL-25-and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 2011, 12, 1055–1062. [Google Scholar] [CrossRef]
- Bie, Q.; Zhang, P.; Su, Z.; Zheng, D.; Ying, X.; Wu, Y.; Yang, H.; Chen, D.; Wang, S.; Xu, H. Polarization of ILC2s in peripheral blood might contribute to immunosuppressive microenvironment in patients with gastric cancer. J. Immunol. Res. 2014, 2014, 923135. [Google Scholar] [CrossRef]
- Oya, Y.; Hayakawa, Y.; Koike, K. Tumor microenvironment in gastric cancers. Cancer Sci. 2020, 111, 2696–2707. [Google Scholar] [CrossRef]
- Khaled, Y.S.; Ammori, B.J.; Elkord, E. Myeloid-derived suppressor cells in cancer: Recent progress and prospects. Immunol. Cell Biol. 2013, 91, 493–502. [Google Scholar] [CrossRef]
- Okwan-Duodu, D.; Umpierrez, G.E.; Brawley, O.W.; Diaz, R. Obesity-driven inflammation and cancer risk: Role of myeloid derived suppressor cells and alternately activated macrophages. Am. J. Cancer Res. 2013, 3, 21. [Google Scholar]
- Meyer, A.R.; Engevik, A.C.; Madorsky, T.; Belmont, E.; Stier, M.T.; Norlander, A.E.; Pilkinton, M.A.; McDonnell, W.J.; Weis, J.A.; Jang, B. Group 2 innate lymphoid cells coordinate damage response in the stomach. Gastroenterology 2020, 159, 2077–2091.e8. [Google Scholar] [CrossRef]
- Busser, B.; Sancey, L.; Brambilla, E.; Coll, J.-L.; Hurbin, A. The multiple roles of amphiregulin in human cancer. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2011, 1816, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ding, G.; Wen, J.; Tang, Q.; Yong, H.; Zhu, H.; Zhang, S.; Qiu, Z.; Feng, Z.; Zhu, J. Correlation between Trop2 and amphiregulin coexpression and overall survival in gastric cancer. Cancer Med. 2017, 6, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Babaie, D.; Rasouli, S.; Darougar, S.; Daneshmandii, Z.; Mesdaghi, M.; Ghadimi, F. Serum interleukin-17 evaluation in patients with eosinophilic gastrointestinal disease. Immunoregulation 2020, 3, 61–66. [Google Scholar] [CrossRef]
- Nussbaum, J.C.; Van Dyken, S.J.; Von Moltke, J.; Cheng, L.E.; Mohapatra, A.; Molofsky, A.B.; Thornton, E.E.; Krummel, M.F.; Chawla, A.; Liang, H.-E. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 2013, 502, 245–248. [Google Scholar] [CrossRef]
- Doherty, T.A.; Baum, R.; Newbury, R.O.; Yang, T.; Dohil, R.; Aquino, M.; Doshi, A.; Walford, H.H.; Kurten, R.C.; Broide, D.H. Group 2 innate lymphocytes (ILC2) are enriched in active eosinophilic esophagitis. J. Allergy Clin. Immunol. 2015, 136, 792–794.e3. [Google Scholar] [CrossRef]
- Judd, L.M.; Heine, R.G.; Menheniott, T.R.; Buzzelli, J.; O’Brien-Simpson, N.; Pavlic, D.; O’Connor, L.; Al Gazali, K.; Hamilton, O.; Scurr, M. Elevated IL-33 expression is associated with pediatric eosinophilic esophagitis, and exogenous IL-33 promotes eosinophilic esophagitis development in mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2016, 310, G13–G25. [Google Scholar] [CrossRef]
- Kortekaas Krohn, I.; Bal, S.M.; Golebski, K. The role of innate lymphoid cells in airway inflammation: Evolving paradigms. Curr. Opin. Pulm. Med. 2018, 24, 11–17. [Google Scholar] [CrossRef]
- O’Keefe, R.N.; Carli, A.L.; Baloyan, D.; Afshar-Sterle, S.; Eissmann, M.F.; Poh, A.R.; Seillet, C.; Locksley, R.M.; Ernst, M.; Buchert, M. Inhibition of the tuft cell/ILC2 axis reduces gastric tumor development in mice. bioRxiv 2022, bioRxiv:2022.02.16.480779. [Google Scholar]
- Busada, J.T.; Peterson, K.N.; Khadka, S.; Xu, X.; Oakley, R.H.; Cook, D.N.; Cidlowski, J.A. Glucocorticoids and Androgens Protect From Gastric Metaplasia by Suppressing Group 2 Innate Lymphoid Cell Activation. Gastroenterology 2021, 161, 637–652.e4. [Google Scholar] [CrossRef]
- Bernink, J.H.; Peters, C.P.; Munneke, M.; Te Velde, A.A.; Meijer, S.L.; Weijer, K.; Hreggvidsdottir, H.S.; Heinsbroek, S.E.; Legrand, N.; Buskens, C.J. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 2013, 14, 221–229. [Google Scholar] [CrossRef]
- Klose, C.S.; Artis, D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 2016, 17, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Marchalot, A.; Mjosberg, J. Innate lymphoid cells in colorectal cancer. Scand. J. Immunol. 2022, 95, e13156. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, Y.; Lin, D.; Lei, L.; Mei, Y.; Jin, Z.; Gong, H.; Zhu, Y.; Hu, B.; Zhang, Y. NCR− group 3 innate lymphoid cells orchestrate IL-23/IL-17 axis to promote hepatocellular carcinoma development. EBioMedicine 2019, 41, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Zhou, J.; Tian, Z.; Lin, Y.; Song, J.; Ruan, Z.; Ni, B.; Zhao, H.; Yang, W. ILC3 cells promote the proliferation and invasion of pancreatic cancer cells through IL-22/AKT signaling. Clin. Transl. Oncol. 2020, 22, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Kirchberger, S.; Royston, D.J.; Boulard, O.; Thornton, E.; Franchini, F.; Szabady, R.L.; Harrison, O.; Powrie, F. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J. Exp. Med. 2013, 210, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Rasul, F.; Nashan, B.; Sun, C. Innate lymphoid cells and cancer: Role in tumor progression and inhibition. Eur. J. Immunol. 2021, 51, 2188–2205. [Google Scholar] [CrossRef]
- Fu, W.; Wang, W.; Zhang, J.; Zhao, Y.; Chen, K.; Wang, Y.; Zhang, J.; Xiong, Y.; Guo, X.; Ding, S. Dynamic change of circulating innate and adaptive lymphocytes subtypes during a cascade of gastric lesions. J. Leukoc. Biol. 2022, 112, 931–938. [Google Scholar] [CrossRef]
| Cell Type | Function | |
|---|---|---|
| Anti-Tumor | Pro-Tumor | |
| NK cell |
| |
| ILC1 |
| N.A. |
| ILC2 | N.A. |
|
| ILC3 | N.A. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Y.; Yan, Z.; Yang, A. The Roles of Innate Lymphoid Cells in the Gastric Mucosal Immunology and Oncogenesis of Gastric Cancer. Int. J. Mol. Sci. 2023, 24, 6652. https://doi.org/10.3390/ijms24076652
Jiao Y, Yan Z, Yang A. The Roles of Innate Lymphoid Cells in the Gastric Mucosal Immunology and Oncogenesis of Gastric Cancer. International Journal of Molecular Sciences. 2023; 24(7):6652. https://doi.org/10.3390/ijms24076652
Chicago/Turabian StyleJiao, Yuhao, Zhiyu Yan, and Aiming Yang. 2023. "The Roles of Innate Lymphoid Cells in the Gastric Mucosal Immunology and Oncogenesis of Gastric Cancer" International Journal of Molecular Sciences 24, no. 7: 6652. https://doi.org/10.3390/ijms24076652
APA StyleJiao, Y., Yan, Z., & Yang, A. (2023). The Roles of Innate Lymphoid Cells in the Gastric Mucosal Immunology and Oncogenesis of Gastric Cancer. International Journal of Molecular Sciences, 24(7), 6652. https://doi.org/10.3390/ijms24076652








