Relationship between Excreted Uremic Toxins and Degree of Disorder of Children with ASD
Abstract
1. Introduction
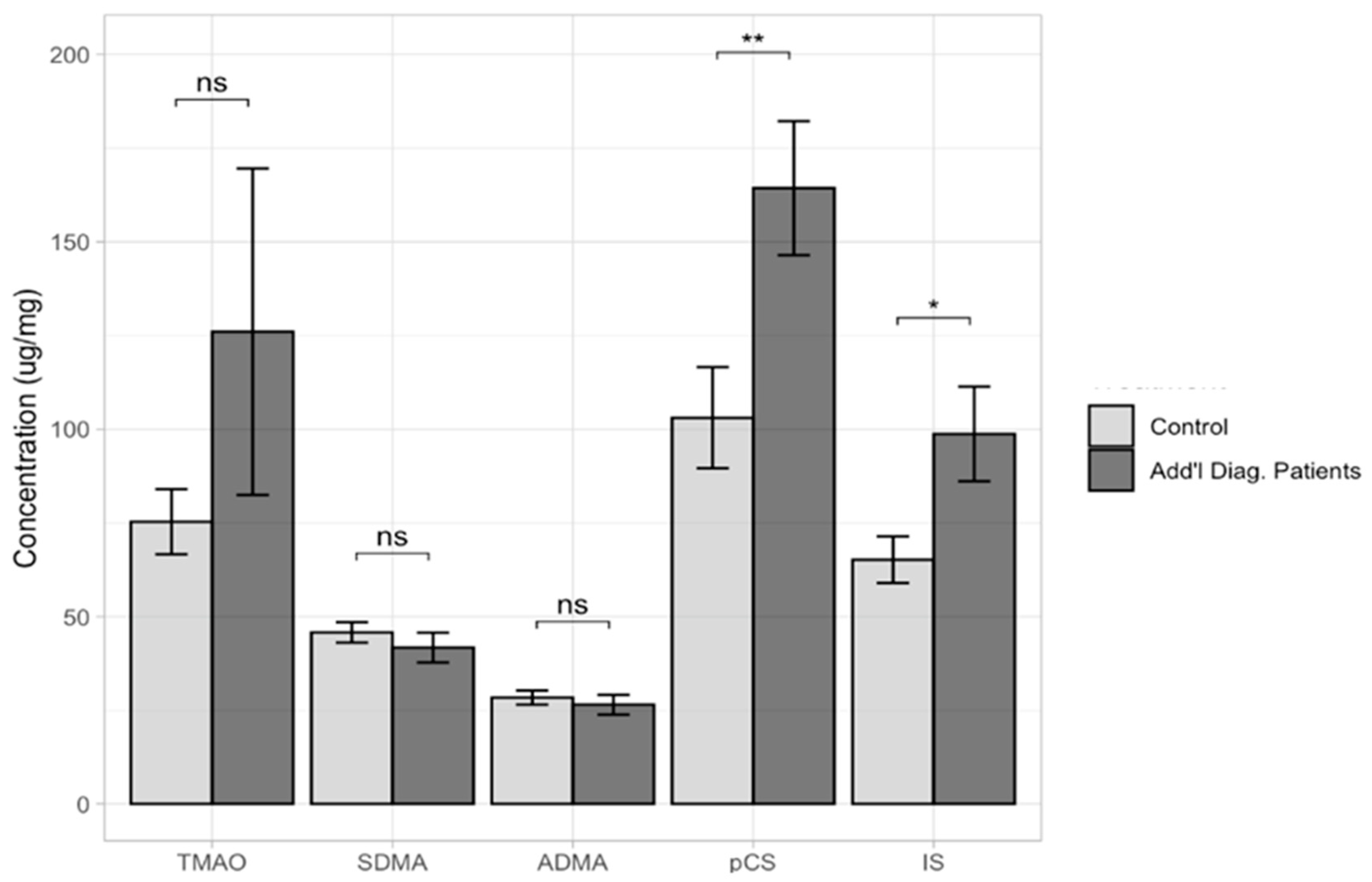
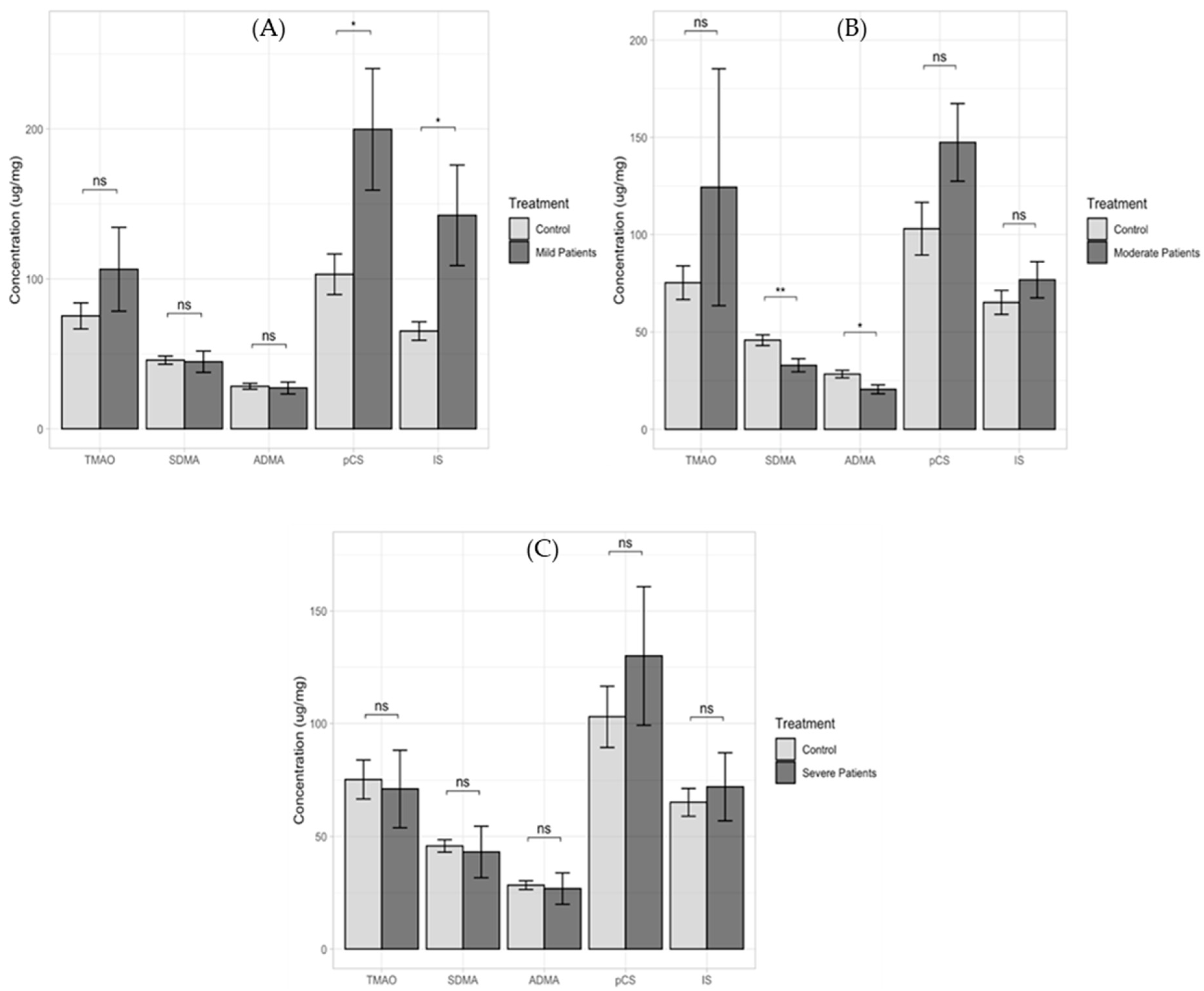
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Samples
4.2. Analytical Method
4.3. Statistical Analysis
5. Strengths and Weaknesses of Our Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masi, A.; DeMayo, M.M.; Glozier, N.; Guastella, A.J. An Overview of Autism Spectrum Disorder, Heterogeneity and Treatment Options. Neurosci. Bull. 2017, 33, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Autism Spectrum Disorder (ASD). Data & Statistics on Autism Spectrum Disorder. Available online: https://www.cdc.gov/ncbddd/autism/data.html (accessed on 8 January 2023).
- NIHM. Mental Health Information. Autism Spectrum Disorder. Available online: https://www.nimh.nih.gov/health/topics/autism-spectrum-disorders-asd#part_145438 (accessed on 8 January 2023).
- Bourgeron, T. Current Knowledge on the Genetics of Autism and Propositions for Future Research. C. R. Biol. 2016, 339, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Lombardo, M.V.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Esnafoglu, E.; Cırrık, S.; Ayyıldız, S.N.; Erdil, A.; Ertürk, E.Y.; Daglı, A.; Noyan, T. Increased Serum Zonulin Levels as an Intestinal Permeability Marker in Autistic Subjects. J. Pediatr. 2017, 188, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Magistris, L.; Familiari, V.; Pascotto, A.; Sapone, A.; Frolli, A.; Iardino, P.; Carteni, M.; De Rosa, M.; Francavilla, R.; Riegler, G.; et al. Alterations of the Intestinal Barrier in Patients with Autism Spectrum Disorders and in Their First-Degree Relatives. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 418–424. [Google Scholar] [CrossRef]
- McElhanon, B.O.; McCracken, C.; Karpen, S.; Sharp, W.G. Gastrointestinal Symptoms in Autism Spectrum Disorder: A Meta-Analysis. Pediatrics 2014, 133, 872–883. [Google Scholar] [CrossRef]
- Chaidez, V.; Hansen, R.L.; Hertz-Picciotto, I. Gastrointestinal Problems in Children with Autism, Developmental Delays or Typical Development. J. Autism Dev. Disord. 2014, 44, 1117–1127. [Google Scholar] [CrossRef]
- Mu, Q.; Kirby, J.; Reilly, C.; Luo, X.M. Leaky Gut as a Danger Signal for Autoimmune Diseases. Front. Immunol. 2017, 8, 598. [Google Scholar] [CrossRef]
- Gabriele, S.; Sacco, R.; Altieri, L.; Neri, C.; Urbani, A.; Bravaccio, C.; Riccio, M.P.; Iovene, M.R.; Bombace, F.; Magistris, L.D.; et al. Slow Intestinal Transit Contributes to Elevate Urinary p-Cresol Level in Italian Autistic Children. Autism Res. 2016, 9, 752–759. [Google Scholar] [CrossRef]
- Mair, R.D.; Sirich, T.L.; Plummer, N.S.; Meyer, T.W. Characteristics of Colon-Derived Uremic Solutes. Clin. J. Am. Soc. Nephrol. 2018, 13, 1398–1404. [Google Scholar] [CrossRef]
- Kikuchi, M.; Ueno, M.; Itoh, Y.; Suda, W.; Hattori, M. Uremic Toxin-Producing Gut Microbiota in Rats with Chronic Kidney Disease. Nephron 2017, 135, 51–60. [Google Scholar] [CrossRef]
- Ramezani, A.; Massy, Z.A.; Meijers, B.; Evenepoel, P.; Vanholder, R.; Raj, D.S. Role of the Gut Microbiome in Uremia: A Potential Therapeutic Target. Am. J. Kidney Dis. 2016, 67, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Gryp, T.; Paepe, K.D.; Vanholder, R.; Kerckhof, F.M.; Biesen, W.V.; Wiele, T.V.; Verbeke, F.; Speeckaert, M.; Joossens, M.; Couttenye, M.M.; et al. Gut Microbiota Generation of Protein-bound Uremic Toxins and Related Metabolites is not Altered at Different Stages of Chronic Kidney Disease. Kidney Int. 2020, 97, 1230–1242. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Chen, G.C.; Wang, Z.; Usyk, M.; Yu, B.; Baeza, Y.V.; Humphrey, G.; Benitez, R.S.; Li, J.; Nguyen, J.S.W.; et al. Dietary Factors, Gut Microbiota, and Serum Trimethylamine-N-oxide Associated with Cardiovascular Disease in the Hispanic Community Health Study/Study of Latinos. Am. J. Clin. Nutr. 2021, 113, 1503–1514. [Google Scholar] [CrossRef] [PubMed]
- Govindarajulu, M.; Pinky, P.D.; Steinke, I.; Bloemer, J.; Ramesh, S.; Kariharan, T.; Rella, R.T.; Bhattacharya, S.; Dhanasekaran, M.; Suppiramaniam, V.; et al. Gut Metabolite TMAO Induces Synaptic Plasticity Deficits by Promoting Endoplasmic Reticulum Stress. Front. Mol. Neurosci. 2020, 13, 138. [Google Scholar] [CrossRef]
- Vernetti, L.; Gough, A.; Baetz, N.; Blutt, S.; Broughman, J.R.; Brown, J.A.; Abel, J.F.; Hasan, N.; In, J.; Kelly, E.; et al. Functional Coupling of Human Microphysiology Systems: Intestine, Liver, Kidney Proximal Tubule, Blood-Brain Barrier and Skeletal Muscle. Sci. Rep. 2017, 8, 42296. [Google Scholar] [CrossRef]
- Rio, D.D.; Zimetti, F.; Caffarra, P.; Tassotti, M.; Bernini, F.; Brighenti, F.; Zini, A.; Zanotti, I. The Gut Microbial Metabolite Trimethylamine-N-Oxide Is Present in Human Cerebrosponal Fluid. Nutrients 2017, 9, 1053. [Google Scholar] [CrossRef]
- Vogt, N.M.; Romano, K.A.; Darst, B.F.; Engelman, C.D.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Blennow, K.; Zetterberg, H.; Bendlin, B.B.; et al. The Gut Microbiota-derived Metabolite Trimethylamine N-oxide is Elevated in Alzheimer’s Disease. Alzheimers Res. Ther. 2018, 10, 124. [Google Scholar] [CrossRef]
- Yu, L.; Meng, G.; Huang, B.; Zhou, X.; Stavrakis, S.; Wang, M.; Li, X.; Zhou, L.; Yuhong, W.; Wang, M.; et al. A Potential Relationship Between Gut Microbes and Atrial Fibrillation: Trimethylamine-N-oxide, a Gut Microbe-Derived Metabolite, Facilitates the Progression of Atrial Fibrillation. Int. J. Cardiol. 2018, 225, 92–98. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins 2017, 9, 92. [Google Scholar] [CrossRef]
- Vanholder, R.; Pletinck, A.; Schepers, E.; Glorieux, G. Biochemical and Clinical Impact of Organic Uremic Retention Solutes: A Comprehensive Update. Toxins 2018, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.C.; Glorieux, G.; Smet, R.D.; Deyn, P.P.D. Low Water-Soluble Uremic Toxins. Adv. Ren. Replace. Ther. 2003, 10, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Damaso, E.O.; Damaso, N.O.; Esparragon, F.R.; Payan, J.; Laborda, E.B.; Cabrera, F.G.; Estupinan, R.S.; Perez, J.C.R. Asymmetric (ADMA) and Symmetric (SDMA) Dimethylarganines in Chronic Kidney Disease: A Clinical Approach. Int. J. Mol. Sci. 2019, 20, 3668. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.C.; Sirich, T.L. Indoxyl Sulfate-Review of Toxicity and Therapeutic Strategies. Toxins 2016, 8, 358. [Google Scholar] [CrossRef]
- Yu, M.; Kim, Y.J.; Kang, D.H. Indoxyl Sulfate-Induced Endothelial Dysfunction in Patients with Chronic Kidney Disease via an Induction of Oxidative Stress. Clin. J. Am. Soc. Nephrol. 2011, 6, 30–39. [Google Scholar] [CrossRef]
- Lin, Y.T.; Wu, P.H.; Liang, S.S.; Mubanga, M.; Yang, Y.H.; Hsu, Y.L.; Kuo, M.C.; Hwang, S.J.; Kuo, P.L. Protein-Bound Uremic Toxins are Associated with Cognitive Function Among Patients Undergoing Maintenance Hemodialysis. Sci. Rep. 2019, 9, 20388. [Google Scholar] [CrossRef]
- Banoglu, E.; King, R.S. Sulfation of Indoxyl by Human and Rat Aryl (Phenol) Sulfotransferases to Form Indoxyl Sulfate. Eur. J. Drug. Metab. Pharmacokinet. 2002, 27, 135–140. [Google Scholar] [CrossRef]
- Gevi, F.; Zolla, L.; Gabriele, S.; Persico, A.M. Urinary Metabolomics of Young Italian Autistic Children Supports Abnormal Tryptophan and Purine Metabolism. Mol. Autism 2016, 7, 47. [Google Scholar] [CrossRef]
- Cassani, E.; Barichella, M.; Cancello, R.; Cavanna, F.; Iorio, L.; Cereda, E.; Bolliri, C.; Maria, P.Z.; Bianchi, F.; Cestaro, B.; et al. Increased Urinary Indoxyl Sulfate (Indican): New Insight Into Gut Dysbiosis in Parkinson’s Disease. Park. Relat. Disord. 2015, 21, 389–393. [Google Scholar] [CrossRef]
- Ohtsuki, S.; Asaba, H.; Takanaga, H.; Deguchi, T.; Hosoya, K.; Otagiri, M.; Terasaki, T. Role of Blood-Brain Barrier Organic Anion Transporter 3 (OAT3) in the Efux of Indoxyl Sulfate, a Uremic Toxin: Its Involvement in Neurotransmitter Metabolite Clearance from the Brain. J. Neurochem. 2022, 83, 57–66. [Google Scholar] [CrossRef]
- Adesso, S.; Magnus, T.; Cuzzocrea, S.; Campolo, M.; Rissiek, B.; Paciello, O.; Autore, G.; Pinto, A.; Marzocco, S. Indoxyl Sulfate Afects Glial Function Increasing Oxidative Stress and Neuroinfammation in Chronic Kidney Disease: Interaction Between Astrocytes and Microglia. Front. Pharmacol. 2017, 8, 370. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.C.; DiNatale, B.C.; Murray, I.A.; Flaveny, C.A.; Liu, Q.; Laurenzana, E.M.; Lin, J.M.; Strom, S.C.; Omiecinski, C.J.; Amin, S.; et al. The Uremic Toxin 3-Indoxyl Sulfate in s Potent Endogenous Agonist for the Human Aryl Hydrocarbon Receptor. Biochemistry 2010, 49, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Lamas, B.; Natividad, J.M.; Sokol, H. Aryl Hydrocarbon Receptor and Intestinal Immunity. Mucosal Immunol. 2018, 11, 1024–1038. [Google Scholar] [CrossRef] [PubMed]
- Altieri, L.; Neri, C.; Sacco, R.; Curatolo, P. Urinary p-Cresol is Elevated in Small Children with Severe Autism Spectrum Disorder. Biomarkers 2011, 16, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, S.; Sacco, R.; Cerullo, S.; Neri, C.; Urbani, A.; Tripi, G.; Malvy, J.; Barthelemy, C.; Brihault, F.B.; Persico, A.M. Urinary p-Cresol is Elevated in Young French Children with Autism Spectrum Disorder: A Replication Study. Biomarkers 2014, 19, 463–470. [Google Scholar] [CrossRef]
- Angelis, M.D.; Piccolo, M.; Vannini, L.; Siragusa, S.; Giacomo, A.D.; Serrazzanetti, D.I.; Cristofori, F.; Guerzoni, M.E.; Gobbetti, M.; Francavilla, R. Fecal Microbiota and Metabolome of Children with Autism and Pervasive Developmental Disorder not Otherwise Specified. PLoS ONE 2013, 8, e76993. [Google Scholar] [CrossRef]
- Kang, D.W.; Ilhan, Z.E.; Isern, N.G.; Hoyt, D.W.; Howsmon, D.P.; Shaffer, M.; Lozupone, C.A.; Hahn, J.; Adams, J.B.; Brown, R.K. Differences in Fecal Microbial Metabolites and Microbiota of Children with Autism Spectrum Disorders. Anaerobe 2018, 49, 121–131. [Google Scholar] [CrossRef]
- Mouridsen, S.E.; Isager, T.; Rich, B. Diseases of the Gastrointestinal Tract in Individuals Diagnosed as Children with Atypical Autism: A Danish Register Study Based on Hospital Diagnoses. Autism 2013, 17, 55–63. [Google Scholar] [CrossRef]
- Zheng, Y.; Bek, M.K.; Prince, B.Z.; Marzal, L.N.P.; Garssen, J.; Pardo, P.P.; Kraneveld, A.D. The Role of Bacterial-Derived Aromatic Amino Acids Metabolites Relevant in Autism Spectrum Disorders: A Comprehensive Review. Front. Neurosci. 2021, 15, 738220. [Google Scholar] [CrossRef]
- Zheng, Y.; Prince, N.Z.; Marzal, L.N.P.; Ahmed, S.; Garssen, J.; Pardo, P.P.; Kraneveld, A.D. The Autism Spectrum Disorder-Associated Bacterial Metabolite p-Cresol Derails the Neuroimmune Response of Microglial Cells Partially via Reduction of ADAM17 and ADAM10. Int. J. Mol. Sci. 2022, 23, 11013. [Google Scholar] [CrossRef]
- Gabriele, S.; Sacco, R.; Persico, A.M. Blood Serotonin Levels in Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Eur. Neuropsychopharmacol. 2014, 24, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Persico, A.M.; Napolioni, V. Autism Genetics. Behav. Brain Res. 2013, 251, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Adams, J.B.; Vargason, T.; Santiago, M.; Hahn, J.; Krajmalnik-Brown, R. Distinct Fecal and Plasma Metabolites in Children with Autism Spectrum Disorders and Their Modulation after Microbiota Transfer Therapy. mSphere 2020, 5, e00314-20. [Google Scholar] [CrossRef]
- Needham, B.D.; Adame, M.D.; Serena, G.; Rose, D.R.; Preston, G.M.; Conrad, M.C.; Campbell, A.S.; Donabedian, D.H.; Fasano, A.; Ashwood, P.; et al. Plasma and Fecal Metabolite Profiles in Autism Spectrum Disorder. Biol. Psychiatry 2021, 89, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Martin, P.; Becker, J.A.J.; Caramello, N.; Fernandez, S.P.; Costa-Campos, R.; Canaguier, J.; Barbosa, S.; Martinez-Gili, L.; Myridakis, A.; Dumas, M.E.; et al. The Microbial Metabolite p-Cresol Induces Autistic-like Behaviors in Mice by Remodeling the Gut Microbiota. Microbiome 2021, 9, 157. [Google Scholar] [CrossRef]
- Zheng, Y.; Prince, N.; Hattem, C.; Garssen, J.; Pardo, P.P.; Kraneveld, A.D. The Interaction Between Intestinal Bacterial Metabolites and Phosphatase and Tensin Homolog in Autism Spectrum Disorder. Mol. Cell. Neurosci. 2023, 124, 103805. [Google Scholar] [CrossRef] [PubMed]
- Karbowska, M.; Hermanowicz, J.M.; Tankiewicz-Kwedlo, A.; Kalaska, B.; Kaminski, T.W.; Nosek, K.; Wisniewska, R.J.; Pawlak, D. Neurobehavioral Effects of Uremic Toxin–Indoxyl Sulfate in the Rat Model. Sci. Rep. 2020, 10, 9483. [Google Scholar] [CrossRef]
- Olesova, D.; Galba, J.; Piestansky, J.; Celusakova, H.; Repiska, G.; Babinska, K.; Ostatnikova, D.; Katina, S.; Kovac, A. A Novel UHPLC-MS Method Targeting Urinary Metabolomic Markers for Autism Spectrum Disorder. Metabolites 2020, 10, 443. [Google Scholar] [CrossRef]
- Smogorzewski, M.; Campese, V.M.; Massry, S.G. Abnormal norepinephrine uptake and release in brain synaptosomes in chronic renal failure. Kidney Int. 1989, 36, 458–465. [Google Scholar] [CrossRef]
- Ma, K.; Zheng, Z.-R.; Meng, Y. Pathogenesis of Chronic Kidney Disease Is Closely Bound up with Alzheimer’s Disease, Especially via the Renin-Angiotensin System. J. Clin. Med. 2023, 12, 1459. [Google Scholar] [CrossRef]
- Pieniazek, A.; Bernasinska-Slomczewska, J.; Gwozdzinski, L. Uremic Toxins and Their Relation with Oxidative Stress Induced in Patients with Ckd. Int. J. Mol. Sci. 2021, 22, 6196. [Google Scholar] [CrossRef] [PubMed]
- Liabeuf, S.; Pepin, M.; Franssen, C.F.M.; Viggiano, D.; Carriazo, S.; Gansevoort, R.T.; Gesualdo, L.; Hafez, G.; Malyszko, J.; Mayer, C.; et al. Chronic kidney disease and neurological disorders: Are uraemic toxins the missing piece of the puzzle? Nephrol. Dial. Transplant. 2021, 37 (Suppl. S2), ii33–ii44. [Google Scholar] [CrossRef] [PubMed]
- Lekawanvijit, S. Cardiotoxicity of Uremic Toxins: A Driver of Cardiorenal Syndrome. Toxins 2018, 10, 352. [Google Scholar] [CrossRef]
- Felice, V.D.; O’Mahony, S.M. The Microbiome and Disorders of the Central Nervous System. Pharmacol. Biochem. Behav. 2017, 160, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, J.-M. The Microbiota–Gut–Brain Axis and its Potential Therapeutic Role in Autism Spectrum Disorder. Neuroscience 2016, 324, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Watanabe, T.; Nakayama, M. Cerebro-Renal Interactions: Impact of Uremic Toxins on Cognitive Function. Neurotoxicology 2014, 44, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Diémé, B.; Mavel, S.; Blasco, H.; Tripi, G.; Bonnet-Brilhault, F.; Malvy, J.; Bocca, C.; Andres, C.R.; Nadal-Desbarats, L.; Emond, P. Metabolomics Study of Urine in Autism Spectrum Disorders Using a Multiplatform Analytical Methodology. J. Proteome Res. 2015, 14, 5273–5282. [Google Scholar] [CrossRef]
- Gątarek, P.; Kalużna-Czaplińska, J. Trimethylamine N-Oxide (TMAO) in Human Health. EXCLI J. 2021, 20, 301–319. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef]
- Mu, C.; Corley, M.J.; Lee, R.W.Y.; Wong, M.; Pang, A.; Arakaki, G.; Miyamoto, R.; Rho, J.M.; Mickiewicz, B.; Dowlatabadi, R.; et al. Metabolic Framework for the Improvement of Autism Spectrum Disorders by a Modified Ketogenic Diet: A Pilot Study. J. Proteome Res. 2019, 19, 382–390. [Google Scholar] [CrossRef]
- Quan, L.; Yi, J.; Zhao, Y.; Zhang, F.; Shi, X.-T.; Feng, Z.; Miller, H.L. Plasma Trimethylamine N-Oxide, a Gut Microbe-Generated Phosphatidylcholine Metabolite, is Associated with Autism Spectrum Disorders. Neurotoxicology 2020, 76, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ke, Y.; Zhan, R.; Liu, C.; Zhao, M.; Zeng, A.; Shi, X.; Ji, L.; Cheng, S.; Pan, B.; et al. Trimethylamine-N -Oxide Promotes Brain Aging and Cognitive Impairment in Mice. Aging Cell. 2018, 17, e12768. [Google Scholar] [CrossRef] [PubMed]
- Molnar, T.; Pusch, G.; Nagy, L.; Keki, S.; Berki, T.; Illes, Z. Correlation of the L-Arginine Pathway with Thrombo-Inflammation May Contribute to the Outcome of Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2016, 25, 2055–2060. [Google Scholar] [CrossRef] [PubMed]
- Sankowski, B.; Księżarczyk, K.; Raćkowska, E.; Szlufik, S.; Koziorowski, D.; Giebułtowicz, J. Higher Cerebrospinal Fluid to Plasma Ratio of p-Cresol Sulfate and Indoxyl Sulfate in Patients with Parkinson’s Disease. Clin. Chim. Acta 2020, 501, 165–173. [Google Scholar] [CrossRef]
- Reyhani, A.; Celik, Y.; Karadag, H.; Gunduz, O.; Asil, T.; Sut, N. High Asymmetric Dimethylarginine, Symmetric Dimethylarginine and L-Arginine Levels in Migraine Patients. Neurol. Sci. 2017, 38, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Mommersteeg, P.M.C.; Schoemaker, R.G.; Eisel, U.L.M.; Garrelds, I.M.; Schalkwijk, C.G.; Kop, W.J. Nitric Oxide Dysregulation in Patients with Heart Failure: The Association of Depressive Symptoms with L-Arginine, Asymmetric Simethylarginine, Symmetric Dimethylarginine, and Isoprostane. Psychosom. Med. 2015, 77, 292–302. [Google Scholar] [CrossRef]
- Bugnicourt, J.M.; Godefroy, O.; Chillon, J.C.; Choukroun, G.; Massy, Z.A. Cognitive Disorders and Dementia in CKD: The Neglected Kidney-Brain Axis. J. Am. Soc. Nephrol. 2013, 24, 353–363. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; DSM-5; American Psychiatric Publishing: Philadelphia, PA, USA, 2013; ISBN 978-08-9042-555-8. [Google Scholar]
- Doernberg, E.; Hollander, E. Neurodevelopmental Disorders (ASD and ADHD): DSM-5, ICD-10, and ICD-11. CNS Spectr. 2016, 21, 295–299. [Google Scholar] [CrossRef]
- Lord, C.; Bishop, S.L. Recent Advances in Autism Research as Reflected in DSM-5 Criteria for Autism Spectrum Disorder. Annu. Rev. Clin. Psychol. 2015, 11, 53–70. [Google Scholar] [CrossRef]
- Autism Speaks. Autism Diagnosis Criteria: DSM-5. Available online: https://www.autismspeaks.org/autism-diagnosis-criteria-dsm-5 (accessed on 10 February 2023).
- Ožek, S.R.; Werdonig, A.; Kaučič, K.S.; Haskić, A.T.; Maček, J. Otroci z avtističnimi motnjami. In Kriteriji za Opredelitev Vrste in Stopnje Primanjkljajev, Over oz. Motenj Otrok s Posebnimi Potrebami, 2nd ed.; Ornik, N.V., Ed.; Zavod RS za šolstvo: Ljubljana, Slovenija, 2015; pp. 36–38. ISBN 978-961-03-0316-9. [Google Scholar]
- Osredkar, J.; Gosar, D.; Maček, J.; Kumer, K.; Fabjan, T.; Finderle, P.; Šterpin, S.; Zupan, M.; Vrhovšek, M.J. Urinary Markers of Oxidative Stress in Children with Autism Spectrum Disorder (ASD). Antioxidants 2019, 8, 187. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority Over-Sampling Technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Vovk-Ornik, N. Kriteriji za Opredelitev Vrste in Stopnje Primanjkljajev, Ovir oz. Motenj Otrok s Posebnimi Potrebami; Zavod RS za Šolstvo: Ljubljana, Slovenija, 2015; ISBN 978-96-1030-249-0. [Google Scholar]

| Unit [µg/mg] | Min | Max | Media | SD | RSD | 25–75 P | Normal Distr. |
|---|---|---|---|---|---|---|---|
| TMAO | 3.14 | 3689.53 | 53.96 | 346.49 | 3.12 | 33.56–81.70 | <0.0001 |
| c–TMAO | 15.53 | 296.80 | 56.94 | 59.99 | 0.79 | 32.71–103.30 | <0.0001 |
| ADMA | 1.01 | 179.64 | 15.93 | 27.05 | 1.10 | 10.38–27.41 | <0.0001 |
| c–ADMA | 7.07 | 69.24 | 24.62 | 13.40 | 0.47 | 19.89–31.34 | 0.0003 |
| SDMA | 2.06 | 360.46 | 26.37 | 46.81 | 1.18 | 18.16–41.95 | <0.0001 |
| c–SDMA | 15.44 | 100.31 | 38.95 | 19.05 | 0.41 | 33.19–58.67 | 0.0008 |
| IS | 3.15 | 1689.66 | 55.64 * | 206.87 | 1.89 | 32.76–112.09 | <0.0001 |
| c–IS | 4.37 | 211.55 | 52.62 | 42.67 | 0.65 | 35.33–86.15 | 0.0002 |
| pCS | 0.37 | 2161.24 | 93.68 * | 262.81 | 1.52 | 41.08–206.85 | <0.0001 |
| c–pCS | 1.29 | 398.93 | 73.99 | 93.75 | 0.90 | 39.82–117.80 | <0.0001 |
| Compound | Lower Limit | Upper Limit |
|---|---|---|
| TMAO | 15.84 | 285.47 |
| SDMA | 16.85 | 97.09 |
| ADMA | 7.97 | 66.07 |
| IS | 6.28 | 198.91 |
| pCS | 1.77 | 382.35 |
| Patients | Controls | |
|---|---|---|
| N | 143 | 48 |
| Female | 17 | 25 |
| Male | 126 | 23 |
| Age | ||
| Average | 9.9 ± 3.5 | 9.2 ± 3.9 |
| Under 8 Years | 46 | 20 |
| Above 8 Years | 97 | 28 |
| Classification 1 | 48 | |
| Mild | 71 | |
| Moderate | 60 | |
| Severe | 12 | |
| Classification 2 | 48 | |
| ASD 1 | 105 | |
| HFA 2 | 38 | |
| Additional Diagnosis | 48 | |
| No | 57 | |
| Yes 3 | 86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osredkar, J.; Baškovič, B.Ž.; Finderle, P.; Bobrowska-Korczak, B.; Gątarek, P.; Rosiak, A.; Giebułtowicz, J.; Vrhovšek, M.J.; Kałużna-Czaplińska, J. Relationship between Excreted Uremic Toxins and Degree of Disorder of Children with ASD. Int. J. Mol. Sci. 2023, 24, 7078. https://doi.org/10.3390/ijms24087078
Osredkar J, Baškovič BŽ, Finderle P, Bobrowska-Korczak B, Gątarek P, Rosiak A, Giebułtowicz J, Vrhovšek MJ, Kałużna-Czaplińska J. Relationship between Excreted Uremic Toxins and Degree of Disorder of Children with ASD. International Journal of Molecular Sciences. 2023; 24(8):7078. https://doi.org/10.3390/ijms24087078
Chicago/Turabian StyleOsredkar, Joško, Barbara Žvar Baškovič, Petra Finderle, Barbara Bobrowska-Korczak, Paulina Gątarek, Angelina Rosiak, Joanna Giebułtowicz, Maja Jekovec Vrhovšek, and Joanna Kałużna-Czaplińska. 2023. "Relationship between Excreted Uremic Toxins and Degree of Disorder of Children with ASD" International Journal of Molecular Sciences 24, no. 8: 7078. https://doi.org/10.3390/ijms24087078
APA StyleOsredkar, J., Baškovič, B. Ž., Finderle, P., Bobrowska-Korczak, B., Gątarek, P., Rosiak, A., Giebułtowicz, J., Vrhovšek, M. J., & Kałużna-Czaplińska, J. (2023). Relationship between Excreted Uremic Toxins and Degree of Disorder of Children with ASD. International Journal of Molecular Sciences, 24(8), 7078. https://doi.org/10.3390/ijms24087078