Integrated QTL Mapping, Meta-Analysis, and RNA-Sequencing Reveal Candidate Genes for Maize Deep-Sowing Tolerance
Abstract
1. Introduction
2. Results
2.1. Phenotypic Variations of Six Deep-Sowing Tolerant Traits
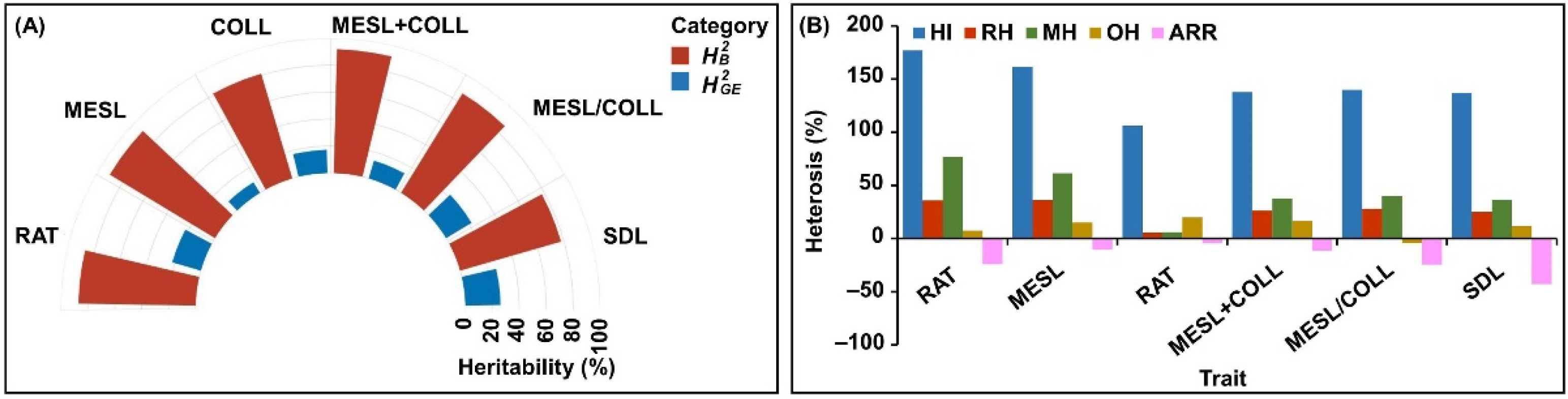
2.2. Heterosis Analysis of Six Deep-Sowing Toletant Traits
2.3. Framework for Relationships among Six Deep-Sowing Toletant Traits
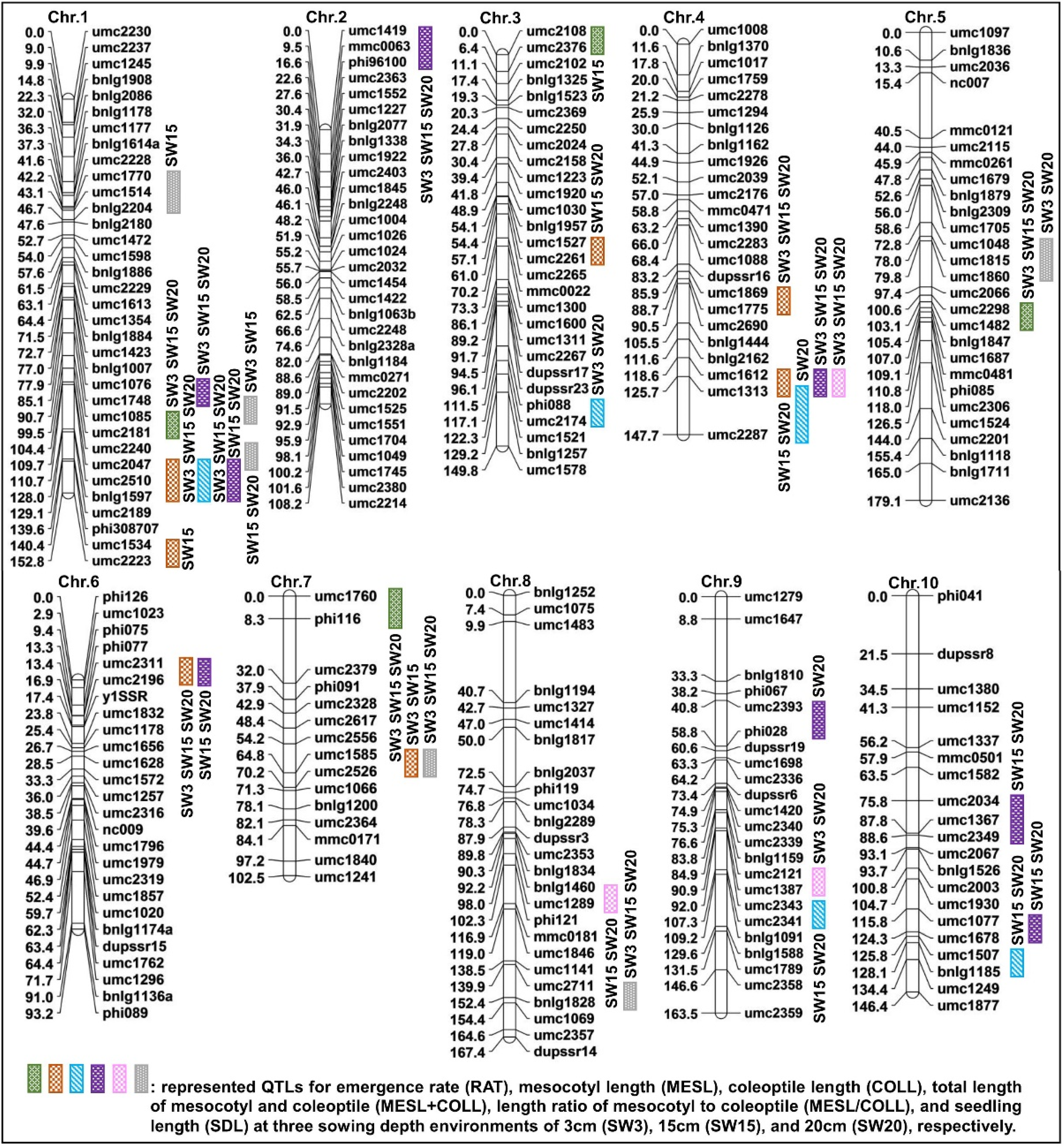
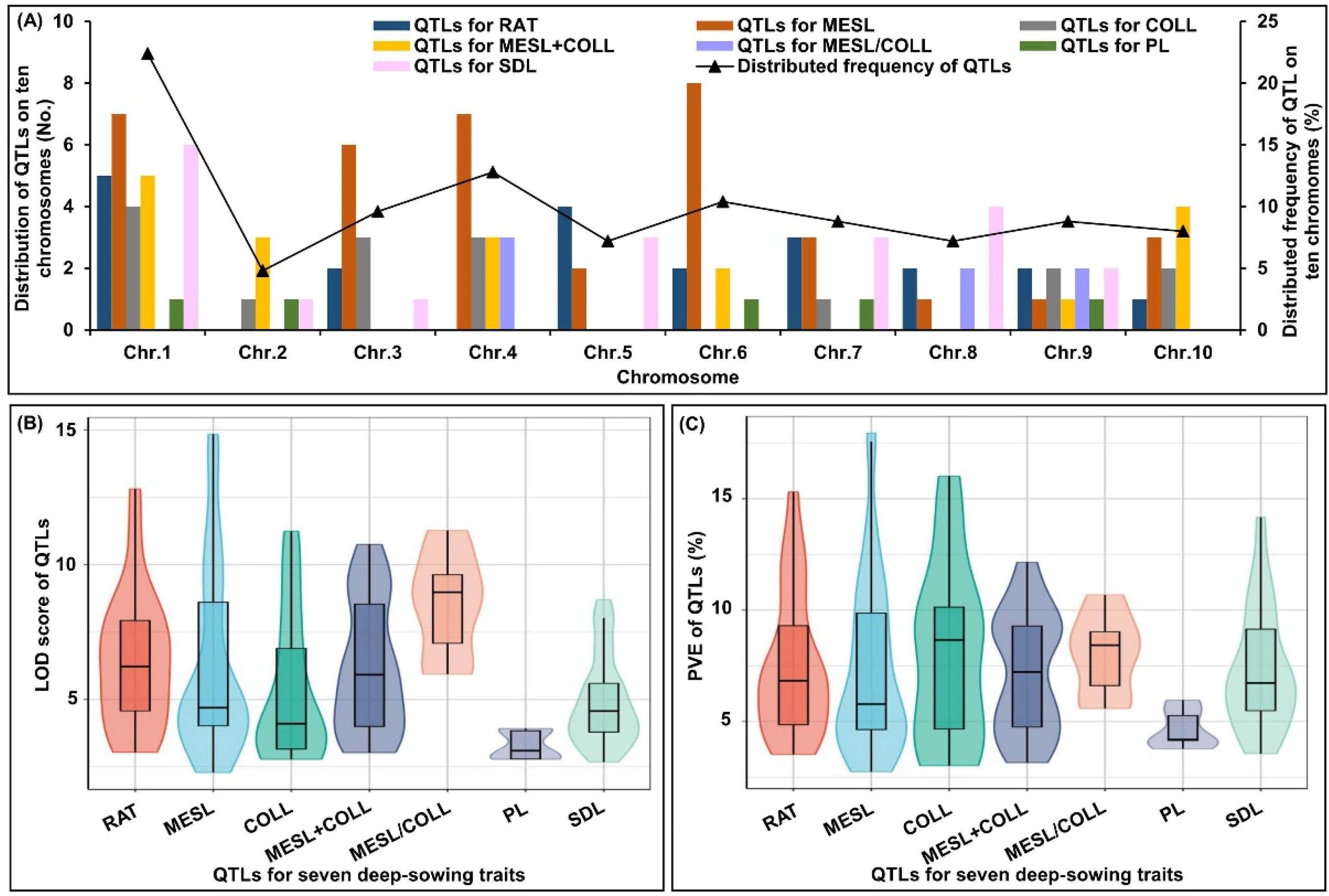
2.4. QTL Analysis of Six Deep-Sowing Toletant Traits
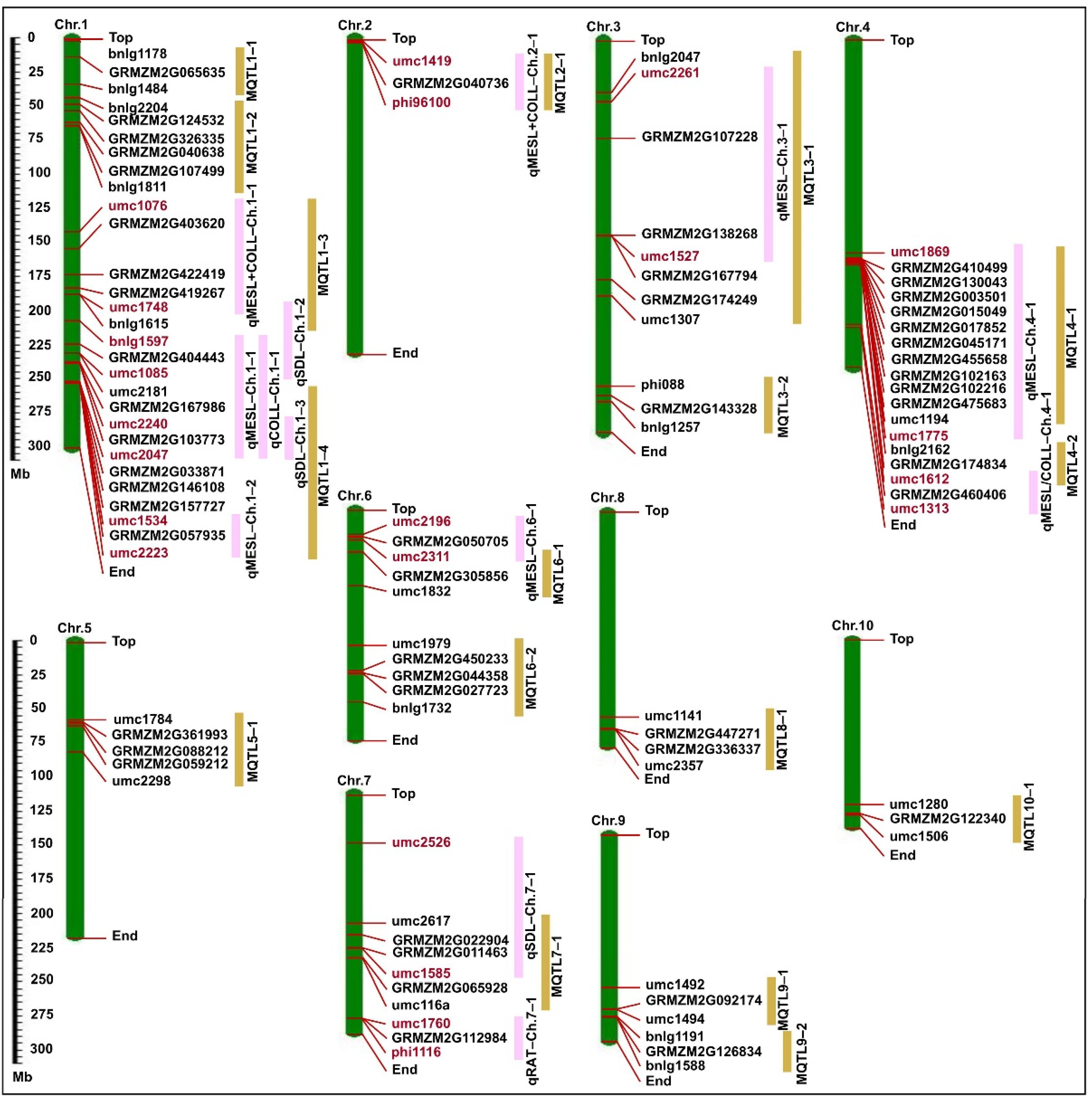
2.5. Consensus Map Development and Meta-QTL Analysis
2.6. Identification of Candidate Genes via RNA-Seq
2.7. Gene Expression Validation by qRT-PCR
3. Discussion
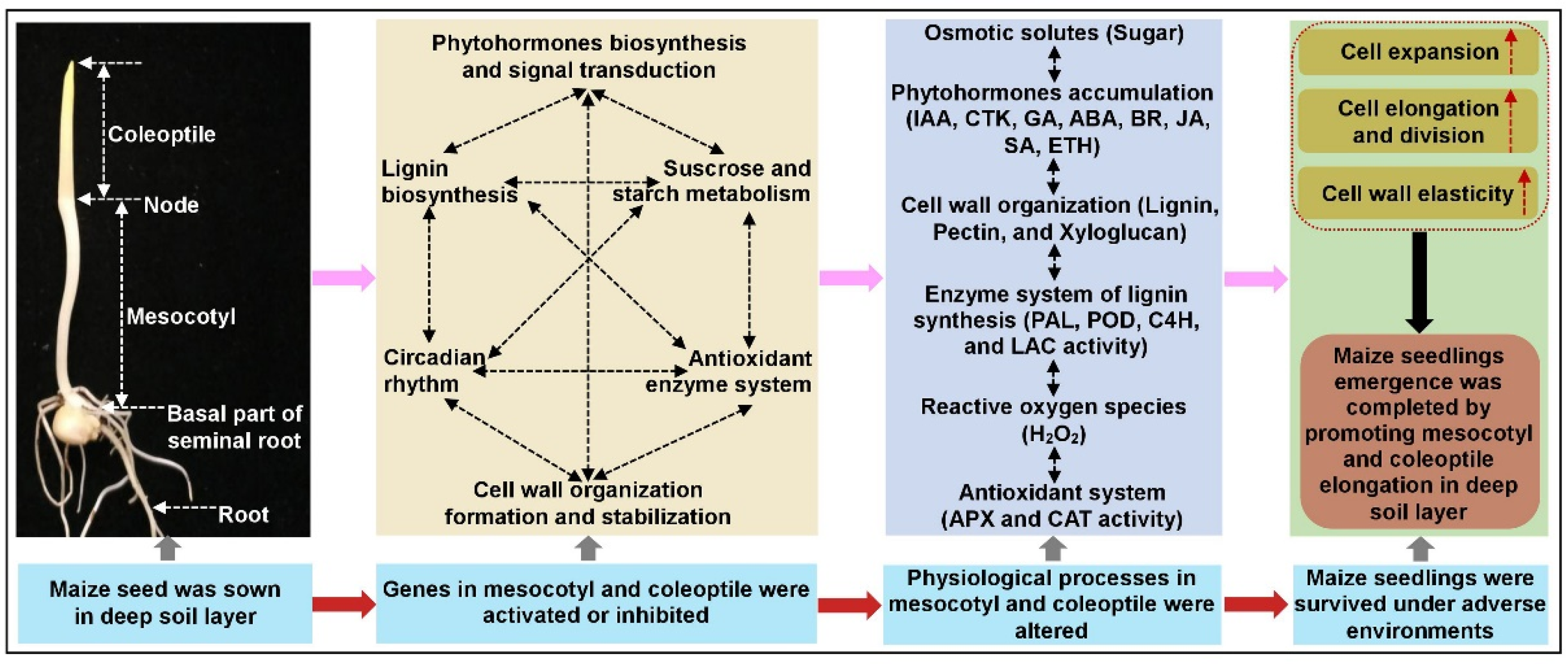
3.1. Adaptive Changes of Maize in Response to Deep-Sowing Stress
3.2. Pleiotropic QTLs Related to Multiple Deep-Sowing Tolerant Traits in Maize
3.3. Candidate Genes in Major QTLs and MQTLs Regions
4. Materials and Methods
4.1. Plant Materials
4.2. Measurement of Six Deep-Sowing Tolerant Traits
4.3. Genetic Linkage Map Construction and QTL Analysis
4.4. Consensus Linkage Map Construction and Meta-Analysis
4.5. Candidate Genes Identification via RNA-Seq
4.6. qRT-PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| APX | Ascorbate peroxidase |
| ARR | F2:3 advantage reduction rate |
| BR | Brassinosteroid |
| BSA | Bulked-segregant analysis |
| CAT | Catalase |
| CI | Confidence interval |
| CIM | Composite interval mapping |
| C4H | Trans-cinnamate 4-monooxygenase |
| COLL | Coleoptile length |
| CTK | Cytokinin |
| CV | Coefficient of variation |
| EBR | 24-Epibrassinolide |
| ETH | Ethylene |
| SA | Salicylic acid |
| FPKM | Fragments per kilobase of transcript per million mapped read |
| GA3 | Gibberellin 3 |
| GO | Gene ontology |
| GWAS | Genome-wide association studies |
| Broad-sense heritability | |
| Genotype × environment interaction heritability | |
| HI | F1 heterosis index |
| IAA | Indole-3-acetic acid |
| JA | Jasmonic acid |
| LAC | Laccase |
| LOD | Threshold logarithm of odds |
| MAS | Marker-assisted selection |
| MESL | Mesocotyl length |
| MESL + COLL | Total length of mesocotyl and coleoptile |
| MESL/COLL | length ratio of mesocotyl to coleoptile |
| MH | Mid-parent heterosis |
| MQTLs | Meta-QTLs |
| OH | Over-parent heterosis |
| PAL | Phenylalanine ammonia-lyase |
| PL | Plumule length |
| POD | Peroxidase |
| PVE | Phenotypic variation explained |
| qRT-PCR | Quantitative real-time PCR |
| QTLs | Quantitative trait loci |
| RAT | Emergence rate |
| RH | Relative heterosis |
| RILs | Recombinant inbred lines |
| RNA-Seq | RNA-sequencing |
| SA | Salicylic acid |
| SDL | Seedling length |
| SSRs | Simple sequence repeats |
| UPLC-MS/MS | Ultra-high-performance liquid chromatography-tandem mass spectrometry |
| ZT | Zeatin |
References
- Greveniotis, V.; Sioki, E.; Ipsilandis, C.G. Frequency distribution analysis of a maize population as a tool in maize breeding. Cereal Res. Commun. 2017, 45, 687–698. [Google Scholar] [CrossRef]
- Feng, P.Y.; Wang, B.; Harrison, M.T.; Wang, J.; Liu, K.; Huang, M.X.; Liu, D.L.; Yu, Q.; Hu, K.L. Soil properties resulting in superior maize yields upon climate warming. Agron. Sustain. Dev. 2022, 42, 85. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Zhong, Y. 24-Epibrassinolide mediated interaction among antioxidant defense, lignin metabolism, and phytohormones signaling promoted better cooperative elongation of maize mesocotyl and coleoptile under deep-seeding stress. Russ. J. Plant Physiol. 2021, 68, 1194–1207. [Google Scholar] [CrossRef]
- Troyer, A.F. The location of genes governing long first internode of corn. Genetics 1997, 145, 1149–1154. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, Y.T.; Liu, Y.Y.; Demar, L.; Li, D.D.; Wang, P.X.; Xu, J.L.; Zhen, S.H.; Lu, J.W.; Peng, Y.L.; et al. Dissecting the genetic basis of maize deep-sowing tolerance by combining association mapping and gene expression analysis. J. Integr. Agr. 2022, 21, 1266–1277. [Google Scholar] [CrossRef]
- Grzybowski, M.; Adamczyk, J.; Jończyk, M.; Sobkowiak, A.; Szczepanik, J.; Frankiewicz, K.; Fronk, J.; Sowiński, P. Increased photosensitivity at early growth as a possible mechanism of maize adaptation to cold springs. J. Exp. Bot. 2019, 70, 2887–2904. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, X.Q.; Niu, Y.N.; Chen, X.J.; Ren, X.W. Brassinosteroid affects the elongation of mesocotyl and coleoptile in Zea mays L. by regulating the network of circadian rhythm under severe stress. Russ. J. Plant Physiol. 2022, 69, 90–99. [Google Scholar] [CrossRef]
- Leng, B.Y.; Li, M.; Mu, C.H.; Yan, Z.W.; Yao, G.Q.; Kong, X.P.; Ma, C.L.; Zhang, F.J.; Liu, X. Molecular mechanism of gibberellins in mesocotyl elongation response to deep-sowing stress in sweet maize. Curr. Issues Mol. Biol. 2023, 45, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Zhong, T.; Zhao, G. Promoting deep-sowing germinability of corn (Zea mays) by seed soaking with gibberellic acid. Arch. Agron. Soil Sci. 2016, 63, 1314–1323. [Google Scholar] [CrossRef]
- Wang, Y.M.; Liu, J.D.; Meng, Y.; Liu, H.Y.; Liu, C.; Ye, G.Y. Rapid identification of QTL for mesocotyl length in rice through combining QTL-seq and genome-wide association analysis. Front. Genet. 2021, 12, 713446. [Google Scholar]
- Blackburn, A.; Sidhu, G.; Schillinger, W.F.; Skinner, D.; Gill, K. QTL mapping using GBS and SSR genotyping reveals genomic regions controlling wheat coleoptile length and seedling emergence. Euphytica 2021, 217, 45. [Google Scholar] [CrossRef]
- Paynter, B.H.; Clarke, G.P.Y. Coleoptile length of barely (Hordeum vulgare L.) cultivars. Genet. Resour. Crop Evol. 2010, 57, 395–403. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Zhong, Y.; Zhou, W.Q. Molecular mechanisms of mesocotyl elongation induced by brassinosteroid observation, and physiological metabolism. Genomics 2021, 113, 3565–3581. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q.; Zhong, Y.; Shi, J.; Zhou, W.Q. 24-Epibrassinolide confers tolerance against deep-seeding stress in Zea mays L. coleoptile development by phytohormones signaling transduction and their interaction network. Plant Signal. Behav. 2021, 16, 1963583. [Google Scholar] [CrossRef]
- Rodríguez, M.N.S.; Cassab, G.I. Primary root and mesocotyl elongation in maize seedlings: Two organs with antagonistic growth below the soil surface. Plants 2021, 10, 1274. [Google Scholar] [CrossRef]
- Markelz, N.H.; Costich, D.E.; Brutnell, T.P. Photomorphogenic responses in maize seedling development. Plant Physiol. 2003, 133, 1578–1591. [Google Scholar] [CrossRef]
- Huang, Q.; Ju, C.; Cheng, Y.; Cui, D.; Han, B.; Zhao, Z.; Ma, X.; Han, L. Mapping of mesocotyl elongation and confirmation of a QTL in Dongxiang common wild rice in China. Agronomy 2022, 12, 1800. [Google Scholar] [CrossRef]
- Zhang, H.W.; Ma, P.; Zhao, Z.N.; Zhao, G.W.; Tian, B.H.; Wang, J.H.; Wang, G.Y. Mapping QTL controlling maize deep-seeding tolerance-related traits and confirmation of major QTL for mesocotyl length. Theor. Appl. Genet. 2012, 124, 223–232. [Google Scholar] [CrossRef]
- Han, Q.H.; Shen, Y.; Lv, L.; Lee, M.; Lübberstedt, T.; Zhao, G.W. QTL analysis of deep-sowing tolerance during seed germination in the maize IBM Syn4 RIL population. Plant Breed. 2020, 139, 1125–1134. [Google Scholar] [CrossRef]
- Liu, H.J.; Zhang, L.; Wang, J.C.; Li, C.S.; Zeng, X.; Xie, S.P.; Zhang, Y.Z.; Liu, S.S. Quantitative trait locus analysis for deep-sowing germination ability in the maize IBM Syn10 DH population. Front. Plant Sci. 2017, 8, 813. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Niu, Y.N. The combination of Conventional QTL analysis, bulked-segregant analysis, and RNA-sequencing provide new genetic insights into maize mesocotyl elongation under multiple deep-seeding environments. Int. J. Mol. Sci. 2022, 23, 4223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q.; Fang, P.; Zhang, J.W.; Peng, Y.L. QTL mapping for six ear leaf architecture traits under water-stressed and well-watered conditions in maize (Zea mays L.). Plant Breed. 2018, 137, 60–72. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Peng, Y.L.; Zhang, J.W.; Fang, P.; Wu, B.Y. Identification of QTLs and meta-QTLs for seven agronomic traits in multiple maize populations under well-watered and water-stressed conditions. Crop Sci. 2018, 58, 507–520. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Peng, Y.L.; Zhang, J.W.; Fang, P.; Wu, B.Y. Mapping QTLs and meta-QTLs for two inflorescence architecture traits in multiple maize populations under different watering environments. Mol. Breed. 2017, 37, 91. [Google Scholar] [CrossRef]
- Wang, D.P.; Mu, Y.Y.; Hu, X.J.; Ma, B.; Wang, Z.B.; Zhu, L.; Xu, J.; Huang, C.L.; Pan, Y.H. Comparative proteomic analysis reveals that the heterosis of two maize hybrids is related to enhancement of stress response and photosynthesis respectively. BMC Plant Biol. 2021, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q.; Fang, P.; Peng, Y.L.; Zhang, J.W.; Zeng, W.J.; Ren, B.; Gao, Q.H. Genetic analysis and QTL mapping for seven agronomic traits in maize (Zea mays L.) using two connected populations. Acta Prataculturae Sin. 2018, 27, 152–165. [Google Scholar]
- Cetinel, A.H.S.; Yalcinkaya, T.; Akyol, T.Y.; Gokce, A.; Turkan, I. Pretreatment of seeds with hydrogen peroxide improves deep-sowing tolerance of wheat seedlings. Plant Physiol. Bioch. 2021, 167, 321–336. [Google Scholar] [CrossRef]
- Borges, B.C.; Ramirez, M.D.; Venske, E.; Basu, S.; Paulo, Z.D. Application of stress indices for low temperature and deep sowing stress screening of rice genotypes. Pak. J. Biol. Sci. 2013, 16, 1618–1622. [Google Scholar]
- Sáenz-Rodríguez, M.N.; López, G.I.C. Assay system for mesocotyl elongation and hydrotropism of maize primary root in response to low moisture gradient. BioTechniques 2021, 71, 516–527. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, W.P.; Jiang, C.H.; Wang, X.N.; Xiong, H.Y.; Todorovska, E.G.; Yin, Z.G.; Chen, Y.F.; Wang, X.; Xie, J.Y.; et al. Genetic architecture and candidate genes for deep-sowing tolerance in rice revealed by non-syn GWAS. Front. Plant Sci. 2018, 9, 332. [Google Scholar] [CrossRef]
- Simon, A.; Yuri, S.; Hironobu, S.; Kenji, I. Genotypic variation in coleoptile or mesocotyl lengths of upland rice (Oryza sativa L.) and seedling emergence in deep sowing. Afr. J. Agric. Res. 2012, 7, 6239–6248. [Google Scholar] [CrossRef]
- Zhao, G.W.; Wang, J.H. Effect of auxin on mesocotyl elongation of dark-grown maize under different seeding depths. Russ. J. Plant Physiol. 2010, 57, 79–86. [Google Scholar] [CrossRef]
- Lorrai, R.; Boccaccini, A.; Ruta, V.; Possenti, M.; Costantino, P.; Vittorioso, P. Abscisic acid inhibits hypocotyl elongation acting on gibberellins, DELLA proteins and auxin. AoB Plants 2018, 10, ply061. [Google Scholar]
- Zhan, J.H.; Lu, X.; Liu, H.Y.; Zhao, Q.Z.; Ye, G.Y. Mesocotyl elongation, an essential trait for dry-seeded rice (Oryza sativa L.): A review of physiological and genetic basis. Planta 2020, 251, 27. [Google Scholar] [CrossRef]
- Feng, F.J.; Mei, H.W.; Fan, P.Q.; Li, Y.N.; Xu, X.Y.; Wei, H.B.; Yan, M.; Luo, L.J. Dynamic transcriptome and phytohormone profiling along the time of light exposure in the mesocotyl of rice seedlings. Sci. Rep. 2017, 7, 11961. [Google Scholar] [CrossRef]
- Wager, A.; Browse, J. Social network: JAZ protein interactions expand our knowledge of jasmonate signaling. Front. Plant Sci. 2012, 2, 41. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Expansive growth of plant cell walls. Plant Physiol. Bioch. 2000, 38, 108–124. [Google Scholar] [CrossRef]
- Hussain, S.; Iqbal, N.; Pang, T.; Khan, M.N.; Liu, W.G.; Yang, W.Y. Weak stem under shade reveals the lignin reduction behavior. J. Integr. Agr. 2019, 18, 496–505. [Google Scholar] [CrossRef]
- Gho, Y.S.; Kim, S.J.; Jung, K.H. Phenylalanine ammonia-lyase family is closely associated with response to phosphate deficiency in rice. Genes Genom. 2020, 42, 67–76. [Google Scholar] [CrossRef]
- Karpinska, B.; Karlsson, M.; Schinkel, H.; Streller, S.; Süss, K.H.; Melzer, M.; Wingsle, G. A novel superoxide dismutase with a high isoelectric point in higher plants. Expression, regulation, and protein localization. Plant Physiol. 2001, 126, 1668–1677. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Niu, Y.N.; Bai, X.D.; Mao, T.T. Transcriptomic and metabolic profiling reveals a lignin metabolism network involved in mesocotyl elongation during maize seed germination. Plants 2022, 11, 1034. [Google Scholar] [CrossRef] [PubMed]
- Ku, L.X.; Cui, X.J.; Cheng, F.F.; Guo, S.L.; Qi, J.S.; Tian, Z.Q.; Han, T.; Ren, Z.Z.; Zhang, L.K.; Su, H.H. Genetic dissection of seed vigor under artificial ageing conditions using two joined maize recombinant inbred line populations. Plant Breed. 2014, 133, 728–737. [Google Scholar] [CrossRef]
- Tian, R.M.; Zhang, X.H.; Tang, J.H.; Bai, G.H.; Fu, Z.Y. Genome-wide association studies of seed germination related traits in maize. Acta Agron. Sin. 2018, 44, 672–685. [Google Scholar] [CrossRef]
- Yang, G.H.; Dong, Y.B.; Li, Y.L.; Wang, Q.L.; Shi, Q.L.; Zhou, Q. Integrative detection and verification of QTL for plant traits in two connected RIL population of high-oil maize. Euphytica 2015, 206, 203–223. [Google Scholar] [CrossRef]
- Wang, C.L.; Sun, Z.H.; Ku, L.X.; Wang, T.G.; Chen, Y.H. Detection of quantitative trait loci for plant height in different photoperiod environments using an immortalized F2 population in maize. Acta Agron. Sin. 2011, 37, 271–279. [Google Scholar] [CrossRef]
- Guo, H.P.; Sun, G.Y.; Zhang, X.X.; Yan, P.S.; Liu, K.; Xie, H.L.; Tang, J.H.; Dong, D.; Li, W.H. QTL analysis of under-ear internode length based on SSSL population. Acta Agron. Sin. 2018, 44, 522–532. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Li, Z.; Fang, H.; Zhang, M.C.; Duan, L.S. Analysis of the genetic basis of plant height-related traits in response to ethylene by QTL mapping in maize (Zea mays L.). PLoS ONE 2018, 13, e0193072. [Google Scholar] [CrossRef]
- Dilday, R.H.; Mgonja, M.A.; Amonsilpa, S.A.; Collins, F.C.; Wells, B.R. Plant height vs. mesocotyl and coleoptile elongation in rice: Linakge or pleiotropism? Crop Sci. 1990, 30, 815–818. [Google Scholar] [CrossRef]
- Huo, D.G.; Ning, Q.; Shen, X.M.; Liu, L.; Zhang, Z.X. QTL mapping of kernel number-related traits and validation of one major QTL for ear length in maize. PLoS ONE 2016, 11, e0155506. [Google Scholar] [CrossRef]
- Zhou, G.F.; Zhu, Q.L.; Yang, G.L.; Huang, J.; Cheng, S.Y.; Yue, B.; Zhang, Z.X. qEL7.2 is a pleiotropic QTL for kernel number per row, ear length and ear weight in maize (Zea mays L.). Euphytica 2015, 203, 429–436. [Google Scholar] [CrossRef]
- Chen, H.; Pan, X.W.; Wang, F.F.; Liu, C.K.; Wang, X.; Li, Y.S.; Zhang, Q.Y. Novel QTL and meta-QTL mapping for major quality traits in soybean. Front. Plant Sci. 2021, 12, 774270. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Wang, Y.; Yu, Y.J.; He, Y.Q.; Wang, L. Coordinative regulation of plants growth and development by light and circadian clock. aBIOTECH 2021, 2, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Dubois, P.G.; Olsefski, G.T.; Flint-Garcia, S. Physiological and genetic characterization of end-of-day far-red light response in maize seedlings. Plant Physiol. 2010, 154, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.S.; Zhang, J.P.; Wang, M.Y.; Wang, Z.Y.; Li, G.F.; Qu, L.; Wang, G.Y. Expression and functional analysis of ZmDWF4, an ortholog of Arabidopsis DWF4 from maize (Zea mays L.). Plant Cell Rep. 2007, 26, 2091–2099. [Google Scholar] [CrossRef]
- Spartz, A.; Lee, S.H.; Wenger, J.P.; Gonzalez, N.; Itoh, H.; Inz, D.; Wendy, P.; Amgus, M.; Paul, O.; William, G. The SAUR19 subfamily of small auxin up RNA genes promote cell expansion. Plant J. 2012, 70, 978–990. [Google Scholar] [CrossRef]
- Du, L.G.; Jiang, H.Y.; Zhao, G.W.; Ren, J.Y. Gene cloning of ZmMYB59 transcription factor in maize and its expression during seed germination in response to deep-sowing and exogenous hormones. Plant Breed. 2017, 136, 834–844. [Google Scholar] [CrossRef]
- Phelps-Durr, T.L.; Thomas, J.; Vahab, P.; Timmermans, M.C.P. Maize rough sheath2 and its Arabidopsis orthologue ASYMMETRIC LEAVES1 interact with HIRA, a predicted histone chaperone, to maintain knox gene silencing and determinacy during organogenesis. Plant Cell 2005, 17, 2886–2898. [Google Scholar] [CrossRef]
- Oh, S.; Park, S.; Han, K.H. Transcriptional regulation of secondary growth in Arabidopsis thaliana. J. Expt. Bot. 2003, 54, 2709–2722. [Google Scholar] [CrossRef]
- Cass, C.L.; Peraldi, A.; Dowd, P.F.; Mottiar, Y.; Santoro, N.; Karlen, S.D.; Bukhman, Y.V.; Foster, C.E.; Thrower, N.; Bruno, L.C.; et al. Effects of phenylalanine ammonialyase (PAL) knockdown on well wall composition, biomass digestibility, and biotic and abiotic stress response in Brachypodium. J. Exp. Bot. 2015, 66, 4317–4335. [Google Scholar] [CrossRef]
- Monireh, C.; Ali, G. Developmental role of phenylalanineammonia-lyase (Pal) and cinnamate 4-hydroxylase (C4H) genes during adventitious rooting of Juglans regia L. microshoots. Acta Biol. Hung. 2016, 67, 379–392. [Google Scholar]
- Chen, F.; Zhang, J.Y.; Li, L.; Ma, Y.W.; Wang, Z.P.; Duan, Y.P.; Hao, Z.F. Genome-wide association analysis of deep-planting related traits in maize. Crops 2014, 2, 43–47. [Google Scholar]
- Blaschek, L.; Murozuka, E.; Serk, H.; Ménard, D.; Pesquet, E. Different combinations of laccase paralogs nonredundantly control the amount and composition of lignin in specific cell types and cell wall layers in Arabidopsis. Plant Cell 2022, 35, 889–909. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.H.; Xiong, Y.Q.; Williams, D.S.; Pozueta-Romero, D.; Chourey, P.S. Miniature1-encoded cell wall invertase is essential for assembly and function of wall-in-growth in the maize endosperm transfer cell. Plant Physiol. 2010, 151, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- Soga-Morimoto, A.; Soga, K.; Wakabayashi, K.; Kamisaka, S.; Hoson, T. Suppression of sugar accumulation in coleoptile and mesocotyl cells by light irradiation to etiolated maize seedlings. J. Plant Physiol. 2021, 260, 153409. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.N.; Zhao, X.Q.; Chao, W.; Lu, P.N.; Bai, X.D.; Mao, T.T. Genetic variation, DIMBOA accumulation, and candidate genes identification in maize multiple insect-resistance. Int. J. Mol. Sci. 2023, 24, 2138. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Zhang, J.W.; Fang, P.; Peng, Y.L. Comparative QTL analysis for yield components and morphological traits in maize (Zea mays L.) under water-stressed and well-watered conditions. Breed. Sci. 2019, 69, 621–632. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Zhong, Y. Genetic dissection of the photosynthetic parameters of maize (Zea mays L.) in drought-stressed and well-watered environments. Russ. J. Plant Physiol. 2021, 68, 1125–1134. [Google Scholar] [CrossRef]
- Trachsel, S.; Messmer, R.; Stamp, P.; Hund, A. Mapping of QTLs for leteral and axile root growth of tropical maize. Theor. Appl. Genet. 2009, 119, 1413–1424. [Google Scholar] [CrossRef]
- McCouch, S.R.; Cho, Y.G.; Yano, P.E.; Paul, E.; Blinstrub, M.; Morishima, H.; Kinoshita, T. Report on QTL nomenclature. Rice Genet. Newslett. 1997, 14, 11–13. [Google Scholar]
- Stuber, C.W.; Edwards, M.D.; Wendel, J. Molecular marker-facilitated investigations of quantitative trait loci in maize. II. Factors influencing yield and its component traits. Crop Sci. 1987, 27, 639–648. [Google Scholar] [CrossRef]
- Darvasi, A.; Soller, M. A simple method to calculate resolving power and confidence interval of QTL map location. Behav. Genet. 1997, 27, 125–132. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, F.; Kim, T.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Different gene and transcript expression analysis of RNA-Seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q.; Niu, Y.N.; Hossain, Z.; Zhao, B.Y.; Bai, X.D.; Mao, T.T. New insights into light spectral quality inhibits the plasticity elongation of maize mesocotyl and coleoptile during seed germination. Front. Plant Sci. 2023, 14, 1152399. [Google Scholar] [CrossRef]

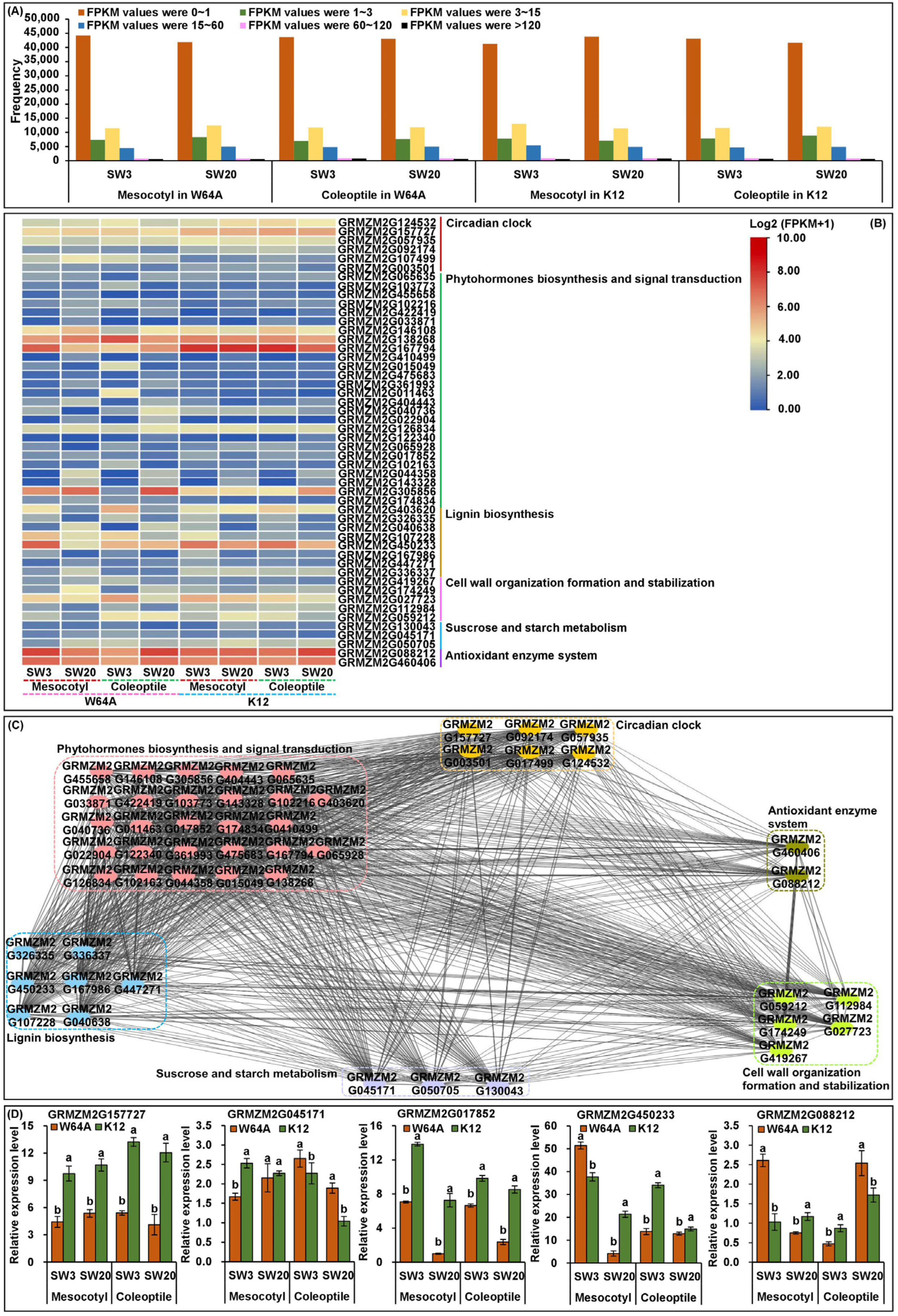
| Trait | Sowing Depth | Female Parent (W64A) | Male Parent (K12) | F1 Hybrid (W64A × K12) | F2:3 Population (346 Families) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | CV (%) | Skewness | Kurtosis | |||||
| RAT (%) | SW3 | 98.67 ± 1.82 | 96.66 ± 3.34 | 100.00 ± 0.00 | 89.18 ± 8.74 | 53.33–100.00 | 9.80 | 0.768 | 0.361 |
| SW15 | 95.32 ± 3.81 | 6.67 ± 2.36 | 99.33 ± 1.49 | 78.21 ± 28.82 | 20.00–100.00 | 25.02 | −0.097 | 0.886 | |
| SW20 | 78.66 ± 1.82 | 0.00 ± 0.00 | 92.00 ± 5.05 | 54.97 ± 19.01 | 13.33–96.67 | 34.58 | 0.290 | −0.618 | |
| MESL (cm) | SW3 | 3.82 ± 0.46 | 2.32 ± 0.29 | 3.85 ± 0.13 | 3.74 ± 0.61 | 1.69–4.79 | 16.31 | 0.112 | 0.110 |
| SW15 | 10.28 ± 1.11 | 3.28 ± 0.11 | 12.85 ± 0.27 | 10.95 ± 2.57 | 2.87–12.62 | 23.47 | 0.450 | 0.301 | |
| SW20 | 12.70 ± 0.42 | 5.20 ± 0.13 | 15.16 ± 0.40 | 13.09 ± 5.88 | 3.91–15.05 | 44.92 | 0.982 | 0.337 | |
| COLL (cm) | SW3 | 3.14 ± 0.35 | 2.94 ± 0.05 | 3.16 ± 0.12 | 2.93 ± 0.43 | 1.18–3.08 | 14.68 | 0.327 | 0.319 |
| SW15 | 4.11 ± 0.63 | 6.20 ± 0.17 | 5.48 ± 0.38 | 5.02 ± 1.12 | 2.25–6.84 | 22.31 | −0.044 | 0.571 | |
| SW20 | 4.87 ± 0.31 | 6.48 ± 0.26 | 6.12 ± 0.16 | 6.24 ± 1.75 | 2.69–6.95 | 28.04 | 1.087 | 0.934 | |
| MESL + COLL (cm) | SW3 | 6.96 ± 0.75 | 5.26 ± 0.32 | 7.00 ± 0.12 | 6.13 ± 1.39 | 2.94–7.98 | 22.82 | 0.578 | −0.113 |
| SW15 | 14.39 ± 1.45 | 9.49 ± 0.20 | 18.32 ± 0.48 | 15.42 ± 5.52 | 5.11–19.06 | 35.80 | 0.832 | 0.974 | |
| SW20 | 17.57 ± 0.24 | 11.68 ± 0.33 | 21.28 ± 0.53 | 19.94 ± 6.71 | 6.65–23.06 | 33.65 | 0.746 | 0.900 | |
| MESL/COLL | SW3 | 1.22 ± 0.11 | 0.79 ± 0.09 | 1.22 ± 0.08 | 1.24 ± 0.15 | 1.05–1.73 | 12.10 | 0.612 | 0.015 |
| SW15 | 2.54 ± 0.44 | 0.53 ± 0.02 | 2.35 ± 0.16 | 1.38 ± 0.32 | 1.07–1.87 | 23.19 | 0.931 | 0.798 | |
| SW20 | 2.62 ± 0.26 | 0.80 ± 0.03 | 2.48 ± 0.05 | 1.64 ± 0.49 | 1.31–2.20 | 29.88 | 0.913 | 1.017 | |
| SDL (cm) | SW3 | 15.64 ± 0.90 | 14.12 ± 0.35 | 16.18 ± 0.83 | 10.86 ± 1.23 | 5.73–16.26 | 11.33 | 0.511 | 0.434 |
| SW15 | 13.25 ± 0.31 | 7.58 ± 0.44 | 15.48 ± 0.48 | 8.33 ± 3.92 | 5.27–15.02 | 47.06 | 0.385 | 0.962 | |
| SW20 | 10.06 ± 0.58 | 5.18 ± 0.59 | 11.58 ± 1.07 | 5.81 ± 1.96 | 3.93–12.94 | 33.73 | −0.683 | −0.790 | |
| Item | Progeny Populations | |||||
|---|---|---|---|---|---|---|
| RAT | MESL | COLL | MESL + COLL | MESL/COLL | SDL | |
| F1 hybrid (W64A × K12) | ||||||
| Female parent (W64A) | 0.775 * | 0.994 *** | 0.985 *** | 0.994 *** | 0.995 *** | 0.167 |
| Male parent (K12) | 0.806 ** | 0.903 ** | 0.928 *** | 0.971 *** | −0.311 | 0.713 * |
| F2:3 population | ||||||
| Female parent (W64A) | 0.937 *** | 0.986 *** | 0.976 *** | 0.987 *** | 0.975 *** | 0.220 |
| Male parent (K12) | 0.782 * | 0.919 **** | 0.922 *** | 0.965 *** | −0.205 | 0.890 ** |
| Population | Sowing Depth | Markers Number | Length (cm) | Identified QTLs by Each Sowing Depth Environment | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Size | RAT | MESL | COLL | MESL + COLL | MESL/COLL | PL | SDL | ||||
| 3681–4 × X178 F2:3 | 221 | SW10 SW20 | 178 | 1865.5 | 6 | 12 | 3 | – | – | – | 4 | [18] |
| B73 × Mo17 IBM Syn4 RILs | 243 | SW12.5 | 1339 | 6242.7 | 7 | 10 | 2 | – | – | 5 | 7 | [19] |
| W64A × K12 F2:3 | 346 | SW3 SW15 SW20 | 253 | 1410.6 | 10 | 16 | 10 | 18 | 7 | – | 13 | This study |
| Trait | Meta–QTL (MQTL) | Major QTLs in This Study | QTLs Number | Bin | Marker Interval | Physical Interval (Mb) | Contig | Candidate Gene | Orthologs |
|---|---|---|---|---|---|---|---|---|---|
| MESL | MQTL1–1 | – | 2 | 1.02–1.03 | bnlg1178–bnlg1484 | 14.07–34.92 | ctg6–ctg11 | GRMZM2G065635 | LOC_Os03g12660 |
| COLL, PL, SDL | MQTL1–2 | – | 4 | 1.03–1.04 | bnlg2204–bnlg1811 | 45.94–70.81 | ctg11–ctg17 | GRMZM2G124532 | LOC_Os03g19590 |
| GRMZM2G326335 | – | ||||||||
| GRMZM2G040638 | LOC_Os03g25330 | ||||||||
| GRMZM2G107499 | LOC_Os03g27019 | ||||||||
| MESL + COLL, MESL, SDL | MQTL1–3 | qMESL + COLL–Ch.1–1 | 6 | 1.05–1.06 | umc1076–bnlg1615 | 143.53–192.99 | ctg30–ctg40 | GRMZM2G403620 | LOC_Os12g38400 |
| GRMZM2G422419 | LOC_Os12g43110 | ||||||||
| GRMZM2G419267 | LOC_Os08g39380.1 | ||||||||
| SDL | – | qSDL–Ch.1–2 | 2 | 1.06–1.08 | umc1748–umc1085 | 191.89–236.21 | ctg40–ctg48 | GRMZM2G404443 | LOC_Os10g34230 |
| RAT, MESL, COLL, MESL + COLL | MQTL1–4 | qMESL–Ch.1–1, qCOLL–Ch.1–1, qSDL–Ch.1–3, qMESL–Ch.1–2 | 13 | 1.08–1.10 | umc2181–umc2223 | 237.72–278.97 | ctg48–ctg58 | GRMZM2G167986 | LOC_Os10g26340 |
| GRMZM2G103773 | LOC_Os03g40540 | ||||||||
| GRMZM2G033871 | LOC_Os03g45850 | ||||||||
| GRMZM2G146108 | LOC_Os03g45850 | ||||||||
| GRMZM2G157727 | LOC_Os03g51030 | ||||||||
| GRMZM2G057935 | LOC_Os03g54084 | ||||||||
| COLL, MESL + COLL | MQTL2–1 | qMESL + COLL–Ch.2–1 | 4 | 2.00–2.01 | umc1419–phi96100 | 1.04–2.83 | ctg68–ctg68 | GRMZM2G040736 | LOC_Os04g57720 |
| RAT, MESL, SDL | MQTL3–1 | qMESL–Ch.3–1 | 7 | 3.04–3.05 | bnlg2047–umc1307 | 30.95–152.88 | ctg117–ctg128 | GRMZM2G107228 | LOC_Os01g22336 |
| GRMZM2G138268 | LOC_Os12g40890 | ||||||||
| GRMZM2G167794 | LOC_Os12g40900 | ||||||||
| GRMZM2G174249 | LOC_Os01g73580 | ||||||||
| COLL, MESL, SDL | MQTL3–2 | – | 4 | 3.08–3.09 | phi088–bnlg1257 | 208.61–217.91 | ctg134–ctg149 | GRMZM2G143328 | LOC_Os01g45090 |
| MESL | MQTL4–1 | qMESL–Ch.4–1 | 4 | 4.06–4.07 | umc1869–umc1194 | 161.53–178.27 | ctg182–ctg182 | GRMZM2G410499 | – |
| GRMZM2G130043 | LOC_Os02g56320 | ||||||||
| GRMZM2G003501 | LOC_Os02g49920 | ||||||||
| GRMZM2G015049 | – | ||||||||
| GRMZM2G017852 | – | ||||||||
| GRMZM2G045171 | LOC_Os02g58480 | ||||||||
| GRMZM2G455658 | – | ||||||||
| GRMZM2G102163 | LOC_Os02g57080 | ||||||||
| GRMZM2G102216 | LOC_Os02g56970 | ||||||||
| GRMZM2G475683 | LOC_Os02g52990 | ||||||||
| COLL, ESL, MESL + COLL MESL/COLL, SDL | MQTL4–2 | – | 13 | 4.08–4.08 | bnlg2162–umc1612 | 185.73–187.53 | ctg184–ctg185 | GRMZM2G174834 | LOC_Os11g03540 |
| MESL/COLL | – | qMESL/COLL–Ch.4–1 | 3 | 4.08–4.08 | umc1612–umc1313 | 187.53–224.58 | ctg185–ctg189 | GRMZM2G460406 | LOC_Os08g43560 |
| MESL, SDL, RAT | MQTL5–1 | – | 6 | 5.03–5.04 | umc1784–umc2298 | 59.17–84.83 | ctg220–ctg225 | GRMZM2G361993 | LOC_Os06g50040 |
| GRMZM2G088212 | LOC_Os06g51150 | ||||||||
| GRMZM2G059212 | LOC_Os06g51270 | ||||||||
| MESL | – | qMESL–Ch.6–1 | 3 | 6.01–6.01 | umc2311–umc2196 | 18.71–24.61 | ctg262–ctg264 | GRMZM2G050705 | – |
| MESL, MESL + COLL | MQTL6–1 | – | 5 | 6.01–6.01 | umc2311–umc1832 | 24.61–60.33 | ctg262–ctg262 | GRMZM2G305856 | LOC_Os05g02420 |
| MESL, RAT | MQTL6–2 | – | 5 | 6.04–6.05 | umc1979–bnlg1732 | 106.23–151.97 | ctg276–ctg287 | GRMZM2G450233 | LOC_Os05g06970 |
| GRMZM2G044358 | LOC_Os05g08540 | ||||||||
| GRMZM2G027723 | LOC_Os05g08370 | ||||||||
| MESL, SDL | MQTL7–1 | qSDL–Ch.7–1 | 5 | 7.02–7.02 | umc2617–umc116a | 100.30–127.13 | ctg310–ctg317 | GRMZM2G022904 | LOC_Os09g23820 |
| GRMZM2G011463 | LOC_Os09g26590 | ||||||||
| GRMZM2G065928 | LOC_Os09g28390 | ||||||||
| RAT | – | qRAT–Ch.7–1 | 3 | 7.06–7.06 | umc1760–phi116 | 174.40–174.61 | ctg325–ctg325 | GRMZM2G112984 | LOC_Os07g49100 |
| SDL | MQTL8–1 | – | 3 | 8.06–8.07 | umc1141–umc2357 | 158.93–169.62 | ctg361–ctg364 | GRMZM2G447271 | LOC_Os01g62480 |
| GRMZM2G336337 | LOC_Os01g61160 | ||||||||
| MESL, COLL, SDL | MQTL9–1 | – | 5 | 9.04–9.05 | umc1492–umc1494 | 119.96–136.63 | ctg383–ctg387 | GRMZM2G092174 | LOC_Os03g19590 |
| RAT, PL | MQTL9–2 | – | 2 | 9.06–9.07 | bnlg1191–bnlg1588 | 143.19–147.21 | ctg389–ctg390 | GRMZM2G126834 | LOC_Os03g12350 |
| COLL, MESL + COLL | MQTL10–1 | – | 4 | 10.04–10.04 | umc1280–umc1506 | 128.12–133.25 | ctg413–ctg414 | GRMZM2G122340 | LOC_Os04g44230 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Niu, Y.; Hossain, Z.; Shi, J.; Mao, T.; Bai, X. Integrated QTL Mapping, Meta-Analysis, and RNA-Sequencing Reveal Candidate Genes for Maize Deep-Sowing Tolerance. Int. J. Mol. Sci. 2023, 24, 6770. https://doi.org/10.3390/ijms24076770
Zhao X, Niu Y, Hossain Z, Shi J, Mao T, Bai X. Integrated QTL Mapping, Meta-Analysis, and RNA-Sequencing Reveal Candidate Genes for Maize Deep-Sowing Tolerance. International Journal of Molecular Sciences. 2023; 24(7):6770. https://doi.org/10.3390/ijms24076770
Chicago/Turabian StyleZhao, Xiaoqiang, Yining Niu, Zakir Hossain, Jing Shi, Taotao Mao, and Xiaodong Bai. 2023. "Integrated QTL Mapping, Meta-Analysis, and RNA-Sequencing Reveal Candidate Genes for Maize Deep-Sowing Tolerance" International Journal of Molecular Sciences 24, no. 7: 6770. https://doi.org/10.3390/ijms24076770
APA StyleZhao, X., Niu, Y., Hossain, Z., Shi, J., Mao, T., & Bai, X. (2023). Integrated QTL Mapping, Meta-Analysis, and RNA-Sequencing Reveal Candidate Genes for Maize Deep-Sowing Tolerance. International Journal of Molecular Sciences, 24(7), 6770. https://doi.org/10.3390/ijms24076770






