Spontaneous Bone Marrow Edema: Perfusion Abnormalities and Treatment with Surgical Decompression
Abstract
1. Introduction
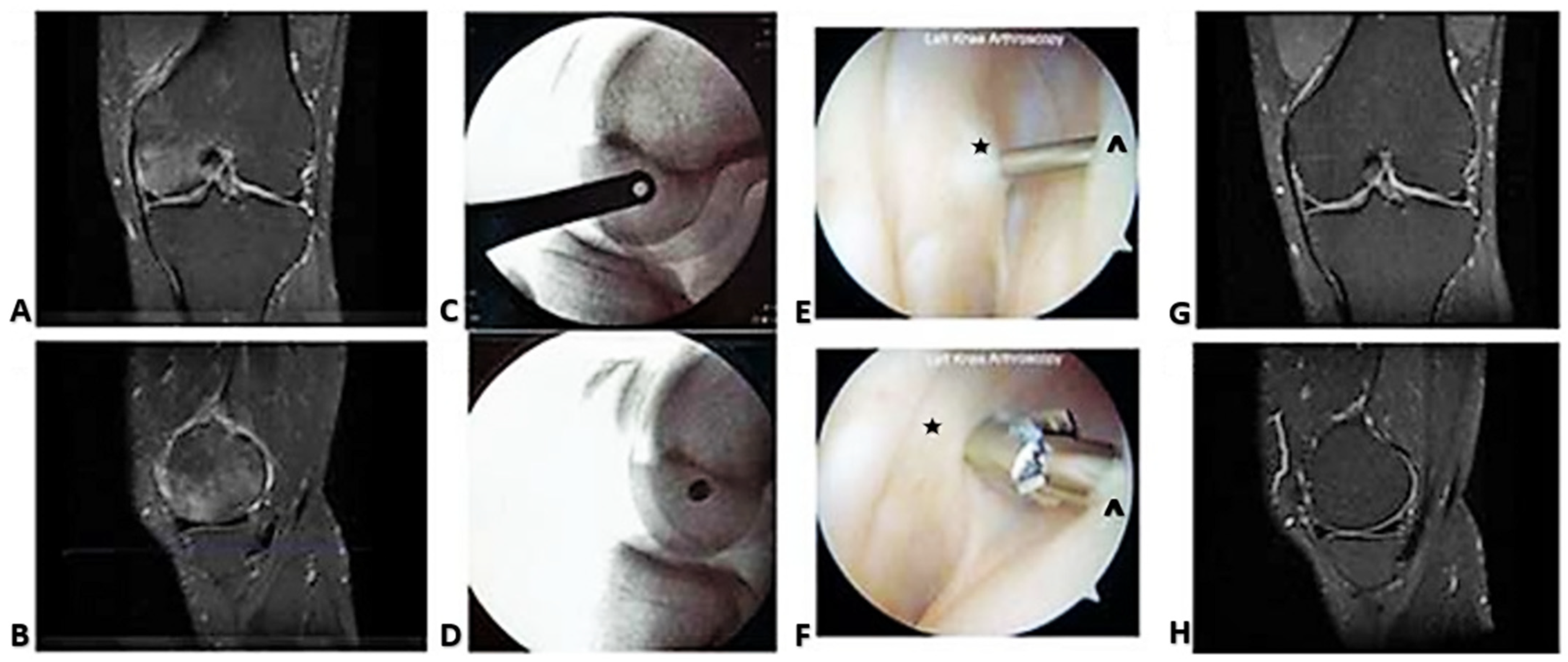
2. Case Reports
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maraghelli, D.; Brandi, M.L.; Cerinic, M.M.; Peired, A.J.; Colagrande, S. Edema-like marrow signal intensity: A narrative review with a pictorial essay. Skelet. Radiol. 2020, 50, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.F. Treatment of bone marrow lesions (bone marrow edema). Bonekey Rep. 2015, 4, 755. [Google Scholar] [CrossRef] [PubMed]
- Gil, H.C.; Levine, S.M.; Zoga, A.C. MRI Findings in the Subchondral Bone Marrow: A Discussion of Conditions Including Transient Osteoporosis, Transient Bone Marrow Edema Syndrome, SONK, and Shifting Bone Marrow Edema of the Knee. Semin. Musculoskelet. Radiol. 2006, 10, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Foti, G.; Serra, G.; Iacono, V.; Marocco, S.; Bertoli, G.; Gori, S.; Zorzi, C. Identification of Non-Traumatic Bone Marrow Oedema: The Pearls and Pitfalls of Dual-Energy CT (DECT). Tomography 2021, 7, 387–396. [Google Scholar] [CrossRef]
- Thiryayi, S.; Freemont, T. Histopathological perspective on bone marrow oedema, reactive bone change and haemorrhage. Eur. J. Radiol. 2008, 67, 62–67. [Google Scholar] [CrossRef]
- Baumbach, S.F.; Pfahler, V.; Pozza, S.B.-D.; Feist-Pagenstert, I.; Fürmetz, J.; Baur-Melnyk, A.; Stumpf, U.C.; Saller, M.M.; Straube, A.; Schmidmaier, R.; et al. How We Manage Bone Marrow Edema—An Interdisciplinary Approach. J. Clin. Med. 2020, 9, 551. [Google Scholar] [CrossRef]
- Starr, A.M.; Wessely, M.A.; Albastaki, U.; Pierre-Jerome, C.; Kettner, N.W. Bone marrow edema: Pathophysiology, differential diagnosis, and imaging. Acta Radiol. 2008, 49, 771–786. [Google Scholar] [CrossRef]
- Guerra, J.J.; Steinberg, M.E. Distinguishing transient osteoporosis from avascular necrosis of the hip. J. Bone Jt. Surg. 1995, 77, 616–624. [Google Scholar] [CrossRef]
- Moosikasuwan, J.B.; Miller, T.T.; Math, K.; Schultz, E. Shifting bone marrow edema of the knee. Skelet. Radiol. 2004, 33, 380–385. [Google Scholar] [CrossRef]
- Guardiano, S.A.; Katz, J.; Schwartz, A.M.; Brindle, K.; Curiel, R. Fracture Complicating the Bone Marrow Edema Syndrome. Am. J. Clin. Oncol. 2004, 10, 269–274. [Google Scholar] [CrossRef]
- Cahir, J.G.; Toms, A.P. Regional migratory osteoporosis. Eur. J. Radiol. 2008, 67, 2–10. [Google Scholar] [CrossRef]
- Arnoldi, C.C.; Linderholm, H.; Müssbichler, H. Venous engorgement and intraosseous hypertension in osteoarthritis of the hip. J. Bone Jt. Surg. 1972, 54, 409–421. [Google Scholar] [CrossRef]
- Arnoldi, C.C. Vascular aspects of degenerative joint disorders. A synthesis. J. Foot Ankle Res. 1994, 261, 1–82. [Google Scholar] [CrossRef]
- Simkin, P.A. Bone Pain and Pressure in Osteoarthritic Joints. In Osteoarthritic Joint Pain: Novartis Foundation Symposium; John Wiley & Sons, Ltd.: Chichester, UK, 2004; Volume 260, pp. 179–190. [Google Scholar] [CrossRef]
- Aaron, R.; Racine, J.; Voisinet, A.; Evangelista, P.; Dyke, J. Subchondral bone circulation in osteoarthritis of the human knee. Osteoarthr. Cartil. 2018, 26, 940–944. [Google Scholar] [CrossRef]
- Aaron, R.K.; Dyke, J.P.; Ciombor, D.M.; Ballon, D.; Lee, J.; Jung, E.; Tung, G.A. Perfusion Abnormalities in Subchondral Bone Associated with Marrow Edema, Osteoarthritis, and Avascular Necrosis. Ann. N. Y. Acad. Sci. 2007, 1117, 124–137. [Google Scholar] [CrossRef]
- Disch, A.C.; Matziolis, G.; Perka, C. The management of necrosis-associated and idiopathic bone-marrow oedema of the proximal femur by intravenous iloprost. J. Bone Jt. Surg. 2005, 87, 560–564. [Google Scholar] [CrossRef]
- Hofmann, S.; Engel, A.; Neuhold, A.; Leder, K.; Krämer, J.; Plenk, H. Bone-marrow oedema syndrome and transient osteoporosis of the hip. An MRI-controlled study of treatment by core decompression. J. Bone Jt. Surg. 1993, 75, 210–216. [Google Scholar] [CrossRef]
- Kiaer, T.; Pedersen, N.; Kristensen, K.; Starklint, H. Intra-osseous pressure and oxygen tension in avascular necrosis and osteoarthritis of the hip. J. Bone Jt. Surg. 1990, 72, 1023–1030. [Google Scholar] [CrossRef]
- Arnoldi, C.C.; Djurhuus, J.C.; Heerfordt, J.; Karle, A. Intraosseous Phlebography, Intraosseous Pressure Measurements and 99mTc-Polyphosphate Scintigraphy in Patients with Various Painful Conditions in the hip and Knee. Acta Orthop. 1980, 51, 19–28. [Google Scholar] [CrossRef]
- Lemperg, R.K.; Arnoldi, C.C. The significance of intraosseous pressure in normal and diseased states with special reference to the intraosseous engorgement-pain syndrome. Clin. Orthop. Relat. Res. 1978, 136, 143–156. [Google Scholar]
- Arnoldi, C.C.; Lemperg, K.; Linderholm, H. Intraosseous hypertension and pain in the knee. J. Bone Jt. Surg. 1975, 57, 360–363. [Google Scholar] [CrossRef]
- Goldie, I.F.; Wetterqvist, H. Intramedullary pressure of the patella in chondromalacia. Arch. Orthop. Trauma Surg. 1980, 97, 81–85. [Google Scholar] [CrossRef]
- Kiaer, T.; Dahl, B.; Lausten, G.S.; Kiær, T. The relationship between inert gas wash-out and radioactive tracer microspheres in measurement of bone blood flow: Effect of decreased arterial supply and venous congestion on bone blood flow in an animal model. J. Orthop. Res. 1993, 11, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Kiaer, T.; Dahl, B.; Lausten, G. Partial pressures of oxygen and carbon dioxide in bone and their correlation with bone-blood flow: Effect of decreased arterial supply and venous congestion on intraosseous oxygen and carbon dioxide in an animal model. J. Orthop. Res. 1992, 10, 807–812. [Google Scholar] [CrossRef]
- Kiaer, T.; Grønlund, J.; Sørensen, K.H. Subchondral pO2, pCO2, Pressure, pH, and Lactate in Human Osteoarthritis of the Hip. Clin. Orthop. Relat. Res. 1988, 229, 149–155. [Google Scholar] [CrossRef]
- Pedersen, N.W.; Kiér, T.; Kristensen, K.D.; Starklint, H. Intraosseous pressure, oxygenation, and histology inarthrosis and osteonecrosis of the hip. Acta Orthop. Scand. 1989, 60, 415–417. [Google Scholar] [CrossRef]
- Tosun, H.B.; Uludağ, A.; Demir, S.; Serbest, S.; Yasar, M.M.; Öznam, K. Effectiveness of Iloprost in the Treatment of Bone Marrow Edema. Cureus 2020, 12, e10547. [Google Scholar] [CrossRef]
- Jager, M.; Tillmann, F.P.; Thornhill, T.S.; Mahmoudi, M.; Blondin, D.; Hetzel, G.R.; Zilkens, C.; Krauspe, R. Rationale for prostaglandin I2 in bone marrow oedema—From theory to application. Arthritis Res. Ther. 2008, 10, R120. [Google Scholar] [CrossRef]
- Meizer, R.; Radda, C.; Stolz, G.; Kotsaris, S.; Petje, G.; Krasny, C.; Wlk, M.; Mayerhöfer, M.; Landsiedl, F.; Aigner, N. MRI-controlled analysis of 104 patients with painful bone marrow edema in different joint localizations treated with the prostacyclin analogue iloprost. Wien. Klin. Wochenschr. 2005, 117, 278–286. [Google Scholar] [CrossRef]
- Aigner, N.; Petje, G.; Schneider, W.; Meizer, R.; Wlk, M.; Kotsaris, S.; Knahr, K.; Landsiedl, F. Bone marrow edema syndrome of the femoral head: Treatment with the prostacyclin analogue iloprost vs. core decompression: An MRI-controlled study. Wien. Klin. Wochenschr. 2005, 117, 130–135. [Google Scholar] [CrossRef]
- Paraskevopoulos, K.; Keskinis, A.; Vasios, I.S.; Makiev, K.G.; Tilkeridis, K.; Drosos, G.I.; Ververidis, A.N. Comparison of various treatment modalities for the management of bone marrow edema syndrome/transient osteoporosis in men and non-pregnant women: A systematic review. Osteoporos. Int. 2022, 34, 269–290. [Google Scholar] [CrossRef]
- Radke, S.; Rader, C.; Kenn, W.; Kirschner, S.; Walther, M.; Eulert, J. Transient marrow edema syndrome of the hip: Results after core decompression. A prospective MRI-controlled study in 22 patients. Arch. Orthop. Trauma Surg. 2003, 123, 223–227. [Google Scholar] [CrossRef]
- Berger, C.E.; Kröner, A.H.; Kristen, K.-H.; Grabmeier, G.F.; Kluger, R.; Minai-Pour, M.B.; Leitha, T.; Engel, A. Transient Bone Marrow Edema Syndrome of the Knee: Clinical and Magnetic Resonance Imaging Results at 5 Years After Core Decompression. Arthrosc. J. Arthrosc. Relat. Surg. 2006, 22, 866–871. [Google Scholar] [CrossRef]
- Ververidis, A.N.; Paraskevopoulos, K.; Tilkeridis, K.; Riziotis, G.; Tottas, S.; Drosos, G.I. Surgical modalities for the management of bone marrow edema of the knee joint. J. Orthop. 2019, 17, 30–37. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Littman, J.; Gil, H.; Aaron, R. Spontaneous Bone Marrow Edema: Perfusion Abnormalities and Treatment with Surgical Decompression. Int. J. Mol. Sci. 2023, 24, 6761. https://doi.org/10.3390/ijms24076761
Littman J, Gil H, Aaron R. Spontaneous Bone Marrow Edema: Perfusion Abnormalities and Treatment with Surgical Decompression. International Journal of Molecular Sciences. 2023; 24(7):6761. https://doi.org/10.3390/ijms24076761
Chicago/Turabian StyleLittman, Jake, Holly Gil, and Roy Aaron. 2023. "Spontaneous Bone Marrow Edema: Perfusion Abnormalities and Treatment with Surgical Decompression" International Journal of Molecular Sciences 24, no. 7: 6761. https://doi.org/10.3390/ijms24076761
APA StyleLittman, J., Gil, H., & Aaron, R. (2023). Spontaneous Bone Marrow Edema: Perfusion Abnormalities and Treatment with Surgical Decompression. International Journal of Molecular Sciences, 24(7), 6761. https://doi.org/10.3390/ijms24076761





