Neonatal Exposure to Valproate Induces Long-Term Alterations in Steroid Hormone Levels in the Brain Cortex of Prepubertal Rats
Abstract
1. Introduction
2. Results
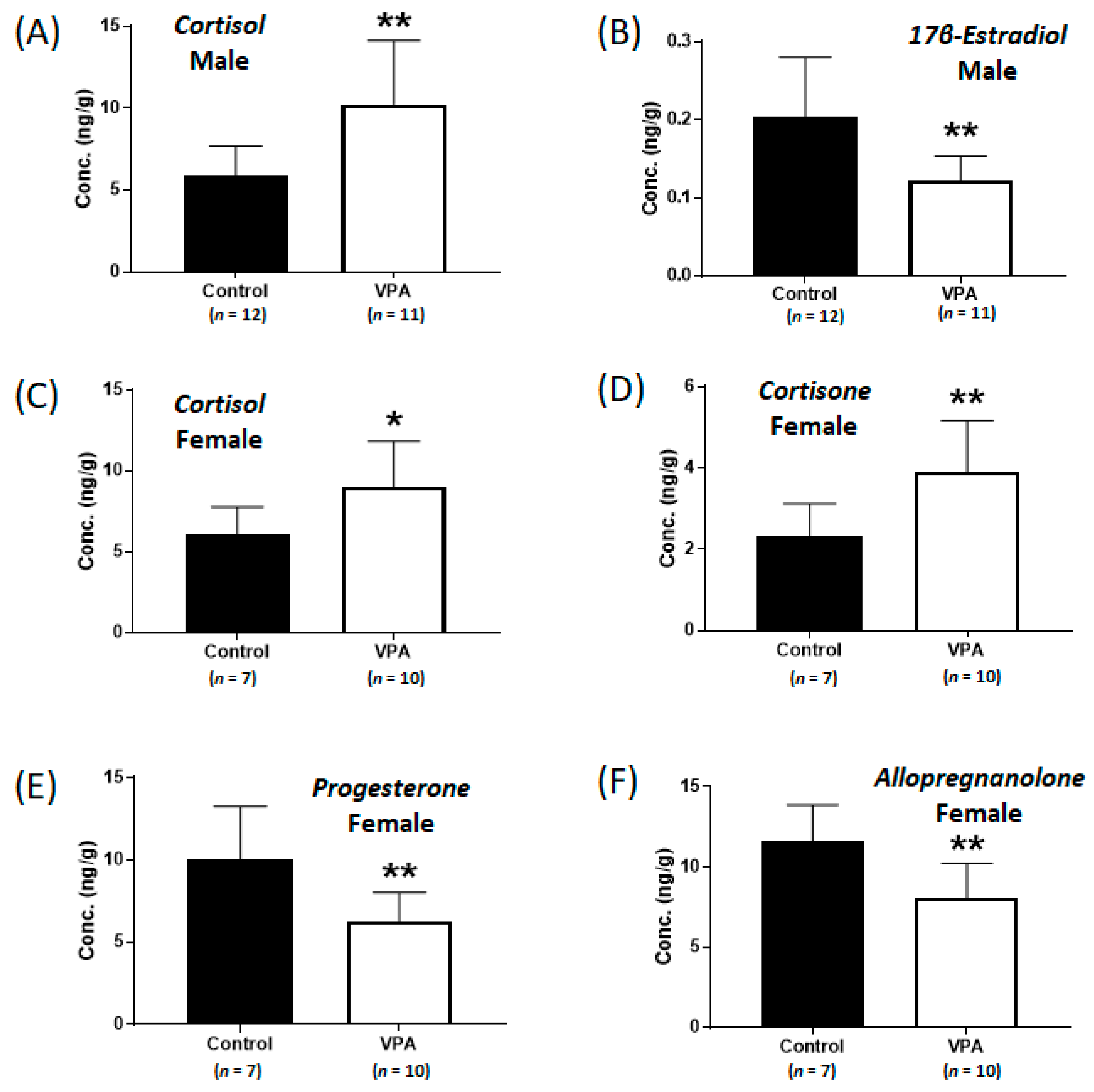
2.1. Increased Cortisol Levels Were Observed in the Cerebral Cortices of Neonatal VPA-Exposed 4-Week-Old Rats
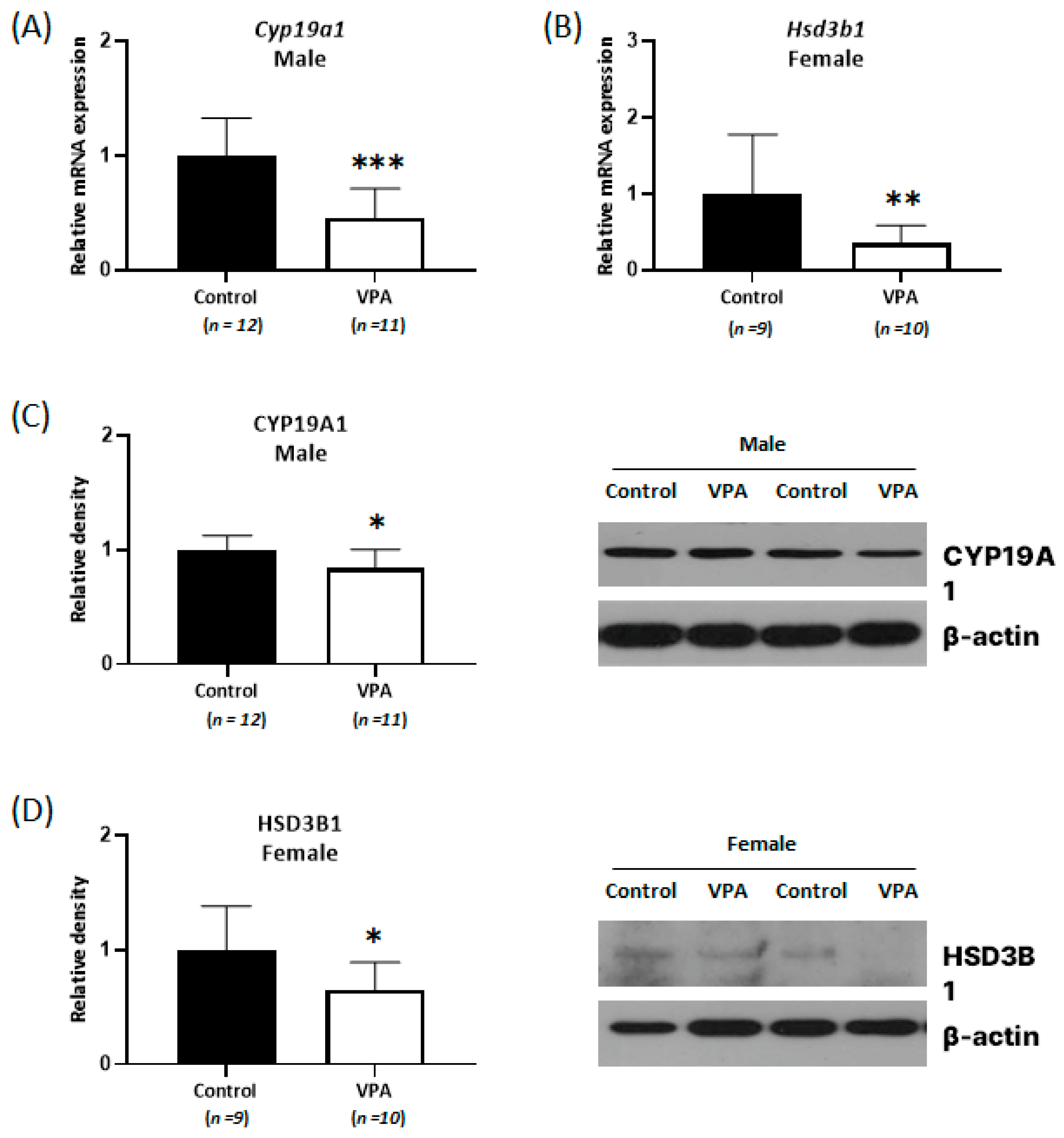
2.2. Decreased Expression of Cyp19a1 and Hsd3b1 According to Sex Was Observed in the Neonatal VPA-Exposed Cortex
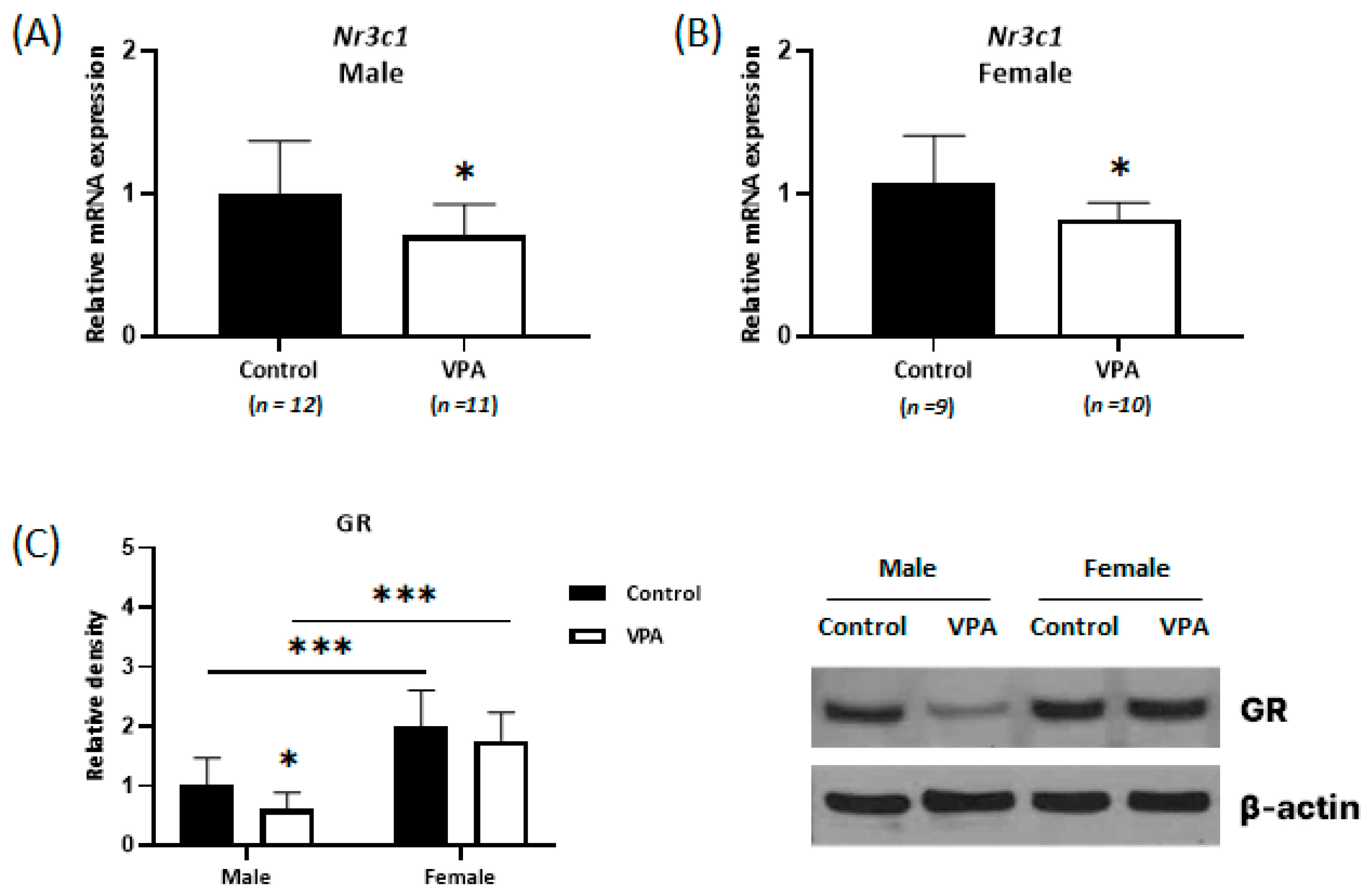
2.3. Decreased Expression of Glucocorticoid Receptors Was Observed Only in the Neonatal VPA-Exposed Male Cortex
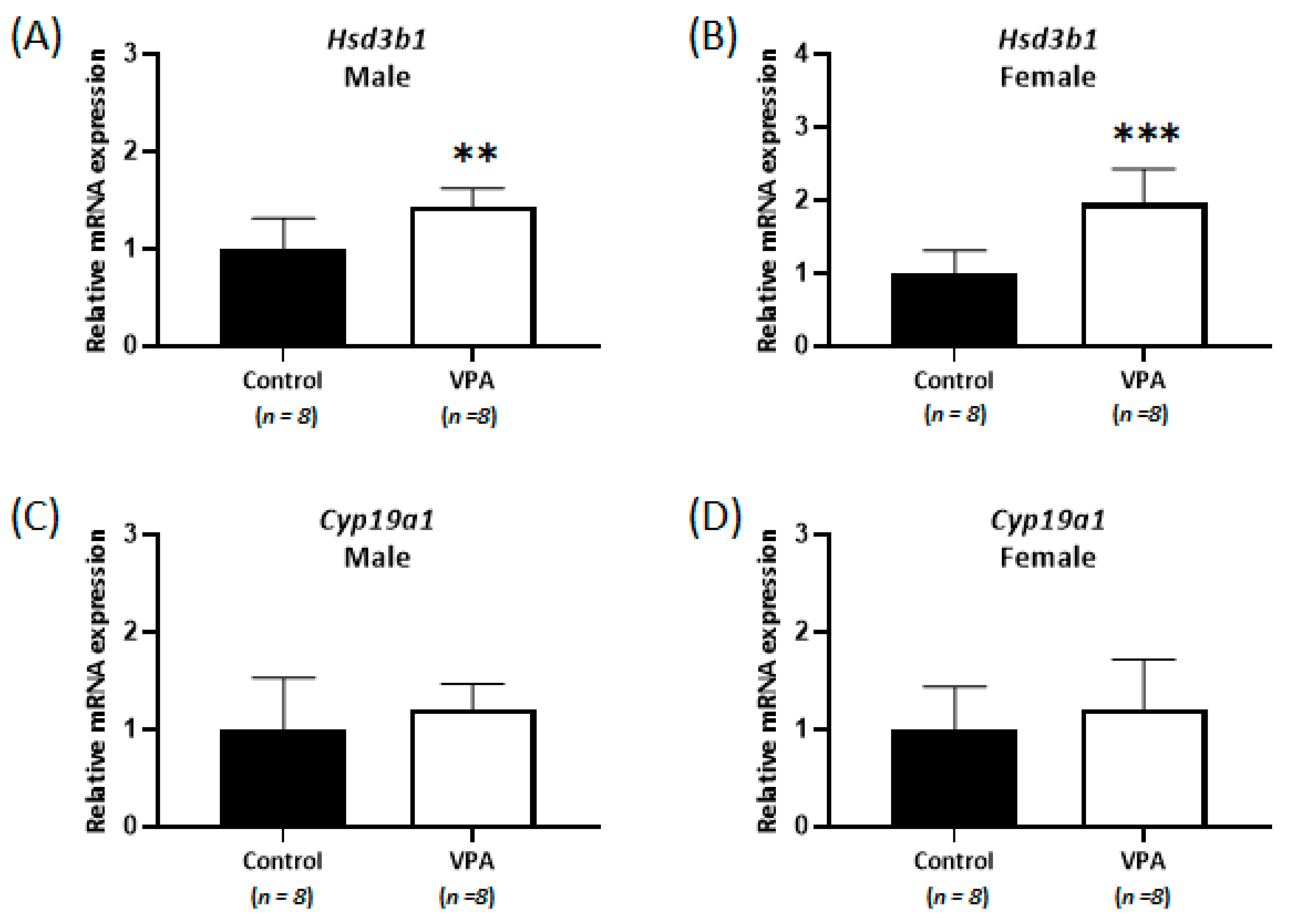
2.4. No Differences Were Observed in the Cortisol Level and Nr3c1 mRNA Expression in the Cerebral Cortices of Prepubertal VPA-Exposed 8-Week-Old Rats
3. Discussion
4. Materials and Methods
4.1. Animals and Treatments
4.2. Analysis of Steroids in Cerebral Cortex Samples Using LC-MS/MS
4.3. RNA Extraction and Quantitative Real-Time qPCR Analysis
4.4. Western Blotting
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASD | autism spectrum disorder |
| BCA | bicinchoninic acid |
| Cyp19a1 | cytochrome P450 19A1 |
| EUIACUC | Institutional Animal Care and Use Committee of Eulji University |
| GABAA | γ-aminobutyric acid A |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GR | glucocorticoid receptor |
| HDAC | histone deacetylase |
| HPA | hypothalamic pituitary adrenal |
| HRP | horseradish peroxidase |
| Hsd3b1 | β-hydroxysteroid dehydrogenase 1 |
| MRI | magnetic resonance imaging |
| mRNA | messenger RNA |
| NMDA | N-methyl-d-aspartate |
| NRF | National Research Foundation |
| Nr3c1 | nuclear receptor subfamily 3 group C member 1 |
| PND | postnatal day |
| RIPA | radioimmunoprecipitation assay |
| sc-RNA-seq | single-cell RNA sequencing |
| SD | Sprague-Dawley |
| TBST | Tris-buffered saline with Tween |
| VPA | valproic acid |
References
- Nicolini, C.; Fahnestock, M. The valproic acid-induced rodent model of autism. Exp. Neurol. 2018, 299, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Chaliha, D.; Albrecht, M.; Vaccarezza, M.; Takechi, R.; Lam, V.; Al-Salami, H.; Mamo, J. A Systematic Review of the Valproic-Acid-Induced Rodent Model of Autism. Dev. Neurosci. 2020, 42, 12–48. [Google Scholar] [CrossRef] [PubMed]
- Mello, M.L.S. Sodium Valproate-Induced Chromatin Remodeling. Front. Cell Dev. Biol. 2021, 9, 645518. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Dreyfus, C.; DiCicco-Bloom, E. Valproic acid stimulates proliferation of glial precursors during cortical gliogenesis in developing rat. Dev. Neurobiol. 2016, 76, 780–798. [Google Scholar] [CrossRef] [PubMed]
- Mony, T.J.; Lee, J.W.; Dreyfus, C.; DiCicco-Bloom, E.; Lee, H.J. Valproic Acid Exposure during Early Postnatal Gliogenesis Leads to Autistic-like Behaviors in Rats, Clin. Psychopharmacol. Neurosci. 2016, 14, 38–344. [Google Scholar] [CrossRef]
- Brion, L.; Gorostizaga, A.; Gómez, N.V.; Podestá, E.J.; Cornejo Maciel, F.; Paz, C. Valproic acid alters mitochondrial cholesterol transport in Y1 adrenocortical cells. Toxicol. In Vitro 2011, 25, 7–12. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Li, S.; Zou, X. Reproductive and metabolic abnormalities in women taking valproate for bipolar disorder: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 202, 26–31. [Google Scholar] [CrossRef]
- Cho, S.H.; Chai, H.H.; Chang, S.Y.; Kim, S.A. Acute Valproate Exposure Induces Sex-Specific Changes in Steroid Hormone Metabolism in the Cerebral Cortex of Juvenile Mice. Neurochem. Res. 2020, 45, 2044–2051. [Google Scholar] [CrossRef]
- Bilo, L.; Meo, R. Polycystic ovary syndrome in women using valproate: A review. Gynecol. Endocrinol. 2008, 24, 562–570. [Google Scholar] [CrossRef]
- Grimm, A.; Schmitt, K.; Lang, U.E.; Mensah-Nyagan, A.G.; Eckert, A. Improvement of neuronal bioenergetics by neurosteroids: Implications for age-related neurodegenerative disorders. Biochim. Biophys. Acta 2014, 1842, 2427–2438. [Google Scholar] [CrossRef]
- Tauboll, E.; Sveberg, L.; Svalheim, S. Interactions between hormones and epilepsy. Seizure 2015, 28, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Hirst, J.J.; Cumberland, A.L.; Shaw, J.C.; Bennett, G.A.; Kelleher, M.A.; Walker, D.W.; Palliser, H.K. Loss of neurosteroid-mediated protection following stress during fetal life. J. Steroid Biochem. Mol. Biol. 2016, 160, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, R. New perspectives in neurosteroid action: Open questions for future research. Front. Cell Neurosci. 2014, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.T. The link between perinatal glucocorticoids exposure and psychiatric disorder. Pediatr. Res. 2011, 69, 19R–25R. [Google Scholar] [CrossRef] [PubMed]
- Danek, P.J.; Bromek, E.; Daniel, W.A. The Influence of Long-Term Treatment with Asenapine on Liver Cytochrome P450 Expression and Activity in the Rat. The Involvement of Different Mechanisms. Pharmaceuticals 2021, 14, 629. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.W.; Grant, P.A.; Rissman, E.F. Sex differences in histone modifications in the neonatal mouse brain. Epigenetics 2009, 4, 47–53. [Google Scholar] [CrossRef]
- Krontira, A.C.; Cruceanu, C.; Binder, E.B. Glucocorticoids as Mediators of Adverse Outcomes of Prenatal Stress. Trends Neurosci. 2020, 43, 394–405. [Google Scholar] [CrossRef]
- Cottrell, E.C.; Seckl, J.R. Prenatal stress, glucocorticoids and the programming of adult disease. Front. Behav. Neurosci. 2009, 3, 19. [Google Scholar] [CrossRef]
- Hamden, J.E.; Gray, K.M.; Salehzadeh, M.; Kachkovski, G.V.; Forys, B.J.; Ma, C.; Austin, S.H.; Soma, K.K. Steroid profiling of glucocorticoids in microdissected mouse brain across development. Dev. Neurobiol. 2021, 81, 189–206. [Google Scholar] [CrossRef]
- Hamden, J.E.; Gray, K.M.; Salehzadeh, M.; Soma, K.K. Isoflurane stress induces region-specific glucocorticoid levels in neonatal mouse brain. J. Endocrinol. 2022, 255, 61–74. [Google Scholar] [CrossRef]
- Shaw, J.C.; Berry, M.J.; Dyson, R.M.; Crombie, G.K.; Hirst, J.J.; Palliser, H.K. Reduced Neurosteroid Exposure Following Preterm Birth and Its’ Contribution to Neurological Impairment: A Novel Avenue for Preventative Therapies. Front. Physiol. 2019, 10, 599. [Google Scholar] [CrossRef] [PubMed]
- Stoye, D.Q.; Blesa, M.; Sullivan, G.; Galdi, P.; Lamb, G.J.; Black, G.S.; Quigley, A.J.; Thrippleton, M.J.; Bastin, M.E.; Reynolds, R.M.; et al. Maternal cortisol is associated with neonatal amygdala microstructure and connectivity in a sexually dimorphic manner. Elife 2020, 9, 1–18. [Google Scholar] [CrossRef]
- Mukherjee, J.; Cardarelli, R.A.; Cantaut-Belarif, Y.; Deeb, T.Z.; Srivastava, D.P.; Tyagarajan, S.K.; Pangalos, M.N.; Triller, A.; Maguire, J.; Brandon, N.J.; et al. Estradiol modulates the efficacy of synaptic inhibition by decreasing the dwell time of GABAA receptors at inhibitory synapses. Proc. Natl. Acad. Sci. USA 2017, 114, 11763–11768. [Google Scholar] [CrossRef] [PubMed]
- Sellers, K.J.; Erli, F.; Raval, P.; Watson, I.A.; Chen, D.; Srivastava, D.P. Rapid modulation of synaptogenesis and spinogenesis by 17beta-estradiol in primary cortical neurons. Front. Cell Neurosci. 2015, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Denley, M.C.S.; Gatford, N.J.F.; Sellers, K.J.; Srivastava, D.P. Estradiol and the Development of the Cerebral Cortex: An Unexpected Role? Front. Neurosci. 2018, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Weaver, I.C.; Meaney, M.J.; Szyf, M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc. Natl. Acad. Sci. USA 2006, 103, 3480–3485. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.K.; Hien, A.; de Vries, G.J.; Forger, N.G. Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology 2009, 150, 4241–4247. [Google Scholar] [CrossRef]
- Choi, C.S.; Gonzales, E.L.; Kim, K.C.; Yang, S.M.; Kim, J.-W.; Mabunga, D.F.; Cheong, J.H.; Han, S.-H.; Bahn, G.H.; Shin, C.Y. The transgenerational inheritance of autism-like phenotypes in mice exposed to valproic acid during pregnancy. Sci. Rep. 2016, 6, 36250. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-A.; Jang, E.-H.; Lee, J.; Cho, S.-H. Neonatal Exposure to Valproate Induces Long-Term Alterations in Steroid Hormone Levels in the Brain Cortex of Prepubertal Rats. Int. J. Mol. Sci. 2023, 24, 6681. https://doi.org/10.3390/ijms24076681
Kim S-A, Jang E-H, Lee J, Cho S-H. Neonatal Exposure to Valproate Induces Long-Term Alterations in Steroid Hormone Levels in the Brain Cortex of Prepubertal Rats. International Journal of Molecular Sciences. 2023; 24(7):6681. https://doi.org/10.3390/ijms24076681
Chicago/Turabian StyleKim, Soon-Ae, Eun-Hye Jang, Jangjae Lee, and Sung-Hee Cho. 2023. "Neonatal Exposure to Valproate Induces Long-Term Alterations in Steroid Hormone Levels in the Brain Cortex of Prepubertal Rats" International Journal of Molecular Sciences 24, no. 7: 6681. https://doi.org/10.3390/ijms24076681
APA StyleKim, S.-A., Jang, E.-H., Lee, J., & Cho, S.-H. (2023). Neonatal Exposure to Valproate Induces Long-Term Alterations in Steroid Hormone Levels in the Brain Cortex of Prepubertal Rats. International Journal of Molecular Sciences, 24(7), 6681. https://doi.org/10.3390/ijms24076681






