Dietary Evodiamine Inhibits Atherosclerosis-Associated Changes in Vascular Smooth Muscle Cells
Abstract
:1. Introduction
2. Results
2.1. Evodiamine Reduces Atherosclerotic Plaque in Mice
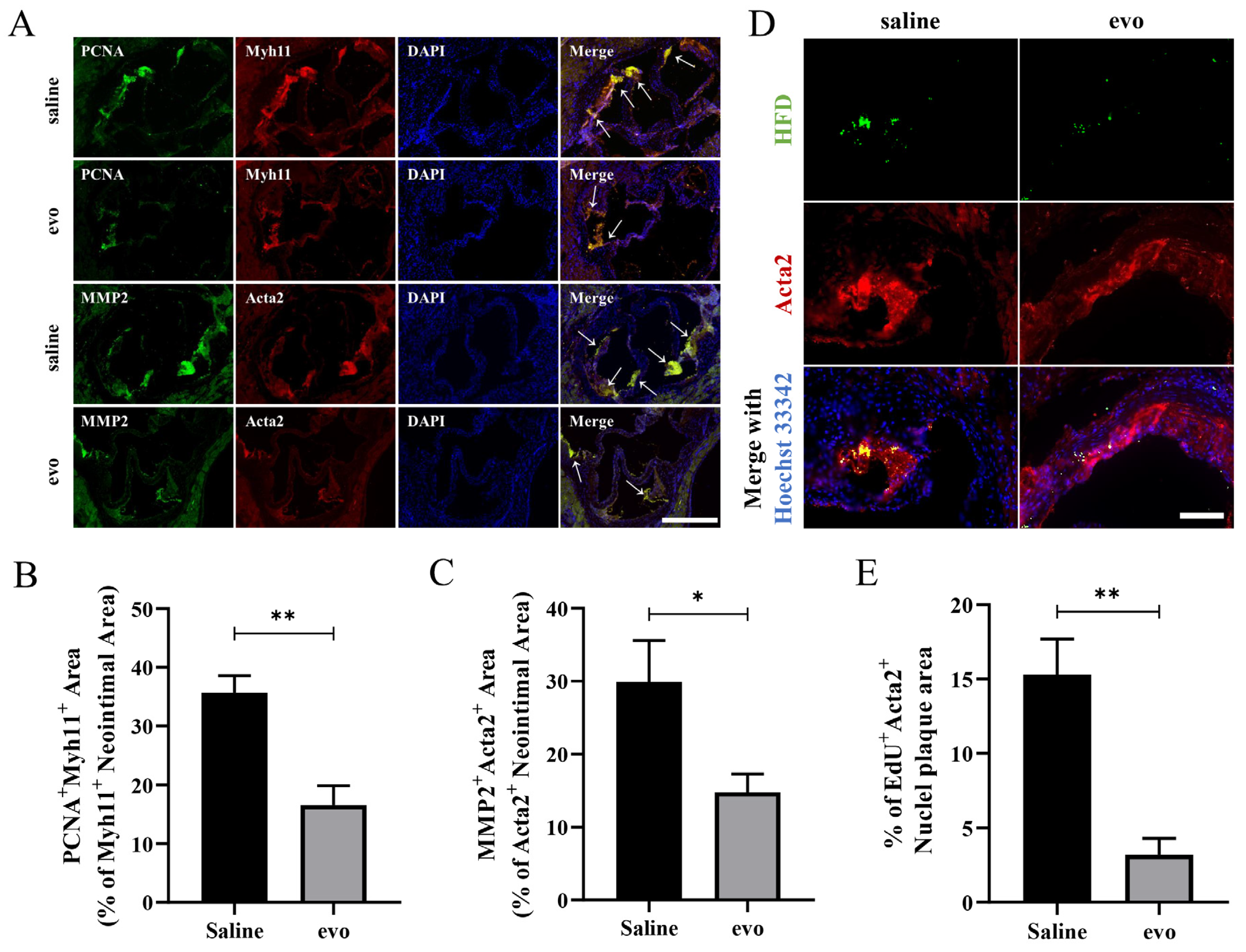
2.2. Evodiamine Reduces Atherosclerotic VSMC Proliferation and Migration in Mice
2.3. Evodiamine Inhibits ox-LDL-Induced VSMC Proliferation and Migration
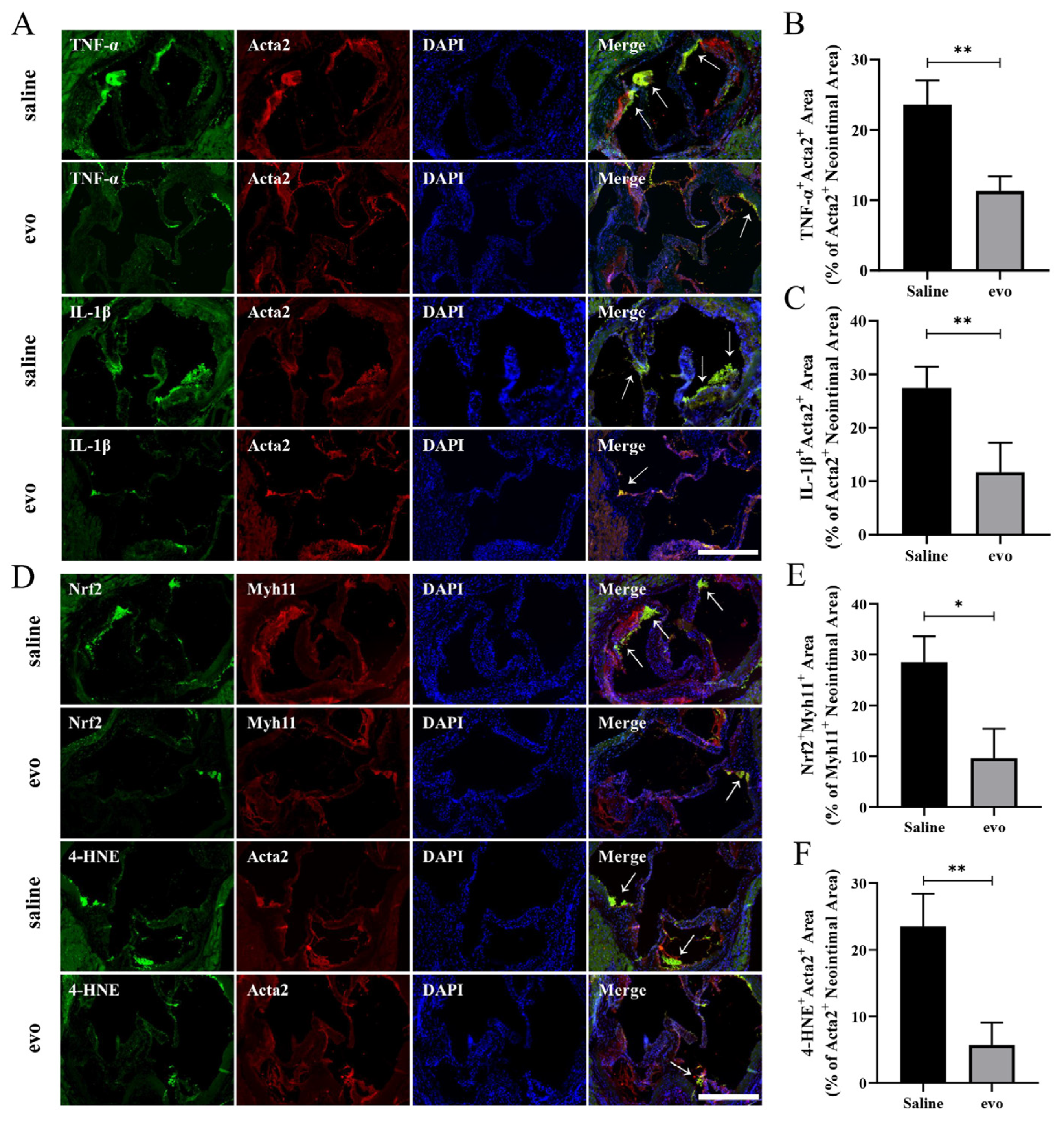
2.4. Evodiamine Reduces Inflammation and Oxidative Stress in Mouse VSMCs
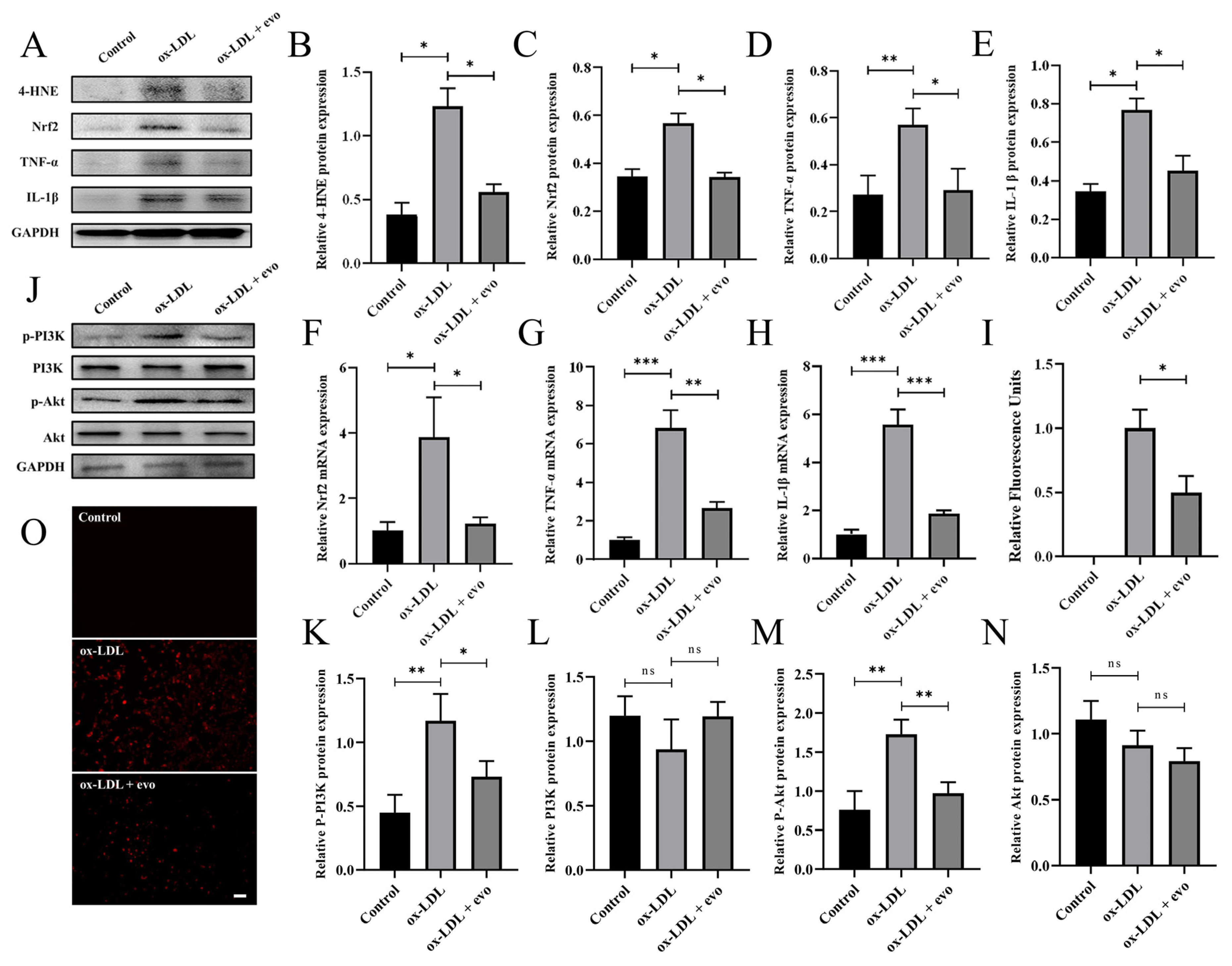
2.5. Evodiamine Attenuates the Inflammatory Response and Oxidative Stress in Atherosclerosis-Induced VSMCs by Inhibiting PI3K/Akt
3. Materials and Methods
3.1. Animals
3.2. Cell Culture and Reagents
3.3. Measurement of Plaque Morphology
3.4. Cell Counting Kit-8 and 5-Ethynyl-2′-Deoxyuridine Assay
3.5. RNA Isolation and qRT-PCR
3.6. Western Blotting and Immunofluorescence Antibodies
3.7. EdU Detection in Tissue
3.8. Detection of Reactive Oxygen Species
3.9. Wound Healing Assay
3.10. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis, Nature Reviews. Dis. Prim. 2019, 5, 56. [Google Scholar]
- Susser, L.I.; Rayner, K.J. Through the layers: How macrophages drive atherosclerosis across the vessel wall. J. Clin. Investig. 2022, 132, e157011. [Google Scholar] [CrossRef]
- Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000, 6, 389–395. [Google Scholar] [CrossRef]
- Miano, J.M.; Fisher, E.A.; Majesky, M.W. Fate and State of Vascular Smooth Muscle Cells in Atherosclerosis. Circulation 2021, 143, 2110–2116. [Google Scholar] [CrossRef]
- Swiatlowska, P.; Sit, B.; Feng, Z.; Marhuenda, E.; Xanthis, I.; Zingaro, S.; Ward, M.; Zhou, X.; Xiao, Q.; Shanahan, C.; et al. Pressure and stiffness sensing together regulate vascular smooth muscle cell phenotype switching. Sci. Adv. 2022, 8, eabm3471. [Google Scholar] [CrossRef]
- Wirka, R.C.; Wagh, D.; Paik, D.T.; Pjanic, M.; Nguyen, T.; Miller, C.L.; Kundu, R.; Nagao, M.; Coller, J.; Koyano, T.K.; et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat. Med. 2019, 25, 1280–1289. [Google Scholar] [CrossRef]
- Basatemur, G.L.; Jørgensen, H.F.; Clarke, M.C.; Bennett, M.R.; Mallat, Z. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef]
- Grootaert, M.O.J.; Moulis, M.; Roth, L.; Martinet, W.; Vindis, C.; Bennett, M.R.; De Meyer, G.R.Y. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc. Res. 2018, 114, 622–634. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Uhrin, P.; Mocan, A.; Waltenberger, B.; Breuss, J.M.; Tewari, D.; Mihaly-Bison, J.; Huminiecki, Ł.; Starzyński, R.R.; Tzvetkov, N.T.; et al. Vascular smooth muscle cell proliferation as a therapeutic target. Part 1: Molecular targets and pathways. Biotechnol. Adv. 2018, 36, 1586–1607. [Google Scholar] [CrossRef] [PubMed]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef] [Green Version]
- Brizzi, M.F.; Formato, L.; Dentelli, P.; Rosso, A.; Pavan, M.; Garbarino, G.; Pegoraro, M.; Camussi, G.; Pegoraro, L. Interleukin-3 stimulates migration and proliferation of vascular smooth muscle cells: A potential role in atherogenesis. Circulation 2001, 103, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Xu, Y.; Qiu, Z.; Jiang, L. Sulforaphane Attenuates Angiotensin II-Induced Vascular Smooth Muscle Cell Migration via Suppression of NOX4/ROS/Nrf2 Signaling. Int. J. Biol. Sci. 2019, 15, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Tan, Q.; Zhang, J. Evodiamine and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 315–328. [Google Scholar] [CrossRef]
- Yang, J.; Kim, J.-B.; Lee, P.; Kim, S.-H. Evodiamine Inhibits Helicobacter pylori Growth and Helicobacter pylori-Induced Inflammation. Int. J. Mol. Sci. 2021, 22, 3385. [Google Scholar] [CrossRef]
- Panda, M.; Tripathi, S.K.; Zengin, G.; Biswal, B.K. Evodiamine as an anticancer agent: A comprehensive review on its therapeutic application, pharmacokinetic, toxicity, and metabolism in various cancers. Cell Biol. Toxicol. 2022, 23, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiong, Y.; Peng, Y.; Zhang, X.; Li, S.; Peng, Y.; Peng, X.; Zhuo, L.; Jiang, W. Natural product evodiamine-inspired medicinal chemistry: Anticancer activity, structural optimization and structure-activity relationship. Eur. J. Med. Chem. 2023, 247, 115031. [Google Scholar] [CrossRef]
- Hou, X.; Malainer, C.; Atanasov, A.G.; Heiß, E.H.; Dirsch, V.M.; Wang, L.; Wang, K. Evodiamine Lowers Blood Lipids by Up-Regulating the PPARgamma/ABCG1 Pathway in High-Fat-Diet-Fed Mice. J. Nat. Prod. 2021, 84, 3110–3116. [Google Scholar] [CrossRef]
- Zhu, B.; Zhao, L.; Liu, Y.; Jin, Y.; Feng, J.; Zhao, F.; Sun, J.; Geng, R.; Wei, Y. Induction of phosphatase shatterproof 2 by evodiamine suppresses the proliferation and invasion of human cholangiocarcinoma. Int. J. Biochem. Cell Biol. 2019, 108, 98–110. [Google Scholar] [CrossRef]
- Jiang, Z.-B.; Huang, J.-M.; Xie, Y.-J.; Zhang, Y.Z.; Chang, C.; Lai, H.-L.; Wang, W.; Yao, X.-J.; Fan, X.-X.; Wu, Q.-B.; et al. Evodiamine suppresses non-small cell lung cancer by elevating CD8+ T cells and downregulating the MUC1-C/PD-L1 axis. J. Exp. Clin. Cancer Res. 2020, 39, 249. [Google Scholar] [CrossRef]
- Zhou, X.; Ren, F.; Wei, H.; Liu, L.; Shen, T.; Xu, S.; Wei, J.; Ren, J.; Ni, H. Combination of berberine and evodiamine inhibits intestinal cholesterol absorption in high fat diet induced hyperlipidemic rats. Lipids Health Dis. 2017, 16, 239. [Google Scholar] [CrossRef] [Green Version]
- Chiou, W.-F.; Liao, J.-F.; Chen, C.-F. Comparative Study on the Vasodilatory Effects of Three Quinazoline Alkaloids Isolated from Evodia rutaecarpa. J. Nat. Prod. 1996, 59, 374–378. [Google Scholar] [CrossRef]
- Wiart, C. A note on Evodia rutaecarpa. Phytomedicine 2012, 19, 1244. [Google Scholar] [CrossRef]
- Tian, K.-M.; Li, J.-J.; Xu, S.-W. Rutaecarpine: A promising cardiovascular protective alkaloid from Evodia rutaecarpa (Wu Zhu Yu). Pharmacol. Res. 2019, 141, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Hu, C. Evodiamine: A Novel Anti-Cancer Alkaloid from Evodia rutaecarpa. Molecules 2009, 14, 1852–1859. [Google Scholar] [CrossRef]
- Su, C.-H.; Cheng, Y.-C.; Chang, Y.-C.; Kung, T.-H.; Chen, Y.-L.; Lai, K.-H.; Hsieh, H.-L.; Chen, C.-Y.; Hwang, T.-L.; Yang, Y.-L. Untargeted LC-MS/MS-Based Multi-Informative Molecular Networking for Targeting the Antiproliferative Ingredients in Tetradium ruticarpum Fruit. Molecules 2022, 27, 4462. [Google Scholar] [CrossRef]
- Yang, C.Q.; Lian, W.Y.; Wang, Y.G.; Gao, Y. Research progress in pharmacology and toxicology of evodiamine. Zhongguo Zhong Yao Za Zhi 2021, 46, 5218–5225. [Google Scholar] [PubMed]
- Sun, Q.; Xie, L.; Song, J.; Li, X. Evodiamine: A review of its pharmacology, toxicity, pharmacokinetics and preparation researches. J. Ethnopharmacol. 2020, 262, 113164. [Google Scholar] [CrossRef]
- Li, X.; Ge, J.; Zheng, Q.; Zhang, J.; Sun, R.; Liu, R. Evodiamine and rutaecarpine from Tetradium ruticarpum in the treatment of liver diseases. Phytomedicine 2020, 68, 153180. [Google Scholar] [CrossRef]
- Lv, Q.; Xue, Y.; Li, G.; Zou, L.; Zhang, X.; Ying, M.; Wang, S.; Guo, L.; Gao, Y.; Li, G.; et al. Beneficial effects of evodiamine on P2X4-mediated inflammatory injury of human umbilical vein endothelial cells due to high glucose. Int. Immunopharmacol. 2015, 28, 1044–1049. [Google Scholar] [CrossRef]
- Zhu, L.-Q.; Zhang, L.; Zhang, J.; Chang, G.-L.; Liu, G.; Yu, D.-D.; Yu, X.-M.; Zhao, M.-S.; Ye, B. Evodiamine inhibits high-fat diet-induced colitis-associated cancer in mice through regulating the gut microbiota. J. Integr. Med. 2020, 19, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-G.; Zeng, Q.-Z.; Chen, M.-Y.; Xu, L.-H.; Zhang, C.-C.; Mai, F.-Y.; Zeng, C.-Y.; He, X.-H.; Ouyang, D.-Y. Evodiamine Augments NLRP3 Inflammasome Activation and Anti-bacterial Responses Through Inducing α-Tubulin Acetylation. Front. Pharmacol. 2019, 10, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Jin, H.; Gong, W.; Wang, Z.; Liang, H. Pharmacological Actions of Multi-Target-Directed Evodiamine. Molecules 2013, 18, 1826–1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Deng, J.; Shi, T.; Wen, H.; Li, J.; Liang, Z.; Lei, F.; Liu, D.; Zhang, H.; Liang, Y.; et al. Design, synthesis and bioactivity study of evodiamine derivatives as multifunctional agents for the treatment of hepatocellular carcinoma. Bioorg. Chem. 2021, 114, 105154. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Pan, S.-L.; Liao, C.-H.; Huang, D.-Y.; Guh, J.-H.; Peng, C.-Y.; Chang, Y.-L.; Teng, C.-M. Evodiamine represses hypoxia-induced inflammatory proteins expression and hypoxia-inducible factor 1α accumulation in RAW264.7. Shock 2009, 32, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Eftekhari, P.; Schachner, D.; Ignatova, I.D.; Palme, V.; Schilcher, N.; Ladurner, A.; Heiss, E.H.; Stangl, H.; Dirsch, V.M.; et al. Novel interactomics approach identifies ABCA1 as direct target of evodiamine, which increases macrophage cholesterol efflux. Sci. Rep. 2018, 8, 11061. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.-H.; Wu, Q.-Q.; Xiao, Y.; Yuan, Y.; Yang, Z.; Bian, Z.-Y.; Chang, W.; Tang, Q.-Z. Evodiamine Prevents Isoproterenol-Induced Cardiac Fibrosis by Regulating Endothelial-to-Mesenchymal Transition. Planta Med. 2016, 83, 761–769. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Cho, L.; Davis, M.; Elgendy, I.; Epps, K.; Lindley, K.J.; Mehta, P.K.; Michos, E.D.; Minissian, M.; Pepine, C.; Vaccarino, V.; et al. Summary of Updated Recommendations for Primary Prevention of Cardiovascular Disease in Women: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2602–2618. [Google Scholar] [CrossRef]
- Stone, N.J.; Smith, S.C., Jr.; Orringer, C.E.; Rigotti, N.A.; Navar, A.M.; Khan, S.S.; Jones, D.W.; Goldberg, R.; Mora, S.; Blaha, M.; et al. Managing Atherosclerotic Cardiovascular Risk in Young Adults: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 819–836. [Google Scholar] [CrossRef]
- Yang, X.; Li, J.; Hu, D.; Chen, J.; Li, Y.; Huang, J.; Liu, X.; Liu, F.; Cao, J.; Shen, C.; et al. Predicting the 10-Year Risks of Atherosclerotic Cardiovascular Disease in Chinese Population: The China-PAR Project (Prediction for ASCVD Risk in China). Circulation 2016, 134, 1430–1440. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Hong, Z.; Wang, Z.; Zhou, B.; Wang, J.; Tong, H.; Liao, Y.; Zheng, P.; Jamshed, M.B.; Zhang, Q.; Chen, H. Effects of evodiamine on PI3K/Akt and MAPK/ERK signaling pathways in pancreatic cancer cells. Int. J. Oncol. 2020, 56, 783–793. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Martinez, M.; Cabail, M.Z. The PI3K/Akt Pathway in Meta-Inflammation. Int. J. Mol. Sci. 2022, 23, 15330. [Google Scholar] [CrossRef]
- Jiang, R.; Tang, J.; Zhang, X.; He, Y.; Yu, Z.; Chen, S.; Xia, J.; Lin, J.; Ou, Q. CCN1 Promotes Inflammation by Inducing IL-6 Production via alpha6beta1/PI3K/Akt/NF-kappaB Pathway in Autoimmune Hepatitis. Front. Immunol. 2022, 13, 810671. [Google Scholar] [CrossRef]
- Liu, Y.; Tie, L. Apolipoprotein M and sphingosine-1-phosphate complex alleviates TNF-α-induced endothelial cell injury and inflammation through PI3K/AKT signaling pathway. BMC Cardiovasc. Disord. 2019, 19, 279. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zhang, T.; Yang, H.; Xiao, X.; Bian, Y.; Si, D.; Liu, C. Preparation of Evodiamine Solid Dispersions and Its Pharmacokinetics. Indian J. Pharm. Sci. 2011, 73, 276–281. [Google Scholar] [PubMed]
- Tan, Q.; Liu, S.; Chen, X.; Wu, M.; Wang, H.; Yin, H.; He, D.; Xiong, H.; Zhang, J. Design and Evaluation of a Novel Evodiamine-Phospholipid Complex for Improved Oral Bioavailability. AAPS PharmSciTech 2012, 13, 534–547. [Google Scholar] [CrossRef] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zha, Y.; Yang, Y.; Zhou, Y.; Ye, B.; Li, H.; Liang, J. Dietary Evodiamine Inhibits Atherosclerosis-Associated Changes in Vascular Smooth Muscle Cells. Int. J. Mol. Sci. 2023, 24, 6653. https://doi.org/10.3390/ijms24076653
Zha Y, Yang Y, Zhou Y, Ye B, Li H, Liang J. Dietary Evodiamine Inhibits Atherosclerosis-Associated Changes in Vascular Smooth Muscle Cells. International Journal of Molecular Sciences. 2023; 24(7):6653. https://doi.org/10.3390/ijms24076653
Chicago/Turabian StyleZha, Yiwen, Yongqi Yang, Yue Zhou, Bingqian Ye, Hongliang Li, and Jingyan Liang. 2023. "Dietary Evodiamine Inhibits Atherosclerosis-Associated Changes in Vascular Smooth Muscle Cells" International Journal of Molecular Sciences 24, no. 7: 6653. https://doi.org/10.3390/ijms24076653




