Osteomalacia Is Not a Single Disease
Abstract
:1. Introduction
2. Physiopathology of Defective Mineralization and Proposed Pathogenetic Classification of Mineralization Disorders
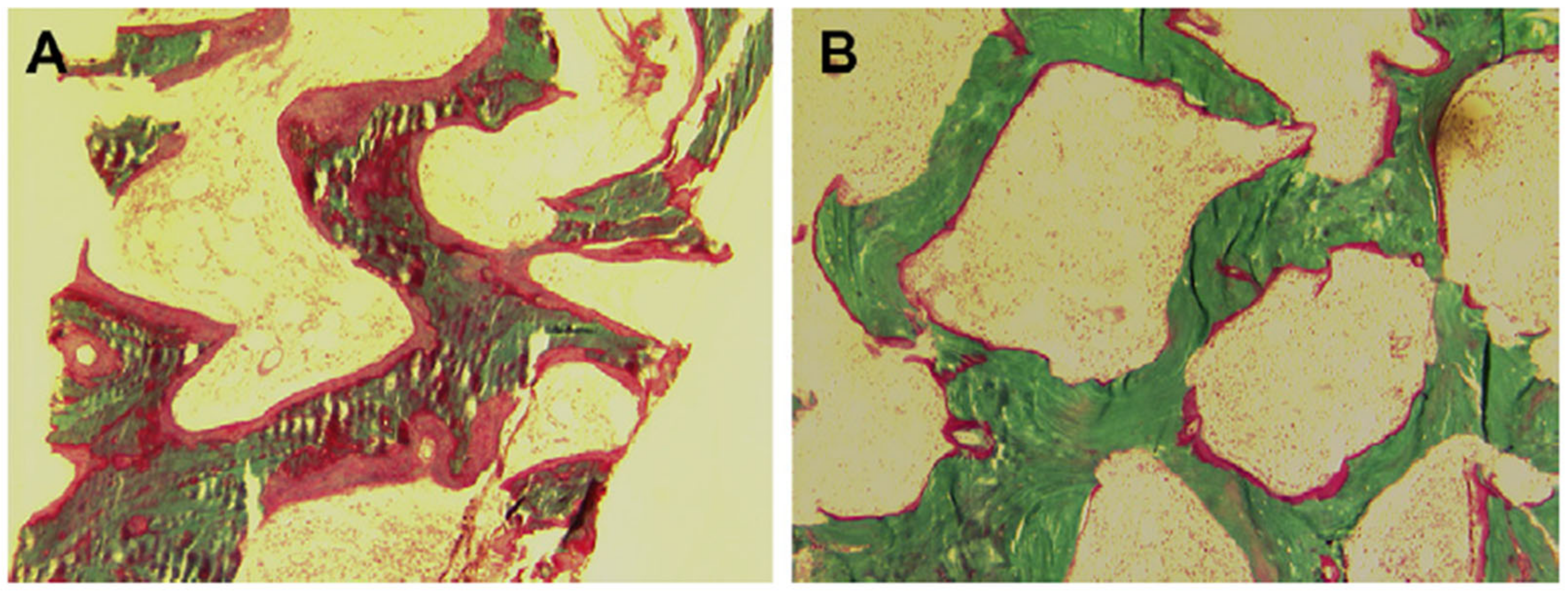
3. Histopathological Features of Osteomalacia
4. Clinical and Radiological Manifestations
5. Biochemical Features and Differential Diagnosis
6. More Frequent Forms of Osteomalacia in Clinical Practice
6.1. Acquired Osteomalacia
6.2. Inherited Osteomalacia
7. Prevention and Treatment
8. Research Gaps and Potential Development in the Field
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fukumoto, S.; Ozono, K.; Michigami, T.; Minagawa, M.; Okazaki, R.; Sugimoto, T.; Takeuchi, Y.; Matsumoto, T. Pathogenesis and Diagnostic Criteria for Rickets and Osteomalacia—Proposal by an Expert Panel Supported by the Ministry of Health, Labour and Welfare, Japan, the Japanese Society for Bone and Mineral Research, and the Japan Endocrine Society. J. Bone Miner. Metab. 2015, 33, 467–473. [Google Scholar] [CrossRef] [PubMed]
- McKee, M.D.; Buss, D.J.; Reznikov, N. Mineral Tessellation in Bone and the Stenciling Principle for Extracellular Matrix Mineralization. J. Struct. Biol. 2022, 214, 107823. [Google Scholar] [CrossRef] [PubMed]
- Jannin, A.; Kerlan, V.; Desailloud, R. Endocrinology of Bone Mineralization: An Update. Ann. Endocrinol. 2022, 83, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.S.; Chaussain, C.; Osdoby, P.; Brandi, M.L.; Clarke, B.; Thakker, R.V. The Role of Biomineralization in Disorders of Skeletal Development and Tooth Formation. Nat. Rev. Endocrinol. 2021, 17, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Bhan, A.; Rao, A.D.; Rao, D.S. Osteomalacia as a Result of Vitamin D Deficiency. Endocrinol. Metab. Clin. 2010, 39, 321–331. [Google Scholar] [CrossRef]
- Bouillon, R.; Antonio, L.; Olarte, O.R. Calcifediol (25OH Vitamin D3) Deficiency: A Risk Factor from Early to Old Age. Nutrients 2022, 14, 1168. [Google Scholar] [CrossRef]
- Vieth, R. Weaker Bones and White Skin as Adaptions to Improve Anthropological “Fitness” for Northern Environments. Osteoporos. Int. 2020, 31, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Janoušek, J.; Pilařová, V.; Macáková, K.; Nomura, A.; Veiga-Matos, J.; da Silva, D.D.; Remião, F.; Saso, L.; Malá-Ládová, K.; Malý, J.; et al. Vitamin D: Sources, Physiological Role, Biokinetics, Deficiency, Therapeutic Use, Toxicity, and Overview of Analytical Methods for Detection of Vitamin D and Its Metabolites. Crit. Rev. Clin. Lab. Sci. 2022, 2070595. [Google Scholar] [CrossRef]
- Bhadada, S.K.; Rao, S.D. Role of Phosphate in Biomineralization. Calcif. Tissue Int. 2021, 108, 32–40. [Google Scholar] [CrossRef]
- Gagnon, C.; Schafer, A.L. Bone Health After Bariatric Surgery. JBMR Plus 2018, 2, 121–133. [Google Scholar] [CrossRef]
- Allan, P.J.; Lal, S. Metabolic Bone Diseases in Intestinal Failure. J. Hum. Nutr. Diet 2020, 33, 423–430. [Google Scholar] [CrossRef]
- Dodamani, M.H.; Sehemby, M.; Memon, S.S.; Sarathi, V.; Lila, A.R.; Chapla, A.; Bhandare, V.V.; Patil, V.A.; Shah, N.S.; Thomas, N.; et al. Genotype and Phenotypic Spectrum of Vitamin D Dependent Rickets Type 1A: Our Experience and Systematic Review. J. Pediatr. Endocrinol. Metab. 2021, 34, 1505–1513. [Google Scholar] [CrossRef]
- Michigami, T. Advances in Understanding of Phosphate Homeostasis and Related Disorders. Endocr. J. 2022, 69, 881–896. [Google Scholar] [CrossRef]
- Peacock, M. Phosphate Metabolism in Health and Disease. Calcif. Tissue Int. 2021, 108, 3–15. [Google Scholar] [CrossRef]
- Trombetti, A.; Al-Daghri, N.; Brandi, M.L.; Cannata-Andía, J.B.; Cavalier, E.; Chandran, M.; Chaussain, C.; Cipullo, L.; Cooper, C.; Haffner, D.; et al. Interdisciplinary Management of FGF23-Related Phosphate Wasting Syndromes: A Consensus Statement on the Evaluation, Diagnosis and Care of Patients with X-Linked Hypophosphataemia. Nat. Rev. Endocrinol. 2022, 18, 366–384. [Google Scholar] [CrossRef]
- Millán, J.L. The Role of Phosphatases in the Initiation of Skeletal Mineralization. Calcif. Tissue Int. 2013, 93, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Borza, R.; Salgado-Polo, F.; Moolenaar, W.H.; Perrakis, A. Structure and Function of the Ecto-Nucleotide Pyrophosphatase/Phosphodiesterase (ENPP) Family: Tidying up Diversity. J. Biol. Chem. 2022, 298, 101526. [Google Scholar] [CrossRef]
- Briolay, A.; Bessueille, L.; Magne, D. TNAP: A New Multitask Enzyme in Energy Metabolism. Int. J. Mol. Sci. 2021, 22, 10470. [Google Scholar] [CrossRef]
- Villa-Suárez, J.M.; García-Fontana, C.; Andújar-Vera, F.; González-Salvatierra, S.; de Haro-Muñoz, T.; Contreras-Bolívar, V.; García-Fontana, B.; Muñoz-Torres, M. Hypophosphatasia: A Unique Disorder of Bone Mineralization. Int. J. Mol. Sci. 2021, 22, 4303. [Google Scholar] [CrossRef]
- Tiosano, D.; Abrams, S.A.; Weisman, Y. Lessons Learned from Hereditary 1,25-Dihydroxyvitamin D-Resistant Rickets Patients on Vitamin D Functions. J. Nutr. 2021, 151, 473–481. [Google Scholar] [CrossRef]
- Chavassieux, P.; Chapurlat, R. Interest of Bone Histomorphometry in Bone Pathophysiology Investigation: Foundation, Present, and Future. Front. Endocrinol. 2022, 13, 907914. [Google Scholar] [CrossRef] [PubMed]
- Bhan, A.; Qiu, S.; Rao, S.D. Bone Histomorphometry in the Evaluation of Osteomalacia. Bone Rep. 2018, 8, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, A.M.; Qiu, S.; Rao, D.S. The Mineralization Index—A New Approach to the Histomorphometric Appraisal of Osteomalacia. Bone 2004, 35, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, A.M.; Rao, D.S.; Stanciu, J.; Villanueva, A.R.; Kleerekoper, M.; Frame, B. Irreversible Bone Loss in Osteomalacia. Comparison of Radial Photon Absorptiometry with Iliac Bone Histomorphometry during Treatment. J. Clin. Investig. 1985, 76, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Lecoq, A.-L.; Brandi, M.L.; Linglart, A.; Kamenický, P. Management of X-Linked Hypophosphatemia in Adults. Metabolism 2020, 103S, 154049. [Google Scholar] [CrossRef]
- Brandi, M.L.; Clunie, G.P.R.; Houillier, P.; Jan de Beur, S.M.; Minisola, S.; Oheim, R.; Seefried, L. Challenges in the Management of Tumor-Induced Osteomalacia (TIO). Bone 2021, 152, 116064. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Gao, Y.-S. Idiopathic Hypophosphatemic Osteomalacia: Recurrent Pseudofracture of the Proximal Femur in a 65-Year-Old Man. Endocrine 2017, 55, 651–652. [Google Scholar] [CrossRef]
- Panda, A.; Das, C.J.; Baruah, U. Imaging of Vertebral Fractures. Indian J. Endocrinol. Metab. 2014, 18, 295–303. [Google Scholar] [CrossRef]
- Wáng, Y.X.J.; Santiago, F.R.; Deng, M.; Nogueira-Barbosa, M.H. Identifying Osteoporotic Vertebral Endplate and Cortex Fractures. Quant. Imaging Med. Surg. 2017, 7, 555–591. [Google Scholar] [CrossRef] [Green Version]
- Grigoryan, M.; Guermazi, A.; Roemer, F.W.; Delmas, P.D.; Genant, H.K. Recognizing and Reporting Osteoporotic Vertebral Fractures. Eur. Spine J. 2003, 12 (Suppl. 2), S104–S112. [Google Scholar] [CrossRef]
- Glerup, H.; Mikkelsen, K.; Poulsen, L.; Hass, E.; Overbeck, S.; Andersen, H.; Charles, P.; Eriksen, E.F. Hypovitaminosis D Myopathy without Biochemical Signs of Osteomalacic Bone Involvement. Calcif. Tissue Int. 2000, 66, 419–424. [Google Scholar] [CrossRef]
- Roschger, P.; Paschalis, E.P.; Fratzl, P.; Klaushofer, K. Bone Mineralization Density Distribution in Health and Disease. Bone 2008, 42, 456–466. [Google Scholar] [CrossRef]
- Abdelrazek, S.; Szumowski, P.; Rogowski, F.; Kociura-Sawicka, A.; Mojsak, M.; Szorc, M. Bone Scan in Metabolic Bone Diseases. Review. Nucl. Med. Rev. Cent. East Eur. 2012, 15, 124–131. [Google Scholar]
- Collins, M.T.; Marcucci, G.; Anders, H.-J.; Beltrami, G.; Cauley, J.A.; Ebeling, P.R.; Kumar, R.; Linglart, A.; Sangiorgi, L.; Towler, D.A.; et al. Skeletal and Extraskeletal Disorders of Biomineralization. Nat. Rev. Endocrinol. 2022, 18, 473–489. [Google Scholar] [CrossRef]
- Vasikaran, S.D.; Miura, M.; Pikner, R.; Bhattoa, H.P.; Cavalier, E. IOF-IFCC Joint Committee on Bone Metabolism (C-BM) Practical Considerations for the Clinical Application of Bone Turnover Markers in Osteoporosis. Calcif. Tissue Int. 2021, 1–10. [Google Scholar] [CrossRef]
- Shapiro, J.R.; Lewiecki, E.M. Hypophosphatasia in Adults: Clinical Assessment and Treatment Considerations. J. Bone Miner. Res. 2017, 32, 1977–1980. [Google Scholar] [CrossRef] [Green Version]
- Koumakis, E.; Cormier, C.; Roux, C.; Briot, K. The Causes of Hypo- and Hyperphosphatemia in Humans. Calcif. Tissue Int. 2021, 108, 41–73. [Google Scholar] [CrossRef]
- Chande, S.; Bergwitz, C. Role of Phosphate Sensing in Bone and Mineral Metabolism. Nat. Rev. Endocrinol. 2018, 14, 637–655. [Google Scholar] [CrossRef]
- Cupisti, A.; Gallieni, M. Urinary Phosphorus Excretion: Not What We Have Believed It to Be? Clin. J. Am. Soc. Nephrol. 2018, 13, 973–974. [Google Scholar] [CrossRef] [Green Version]
- Payne, R.B. Renal Tubular Reabsorption of Phosphate (TmP/GFR): Indications and Interpretation. Ann. Clin. Biochem. 1998, 35 Pt 2, 201–206. [Google Scholar] [CrossRef]
- Fauconnier, C.; Roy, T.; Gillerot, G.; Roy, C.; Pouleur, A.-C.; Gruson, D. FGF23: Clinical Usefulness and Analytical Evolution. Clin. Biochem. 2019, 66, 2. [Google Scholar] [CrossRef]
- Souberbielle, J.-C.; Prié, D.; Piketty, M.-L.; Rothenbuhler, A.; Delanaye, P.; Chanson, P.; Cavalier, E. Evaluation of a New Fully Automated Assay for Plasma Intact FGF23. Calcif. Tissue Int. 2017, 101, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O.; et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415. [Google Scholar] [CrossRef] [PubMed]
- Uday, S.; Högler, W. Nutritional Rickets and Osteomalacia in the Twenty-First Century: Revised Concepts, Public Health, and Prevention Strategies. Curr. Osteoporos. Rep. 2017, 15, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iguacel, I.; Miguel-Berges, M.L.; Gómez-Bruton, A.; Moreno, L.A.; Julián, C. Veganism, Vegetarianism, Bone Mineral Density, and Fracture Risk: A Systematic Review and Meta-Analysis. Nutr. Rev. 2019, 77, 45. [Google Scholar] [CrossRef]
- De Prisco, C.; Levine, S.N. Metabolic Bone Disease after Gastric Bypass Surgery for Obesity. Am. J. Med. Sci. 2005, 329, 57–61. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, L.; Zhang, J.; Liu, S.; Han, J.; Liu, Y. Adverse Impact of Heavy Metals on Bone Cells and Bone Metabolism Dependently and Independently through Anemia. Adv. Sci. 2020, 7, 2000383. [Google Scholar] [CrossRef]
- Igbokwe, I.O.; Igwenagu, E.; Igbokwe, N.A. Aluminium Toxicosis: A Review of Toxic Actions and Effects. Interdiscip. Toxicol. 2019, 12, 45–70. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Ran, D.; Shi, X.; Zhao, H.; Liu, Z. Cadmium Toxicity: A Role in Bone Cell Function and Teeth Development. Sci. Total Environ. 2021, 769, 144646. [Google Scholar] [CrossRef]
- Minisola, S.; Barlassina, A.; Vincent, S.-A.; Wood, S.; Williams, A. A Literature Review to Understand the Burden of Disease in People Living with Tumour-Induced Osteomalacia. Osteoporos. Int. 2022, 33, 1845–1857. [Google Scholar] [CrossRef]
- Minisola, S.; Peacock, M.; Fukumoto, S.; Cipriani, C.; Pepe, J.; Tella, S.H.; Collins, M.T. Tumour-Induced Osteomalacia. Nat. Rev. Dis. Primers 2017, 3, 17044. [Google Scholar] [CrossRef]
- Minisola, S.; Fukumoto, S.; Xia, W.; Corsi, A.; Colangelo, L.; Scillitani, A.; Pepe, J.; Cipriani, C.; Thakker, R.V. Tumor-Induced Osteomalacia: A Comprehensive Review. Endocr. Rev. 2022, bnac026. [Google Scholar] [CrossRef]
- Gohil, A.; Imel, E.A. FGF23 and Associated Disorders of Phosphate Wasting. Pediatr. Endocrinol. Rev. 2019, 17, 17–34. [Google Scholar] [CrossRef]
- Bitzan, M.; Goodyer, P.R. Hypophosphatemic Rickets. Pediatr. Clin. North Am. 2019, 66, 179–207. [Google Scholar] [CrossRef]
- Macica, C.M.; Luo, J.; Tommasini, S.M. The Enthesopathy of XLH Is a Mechanical Adaptation to Osteomalacia: Biomechanical Evidence from Hyp Mice. Calcif. Tissue Int. 2022, 111, 313–322. [Google Scholar] [CrossRef]
- Linglart, A.; Biosse-Duplan, M. Hypophosphatasia. Curr. Osteoporos. Rep. 2016, 14, 95–105. [Google Scholar] [CrossRef]
- Bouillon, R. Comparative Analysis of Nutritional Guidelines for Vitamin D. Nat. Rev. Endocrinol. 2017, 13, 466–479. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Collection: Reports funded by National Institutes of Health; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Sosa Henríquez, M.; Gómez de Tejada Romero, M.J. Cholecalciferol or Calcifediol in the Management of Vitamin D Deficiency. Nutrients 2020, 12, 1617. [Google Scholar] [CrossRef]
- Cianferotti, L.; Cricelli, C.; Kanis, J.A.; Nuti, R.; Reginster, J.-Y.; Ringe, J.D.; Rizzoli, R.; Brandi, M.L. The Clinical Use of Vitamin D Metabolites and Their Potential Developments: A Position Statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the International Osteoporosis Foundation (IOF). Endocrine 2015, 50, 12–26. [Google Scholar] [CrossRef]
- Quesada-Gomez, J.M.; Castillo, M.E.; Bouillon, R. Vitamin D Receptor Stimulation to Reduce Acute Respiratory Distress Syndrome (ARDS) in Patients with Coronavirus SARS-CoV-2 Infections: Revised Ms SBMB 2020_166. J. Steroid Biochem. Mol. Biol. 2020, 202, 105719. [Google Scholar] [CrossRef]
- Cesareo, R.; Falchetti, A.; Attanasio, R.; Tabacco, G.; Naciu, A.M.; Palermo, A. Hypovitaminosis D: Is It Time to Consider the Use of Calcifediol? Nutrients 2019, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Magarey, A.; Baulderstone, L.; Yaxley, A.; Markow, K.; Miller, M. Evaluation of Tools Used to Measure Calcium and/or Dairy Consumption in Adults. Public Health Nutr. 2015, 18, 1225–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palermo, A.; Naciu, A.M.; Tabacco, G.; Manfrini, S.; Trimboli, P.; Vescini, F.; Falchetti, A. Calcium Citrate: From Biochemistry and Physiology to Clinical Applications. Rev. Endocr. Metab. Disord. 2019, 20, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, G.; Brandi, M.L. Congenital Conditions of Hypophosphatemia Expressed in Adults. Calcif. Tissue Int. 2021, 108, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Athonvarangkul, D.; Insogna, K.L. New Therapies for Hypophosphatemia-Related to FGF23 Excess. Calcif. Tissue Int. 2021, 108, 143–157. [Google Scholar] [CrossRef]
- Jan de Beur, S.M.; Miller, P.D.; Weber, T.J.; Peacock, M.; Insogna, K.; Kumar, R.; Rauch, F.; Luca, D.; Cimms, T.; Roberts, M.S.; et al. Burosumab for the Treatment of Tumor-Induced Osteomalacia. J. Bone Miner. Res. 2021, 36, 627–635. [Google Scholar] [CrossRef]
- Weber, T.J.; Imel, E.A.; Carpenter, T.O.; Peacock, M.; Portale, A.A.; Hetzer, J.; Merritt, J.L.; Insogna, K. Long-Term Burosumab Administration Is Safe and Effective in Adults With X-Linked Hypophosphatemia (XLH). J. Clin. Endocrinol. Metab. 2022, 2022, dgac518. [Google Scholar] [CrossRef]
- Bowden, S.A.; Foster, B.L. Alkaline Phosphatase Replacement Therapy for Hypophosphatasia in Development and Practice. Adv. Exp. Med. Biol. 2019, 1148, 279–322. [Google Scholar] [CrossRef]
- Whyte, M.P.; Simmons, J.H.; Moseley, S.; Fujita, K.P.; Bishop, N.; Salman, N.J.; Taylor, J.; Phillips, D.; McGinn, M.; McAlister, W.H. Asfotase Alfa for Infants and Young Children with Hypophosphatasia: 7 Year Outcomes of a Single-Arm, Open-Label, Phase 2 Extension Trial. Lancet Diabetes Endocrinol. 2019, 7, 93–105. [Google Scholar] [CrossRef]
- Kishnani, P.S.; Rockman-Greenberg, C.; Rauch, F.; Bhatti, M.T.; Moseley, S.; Denker, A.E.; Watsky, E.; Whyte, M.P. Five-Year Efficacy and Safety of Asfotase Alfa Therapy for Adults and Adolescents with Hypophosphatasia. Bone 2019, 121, 149–162. [Google Scholar] [CrossRef]
- Krohn, K.; Schwartz, E.N.; Chung, Y.-S.; Lewiecki, E.M. Dual-Energy X-ray Absorptiometry Monitoring with Trabecular Bone Score: 2019 ISCD Official Position. J. Clin. Densitom. 2019, 22, 501–505. [Google Scholar] [CrossRef]
- Ni, X.; Feng, Y.; Guan, W.; Chi, Y.; Li, X.; Gong, Y.; Zhao, N.; Pang, Q.; Yu, W.; Wu, H.; et al. Bone Impairment in a Large Cohort of Chinese Patients with Tumor-Induced Osteomalacia Assessed by HR-PQCT and TBS. J. Bone Miner. Res. 2022, 37, 454–464. [Google Scholar] [CrossRef]
- Bravo Vázquez, L.A.; Moreno Becerril, M.Y.; Mora Hernández, E.O.; de León Carmona, G.G.; Aguirre Padilla, M.E.; Chakraborty, S.; Bandyopadhyay, A.; Paul, S. The Emerging Role of MicroRNAs in Bone Diseases and Their Therapeutic Potential. Molecules 2021, 27, 211. [Google Scholar] [CrossRef]
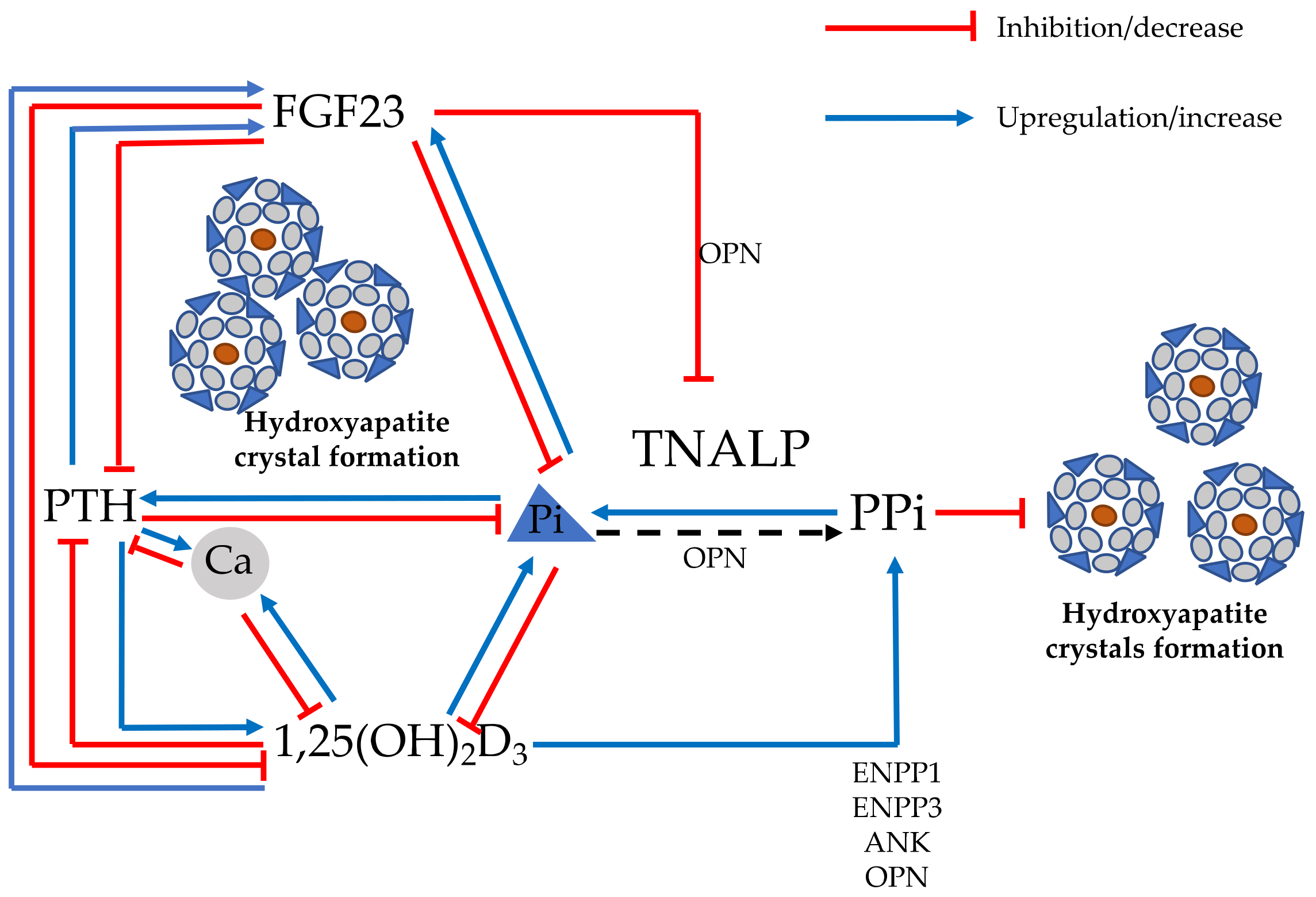


| 1A—Classification of osteomalacia based on response to treatment with vitamin D metabolites. |
| Vitamin D-Sensitive Osteomalacia |
| Vitamin D deficiency Malabsorption syndromes (e.g., celiac disease, bariatric surgery) Malnutrition (e.g., unbalanced vegan diet) Chronic liver diseases Chronic kidney diseases Drugs interfering with vitamin D metabolism Vitamin D-dependent rickets
|
| Vitamin D-Resistant Osteomalacia |
Vitamin D-dependent rickets
|
| 1B—Classification of osteomalacia based on the time-of-onset. |
| Early-onset Rickets/Osteomalacia |
Genetically determined hypophosphatemia
|
| Early- or Late-onset Rickets/osteomalacia |
FGF23-dependent
|
| 1C—Classification of osteomalacia based on the pathogenesis. |
| Congenital Osteomalacia |
Genetically determined hypophosphatemia
|
| Acquired Osteomalacia |
FGF23-independent
|
| ALP | Pi | TmP/GFR | Ca | PTH | 25(OH)D | 1,25(OH)2D | FGF23 | |
|---|---|---|---|---|---|---|---|---|
| Vitamin D deficiency | =↑ | =↓ | =↑ | =↓ | =↑ | ↓↓ | =↑↓ | =↓ |
| Malabsorption syndromes, other nutritional deficiencies | =↑ | =↓ | =↑ | =↓ | ↑/↑↑ | ↓ | =↑↓ | =↓ |
| Vitamin D-dependent rickets type 1 | ↑ | ↓↓ | ↑ | ↓↓ | ↑ | ↑↓ | ↓↓ | =↓ |
| Vitamin D-dependent rickets type 2 | ↑ | ↓↓ | ↑ | ↓↓ | ↑ | =↑↓ | ↑↑ | =↓ |
| Drugs inhibiting mineralization | =↑ | = | = | = | = | = | = | = |
| Hypophosphatasia | ↓↓ | = | = | = | = | = | = | = |
| FGF23-unrelated hypophosphatemic disorders | ↑ | ↓↓ | =↑ | = | =↑ | = | =↑ | =↓ |
| FGF23-related hypophosphatemic disorders | ↑ | ↓↓ | ↓↓ | = | =↑ | = | ↓ | ↑↑ |
| Disease | Gene | OMIM Phenotype Number | OMIM Gene Number | Inheritance |
|---|---|---|---|---|
| ||||
| X-linked dominant hypophosphatemic rickets/osteomalacia (XLH) | PHEX | # 307800 | 300550 | XLD |
| Autosomal dominant hypophosphatemic rickets/osteomalacia (ADRH) | FGF23 | # 193100 | 605380 | AD |
| Autosomal recessive hypophosphatemic rickets/osteomalacia 1 (ARHR1) | DMP1 | # 241520 | 600980 | AR |
| Autosomal recessive hypophosphatemic rickets/osteomalacia 2 (ARHR2) | ENPP1 | # 613312 | 173335 | AR |
| Hypophosphatemic disease with dental anomalies and ectopic calcification | FAM20C | # 259775 | 611061 | AR |
| Hereditary hypophosphatemic rickets with hypercalciuria (HHRH) | SLC34A3 | # 241530 | 609826 | AR |
| McCune–Albright syndrome/fibrous dysplasia (MAS) | GNAS | # 174800 | 139320 | (postzygotic somatic mutations) |
| Cutaneous skeletal hypophosphatemia syndrome | NRAS HRAS | # 162900 | 164790 190020 | (somatic mutations) |
| ||||
| Fanconi renotubular syndrome 1 (FRTS1) | - | # 134600 | - | AD |
| Fanconi renotubular syndrome 2 (FRTS2) | SLC34A1 | # 613388 | 182309 | AR |
| Fanconi renotubular syndrome 3 (FRTS3) | EHHADH | # 615605 | 607037 | AD |
| Fanconi renotubular syndrome 4 (FRTS4) | HNF4A | # 616026 | 600281 | AD |
| Dent’s disease 1 | CLCN5 | # 300009 | 300008 | XLR |
| Dent’s disease 2 | OCRL | # 300555 | 300535 | XLR |
| Lowe syndrome | OCRL | # 309000 | 300535 | XLR |
| ||||
| Vitamin D-dependent rickets/osteomalacia type 1A (VDDR1A) | CYP27B1 | # 264700 | 609506 | AR |
| Vitamin D-dependent rickets/osteomalacia type 1B (VDDR1B) | CYP2R1 | # 600081 | 608713 | AR |
| Vitamin D-dependent rickets/osteomalacia type 2A (VDDR2A) | VDR | # 277440 | 601769 | AR |
| Vitamin D-dependent rickets/osteomalacia type 2B (VDDR2B) | - | # 600785 | - | AD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cianferotti, L. Osteomalacia Is Not a Single Disease. Int. J. Mol. Sci. 2022, 23, 14896. https://doi.org/10.3390/ijms232314896
Cianferotti L. Osteomalacia Is Not a Single Disease. International Journal of Molecular Sciences. 2022; 23(23):14896. https://doi.org/10.3390/ijms232314896
Chicago/Turabian StyleCianferotti, Luisella. 2022. "Osteomalacia Is Not a Single Disease" International Journal of Molecular Sciences 23, no. 23: 14896. https://doi.org/10.3390/ijms232314896





