Gender Differences in Cortisol and Cortisol Receptors in Depression: A Narrative Review
Abstract
:1. Introduction
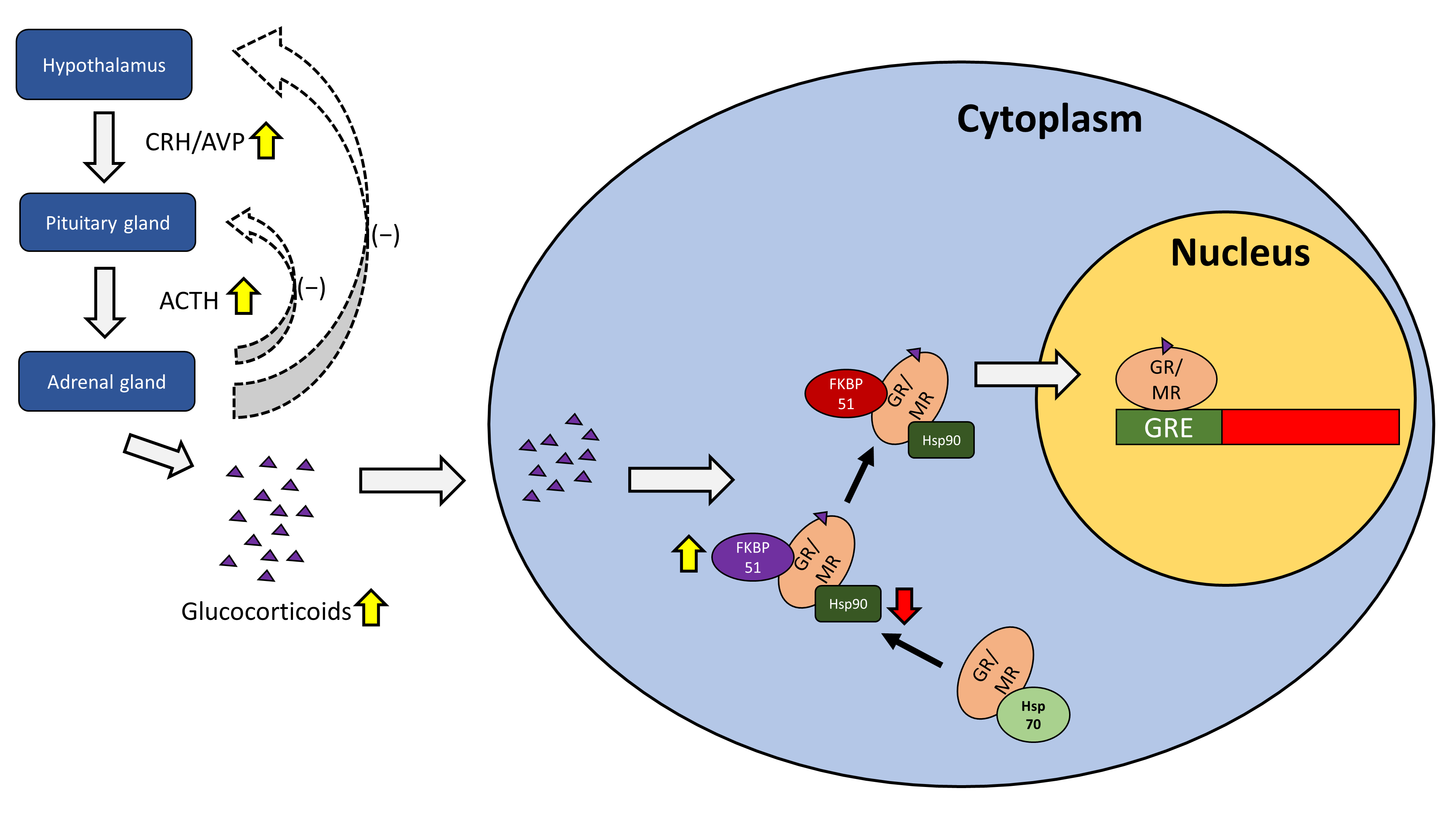
2. Structure and Function of Glucocorticoid and Mineralocorticoid Receptors in the HPA Axis
3. Gender Differences of Cortisol in Clinical Studies
3.1. Recent Findings in Gender Differences of Cortisol
| Reference Number | Country | Type of Study | Duration | Sample Size | Age Group (year) | Type of Cortisol Sample | Main Conclusions |
|---|---|---|---|---|---|---|---|
| [8] | Nigeria | Cross-sectional | 3 months | 88 | 17–25 | Saliva | No significant correlation between salivary parameters and depression scores among both genders. |
| [35] | United Kingdom | Longitudinal | 3 days | 841 | 15 | Saliva | No difference in association of cortisol awakening pattern and depression according to gender. |
| [36] | Ecuador | Cross-sectional | 4 months | 522 | 11–17 | Saliva | No significant association between depression and cortisol according to gender. |
| [37] | Canada | Longitudinal | 2 years | 409 | 3–5 | Saliva | Lower cortisol reactivity in female children and high cortisol reactivity in male children showed negative correlation between depressive symptoms and time. |
| [38] | China | Longitudinal | 3 months | 85 | 10–12 | Hair, Saliva | Positive correlation among depressive symptoms and hair cortisol seen in males but not females. |
| [39] | Canada | Cross-sectional | 1 week | 111 | 12–18 | Saliva | Higher cortisol reactivity seen in depressed males but not females. |
| [40] | United Kingdom | Longitudinal | 3 years | 1858 | Mean = 13 | Saliva | High morning cortisol levels increases risk of depressive disorder among male children with high depression but not females. |
| [41] | China | Longitudinal | 1 year | 712 | 8–11 | Saliva | High level of cortisol associated with risk of depressive symptoms in both male and female children, but moderate level of cortisol only shows the same association for males and not females. Low cortisol levels seem to be a protective factor only for male children. |
| [42] | United States | Longitudinal | 3 days | 300 | 16–18 | Saliva | Measurement of cortisol awakening response and rhythmicity; no gender differences noted. |
| [43] | Canada | Longitudinal | 2 days | 120 | 20–45 | Saliva | Gender does not play a role between both groups. Depression associated with lower morning cortisol. |
| [44] | United States, Belgium | Continuous-time process model | 30 days | 621 | 18–61 | Saliva | No relationship between gender, cortisol, and depression. Variability of cortisol associated with gender but not depression. Regulation strength of cortisol associated with depression (specifically chronic depression) but not gender. |
| [45] | Pakistan | Cross-sectional | 8 months | 60 | >17 | Saliva | Mean cortisol levels are not significantly different among depressed males and females. |
| [46] | United States | Exploratory | 1 month | 162 | 12–19 | Saliva | No difference in cortisol pattern in depressed males and females. |
| [47] | United States | Exploratory | 2 days | 60 | Mean = 43.84 | Saliva | No significant difference in females and males between depressive symptoms and diurnal cortisol slopes. |
| [48] | Brazil | Cross-sectional | Unavailable | 256 | >65 | Saliva | No significance in cortisol and depression relation to gender. |
| [49] | United States | Cross-sectional | 2 weeks | 196 | 18–21 | Saliva | Lower cortisol level in depressed females but higher cortisol levels seen in depressed males. |
| [50] | China | Longitudinal | 3 days | 80 | 18–45 | Saliva, Serum | Measurement of cortisol awakening response and rhythmicity; no gender differences noted. |
| [51] | United Kingdom | Cross-sectional | 1 days | 80 | 20–65 | Saliva | No gender differences noted in salivary cortisol. |
| [52] | Germany | Longitudinal | 3 weeks | 50 | 18–65 | Saliva | No gender differences noted in salivary cortisol. |
| [57] | Netherlands | Systematic review and meta-analysis | 24 years | 1270 | Mean = 30.5 | Saliva, Serum | Females with depressive disorder had blunted cortisol response while males had increased cortisol response. |
| Reference Number | Country | Type of Study | Duration | Sample Size | Age Group (Year) | Type of Cortisol Sample | Main Conclusions |
|---|---|---|---|---|---|---|---|
| [53] | Russia | Cohort | 2 days | 363 | 18–45 | Serum | No significant difference between depressive disorders and cortisol levels between gender. |
| [54] | Indonesia | Cross-sectional | 3 months | 79 | 19–68 | Serum | There was a significant correlation between gender and cortisol level with males showing higher cortisol levels. |
| [55] | United States | Cross-sectional | 1 day | 131 | Mean = 38 | Serum | Male patients had significantly higher cortisol levels, but females did not show similar findings. |
| [56] | Poland | Prospective | 6 months | 37 | Mean = 54.7 | Serum | No significant difference between cortisol concentration and depression between females and males. |
| [57] | Netherlands | Systematic review and meta-analysis | 24 years | 1270 | Mean = 30.5 | Saliva, Serum | Females with depressive disorder had blunted cortisol response, while males had increased cortisol response. |
| [58] | Japan | Cross-sectional | 1 day | 87 | Mean = 50.7 | Serum | Males exhibited higher cortisol levels in the control group; females that were depressed exhibited higher cortisol levels than females in the control group. |
3.2. Differences in Sampling Methodology
4. Gender Differences in Glucocorticoid and Mineralocorticoid Receptors in Mental Health
4.1. Gene Expression of Glucocorticoid Receptor- and Mineralocorticoid Receptor-Related Genes
4.2. Gender Differences in Animal Studies of Glucocorticoid and Mineralocorticoid Receptor Expression
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Bio Med. Atenei Parm. 2020, 91, 157–160. [Google Scholar] [CrossRef]
- Pierce, M.; Hope, H.; Ford, T.; Hatch, S.; Hotopf, M.; John, A.; Kontopantelis, E.; Webb, R.; Wessely, S.; McManus, S.; et al. Mental health before and during the COVID-19 pandemic: A longitudinal probability sample survey of the UK population. Lancet Psychiatry 2020, 7, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pan, R.; Wan, X.; Tan, Y.; Xu, L.; Ho, C.S.; Ho, R.C. Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. Int. J. Environ. Res. Public Health 2020, 17, 1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, J.; Lipsitz, O.; Nasri, F.; Lui, L.M.W.; Gill, H.; Phan, L.; Chen-Li, D.; Iacobucci, M.; Ho, R.; Majeed, A.; et al. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J. Affect. Disord. 2020, 277, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Al Dhaheri, A.S.; Bataineh, M.F.; Mohamad, M.N.; Ajab, A.; Al Marzouqi, A.; Jarrar, A.H.; Habib-Mourad, C.; Abu Jamous, D.O.; Ali, H.I.; Al Sabbah, H.; et al. Impact of COVID-19 on mental health and quality of life: Is there any effect? A cross-sectional study of the MENA region. PLoS ONE 2021, 16, e0249107. [Google Scholar] [CrossRef]
- Adam, E.K.; Quinn, M.E.; Tavernier, R.; McQuillan, M.T.; Dahlke, K.A.; Gilbert, K.E. Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology 2017, 83, 25–41. [Google Scholar] [CrossRef]
- Hidalgo, V.; Pulopulos, M.M.; Puig-Perez, S.; Montoliu, T.; Salvador, A. Diurnal cortisol secretion and health-related quality of life in healthy older people. Int. J. Psychophysiol. 2021, 166, 127–133. [Google Scholar] [CrossRef]
- Biliaminu, S.A.; Saka, M.J.; Sanni, E.O.; Imran, J.; Oluwatosin, I.O.; Dane, S. Gender-related Differences in Correlations among BMI, Salivary Testosterone and Cortisol and Depression and Alexithymia Scores in University Students. J. Res. Med. Dent. Sci. 2020, 8, 152–157. [Google Scholar]
- Altemus, M. Sex differences in depression and anxiety disorders: Potential biological determinants. Horm. Behav. 2006, 50, 534–538. [Google Scholar] [CrossRef]
- McEwen, B.S. Glucocorticoids, depression, and mood disorders: Structural remodeling in the brain. Metabolism 2005, 54, 20–23. [Google Scholar] [CrossRef]
- Kumar, R.; Thompson, E.B. Gene regulation by the glucocorticoid receptor: Structure:function relationship. J. Steroid Biochem. Mol. Biol. 2005, 94, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Trapp, T.; Holsboer, F. Heterodimerization between mineralocorticoid and glucocorticoid receptors increases the functional diversity of corticosteroid action. Trends Pharmacol. Sci. 1996, 17, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Oldehinkel, A.J.; Bouma, E.M.C. Sensitivity to the depressogenic effect of stress and HPA-axis reactivity in adolescence: A review of gender differences. Neurosci. Biobehav. Rev. 2011, 35, 1757–1770. [Google Scholar] [CrossRef]
- Medina, A.; Seasholtz, A.F.; Sharma, V.; Burke, S.; Bunney, W., Jr.; Myers, R.M.; Schatzberg, A.; Akil, H.; Watson, S.J. Glucocorticoid and mineralocorticoid receptor expression in the human hippocampus in major depressive disorder. J. Psychiatr. Res. 2013, 47, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Knorr, U.; Vinberg, M.; Kessing, L.V.; Wetterslev, J. Salivary cortisol in depressed patients versus control persons: A systematic review and meta-analysis. Psychoneuroendocrinology 2010, 35, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.; Kraeuter, A.K.; McDermott, B.; Lupien, S.; Sarnyai, Z. Human nail cortisol as a retrospective biomarker of chronic stress: A systematic review. Psychoneuroendocrinology 2021, 123, 104903. [Google Scholar] [CrossRef] [PubMed]
- Stalder, T.; Steudte-Schmiedgen, S.; Alexander, N.; Klucken, T.; Vater, A.; Wichmann, S.; Kirschbaum, C.; Miller, R. Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology 2017, 77, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Hopf, D.; Eckstein, M.; Aguilar-Raab, C.; Warth, M.; Ditzen, B. Neuroendocrine mechanisms of grief and bereavement: A systematic review and implications for future interventions. J. Neuroendocrinol. 2020, 32, e12887. [Google Scholar] [CrossRef]
- Meewisse, M.L.; Reitsma, J.B.; de Vries, G.J.; Gersons, B.P.; Olff, M. Cortisol and post-traumatic stress disorder in adults: Systematic review and meta-analysis. Br. J. Psychiatry 2007, 191, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Turk, M.C.; Bakker, C.J.; Spencer, S.M.; Lofgren, S.M. Systematic review of sex differences in the relationship between hormones and depression in HIV. Psychoneuroendocrinology 2022, 138, 105665. [Google Scholar] [CrossRef]
- Gehring, U. The structure of glucocorticoid receptors. J. Steroid Biochem. Mol. Biol. 1993, 45, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Duma, D.; Jewell, C.M.; Cidlowski, J.A. Multiple glucocorticoid receptor isoforms and mechanisms of post-translational modification. J. Steroid Biochem. Mol. Biol. 2006, 102, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao-Lei, L.; Suwansirikul, S.; Jutavijittum, P.; Mériaux, S.B.; Turner, J.D.; Muller, C.P. Glucocorticoid receptor gene expression and promoter CpG modifications throughout the human brain. J. Psychiatr. Res. 2013, 47, 1597–1607. [Google Scholar] [CrossRef]
- Wang, Q.; Van Heerikhuize, J.; Aronica, E.; Kawata, M.; Seress, L.; Joels, M.; Swaab, D.F.; Lucassen, P.J. Glucocorticoid receptor protein expression in human hippocampus; stability with age. Neurobiol. Aging 2013, 34, 1662–1673. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Verweij, E.; Krugers, H.; Joels, M.; Swaab, D.F.; Lucassen, P. Distribution of the glucocorticoid receptor in the human amygdala; changes in mood disorder patients. Brain Struct. Funct. 2014, 219, 1615–1626. [Google Scholar] [CrossRef]
- Koning, A.-S.C.A.M.; Buurstede, J.C.; van Weert, L.T.C.M.; Meijer, O.C. Glucocorticoid and Mineralocorticoid Receptors in the Brain: A Transcriptional Perspective. J. Endocr. Soc. 2019, 3, 1917–1930. [Google Scholar] [CrossRef]
- Rogerson, F.M.; Brennan, F.E.; Fuller, P.J. Mineralocorticoid receptor binding, structure and function. Mol. Cell. Endocrinol. 2004, 217, 203–212. [Google Scholar] [CrossRef]
- Reul, J.M.H.M.; Gesing, A.; Droste, S.; Stec, I.S.M.; Weber, A.; Bachmann, C.; Bilang-Bleuel, A.; Holsboer, F.; Linthorst, A.C.E. The brain mineralocorticoid receptor: Greedy for ligand, mysterious in function. Eur. J. Pharmacol. 2000, 405, 235–249. [Google Scholar] [CrossRef]
- Reul, J.M.; van den Bosch, F.R.; de Kloet, E.R. Relative occupation of type-I and type-II corticosteroid receptors in rat brain following stress and dexamethasone treatment: Functional implications. J. Endocrinol. 1987, 115, 459–467. [Google Scholar] [CrossRef]
- Kirschke, E.; Goswami, D.; Southworth, D.; Griffin, P.R.; Agard, D.A. Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell 2014, 157, 1685–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatro, E.T.; Everall, I.P.; Kaul, M.; Achim, C.L. Modulation of glucocorticoid receptor nuclear translocation in neurons by immunophilins FKBP51 and FKBP52: Implications for major depressive disorder. Brain Res. 2009, 1286, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galigniana, M.D.; Erlejman, A.G.; Monte, M.; Gomez-Sanchez, C.; Piwien-Pilipuk, G. The hsp90-FKBP52 complex links the mineralocorticoid receptor to motor proteins and persists bound to the receptor in early nuclear events. Mol. Cell Biol. 2010, 30, 1285–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, B.; Dunbar, M.; Shelton, R.C.; Dwivedi, Y. Identification of microRNA-124-3p as a putative epigenetic signature of major depressive disorder. Neuropsychopharmacology 2017, 42, 864–875. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-S.; Mu, R.-H.; Li, C.-F.; Dong, S.-Q.; Geng, D.; Liu, Q.; Yi, L.-T. microRNA-124 targets glucocorticoid receptor and is involved in depression-like behaviors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 417–425. [Google Scholar] [CrossRef]
- Farrell, C.; Doolin, K.; O’Leary, N.; Jairaj, C.; Roddy, D.; Tozzi, L.; Morris, D.; Harkin, A.; Frodl, T.; Nemoda, Z. DNA methylation differences at the glucocorticoid receptor gene in depression are related to functional alterations in hypothalamic–pituitary–adrenal axis activity and to early life emotional abuse. Psychiatry Res. 2018, 265, 341–348. [Google Scholar] [CrossRef]
- Plieger, T.; Felten, A.; Splittgerber, H.; Duke, É.; Reuter, M. The role of genetic variation in the glucocorticoid receptor (NR3C1) and mineralocorticoid receptor (NR3C2) in the association between cortisol response and cognition under acute stress. Psychoneuroendocrinology 2018, 87, 173–180. [Google Scholar] [CrossRef]
- Rao, S.; Yao, Y.; Ryan, J.; Li, T.; Wang, D.; Zheng, C.; Xu, Y.; Xu, Q. Common variants in FKBP5 gene and major depressive disorder (MDD) susceptibility: A comprehensive meta-analysis. Sci. Rep. 2016, 6, 32687. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Ba, Y.; Han, W.; Zhang, H.; Zhu, L.; Jiang, P. Association of heat shock protein polymorphisms with patient susceptibility to coronary artery disease comorbid depression and anxiety in a Chinese population. PeerJ 2021, 9, e11636. [Google Scholar] [CrossRef]
- Tatro, E.T.; Everall, I.P.; Masliah, E.; Hult, B.J.; Lucero, G.; Chana, G.; Soontornniyomkij, V.; Achim, C.L. Differential expression of immunophilins FKBP51 and FKBP52 in the frontal cortex of HIV-infected patients with major depressive disorder. J. Neuroimmune Pharmacol. 2009, 4, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Adams, G.C.; Wrath, A.J.; von Dewitz, B.; Marciniuk, K.; Roesler, A.; Napper, S. Attachment impacts cortisol awakening response in chronically depressed individuals. Psychoneuroendocrinology 2020, 120, 104778. [Google Scholar] [CrossRef]
- Carnegie, R.; Araya, R.; Ben-Shlomo, Y.; Glover, V.; O’Connor, T.G.; O’Donnell, K.J.; Pearson, R.; Lewis, G. Cortisol awakening response and subsequent depression: Prospective longitudinal study. Br. J. Psychiatry 2014, 204, 137–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chronister, B.N.; Gonzalez, E.; Lopez-Paredes, D.; Suarez-Torres, J.; Gahagan, S.; Martinez, D.; Barros, J.; Jacobs, D.R., Jr.; Checkoway, H.; Suarez-Lopez, J.R. Testosterone, estradiol, DHEA and cortisol in relation to anxiety and depression scores in adolescents. J. Affect. Disord. 2021, 294, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Doane, L.D.; Mineka, S.; Zinbarg, R.E.; Craske, M.; Griffith, J.W.; Adam, E.K. Are flatter diurnal cortisol rhythms associated with major depression and anxiety disorders in late adolescence? The role of life stress and daily negative emotion. Dev. Psychopathol. 2013, 25, 629–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinkelmann, K.; Moritz, S.; Botzenhardt, J.; Muhtz, C.; Wiedemann, K.; Kellner, M.; Otte, C. Changes in cortisol secretion during antidepressive treatment and cognitive improvement in patients with major depression: A longitudinal study. Psychoneuroendocrinology 2012, 37, 685–692. [Google Scholar] [CrossRef]
- Khan, Q.U. Relationship of Salivary Cortisol Level with Severe Depression and Family History. Cureus 2020, 12, e11548. [Google Scholar] [CrossRef]
- Klimes-Dougan, B.; Begnel, E.; Almy, B.; Thai, M.; Schreiner, M.W.; Cullen, K.R. Hypothalamic-pituitary-adrenal axis dysregulation in depressed adolescents with non-suicidal self-injury. Psychoneuroendocrinology 2019, 102, 216–224. [Google Scholar] [CrossRef]
- Lu, S.; Gao, W.; Huang, M.; Li, L.; Xu, Y. In search of the HPA axis activity in unipolar depression patients with childhood trauma: Combined cortisol awakening response and dexamethasone suppression test. J. Psychiatr. Res. 2016, 78, 24–30. [Google Scholar] [CrossRef]
- Suzuki, A.; Poon, L.; Papadopoulos, A.S.; Kumari, V.; Cleare, A.J. Long term effects of childhood trauma on cortisol stress reactivity in adulthood and relationship to the occurrence of depression. Psychoneuroendocrinology 2014, 50, 289–299. [Google Scholar] [CrossRef]
- Daoust, A.R.; Kotelnikova, Y.; Kryski, K.R.; Sheikh, H.I.; Singh, S.M.; Hayden, E.P. Child sex moderates the relationship between cortisol stress reactivity and symptoms over time. Compr. Psychiatry 2018, 87, 161–170. [Google Scholar] [CrossRef]
- Mazurka, R.; Wynne-Edwards, K.E.; Harkness, K.L. Sex Differences in the Cortisol Response to the Trier Social Stress Test in Depressed and Nondepressed Adolescents. Clin. Psychol. Sci. 2018, 6, 301–314. [Google Scholar] [CrossRef]
- Owens, M.; Herbert, J.; Jones, P.B.; Sahakian, B.J.; Wilkinson, P.O.; Dunn, V.J.; Croudace, T.J.; Goodyer, I.M. Elevated morning cortisol is a stratified populationlevel biomarker for major depression in boys only with high depressive symptoms. Proc. Natl. Acad. Sci. USA 2014, 111, 3638–3643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, S.I.; Laurent, H.K.; Gunlicks-Stoessel, M.; Balaban, S.; Bent, E. Depression and anxiety predict sex-specific cortisol responses to interpersonal stress. Psychoneuroendocrinology 2016, 69, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Y.; Yan, S.Q.; Hu, J.J.; Xu, G.; Liu, J.; Tao, F.B. Longitudinal pattern of early maturation on morning cortisol and depressive symptoms: Sex-specific effects. Psychoneuroendocrinology 2016, 71, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Druzhkova, T.A.; Pochigaeva, K.I.; Yakovlev, A.A.; Gersamia, A.G.; Guekht, A.B.; Gulyaeva, N.V. Effects of Childhood Trauma on the Biological Correlates of Stress in Men and Women with Borderline Mental Disorders. Neurosci. Behav. Physiol. 2019, 49, 916–920. [Google Scholar] [CrossRef]
- Wilkowska, A.; Rynkiewicz, A.; Wdowczyk, J.; Landowski, J. Morning and afternoon serum cortisol level in patients with post-myocardial infarction depression. Cardiol. J. 2019, 26, 550–554. [Google Scholar] [CrossRef] [Green Version]
- Fitrikasari, A.; Wardani, N.D.; Sumekar, T.A.; Saktini, F.; Asikin, H.G.; Sulchan, M. The role of psychosocial stressors, carbohydrate and protein intake on serum serotonin and cortisol levels in patients with depression: A preliminary evaluation. Bali Med. J. 2021, 10, 137–141. [Google Scholar] [CrossRef]
- Zorn, J.V.; Schur, R.R.; Boks, M.P.; Kahn, R.S.; Joels, M.; Vinkers, C.H. Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology 2017, 77, 25–36. [Google Scholar] [CrossRef]
- Matsuzaka, H.; Maeshima, H.; Kida, S.; Kurita, H.; Shimano, T.; Nakano, Y.; Baba, H.; Suzuki, T.; Arai, H. Gender Differences in Serum Testosterone and Cortisol in Patients with Major Depressive Disorder Compared with Controls. Int. J. Psychiatry Med. 2013, 46, 203–221. [Google Scholar] [CrossRef]
- Lu, Q.; Pan, F.; Ren, L.; Xiao, J.; Tao, F. Sex differences in the association between internalizing symptoms and hair cortisol level among 10–12 year-old adolescents in China. PLoS ONE 2018, 13, e0192901. [Google Scholar] [CrossRef] [Green Version]
- Berger, M.; Taylor, S.; Harriss, L.; Campbell, S.; Thompson, F.; Jones, S.; Sushames, A.; Amminger, G.P.; Sarnyai, Z.; McDermott, R. Hair cortisol, allostatic load, and depressive symptoms in Australian Aboriginal and Torres Strait Islander people. Stress 2019, 22, 312–320. [Google Scholar] [CrossRef]
- Gerber, M.; Kalak, N.; Elliot, C.; Holsboer-Trachsler, E.; Pühse, U.; Brand, S. Both Hair Cortisol Levels and Perceived Stress Predict Increased Symptoms of Depression: An Exploratory Study in Young Adults. Neuropsychobiology 2013, 68, 100–109. [Google Scholar] [CrossRef] [Green Version]
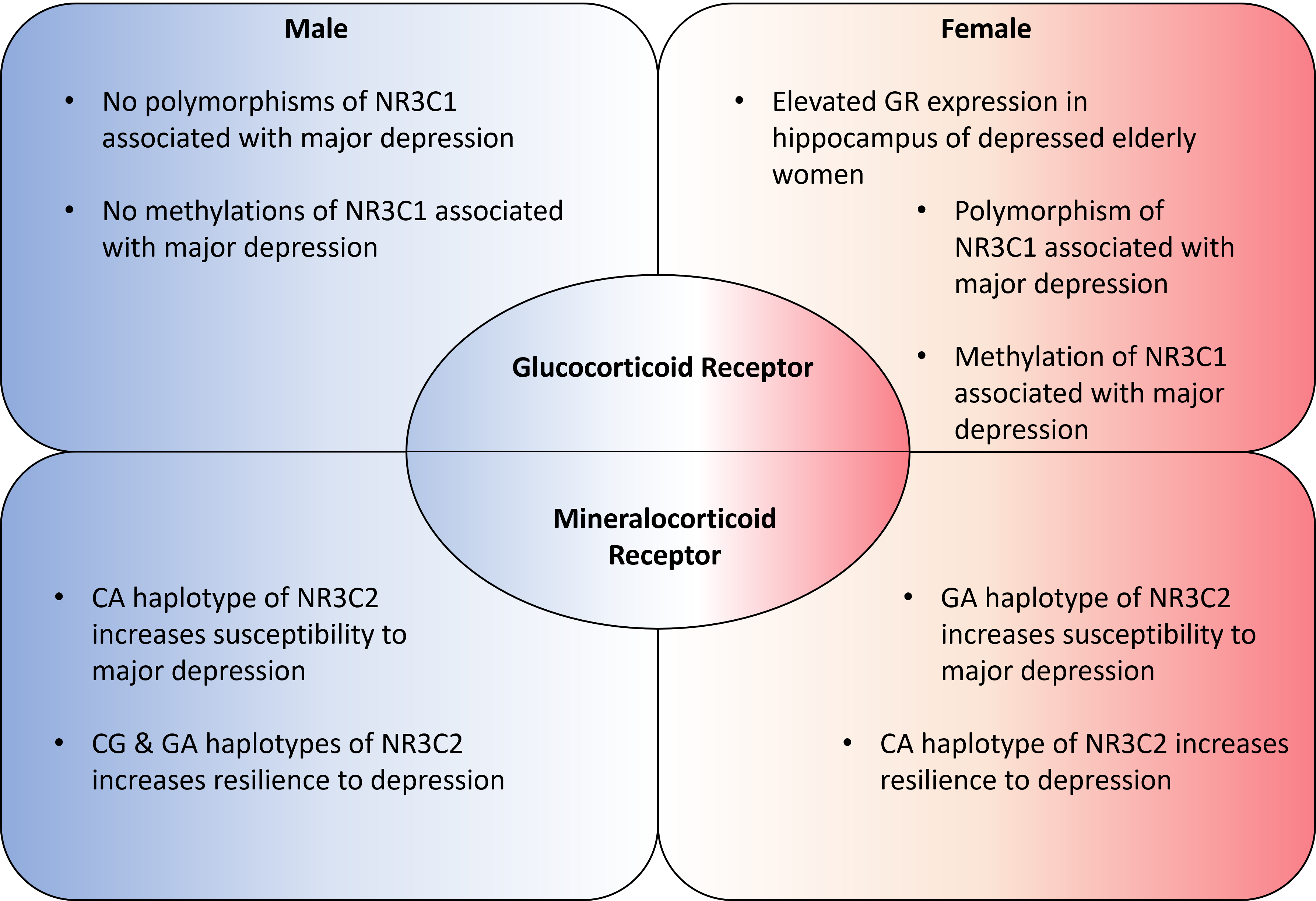
- Sarubin, N.; Hilbert, S.; Naumann, F.; Zill, P.; Wimmer, A.-M.; Nothdurfter, C.; Rupprecht, R.; Baghai, T.C.; Bühner, M.; Schüle, C. The sex-dependent role of the glucocorticoid receptor in depression: Variations in the NR3C1 gene are associated with major depressive disorder in women but not in men. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 123–133. [Google Scholar] [CrossRef]
- Nantharat, M.; Wanitchanon, T.; Amesbutr, M.; Tammachote, R.; Praphanphoj, V. Glucocorticoid receptor gene (NR3C1) promoter is hypermethylated in Thai females with major depressive disorder. Genet. Mol. Res. 2015, 14, 19071. [Google Scholar] [CrossRef]
- Klok, M.D.; Giltay, E.J.; Van der Does, A.J.W.; Geleijnse, J.M.; Antypa, N.; Penninx, B.W.J.H.; de Geus, E.J.C.; Willemsen, G.; Boomsma, D.I.; van Leeuwen, N.; et al. A common and functional mineralocorticoid receptor haplotype enhances optimism and protects against depression in females. Transl. Psychiatry 2011, 1, e62. [Google Scholar] [CrossRef] [Green Version]
- Vinkers, C.H.; Joëls, M.; Milaneschi, Y.; Gerritsen, L.; Kahn, R.S.; Penninx, B.W.J.H.; Boks, M.P.M. Mineralocorticoid receptor haplotypes sex-dependently moderate depression susceptibility following childhood maltreatment. Psychoneuroendocrinology 2015, 54, 90–102. [Google Scholar] [CrossRef] [Green Version]
- Büttner, M.; Jezova, D.; Greene, B.; Konrad, C.; Kircher, T.; Murck, H. Target-based biomarker selection—Mineralocorticoid receptor-related biomarkers and treatment outcome in major depression. J. Psychiatr. Res. 2015, 66, 24–37. [Google Scholar] [CrossRef]
- Webster, M.J.; Knable, M.B.; O’Grady, J.; Orthmann, J.; Weickert, C.S. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol. Psychiatry 2002, 7, 985–994. [Google Scholar] [CrossRef] [Green Version]
- Xing, G.-Q.; Russell, S.; Webster, M.J.; Post, R.M. Decreased expression of mineralocorticoid receptor mRNA in the prefrontal cortex in schizophrenia and bipolar disorder. Int. J. Neuropsychopharmacol. 2004, 7, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wu, J.; Qing, L.; Li, J.; Yang, H.; Ji, A.; Yan, M.; Hu, L.; Nie, S. DNA Methylation Analysis of the NR3C1 Gene in Patients with Schizophrenia. J. Mol. Neurosci. 2020, 70, 1177–1185. [Google Scholar] [CrossRef]
- Qing, L.; Liu, L.; Zhou, L.; Zhang, F.; Gao, C.; Hu, L.; Nie, S. Sex-dependent association of mineralocorticoid receptor gene (NR3C2) DNA methylation and schizophrenia. Psychiatry Res. 2020, 292, 113318. [Google Scholar] [CrossRef]
- Misiak, B.; Samochowiec, J.; Konopka, A.; Gawrońska-Szklarz, B.; Beszłej, J.A.; Szmida, E.; Karpiński, P. Clinical Correlates of the NR3C1 Gene Methylation at Various Stages of Psychosis. Int. J. Neuropsychopharmacol. 2021, 24, 322–332. [Google Scholar] [CrossRef]
- Bonapersona, V.; Damsteegt, R.; Adams, M.L.; van Weert, L.T.; Meijer, O.C.; Joëls, M.; Sarabdjitsingh, R.A. Sex-Dependent modulation of acute stress reactivity after early life stress in mice: Relevance of mineralocorticoid receptor expression. Front. Behav. Neurosci. 2019, 13, 181. [Google Scholar] [CrossRef] [PubMed]
- Madison, F.N.; Kesner, A.J.; Alward, B.A.; Ball, G.F. Sex differences in hippocampal mineralocorticoid and glucocorticoid receptor mRNA expression in response to acute mate pair separation in zebra finches (Taeniopygia guttata). Hippocampus 2018, 28, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Ortolaza, D.L.; Doreste-Mendez, R.J.; Alvarado-Torres, J.K.; Torres-Reveron, A. Ovarian hormones modify anxiety behavior and glucocorticoid receptors after chronic social isolation stress. Behav. Brain Res. 2017, 328, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Boero, G.; Pisu, M.G.; Biggio, F.; Muredda, L.; Carta, G.; Banni, S.; Paci, E.; Follesa, P.; Concas, A.; Porcu, P. Impaired glucocorticoid-mediated HPA axis negative feedback induced by juvenile social isolation in male rats. Neuropharmacology 2018, 133, 242–253. [Google Scholar] [CrossRef]
- Solomon, M.B.; Furay, A.R.; Jones, K.; Packard, A.E.B.; Packard, B.A.; Wulsin, A.C.; Herman, J.P. Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience 2012, 203, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Joels, M.; Swaab, D.F.; Lucassen, P.J. Hippocampal GR expression is increased in elderly depressed females. Neuropharmacology 2012, 62, 527–533. [Google Scholar] [CrossRef]
- Tyrka, A.R.; Lee, J.K.; Graber, J.A.; Clement, A.M.; Kelly, M.M.; DeRose, L.; Warren, M.P.; Brooks-Gunn, J. Neuroendocrine predictors of emotional and behavioral adjustment in boys: Longitudinal follow-up of a community sample. Psychoneuroendocrinology 2012, 37, 2042–2046. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Li, J.; Xie, Q.; Deng, H. Hair cortisol levels and symptoms of anxiety and depression in Chinese adolescents: Comparison between incarcerated and community populations. PsyCH J. 2019, 8, 147–157. [Google Scholar] [CrossRef] [Green Version]
- De Rezende, M.G.; Garcia-Leal, C.; de Figueiredo, F.P.; Cavalli Rde, C.; Spanghero, M.S.; Barbieri, M.A.; Bettiol, H.; de Castro, M.; Del-Ben, C.M. Altered functioning of the HPA axis in depressed postpartum women. J. Affect. Disord. 2016, 193, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Hampson, E.; Phillips, S.D.; Duff-Canning, S.J.; Evans, K.L.; Merrill, M.; Pinsonneault, J.K.; Sadée, W.; Soares, C.N.; Steiner, M. Working memory in pregnant women: Relation to estrogen and antepartum depression. Horm. Behav. 2015, 74, 218–227. [Google Scholar] [CrossRef]
- Jarcho, M.R.; Slavich, G.M.; Tylova-Stein, H.; Wolkowitz, O.M.; Burke, H.M. Dysregulated diurnal cortisol pattern is associated with glucocorticoid resistance in women with major depressive disorder. Biol. Psychol. 2013, 93, 150–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasap, E.; Aksu, E.E.; Gur, E.B.; Genc, M.; Eskicioğlu, F.; Gökduman, A.; Güçlü, S. Investigation of the relationship between salivary cortisol, dehydroepiandrosterone sulfate, anxiety, and depression in patients with hyperemesis gravidarum. J. Matern. Fetal Neonatal Med. 2016, 29, 3686–3689. [Google Scholar] [CrossRef] [PubMed]
- Orta, O.R.; Gelaye, B.; Bain, P.A.; Williams, M.A. The association between maternal cortisol and depression during pregnancy, a systematic review. Arch. Womens Ment. Health 2018, 21, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Szpunar, M.J.; Parry, B.L. A systematic review of cortisol, thyroid-stimulating hormone, and prolactin in peripartum women with major depression. Arch. Womens Ment. Health 2018, 21, 149–161. [Google Scholar] [CrossRef] [PubMed]
| Reference Number | Country | Type of Study | Duration | Sample Size | Age Group (Year) | Type of Cortisol Sample | Salient Points |
|---|---|---|---|---|---|---|---|
| [38] | China | Longitudinal | 3 months | 85 | 10–12 | Hair, Saliva | Positive correlation among depressive symptoms and hair cortisol only seen in males and not females. |
| [59] | Australia | Cross-sectional | 2–3 months | 329 | 15–24 | Hair | No differences in hair cortisol among gender and depressed groups. |
| [60] | Switzerland | Cross-sectional | 7 days | 46 | Mean = 21.17 | Hair | Increased hair cortisol correlated with lower reported self-perceived stress and anxiety; no gender differences observed. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teo, C.H.; Wong, A.C.H.; Sivakumaran, R.N.; Parhar, I.; Soga, T. Gender Differences in Cortisol and Cortisol Receptors in Depression: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 7129. https://doi.org/10.3390/ijms24087129
Teo CH, Wong ACH, Sivakumaran RN, Parhar I, Soga T. Gender Differences in Cortisol and Cortisol Receptors in Depression: A Narrative Review. International Journal of Molecular Sciences. 2023; 24(8):7129. https://doi.org/10.3390/ijms24087129
Chicago/Turabian StyleTeo, Chuin Hau, Ally Chai Hui Wong, Rooba Nair Sivakumaran, Ishwar Parhar, and Tomoko Soga. 2023. "Gender Differences in Cortisol and Cortisol Receptors in Depression: A Narrative Review" International Journal of Molecular Sciences 24, no. 8: 7129. https://doi.org/10.3390/ijms24087129
APA StyleTeo, C. H., Wong, A. C. H., Sivakumaran, R. N., Parhar, I., & Soga, T. (2023). Gender Differences in Cortisol and Cortisol Receptors in Depression: A Narrative Review. International Journal of Molecular Sciences, 24(8), 7129. https://doi.org/10.3390/ijms24087129





