Cyanogenesis, a Plant Defence Strategy against Herbivores
Abstract
1. Introduction
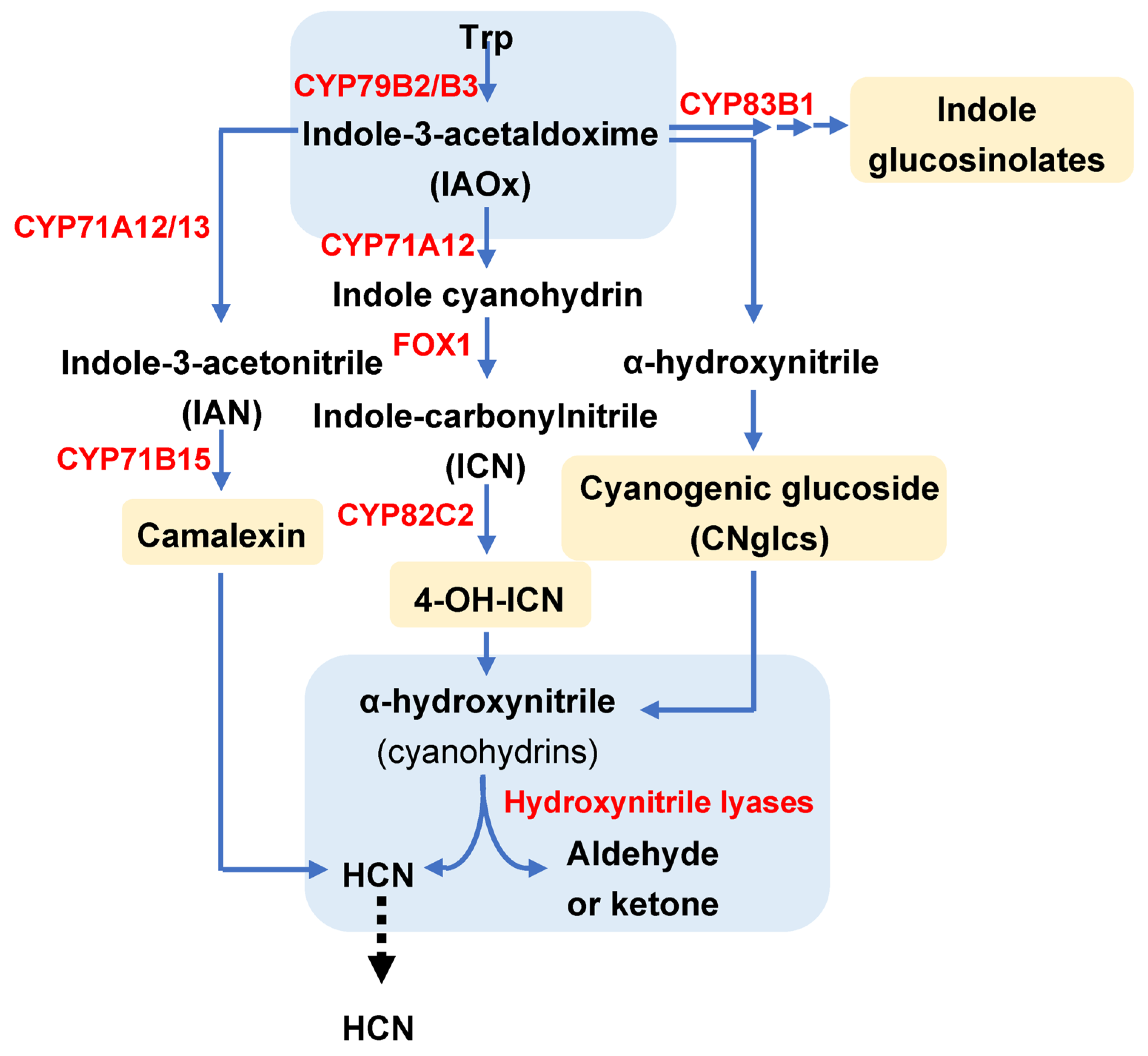
2. Metabolic Pathways Linked to Cyanogenesis
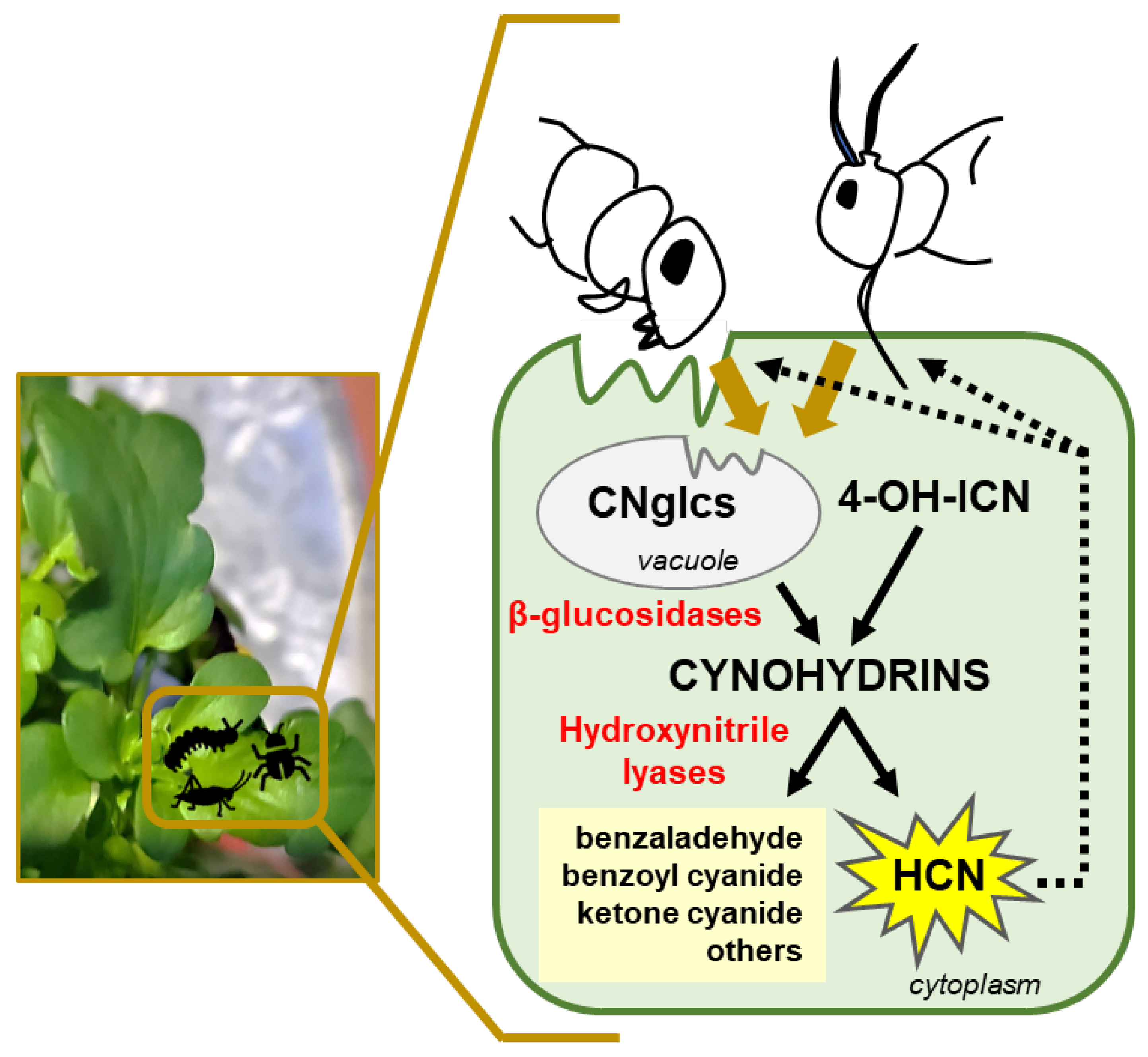
2.1. Cyanogenic Glucosides (CNglcs): Biosynthesis and Catabolism
2.2. 4-Hydroxy-Indole-3-Carbonyl Nitrile (4-OH-ICN) Pathway
2.3. Crosstalk of Indole Metabolic Related Pathways and Cyanogenesis Involved in Defence to Herbivores
3. Cyanohydrins, Cyanogenesis and the Control of Phytophagous Arthropods (Insects and Mites)
4. Herbivore Responses to Cyanogenic Metabolites
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CNglcs | Cyanogenic glucosides |
| HAMPS | Herbivore associated molecular patterns |
| HCN | Hydrogen cyanide |
| IAOx | indole-3-acetaldoxime |
| IAN | Indole-3-acetonitrile |
| ICN | Indole-3-carbonyl nitrile |
| IGs | Indole glucosinaltes |
| MNL | Mandelonitrile lyase |
| 4-OH-ICN | 4-Hydroxy-indole-3-carbonylnitrile |
References
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Plant responses to herbivory, wounding and infection. Int. J. Mol. Sci. 2022, 23, 7031. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M.E.; Arnaiz, A.; Rosa-Diaz, I.; Gonzalez-Melendi, P.; Romero-Hernandez, G.; Ojeda-Martinez, D.A.; Garcia, A.; Contreras, E.; Martinez, M.; Diaz, I. Plant defences against Tretranychus urticae: Mind the gaps. Plants 2020, 9, 464. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M.E.; Arnaiz, A.; Gonzalez-Melendi, P.; Martinez, M.; Diaz, I. Plant perception and short-term responses to phytophagous insects and mites. Int. J. Mol. Sci. 2018, 19, 1356. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Legrarrea, S.; Kant, M.R. Tomato reproductive success is equally affected by herbivores that induce or that suppress defences. Front. Plant Sci. 2017, 8, 2128. [Google Scholar] [CrossRef] [PubMed]
- Hogenhout, S.A.; Bos, J.I.B. Effector proteins that modulate plant-insect interactions. Curr. Opin. Plant Biol. 2011, 14, 422–428. [Google Scholar] [CrossRef]
- Morant, A.V.; Jørgensen, K.; Jørgensen, C.; Paquette, S.M.; Sánchez-Pérez, R.; Møller, B.L.; Bak, S. β-glucosidases as detonators of plant chemical defense. Phytochemistry 2008, 69, 1795–1813. [Google Scholar] [CrossRef]
- Burow, M.; Halkier, B.A. How does a plant orchestrate defense in time and space? Using glucosinolates in Arabidopsis as case study. Curr. Opin. Plant Biol. 2017, 38, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Griengl, H.; Schwab, H.; Fechter, M. The synthesis of chiral cyanohydrins by oxynitrilases. Trends Biotechnol. 2000, 18, 252–256. [Google Scholar] [CrossRef]
- Gleadow, R.M.; Møller, B.L. Cyanogenic glycosides: Synthesis, physiology, and phenotypic plasticity. Ann. Rev. Plant Biol. 2014, 65, 155–185. [Google Scholar] [CrossRef]
- Brattsten, L.B.; Samuelian, J.H.; Long, K.Y.; Kinkcaid, S.A.; Evans, C.K. Cyanide as a feeding stimulant for the southern armyworm, Spodoptera eridania. Ecol. Entomol. 1983, 8, 125–132. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Yamaguchi, T.; Ichiki, Y.; Tanabe, T.; Asano, Y. Hydrogen peroxide as a new defensive compound in benzoyl cyanide producing polydesmid millipedes. Sci. Nat. 2017, 104, 19. [Google Scholar] [CrossRef]
- Santamaria, M.E.; Martinez, M.; Arnaiz, A.; Ortego, F.; Grbic, V.; Diaz, I. MATI, a novel protein involved in the regulation of herbivore-associated signalling pathways. Front. Plant Sci. 2017, 8, 975. [Google Scholar] [CrossRef]
- Park, D.-S.; Peterson, C.; Zhao, S.; Coats, J.R. Fumigation toxicity of volatile natural and synthetic cyanohydrins to stored-product pests and activity as soil fumigants. Pest Manag. Sci. 2004, 60, 833–838. [Google Scholar] [CrossRef]
- Rajniak, J.; Barco, B.; Clay, N.K.; Sattely, E.S. A new cyanogenic metabolite in Arabidopsis required for inducible pathogen defence. Nature 2015, 525, 376–379. [Google Scholar] [CrossRef]
- Peiser, G.D.; Wang, T.T.; Hoffman, N.E.; Yang, S.F.; Liu, H.W.; Walsh, C.T. Formation of cyanide from carbon 1 of 1-amino-cyclopropane-1-carboxylic acid during its conversion to ethylene. Proc. Natl. Acad. Sci. USA 1984, 81, 3059–3063. [Google Scholar] [CrossRef]
- García, I.; Arenas-Alfonseca, L.; Moreno, I.; Gotor, C.; Romero, L.C. HCN regulates cellular processes through posttranslational modification of proteins by S-cyanylation. Plant Physiol. 2019, 179, 107–123. [Google Scholar] [CrossRef]
- Garcia, I.; Castellano, J.M.; Vioque, B.; Solano, R.; Gotor, C.; Romero, L.C. Mitochondrial b-cyanoalanine synthase is essential for root hair formation in Arabidopsis thaliana. Plant Cell 2010, 22, 3268–3279. [Google Scholar] [CrossRef]
- Yulvianti, M.; Zidorn, C. Chemical diversity of plant cyanogenic glycosides: An overview of reported natural products. Molecules 2021, 26, 719. [Google Scholar] [CrossRef]
- Zagrobelny, M.; Bak, S.; Rasmussen, A.V.; Jørgensen, B.; Naumann, C.M.; Møller, B.L. Cyanogenic glucosides and plant-insect interactions. Phytochemistry 2004, 65, 293–306. [Google Scholar] [CrossRef]
- Arnaiz, A.; Santamaria, M.E.; Rosa-Diaz, I.; Garcia, I.; Dixit, S.; Vallejos, S.; Gotor, C.; Martinez, M.; Gribc, V.; Diaz, I. Hydroxynitrile lyase defends Arabidopsis against Tetranychus urticae. Plant Physiol. 2022, 189, 2244–2258. [Google Scholar] [CrossRef]
- Thayer, S.S.; Coon, E.E. Subcellular localization of dhurrin β-glucoside and hydroxinitrile lyase in the mesophyll cells of sorghum leaf blades. Plant Physiol. 1981, 67, 617–622. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, K.; Chen, C.; Wu, Y.; Tang, Y.; Georgiev, M.I.; Zhang, X.; Lin, M.; Zhou, M. Biosynthesis and regulation of cyanogenic glycoside production in forage plants. Appl. Microbiol. Biotechnol. 2018, 102, 9–16. [Google Scholar] [CrossRef]
- Bak, S.; Olsen, C.E.; Halkier, B.A.; Møller, B.L. Transgenic tobacco and Arabidopsis plants expressing the two multifunctional Sorghum cytochrome P450 enzymes, CYP79A1 and CYP71E1, are cyanogenic and accumulate metabolites derived from intermediates in dhurrin biosynthesis. Plant Phsyiol. 2000, 123, 1437–1448. [Google Scholar] [CrossRef]
- Lai, D.; Maimann, A.B.; Macea, E.; Ocampo, C.H.; Cardona, G.; Pičmanová, M.; Darbani, B.; Olsen, C.E.; Debouck, D.; Raatz, B.; et al. Biosynthesis of cyanogenic glucosides in Phaseolus lunatus and evolution of oximes-based defenses. Plant Direct 2020, 4, e00244. [Google Scholar] [CrossRef]
- Lai, D.; Pičmanová, M.; Hachem, M.A.; Motawia, M.S.; Olsen, C.E.; Møller, B.L.; Rook, F.; Takos, A.M. Lotus japonicus flowers are defended by cyanogenic β-glucosidase with highly restricted expression to essential reproductive organs. Plant Mol. Biol. 2015, 89, 21–34. [Google Scholar] [CrossRef]
- Juma, B.S.; Mukami, A.; Mweu, C.; Ngugi, M.P.; Mbinda, W. Target mutagenesis of the CYP79D1 gene via CRISPR/cas9-mediated genome editing results in lower levels cyanide in cassava. Front Plant Sci. 2022, 13, 1009860. [Google Scholar] [CrossRef]
- Siritunga, D.; Arias-Garzon, D.; White, W.; Sayre, R.T. Over-expression of hdroxynitrile lyase in transgenic cassava roots accelerates cyanogenesis and food detoxification. Plant Biotechnol. J. 2004, 2, 37–43. [Google Scholar] [CrossRef]
- Narayahan, N.N.; Ihemere, U.; Ellery, C.; Sayre, R.T. Overexpression of hydroxynitrile lyase in cassava roots elevates protein and free amino acids while reducing residual cyanogenic levels. PLoS ONE 2011, 6, e21996. [Google Scholar]
- Zevallos, D.M.P.; Querol, M.P.; Ambrogi, B.G. Cassava wastewater as a natural pesticide: Current knowledge and challenges for broader utilization. Ann. Appl. Biol. 2018, 173, 191–201. [Google Scholar] [CrossRef]
- Cuny, M.A.C.; La Fogia, D.; Desurmont, G.A.; Glauser, G.; Benrey, B. Role of cyanogenic glycosides in the seeds of wild lima bean, Phaseolus lunatus: Defense, plant nutrition or both? Planta 2019, 250, 1281–1292. [Google Scholar] [CrossRef]
- Møller, B.L. Functional diversifications of cyanogenic glucosides. Curr. Opin. Plant Biol. 2010, 13, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Pastorczyk, M.; Kosaka, A.; Piślewska-Bednarek, M.; López, G.; Frerigmann, H.; Kułak, K.; Glawischnig, E.; Molina, A.; Takano, Y.; Bednarek, P. The role of CYP71A12 monooxygenase in pathogen-triggered tryptophan metabolism and Arabidopsis immunity. New Phytol. 2020, 225, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, M.; Neilson, E.H.J.; Møller, B.L. Oximes: Unrecognized chameleons in general and specialized plant metabolism. Mol. Plant 2018, 11, 95–117. [Google Scholar] [CrossRef]
- Böttcher, C.; Westphal, L.; Schmotz, C.; Prade, E.; Scheed, D.; Glawisching, E. The multifunctional enzyme CYP71B15 (PHYTOALEXIN DEFICIENT3) converts cysteine-indloe-3-ccetonitrile to camalexin in the indole-3-acetonitrile metabolic network of Arabidopsis thaliana. Plant Cell 2009, 21, 1830–1845. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Trotel-Aziz, P.; Clément, C.; Jeandet, P.; Baillieul, F.; Aziz, A. Camaleixn accumulation as a component of plant immunity during interaction with pathogens and beneficial microbes. Planta 2022, 255, 116–123. [Google Scholar] [CrossRef]
- Kettles, G.J.; Drurey, C.; Schoonbeek, H.J.; Maule, A.J.; Hogenhout, S.A. Resistance of Arabidopsis thaliana to the green peach aphid, Myzus persicae, involves camalexin and is regulated by microRNAS. New Phytol. 2013, 198, 1178–1190. [Google Scholar] [CrossRef]
- Widemann, E.; Bruisma, K.; Walshe-Roussel, B.; Saha, R.K.; Letwin, D.; Zhurov, V.; Bernards, M.A.; Grbić, M.; Grbić, V. Multiple indole glucosinolates and myrosinases defend Arabidopsis against Tetranychus urticae herbivory. Plant Physiol. 2021, 187, 116–132. [Google Scholar] [CrossRef]
- Yadav, M.; Singh, I.K.; Singh, A. Dhurrin: A naturally occurring phytochemical as a weapon against insect herbivores. Phytochemistry 2023, 205, 113483. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Heil, M.; Pietrowski, A.; Lieberei, R. Quantitative effects of cyanogenesis on an adapted herbivore. J. Chem. Ecol. 2007, 33, 2195–2208. [Google Scholar] [CrossRef]
- Riis, L.; Bellotti, A.C.; Bonierbale, M.; O´Brien, G.M. Cyanogenic potential in cassava and its influence on a generalist insect herbivore Cytomenus bergi (Hemiptera: Cydnidae). J. Econ. Entomol. 2003, 96, 1905–1914. [Google Scholar] [CrossRef]
- Torto, B.; Hassanali, A.; Saxena, K.N.; Nokoe, S. Feeding responses of Chilo partellus (Swinhoe) (Lepidoptera: Pyralidae) larvae to sorghum plant phenolics and their analogs. J. Chem. Ecol. 1991, 17, 67–78. [Google Scholar] [CrossRef]
- Woodhead, S.; Bernays, E.A. The chemical bases of resistance of Sorghum bicolor to attack by Locustra migratoria. Entomol. Exp. Appl. 1978, 24, 123–144. [Google Scholar] [CrossRef]
- Krothapalli, K.; Buescher, E.M.; Li, X.; Brown, E.; Chapple, C.; Dilkes, B.P.; Tuinstra, M.R. Forward genetics by genome sequencing reveals that rapid cyanide release deters insect herbivore of Sorghum bicolor. Genetics 2013, 195, 309–318. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Lieberei, R. Oviposition choice of Mexican bean beetle (Epilachna varivestis) depends on host plants cyanogenic capacity. J. Chem. Ecol. 2006, 32, 1861–1865. [Google Scholar] [CrossRef]
- Dreyer, D.L.; Reese, J.C.; Jones, K.C. Aphid feeding deterrents in sorghum: Bioassay isolation and characterization. J. Chem. Ecol. 1981, 7, 273–284. [Google Scholar] [CrossRef]
- Hay-Roe, M.M.; Meagher, R.L.; Nagoshi, R.N. Effects of cyanogenic plants on fitness in two host strains of the fall armyworm (Spodoptera frugiperda). J. Chem. Ecol. 2011, 37, 1314–1322. [Google Scholar] [CrossRef]
- Mora, C.A.; Halter, J.G.; Adler, C.; Hund, A.; Anders, H.; Yu, K.; Stark, W.J. Application of the Prunus spp. cyanide seed-defense system onto wheat: Reduced insect feeding and field growth test. J. Agric. Food Chem. 2016, 64, 3501–3507. [Google Scholar] [CrossRef]
- Gruss, S.M.; Ghaste, M.; Widhalm, J.R.; Tuinstra, M.R. Seedling growth and fall armyworm feeding preference influenced by dhurrin production in sorghum. Theor. Appl. Genet. 2022, 135, 1037–1047. [Google Scholar] [CrossRef]
- Tattersall, D.B.; Bak, S.; Jones, P.R.; Olsen, C.E.; Nielsen, J.K.; Hansen, M.L.; Høj, P.B.; Møller, B.L. Resistance to an herbivore through engineered cyanogenic glucoside synthesis. Science 2001, 293, 1826–1828. [Google Scholar] [CrossRef]
- Peterson, C.J.; Tsao, R.; Eggler, A.L.; Coats, J.R. Insecticidal activity of cyanohydrin and monoterpenoid compounds. Molecules 2000, 5, 648–654. [Google Scholar] [CrossRef]
- Pentzold, S.; Zagrobelny, M.; Bjarnholt, N.; Kroymann, J.; Vogel, H.; Olsen, C.E.; Møller, B.L.; Bak, S. Metabolism, excretion and avoidance of cyanogenic glucosides in insects with different specialisations. Insect Biochem. Mol. Biol. 2015, 66, 119–128. [Google Scholar] [CrossRef]
- Pentzold, S.; Zagrobelny, M.; Rook, F.; Bak, S. How insects overcome two-component plant chemical defense: Plant β-glucosidases as the main target for herbivore adaptation. Biol. Rev. 2015, 89, 531–551. [Google Scholar] [CrossRef] [PubMed]
- Bensoussan, N.; Santamaria, M.E.; Zhurov, V.; Diaz, I.; Grbić, M.; Grbić, V. Plant-herbivore interaction: Dissections of the cellular pattern of Tetranychus urticae feeding on the host plant. Front. Plant Sci. 2016, 7, 1105. [Google Scholar] [CrossRef] [PubMed]
- Easson, M.L.A.E.; Malka, O.; Paetz, C.; Hojná, A.; Reichelt, M.; Stein, B.; van Brunschot, A.; Feldmesser, E.; Campbell, L.; Colvin, J.; et al. Activation and detoxification of cassava cyanogenic glucosides by the whitefly Bemisia tabaci. Sci. Rep. 2021, 11, 13244. [Google Scholar] [CrossRef] [PubMed]
- Zagrobelny, M.; Bak, S.; Møller, B.L. Cyanogenesis in plant and arthropods. Phytochemistry 2008, 69, 1457–1468. [Google Scholar] [CrossRef]
- Miller, J.M.; Conn, E.E. Metabolism of hydrogen cyanide by higher plants. Plant Physiol. 1980, 65, 1199–1202. [Google Scholar] [CrossRef]
- Qian, D.; Jiang, L.; Lu, L.; Wei, C.; Li, Y. Biochemical and structural properties of cyanases from Arabidopsis thaliana and Oryza sativa. PLoS ONE 2011, 6, e18300. [Google Scholar] [CrossRef]
- Beesley, S.G.; Compton, S.G.; Jones, D.A. Rhodanese in insects. J. Chem. Ecol. 1985, 11, 45–50. [Google Scholar] [CrossRef]
- Stauber, E.J.; Kuczka, P.; van Ohlen, M.; Vogt, B.; Janowitz, T.; Piotrowski, M.; Beuerle, T.; Wittstock, U. Turning the “Mustard Oil Bomb” into “Cyanide Bomb”: Aromatic glucosinolate metabolism in specialist insect herbivore. PLoS ONE 2012, 7, e35545. [Google Scholar] [CrossRef]
- Dixit, S.; Widemann, E.; Bensoussan, N.; Salehipourshirazi, G.; Bruinsma, K.; Milojevic, M.; Shukla, A.; Romero, L.C.; Zhurov, V.; Bernards, M.A.; et al. β-cyanoalanine synthase protects mites against Arabidopsis defences. Plant Physiol. 2022, 189, 1961–1975. [Google Scholar] [CrossRef]


| Insect/Acari | Plant/Assay | Feature | Reference | |
|---|---|---|---|---|
| Order | Species | |||
| Lepidopteran | Chilo partellus | sorghum | cyanogenic | [41] |
| Spodoptera frugiperda | synthetic diet | NaCN | [46] | |
| Spodoptera frugiperda | sorghum | cyanogenic | [43] | |
| Plodia interpunctella | wheat | amygdalin or β-glucosidase | [47] | |
| Spodoptera littoralis | lima bean | cyanogenic | [30] | |
| Spodoptera frugiperda | sorghum | cyanogenic | [48] | |
| Hemipteran | Cyrtomenus bergi | cassava | cyanogenic | [40] |
| Coleopteran | Phyllotreta nemorum | Transgenic arabidopsis | dhurrin | [49] |
| Rizopertha dominica | glass tube fumigant | cyanohydrins | [13] | |
| Tribolium castaneum | glass tube-fumigant | cyanohydrins | [13] | |
| Sitophilus zeamias | glass tube-fumigant | cyanohydrins | [13] | |
| Oryzaephilus surinamensis | glass tube-fumigant | cyanohydrins | [13] | |
| Epilachna varivestis | lima bean | cyanogenic | [39] | |
| Tenebrio molitor | wheat | amygdalin or β-glucosidase | [47] | |
| Rizopertha dominica | wheat | amygdalin or β-glucosidase | [47] | |
| Orthopteran | Locusta migratoria | sorghum | cyanogenic | [42] |
| Homopteran | Schizaphis graminum | synthetic diet | dhurrin | [45] |
| Acari | Tetranychus urticae | transgenic arabidopsis | HNL | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boter, M.; Diaz, I. Cyanogenesis, a Plant Defence Strategy against Herbivores. Int. J. Mol. Sci. 2023, 24, 6982. https://doi.org/10.3390/ijms24086982
Boter M, Diaz I. Cyanogenesis, a Plant Defence Strategy against Herbivores. International Journal of Molecular Sciences. 2023; 24(8):6982. https://doi.org/10.3390/ijms24086982
Chicago/Turabian StyleBoter, Marta, and Isabel Diaz. 2023. "Cyanogenesis, a Plant Defence Strategy against Herbivores" International Journal of Molecular Sciences 24, no. 8: 6982. https://doi.org/10.3390/ijms24086982
APA StyleBoter, M., & Diaz, I. (2023). Cyanogenesis, a Plant Defence Strategy against Herbivores. International Journal of Molecular Sciences, 24(8), 6982. https://doi.org/10.3390/ijms24086982







