Maternal Immune Activation Induced by Prenatal Lipopolysaccharide Exposure Leads to Long-Lasting Autistic-like Social, Cognitive and Immune Alterations in Male Wistar Rats
Abstract
1. Introduction
2. Results
2.1. Reproduction Data
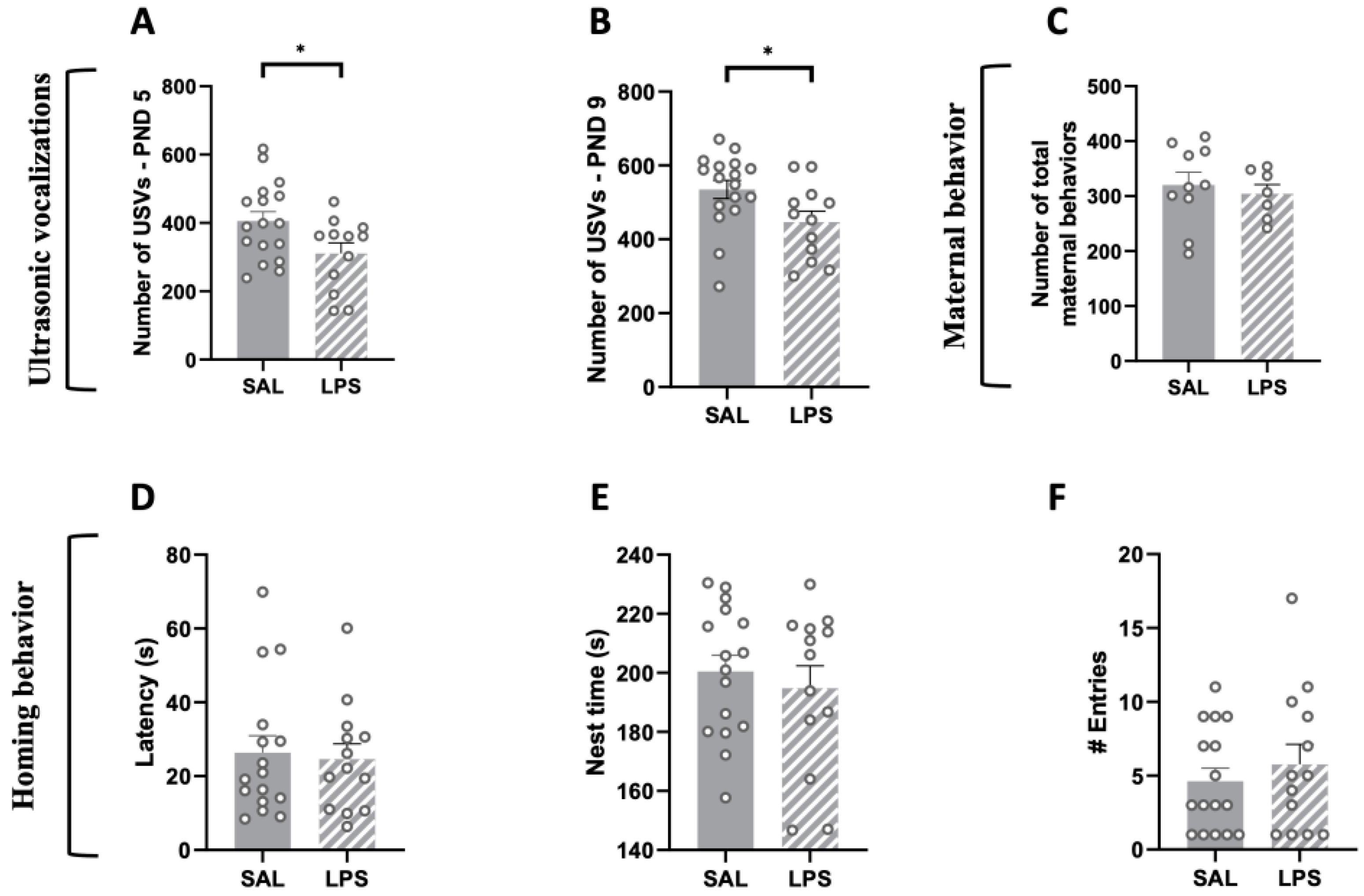
2.2. Prenatal Exposure to LPS Caused Deficits in Social Communication in Infant Rat Offspring, While Maternal Care and Pup Homing Behavior Were Unaffected
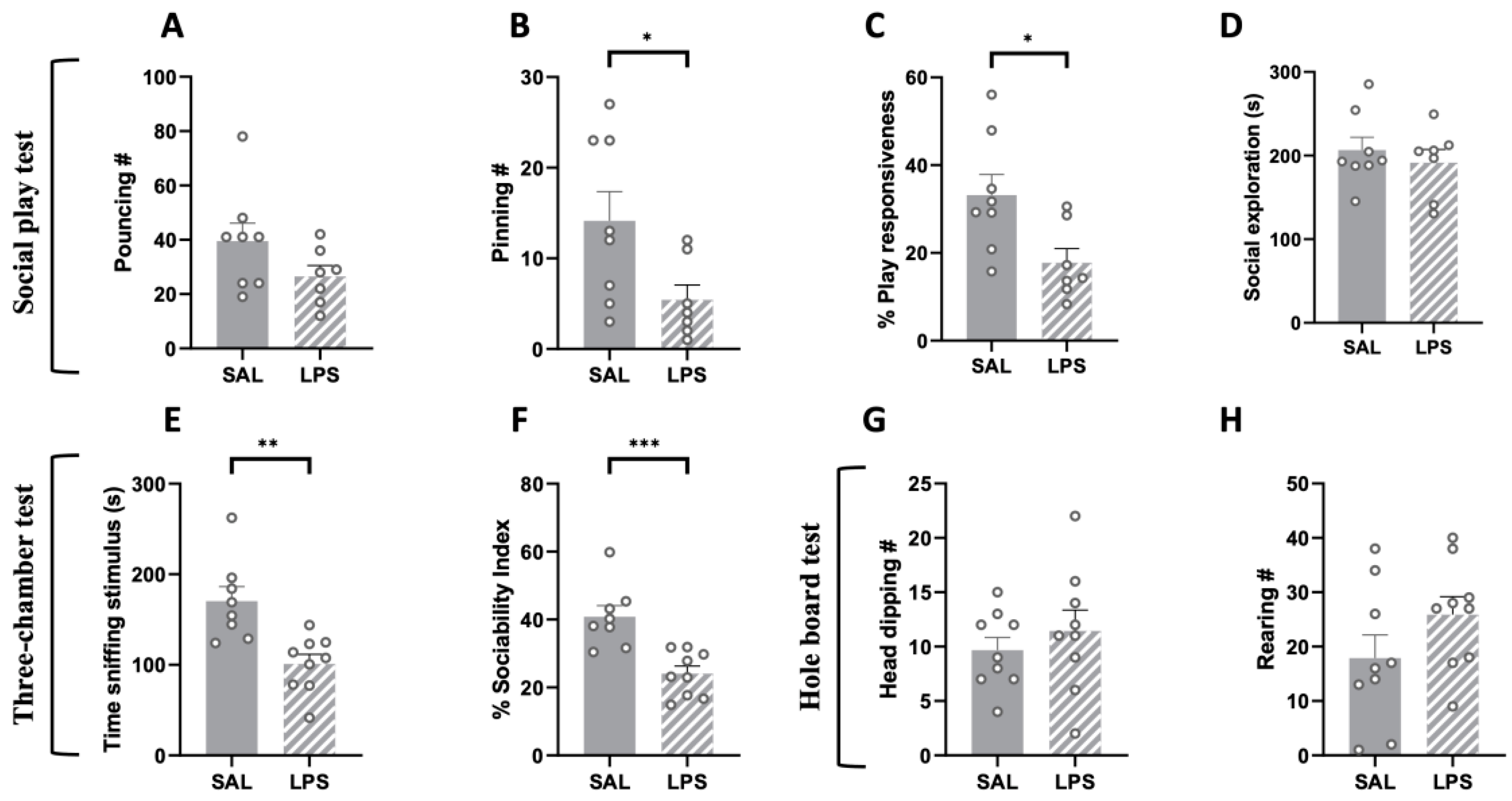
2.3. Prenatal Exposure to LPS Caused Deficits in Social Play Behavior and Sociability, but No Stereotypic Behaviors, in the Adolescent Offspring
2.4. Prenatal Exposure to LPS Induced Deficits in Social Discrimination and Sociability Together with Stereotypic-like Behaviors in the Adult Offspring, with No Changes in the Elevated Plus-Maze Test
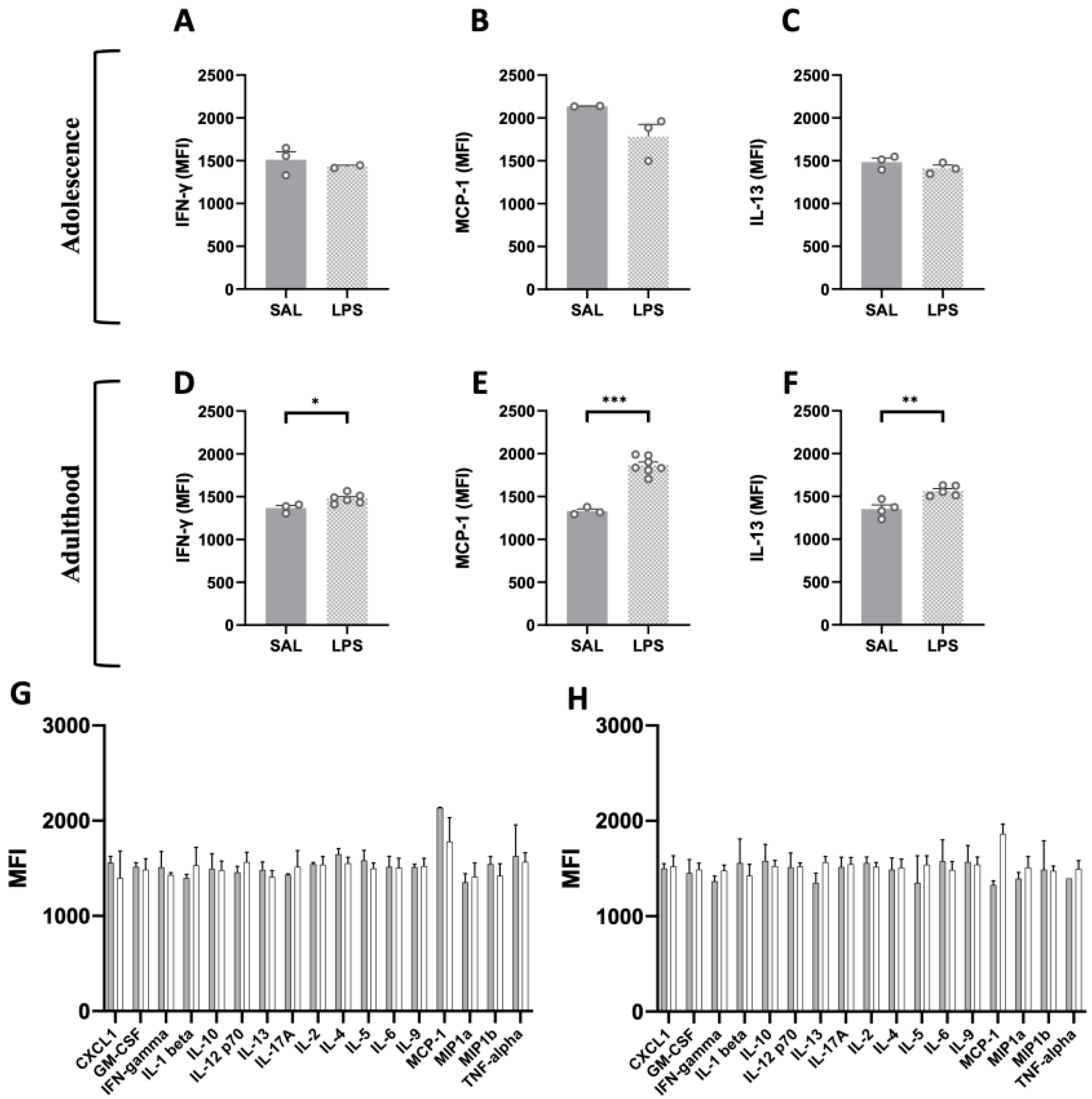
2.5. Flow Cytometric Analysis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Reproduction Data
4.4. Maternal Behavior Observation
4.5. Isolation-Induced Ultrasonic Vocalizations
4.6. Homing Behavior
4.7. Social Play Behavior
4.8. Three-Chamber Test
4.9. Hole Board Test
4.10. Social Discrimination Test
4.11. Elevated Plus-Maze Test
4.12. Flow Cytometric Analysis
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASD | Autism Spectrum Disorder. |
| CNS | Central nervous system. |
| GD | Gestational day. |
| i.p. | Intraperitoneal. |
| LPS | Lipopolysaccharide. |
| MIA | Maternal immune activation. |
| NDDs | Neurodevelopmental disorders. |
| PND | Postnatal day. |
| poly(I:C) | Polyinosinic:polycytidylic. |
| S.D. | Standard Deviation. |
| S.E.M. | Standard Error Mean. |
| SAL | Saline. |
| TLR-4 | Toll-like receptor 4. |
| USV | Isolation-induced ultrasonic vocalizations. |
| VPA | Valproic acid. |
References
- Smith, S.E.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.B.; Yim, Y.S.; Wong, H.; Kim, S.; Kim, H.; Kim, S.V.; Hoeffer, C.A.; Littman, D.R.; Huh, J.R. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 2016, 351, 933–939. [Google Scholar] [CrossRef]
- Lins, B.R.; Marks, W.N.; Zabder, N.K.; Greba, Q.; Howland, J.G. Maternal Immune Activation during Pregnancy Alters the Behavior Profile of Female Offspring of Sprague Dawley Rats. eNeuro 2019, 6, e0437-18. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.V.; Moon, H.M.; Su, J.; Palmer, T.D.; Courchesne, E.; Pramparo, T. Maternal immune activation dysregulation of the fetal brain transcriptome and relevance to the pathophysiology of autism spectrum disorder. Mol. Psychiatry 2018, 23, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Quagliato, L.A.; de Matos, U.; Nardi, A.E. Maternal immune activation generates anxiety in offspring: A translational meta-analysis. Transl. Psychiatry 2021, 11, 245. [Google Scholar] [CrossRef]
- Ganguli, S.; Chavali, P.L. Intrauterine Viral Infections: Impact of Inflammation on Fetal Neurodevelopment. Front. Neurosci. 2021, 15, 771557. [Google Scholar] [CrossRef]
- Kealy, J.; Greene, C.; Campbell, M. Blood-brain barrier regulation in psychiatric disorders. Neurosci. Lett. 2020, 726, 133664. [Google Scholar] [CrossRef]
- Ratnayake, U.; Quinn, T.; Walker, D.W.; Dickinson, H. Cytokines and the neurodevelopmental basis of mental illness. Front. Neurosci. 2013, 7, 180. [Google Scholar] [CrossRef]
- Carbone, E.; Manduca, A.; Cacchione, C.; Vicari, S.; Trezza, V. Healing autism spectrum disorder with cannabinoids: A neuroinflammatory story. Neurosci. Biobehav. Rev. 2021, 121, 128–143. [Google Scholar] [CrossRef]
- Jash, S.; Sharma, S. Pathogenic Infections during Pregnancy and the Consequences for Fetal Brain Development. Pathogens 2022, 11, 193. [Google Scholar] [CrossRef]
- Solek, C.M.; Farooqi, N.; Verly, M.; Lim, T.K.; Ruthazer, E.S. Maternal immune activation in neurodevelopmental disorders. Dev. Dyn. 2018, 247, 588–619. [Google Scholar] [CrossRef]
- Martins-Filho, P.R.; Tanajura, D.M.; Santos, H.P., Jr.; Santos, V.S. COVID-19 during pregnancy: Potential risk for neurodevelopmental disorders in neonates? Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 250, 255–256. [Google Scholar] [CrossRef]
- Onore, C.; Careaga, M.; Ashwood, P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav. Immun. 2012, 26, 383–392. [Google Scholar] [CrossRef]
- Matta, S.M.; Hill-Yardin, E.L.; Crack, P.J. The influence of neuroinflammation in Autism Spectrum Disorder. Brain Behav. Immun. 2019, 79, 75–90. [Google Scholar] [CrossRef]
- Hameete, B.C.; Fernandez-Calleja, J.M.S.; de Groot, M.; Oppewal, T.R.; Tiemessen, M.M.; Hogenkamp, A.; de Vries, R.B.M.; Groenink, L. The poly(I:C)-induced maternal immune activation model; a systematic review and meta-analysis of cytokine levels in the offspring. Brain Behav. Immun. Health 2021, 11, 100192. [Google Scholar] [CrossRef]
- Woods, R.M.; Lorusso, J.M.; Potter, H.G.; Neill, J.C.; Glazier, J.D.; Hager, R. Maternal immune activation in rodent models: A systematic review of neurodevelopmental changes in gene expression and epigenetic modulation in the offspring brain. Neurosci. Biobehav. Rev. 2021, 129, 389–421. [Google Scholar] [CrossRef]
- Oskvig, D.B.; Elkahloun, A.G.; Johnson, K.R.; Phillips, T.M.; Herkenham, M. Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav. Immun. 2012, 26, 623–634. [Google Scholar] [CrossRef]
- Jiang, N.M.; Cowan, M.; Moonah, S.N.; Petri, W.A., Jr. The Impact of Systemic Inflammation on Neurodevelopment. Trends Mol. Med. 2018, 24, 794–804. [Google Scholar] [CrossRef]
- O’Loughlin, E.; Pakan, J.M.P.; Yilmazer-Hanke, D.; McDermott, K.W. Acute in utero exposure to lipopolysaccharide induces inflammation in the pre- and postnatal brain and alters the glial cytoarchitecture in the developing amygdala. J. Neuroinflamm. 2017, 14, 212. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Q.; Wang, J.; Tang, M.; Huang, S.; Peng, K.; Han, Y.; Zhang, J.; Liu, G.; Fang, Q.; et al. Maternal immune activation-induced PPARgamma-dependent dysfunction of microglia associated with neurogenic impairment and aberrant postnatal behaviors in offspring. Neurobiol. Dis. 2019, 125, 1–13. [Google Scholar] [CrossRef]
- Plociennikowska, A.; Hromada-Judycka, A.; Borzecka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. CMLS 2015, 72, 557–581. [Google Scholar] [CrossRef] [PubMed]
- Kirsten, T.B.; Lippi, L.L.; Bevilacqua, E.; Bernardi, M.M. LPS exposure increases maternal corticosterone levels, causes placental injury and increases IL-1Beta levels in adult rat offspring: Relevance to autism. PLoS ONE 2013, 8, e82244. [Google Scholar] [CrossRef] [PubMed]
- Solmaz, V.; Tekatas, A.; Erdogan, M.A.; Erbas, O. Exenatide, a GLP-1 analog, has healing effects on LPS-induced autism model: Inflammation, oxidative stress, gliosis, cerebral GABA, and serotonin interactions. Int. J. Dev. Neurosci. 2020, 80, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Xuan, I.C.; Hampson, D.R. Gender-dependent effects of maternal immune activation on the behavior of mouse offspring. PLoS ONE 2014, 9, e104433. [Google Scholar] [CrossRef]
- Kirsten, T.B.; Queiroz-Hazarbassanov, N.; Bernardi, M.M.; Felicio, L.F. Prenatal zinc prevents communication impairments and BDNF disturbance in a rat model of autism induced by prenatal lipopolysaccharide exposure. Life Sci. 2015, 130, 12–17. [Google Scholar] [CrossRef]
- Kirsten, T.B.; Bernardi, M.M. Prenatal lipopolysaccharide induces hypothalamic dopaminergic hypoactivity and autistic-like behaviors: Repetitive self-grooming and stereotypies. Behav. Brain Res. 2017, 331, 25–29. [Google Scholar] [CrossRef]
- Fernandez de Cossio, L.; Guzman, A.; van der Veldt, S.; Luheshi, G.N. Prenatal infection leads to ASD-like behavior and altered synaptic pruning in the mouse offspring. Brain Behav. Immun. 2017, 63, 88–98. [Google Scholar] [CrossRef]
- Shigemoto-Mogami, Y.; Hoshikawa, K.; Goldman, J.E.; Sekino, Y.; Sato, K. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J. Neurosci. 2014, 34, 2231–2243. [Google Scholar] [CrossRef]
- Musaelyan, K.; Egeland, M.; Fernandes, C.; Pariante, C.M.; Zunszain, P.A.; Thuret, S. Modulation of adult hippocampal neurogenesis by early-life environmental challenges triggering immune activation. Neural Plast. 2014, 2014, 194396. [Google Scholar] [CrossRef]
- Stolp, H.B.; Liddelow, S.A.; Sa-Pereira, I.; Dziegielewska, K.M.; Saunders, N.R. Immune responses at brain barriers and implications for brain development and neurological function in later life. Front. Integr. Neurosci. 2013, 7, 61. [Google Scholar] [CrossRef]
- Werneburg, S.; Feinberg, P.A.; Johnson, K.M.; Schafer, D.P. A microglia-cytokine axis to modulate synaptic connectivity and function. Curr. Opin. Neurobiol. 2017, 47, 138–145. [Google Scholar] [CrossRef]
- Mirabella, F.; Desiato, G.; Mancinelli, S.; Fossati, G.; Rasile, M.; Morini, R.; Markicevic, M.; Grimm, C.; Amegandjin, C.; Termanini, A.; et al. Prenatal interleukin 6 elevation increases glutamatergic synapse density and disrupts hippocampal connectivity in offspring. Immunity 2021, 54, 2611–2631.e8. [Google Scholar] [CrossRef]
- Masi, A.; Quintana, D.S.; Glozier, N.; Lloyd, A.R.; Hickie, I.B.; Guastella, A.J. Cytokine aberrations in autism spectrum disorder: A systematic review and meta-analysis. Mol. Psychiatry 2015, 20, 440–446. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, H.; Liu, S.; Luo, W.; Jiang, Y.; Gao, J. Association of Peripheral Blood Levels of Cytokines With Autism Spectrum Disorder: A Meta-Analysis. Front. Psychiatry 2021, 12, 670200. [Google Scholar] [CrossRef]
- Kirsten, T.B.; Chaves-Kirsten, G.P.; Chaible, L.M.; Silva, A.C.; Martins, D.O.; Britto, L.R.; Dagli, M.L.; Torrao, A.S.; Palermo-Neto, J.; Bernardi, M.M. Hypoactivity of the central dopaminergic system and autistic-like behavior induced by a single early prenatal exposure to lipopolysaccharide. J. Neurosci. Res. 2012, 90, 1903–1912. [Google Scholar] [CrossRef]
- Servadio, M.; Melancia, F.; Manduca, A.; di Masi, A.; Schiavi, S.; Cartocci, V.; Pallottini, V.; Campolongo, P.; Ascenzi, P.; Trezza, V. Targeting anandamide metabolism rescues core and associated autistic-like symptoms in rats prenatally exposed to valproic acid. Transl. Psychiatry 2016, 6, e902. [Google Scholar] [CrossRef]
- Melancia, F.; Schiavi, S.; Servadio, M.; Cartocci, V.; Campolongo, P.; Palmery, M.; Pallottini, V.; Trezza, V. Sex-specific autistic endophenotypes induced by prenatal exposure to valproic acid involve anandamide signalling. Br. J. Pharmacol. 2018, 175, 3699–3712. [Google Scholar] [CrossRef]
- Prieto, M.; Folci, A.; Poupon, G.; Schiavi, S.; Buzzelli, V.; Pronot, M.; Francois, U.; Pousinha, P.; Lattuada, N.; Abelanet, S.; et al. Missense mutation of Fmr1 results in impaired AMPAR-mediated plasticity and socio-cognitive deficits in mice. Nat. Commun. 2021, 12, 1557. [Google Scholar] [CrossRef]
- Schiavi, S.; Carbone, E.; Melancia, F.; Buzzelli, V.; Manduca, A.; Campolongo, P.; Pallottini, V.; Trezza, V. Perinatal supplementation with omega-3 fatty acids corrects the aberrant social and cognitive traits observed in a genetic model of autism based on FMR1 deletion in rats. Nutr. Neurosci. 2022, 25, 898–911. [Google Scholar] [CrossRef]
- Kirsten, T.B.; Chaves, G.P.; Taricano, M.; Martins, D.O.; Florio, J.C.; Britto, L.R.; Torrao, A.S.; Palermo-Neto, J.; Bernardi, M.M. Prenatal LPS exposure reduces olfactory perception in neonatal and adult rats. Physiol. Behav. 2011, 104, 417–422. [Google Scholar] [CrossRef]
- Trezza, V.; Vanderschuren, L.J. Cannabinoid and opioid modulation of social play behavior in adolescent rats: Differential behavioral mechanisms. Eur. Neuropsychopharmacol. 2008, 18, 519–530. [Google Scholar] [CrossRef]
- Chan, W.Y.; Kohsaka, S.; Rezaie, P. The origin and cell lineage of microglia: New concepts. Brain Res. Rev. 2007, 53, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Lim, S.; Hoeffel, G.; Low, D.; Huber, T. Origin and differentiation of microglia. Front. Cell. Neurosci. 2013, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Norris, G.T.; Kipnis, J. Immune cells and CNS physiology: Microglia and beyond. J. Exp. Med. 2019, 216, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Manduca, A.; Servadio, M.; Melancia, F.; Schiavi, S.; Manzoni, O.J.; Trezza, V. Sex-specific behavioural deficits induced at early life by prenatal exposure to the cannabinoid receptor agonist WIN55, 212–2 depend on mGlu5 receptor signalling. Br. J. Pharmacol. 2020, 177, 449–463. [Google Scholar] [CrossRef]
- Terry, L.M.; Johanson, I.B. Effects of altered olfactory experiences on the development of infant rats’ responses to odors. Dev. Psychobiol. 1996, 29, 353–377. [Google Scholar] [CrossRef]
- Bignami, G. Economical test methods for developmental neurobehavioral toxicity. Environ. Health Perspect. 1996, 104, 285–298. [Google Scholar]
- Servadio, M.; Vanderschuren, L.J.; Trezza, V. Modeling autism-relevant behavioral phenotypes in rats and mice: Do ’autistic’ rodents exist? Behav. Pharmacol. 2015, 26, 522–540. [Google Scholar] [CrossRef]
- Melancia, F.; Trezza, V. Modelling fragile X syndrome in the laboratory setting: A behavioral perspective. Behav. Brain Res. 2018, 350, 149–163. [Google Scholar] [CrossRef]
- Kirsten, T.B.; Taricano, M.; Maiorka, P.C.; Palermo-Neto, J.; Bernardi, M.M. Prenatal lipopolysaccharide reduces social behavior in male offspring. Neuroimmunomodulation 2010, 17, 240–251. [Google Scholar] [CrossRef]
- Kirsten, T.B.; Chaves-Kirsten, G.P.; Bernardes, S.; Scavone, C.; Sarkis, J.E.; Bernardi, M.M.; Felicio, L.F. Lipopolysaccharide Exposure Induces Maternal Hypozincemia, and Prenatal Zinc Treatment Prevents Autistic-Like Behaviors and Disturbances in the Striatal Dopaminergic and mTOR Systems of Offspring. PLoS ONE 2015, 10, e0134565. [Google Scholar] [CrossRef]
- Lai, M.C.; Lombardo, M.V.; Pasco, G.; Ruigrok, A.N.; Wheelwright, S.J.; Sadek, S.A.; Chakrabarti, B.; Baron-Cohen, S. A behavioral comparison of male and female adults with high functioning autism spectrum conditions. PLoS ONE 2011, 6, e20835. [Google Scholar] [CrossRef]
- Dworzynski, K.; Ronald, A.; Bolton, P.; Happe, F. How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 788–797. [Google Scholar] [CrossRef]
- Head, A.M.; McGillivray, J.A.; Stokes, M.A. Gender differences in emotionality and sociability in children with autism spectrum disorders. Mol. Autism 2014, 5, 19. [Google Scholar] [CrossRef]
- Singh, V.K. Plasma increase of interleukin-12 and interferon-gamma. Pathological significance in autism. J. Neuroimmunol. 1996, 66, 143–145. [Google Scholar] [CrossRef]
- Kordulewska, N.K.; Kostyra, E.; Piskorz-Ogorek, K.; Moszynska, M.; Cieslinska, A.; Fiedorowicz, E.; Jarmolowska, B. Serum cytokine levels in children with spectrum autism disorder: Differences in pro- and anti-inflammatory balance. J. Neuroimmunol. 2019, 337, 577066. [Google Scholar] [CrossRef]
- Toscano, C.V.A.; Barros, L.; Lima, A.B.; Nunes, T.; Carvalho, H.M.; Gaspar, J.M. Neuroinflammation in autism spectrum disorders: Exercise as a “pharmacological” tool. Neurosci. Biobehav. Rev. 2021, 129, 63–74. [Google Scholar] [CrossRef]
- Majerczyk, D.; Ayad, E.G.; Brewton, K.L.; Saing, P.; Hart, P.C. Systemic maternal inflammation promotes ASD via IL-6 and IFN-gamma. Biosci. Rep. 2022, 42, BSR20220713. [Google Scholar] [CrossRef]
- Ellis, T.N.; Beaman, B.L. Interferon-gamma activation of polymorphonuclear neutrophil function. Immunology 2004, 112, 2–12. [Google Scholar] [CrossRef]
- Burke, J.D.; Young, H.A. IFN-gamma: A cytokine at the right time, is in the right place. Semin. Immunol. 2019, 43, 101280. [Google Scholar] [CrossRef]
- Monteiro, S.; Ferreira, F.M.; Pinto, V.; Roque, S.; Morais, M.; de Sa-Calcada, D.; Mota, C.; Correia-Neves, M.; Cerqueira, J.J. Absence of IFNgamma promotes hippocampal plasticity and enhances cognitive performance. Transl. Psychiatry 2016, 6, e707. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.; Hill, M.D.; Rahimi, F.; Warden, L.A.; Halliday, G.M.; Shepherd, C.E. Monocyte chemoattractant protein-1 plays a dominant role in the chronic inflammation observed in Alzheimer’s disease. Brain Pathol 2009, 19, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Sugihara, G.; Ouchi, Y.; Nakamura, K.; Futatsubashi, M.; Takebayashi, K.; Yoshihara, Y.; Omata, K.; Matsumoto, K.; Tsuchiya, K.J.; et al. Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry 2013, 70, 49–58. [Google Scholar] [CrossRef]
- Shen, Y.; Ou, J.; Liu, M.; Shi, L.; Li, Y.; Xiao, L.; Dong, H.; Zhang, F.; Xia, K.; Zhao, J. Altered plasma levels of chemokines in autism and their association with social behaviors. Psychiatry Res. 2016, 244, 300–305. [Google Scholar] [CrossRef]
- Gomez-Fernandez, A.; de la Torre-Aguilar, M.J.; Gil-Campos, M.; Flores-Rojas, K.; Cruz-Rico, M.D.; Martin-Borreguero, P.; Perez-Navero, J.L. Children With Autism Spectrum Disorder With Regression Exhibit a Different Profile in Plasma Cytokines and Adhesion Molecules Compared to Children Without Such Regression. Front. Pediatr. 2018, 6, 264. [Google Scholar] [CrossRef]
- Kolosowska, N.; Keuters, M.H.; Wojciechowski, S.; Keksa-Goldsteine, V.; Laine, M.; Malm, T.; Goldsteins, G.; Koistinaho, J.; Dhungana, H. Peripheral Administration of IL-13 Induces Anti-inflammatory Microglial/Macrophage Responses and Provides Neuroprotection in Ischemic Stroke. Neurotherapeutics 2019, 16, 1304–1319. [Google Scholar] [CrossRef]
- Miao, W.; Zhao, Y.; Huang, Y.; Chen, D.; Luo, C.; Su, W.; Gao, Y. IL-13 Ameliorates Neuroinflammation and Promotes Functional Recovery after Traumatic Brain Injury. J. Immunol. 2020, 204, 1486–1498. [Google Scholar] [CrossRef]
- Konsman, J.P. Cytokines in the Brain and Neuroinflammation: We Didn’t Starve the Fire! Pharmaceuticals 2022, 15, 140. [Google Scholar] [CrossRef]
- Galic, M.A.; Riazi, K.; Pittman, Q.J. Cytokines and brain excitability. Front. Neuroendocrinol. 2012, 33, 116–125. [Google Scholar] [CrossRef]
- Werling, D.M. The role of sex-differential biology in risk for autism spectrum disorder. Biol. Sex Differ. 2016, 7, 58. [Google Scholar] [CrossRef]
- Werling, D.M.; Geschwind, D.H. Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 2013, 26, 146–153. [Google Scholar] [CrossRef]
- Napolitano, A.; Schiavi, S.; La Rosa, P.; Rossi-Espagnet, M.C.; Petrillo, S.; Bottino, F.; Tagliente, E.; Longo, D.; Lupi, E.; Casula, L.; et al. Sex Differences in Autism Spectrum Disorder: Diagnostic, Neurobiological, and Behavioral Features. Front. Psychiatry 2022, 13, 889636. [Google Scholar] [CrossRef]
- Ardalan, M.; Chumak, T.; Vexler, Z.; Mallard, C. Sex-Dependent Effects of Perinatal Inflammation on the Brain: Implication for Neuro-Psychiatric Disorders. Int. J. Mol. Sci. 2019, 20, 2270. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Govindaraj, S.; Shanmuganathan, A.; Rajan, R. Maternal psychological stress-induced developmental disability, neonatal mortality and stillbirth in the offspring of Wistar albino rats. PLoS ONE 2017, 12, e0171089. [Google Scholar] [CrossRef]
- Colucci, P.; De Castro, V.; Peloso, A.; Splendori, M.; Trezza, V.; Campolongo, P. Perinatal exposure to omega-3 fatty acid imbalance leads to early behavioral alterations in rat pups. Behav. Brain Res. 2020, 392, 112723. [Google Scholar] [CrossRef]
- Champagne, F.A.; Francis, D.D.; Mar, A.; Meaney, M.J. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 2003, 79, 359–371. [Google Scholar] [CrossRef]
- van Hasselt, F.N.; Boudewijns, Z.S.; van der Knaap, N.J.; Krugers, H.J.; Joels, M. Maternal care received by individual pups correlates with adult CA1 dendritic morphology and synaptic plasticity in a sex-dependent manner. J. Neuroendocrinol. 2012, 24, 331–340. [Google Scholar] [CrossRef]
- Servadio, M.; Manduca, A.; Melancia, F.; Leboffe, L.; Schiavi, S.; Campolongo, P.; Palmery, M.; Ascenzi, P.; di Masi, A.; Trezza, V. Impaired repair of DNA damage is associated with autistic-like traits in rats prenatally exposed to valproic acid. Eur. Neuropsychopharmacol. 2018, 28, 85–96. [Google Scholar] [CrossRef]
- Vanderschuren, L.J.; Achterberg, E.J.; Trezza, V. The neurobiology of social play and its rewarding value in rats. Neurosci. Biobehav. Rev. 2016, 70, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Schiavi, S.; Manduca, A.; Segatto, M.; Campolongo, P.; Pallottini, V.; Vanderschuren, L.; Trezza, V. Unidirectional opioid-cannabinoid cross-tolerance in the modulation of social play behavior in rats. Psychopharmacology 2019, 236, 2557–2568. [Google Scholar] [CrossRef] [PubMed]
- Niesink, R.J.; Van Ree, J.M. Involvement of opioid and dopaminergic systems in isolation-induced pinning and social grooming of young rats. Neuropharmacology 1989, 28, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Pellis, S.; Pellis, V. The Playful Brain: Venturing to the Limits of Neuroscience, eBook ed.; Oneworld Publications: London, UK, 2013; 258p. [Google Scholar]
- Schiavi, S.; Iezzi, D.; Manduca, A.; Leone, S.; Melancia, F.; Carbone, C.; Petrella, M.; Mannaioni, G.; Masi, A.; Trezza, V. Reward-Related Behavioral, Neurochemical and Electrophysiological Changes in a Rat Model of Autism Based on Prenatal Exposure to Valproic Acid. Front. Cell. Neurosci. 2019, 13, 479. [Google Scholar] [CrossRef] [PubMed]
- Schiavi, S.; Manduca, A.; Carbone, E.; Buzzelli, V.; Rava, A.; Feo, A.; Ascone, F.; Morena, M.; Campolongo, P.; Hill, M.N.; et al. Anandamide and 2-arachidonoylglycerol differentially modulate autistic-like traits in a genetic model of autism based on FMR1 deletion in rats. Neuropsychopharmacology 2022. [Google Scholar] [CrossRef] [PubMed]
- Manduca, A.; Morena, M.; Campolongo, P.; Servadio, M.; Palmery, M.; Trabace, L.; Hill, M.N.; Vanderschuren, L.J.; Cuomo, V.; Trezza, V. Distinct roles of the endocannabinoids anandamide and 2-arachidonoylglycerol in social behavior and emotionality at different developmental ages in rats. Eur. Neuropsychopharmacol. 2015, 25, 1362–1374. [Google Scholar] [CrossRef]
- Schneider, R., Jr.; Bandiera, S.; Souza, D.G.; Bellaver, B.; Caletti, G.; Quincozes-Santos, A.; Elisabetsky, E.; Gomez, R. N-acetylcysteine Prevents Alcohol Related Neuroinflammation in Rats. Neurochem. Res. 2017, 42, 2135–2141. [Google Scholar] [CrossRef]
- Pierozan, P.; Jerneren, F.; Ransome, Y.; Karlsson, O. The Choice of Euthanasia Method Affects Metabolic Serum Biomarkers. Basic Clin. Pharmacol. Toxicol. 2017, 121, 113–118. [Google Scholar] [CrossRef]

| Group | Gestation Length (Days) | Successful Deliveries (%) | Litter Size at Birth | Male/Female Ratio | Pups’ Weight | Postnatal Vitality (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| PND1 | PND5 | PND9 | PND13 | ||||||
| SAL | 22.24 ± 0.14 | 94.44 ± 5.56 | 14.47 ± 0.82 | 1.39 ± 0.26 | 7.42 ± 0.22 | 12.76 ± 0.20 | 22.83 ± 0.60 | 32.94 ± 0.53 | 100 ± 0 |
| LPS | 21.88 ± 0.12 | 60.65 ± 13.52 | 14.06 ± 0.75 | 1.17 ± 0.21 | 7.99 ± 0.33 | 13.33 ± 0.31 | 21.50 ± 0.54 | 33.21 ± 0.53 | 100 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbone, E.; Buzzelli, V.; Manduca, A.; Leone, S.; Rava, A.; Trezza, V. Maternal Immune Activation Induced by Prenatal Lipopolysaccharide Exposure Leads to Long-Lasting Autistic-like Social, Cognitive and Immune Alterations in Male Wistar Rats. Int. J. Mol. Sci. 2023, 24, 3920. https://doi.org/10.3390/ijms24043920
Carbone E, Buzzelli V, Manduca A, Leone S, Rava A, Trezza V. Maternal Immune Activation Induced by Prenatal Lipopolysaccharide Exposure Leads to Long-Lasting Autistic-like Social, Cognitive and Immune Alterations in Male Wistar Rats. International Journal of Molecular Sciences. 2023; 24(4):3920. https://doi.org/10.3390/ijms24043920
Chicago/Turabian StyleCarbone, Emilia, Valeria Buzzelli, Antonia Manduca, Stefano Leone, Alessandro Rava, and Viviana Trezza. 2023. "Maternal Immune Activation Induced by Prenatal Lipopolysaccharide Exposure Leads to Long-Lasting Autistic-like Social, Cognitive and Immune Alterations in Male Wistar Rats" International Journal of Molecular Sciences 24, no. 4: 3920. https://doi.org/10.3390/ijms24043920
APA StyleCarbone, E., Buzzelli, V., Manduca, A., Leone, S., Rava, A., & Trezza, V. (2023). Maternal Immune Activation Induced by Prenatal Lipopolysaccharide Exposure Leads to Long-Lasting Autistic-like Social, Cognitive and Immune Alterations in Male Wistar Rats. International Journal of Molecular Sciences, 24(4), 3920. https://doi.org/10.3390/ijms24043920





