The Influence of Polyphenols on Atherosclerosis Development
Abstract
1. Introduction
2. Atherosclerosis: Risk Factors and the Mechanism of Development
2.1. Atherosclerosis as an Inflammatory Disease
2.2. The Impact of Oxidative Stress on the Development of Atherosclerosis
3. Classes of Phenolic Compounds and Their Role in Human Health
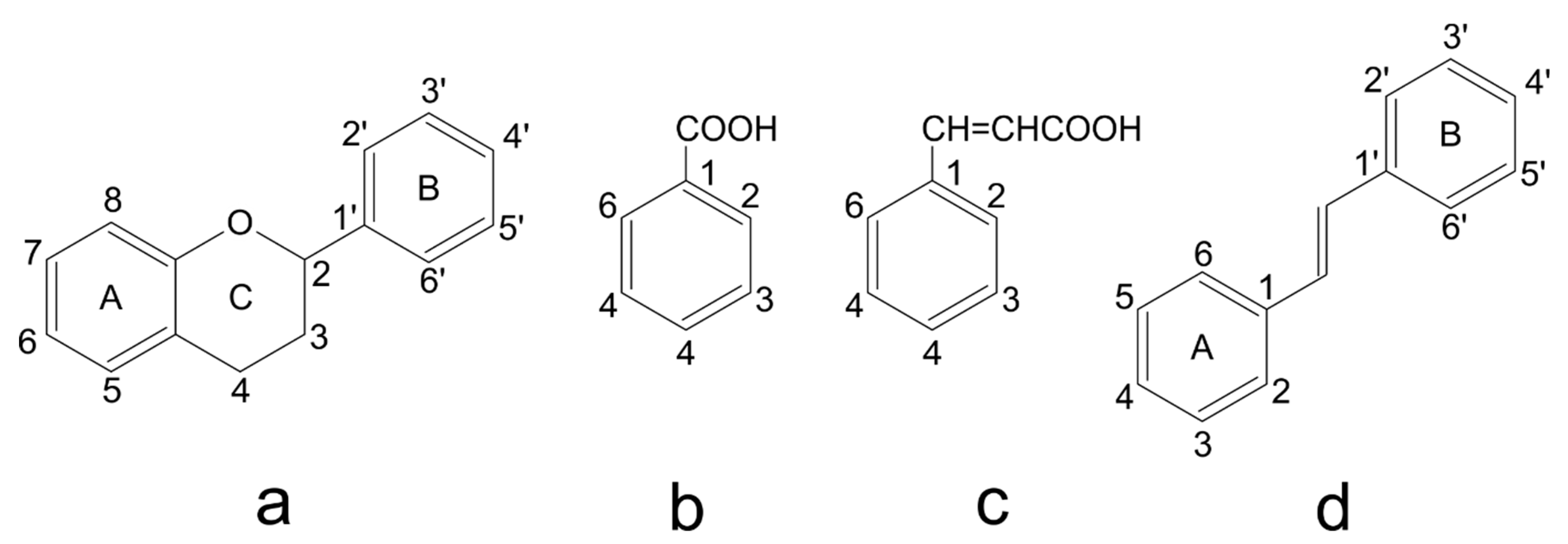
3.1. Structure and Classification of Phenolic Compounds
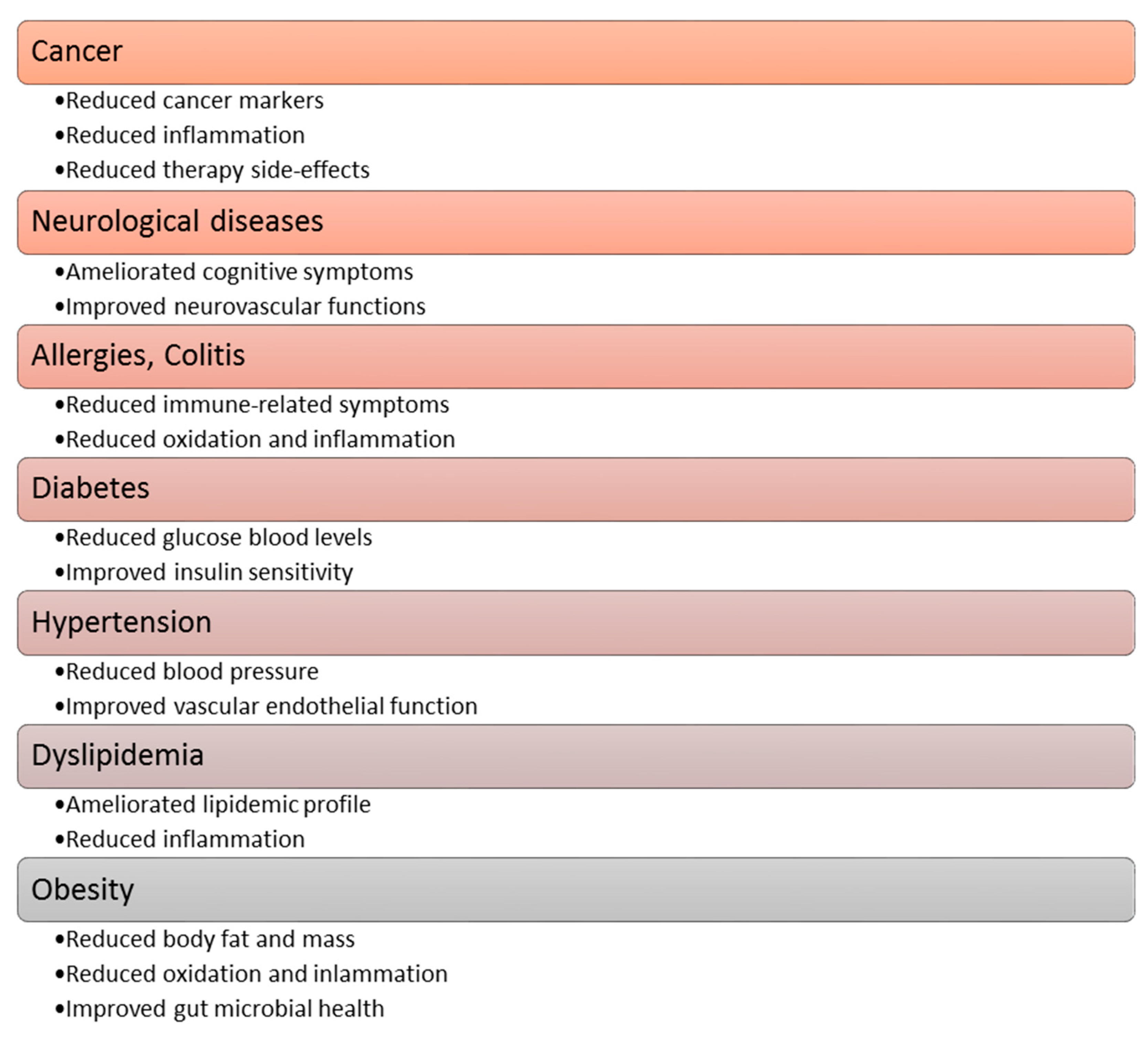
3.2. Health Benefit Properties of Plant Polyphenolic Compounds
4. Polyphenolic Compounds as a Considerable Plant Component of the Daily Diet
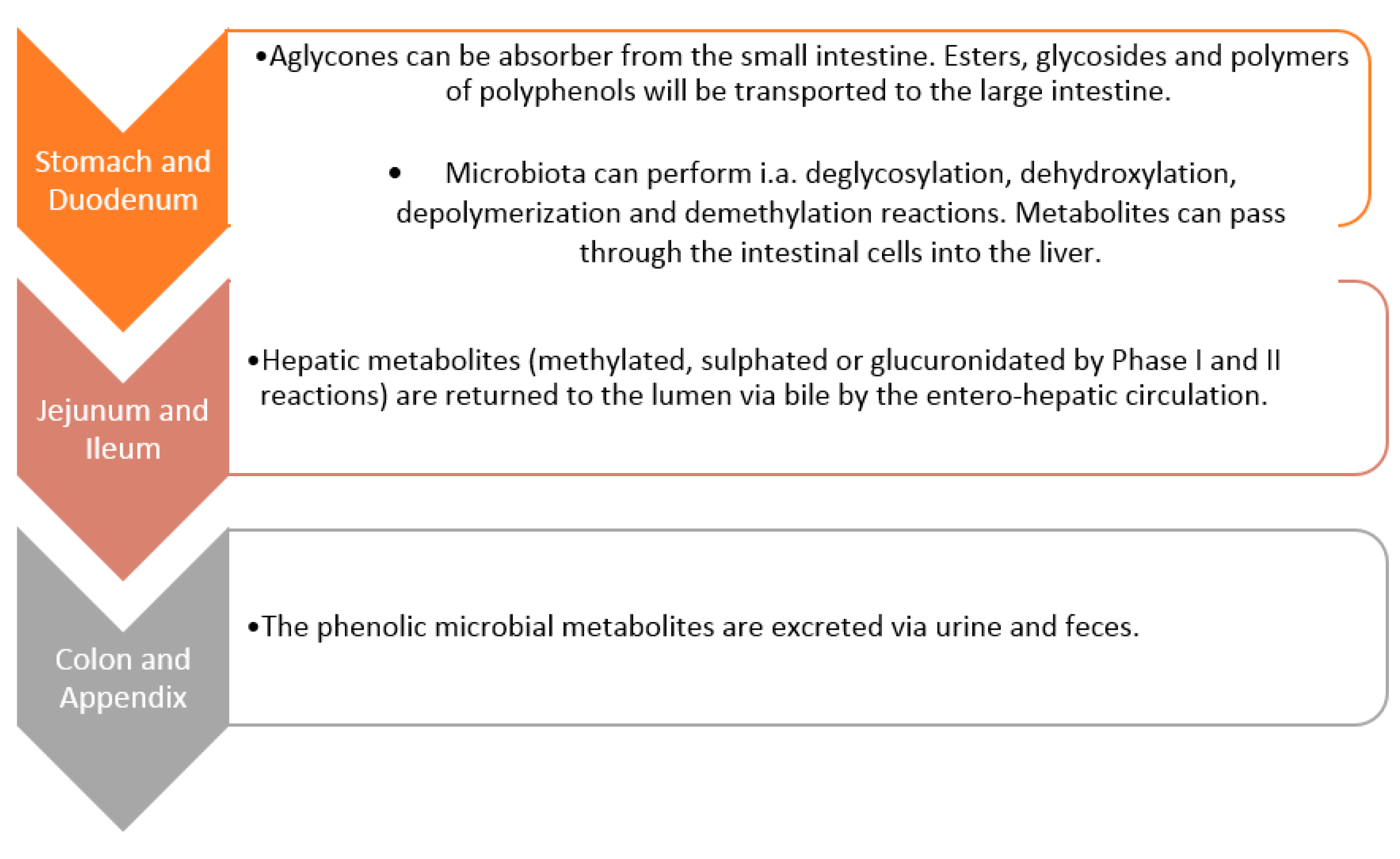
5. Metabolism of Polyphenols by Gut Microbiota
6. Effect of Polyphenols on the Composition of Gut Microbiota
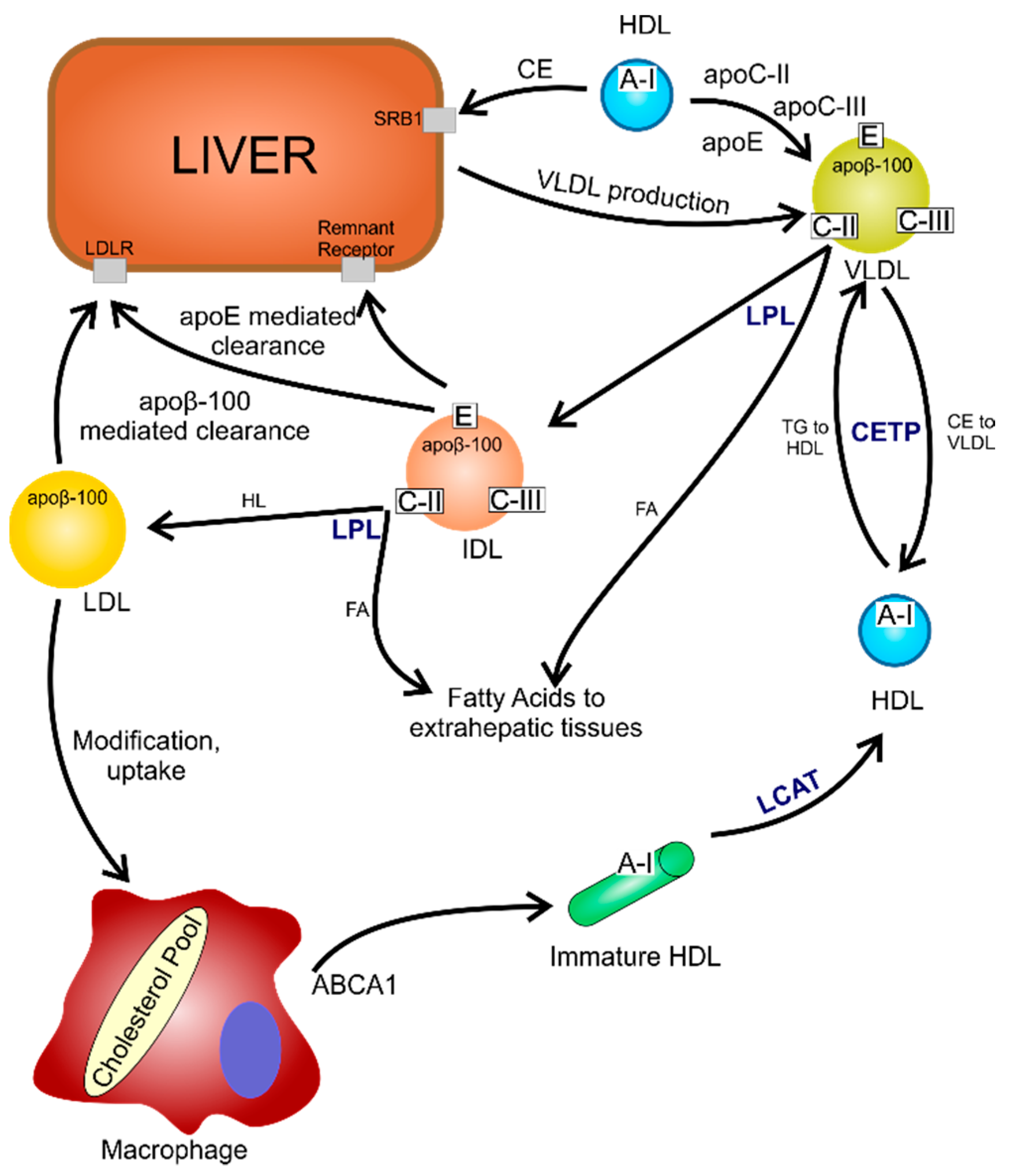
7. Polyphenols in the Cardiovascular System
8. The Impact of Polyphenols and Their Metabolites on the Mechanisms and Factors Causing Atherosclerosis
8.1. Cacao and Green Tea Flavonols
8.2. Resveratrol
8.3. Curcumin
8.4. Quercetin
8.5. Protocatechuic Acid
8.6. Trimethoxycinnamic Acid
8.7. Gallic Acid
8.8. Equol
8.9. Mediterranean Diet
8.10. Other Polyphenol-Rich Foods
9. The Anti-Atherosclerosis Therapeutic Potential of Polyphenol by Gut Microbiota Modulation
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCL3 | chemokine ligand 3 |
| CLP | cacao liquor polyphenols |
| COX | cyclooxygenase |
| CRP | C reactive protein |
| CVD | cardiovascular disease |
| DCs | dendritic cells |
| EGCG | epigallocatechin-3-gallate |
| ESRD | end-stage renal disease |
| FMD | flow-mediated dilation |
| GA | gallic acid |
| GM | gut microbiota |
| HDL | high-density lipoprotein |
| HDLC-C | high-density lipoprotein cholesterol |
| HO-1 | heme-oxygenase-1 |
| IL | interleukin |
| LDL | low-density lipoprotein |
| LDL-C | low-density lipoprotein cholesterol |
| LOX | lipoxygenase |
| MAPK | mitogen-activated protein kinase |
| NF-κB | Nuclear factor-κ |
| NO | nitric oxide |
| OxLDL | oxidised LDL |
| PCA | protocatechuic acid |
| PMFs | polymethoxyflavones |
| PPAR | peroxisome proliferator-activated receptor |
| PUFAs | polyunsaturated fatty acids |
| RONS | reactive oxygen and nitrogen species |
| ROS | reactive oxygen species |
| SIRT | Sirtuin |
| SOD | superoxide dismutase |
| TC | total cholesterol |
| TNF | tumour necrosis factor |
| TRLs | Toll-like receptors |
| VCAM | vascular cell adhesion protein |
References
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus–Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Liu, J.; Zhang, D.; Wang, X.; Li, G.; Ma, R.; Chen, G.; Lin, X.; Guo, X. The Relationship Between Nutrition and Atherosclerosis. Front. Bioeng. Biotechnol. 2021, 9, 635504. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, M.A.; Massaro, M.; Scoditti, E.; Caterina, R. Atherosclerosis and Mediterranean Diet Polyphenols. Polyphen. Hum. Health Dis. 2013, 2, 895–903. [Google Scholar] [CrossRef]
- Kobiyama, K.; Ley, K. Atherosclerosis: A Chronic Inflammatory Disease with an Autoimmune Component. Circ. Res. 2018, 123, 1118. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.-P.; Fullerton, H.J.; Howard, V.J.; et al. Heart Disease and Stroke Statistics—2015 Update. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef]
- Shapiro, M.D.; Fazio, S. From Lipids to Inflammation: New Approaches to Reducing Atherosclerotic Risk. Circ. Res. 2016, 118, 732–749. [Google Scholar] [CrossRef]
- Kingwell, B.A.; Chapman, M.J.; Kontush, A.; Miller, N.E. HDL-Targeted Therapies: Progress, Failures and Future. Nat. Rev. Drug Discov. 2014, 13, 445–464. [Google Scholar] [CrossRef]
- Moss, J.W.E.; Ramji, D.P. Cytokines: Roles in Atherosclerosis Disease Progression and Potential Therapeutic Targets. Future Med. Chem. 2016, 8, 1317–1330. [Google Scholar] [CrossRef]
- Yc, C.; Jm, S.; Wl, H.; Yc, H. Polyphenols and Oxidative Stress in Atherosclerosis-Related Ischemic Heart Disease and Stroke. Oxidative Med. Cell. Longev. 2017, 2017, 8526438. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa Polyphenols and Gut Microbiota Interplay: Bioavailability, Prebiotic Effect, and Impact on Human Health. Nutrients 2020, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Lechner, K.; von Schacky, C.; McKenzie, A.L.; Worm, N.; Nixdorff, U.; Lechner, B.; Kränkel, N.; Halle, M.; Krauss, R.M.; Scherr, J. Lifestyle Factors and High-Risk Atherosclerosis: Pathways and Mechanisms beyond Traditional Risk Factors. Eur. J. Prev. Cardiol. 2020, 27, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Mundi, S.; Massaro, M.; Scoditti, E.; Carluccio, M.A.; van Hinsbergh, V.W.M.; Iruela-Arispe, M.L.; De Caterina, R. Endothelial Permeability, LDL Deposition, and Cardiovascular Risk Factors-a Review. Cardiovasc. Res. 2018, 114, 35–52. [Google Scholar] [CrossRef]
- Stanhope, K.L.; Goran, M.I.; Bosy-Westphal, A.; King, J.C.; Schmidt, L.A.; Schwarz, J.-M.; Stice, E.; Sylvetsky, A.C.; Turnbaugh, P.J.; Bray, G.A.; et al. Pathways and Mechanisms Linking Dietary Components to Cardiometabolic Disease: Thinking beyond Calories. Obes. Rev. 2018, 19, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Csige, I.; Ujvárosy, D.; Szabó, Z.; Lőrincz, I.; Paragh, G.; Harangi, M.; Somodi, S. The Impact of Obesity on the Cardiovascular System. J. Diabetes Res. 2018, 2018, e3407306. [Google Scholar] [CrossRef]
- de Lima, J.C.; Moura-Assis, A.; Cintra, R.M.; Quinaglia, T.; Velloso, L.A.; Sposito, A.C. Central Role of Obesity in Endothelial Cell Dysfunction and Cardiovascular Risk. Rev. Assoc. Med. Bras. 2019, 65, 87–97. [Google Scholar] [CrossRef]
- Katakami, N. Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J. Atheroscler. Thromb. 2018, 25, 27–39. [Google Scholar] [CrossRef]
- Olszewski, W.; Głuszek, J. Role of Magnesium in Hypertension. Arter. Hypertens. 2007, 11, 536–544. [Google Scholar]
- Kostov, K.; Halacheva, L. Role of Magnesium Deficiency in Promoting Atherosclerosis, Endothelial Dysfunction, and Arterial Stiffening as Risk Factors for Hypertension. Int. J. Mol. Sci. 2018, 19, 1724. [Google Scholar] [CrossRef] [PubMed]
- Szymczyk, H. Magnez—Pierwiastek Niezbędny Do Prawidłowego Funkcjonowania Organizmu. Farm Współ 2016, 9, 217–223. [Google Scholar]
- Man, J.J.; Beckman, J.A.; Jaffe, I.Z. Sex as a Biological Variable in Atherosclerosis. Circ. Res. 2020, 126, 1297. [Google Scholar] [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxidative Med. Cell. Longev. 2019, 2019, e8563845. [Google Scholar] [CrossRef] [PubMed]
- Palano, M.T.; Cucchiara, M.; Gallazzi, M.; Riccio, F.; Mortara, L.; Gensini, G.F.; Spinetti, G.; Ambrosio, G.; Bruno, A. When a Friend Becomes Your Enemy: Natural Killer Cells in Atherosclerosis and Atherosclerosis-Associated Risk Factors. Front. Immunol. 2022, 12, 798155. [Google Scholar] [CrossRef]
- Shah, P.K.; Lecis, D. Inflammation in Atherosclerotic Cardiovascular Disease. F1000Research 2019, 8, 1402. [Google Scholar] [CrossRef]
- Kong, P.; Cui, Z.-Y.; Huang, X.-F.; Zhang, D.-D.; Guo, R.-J.; Han, M. Inflammation and Atherosclerosis: Signaling Pathways and Therapeutic Intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef]
- Bi, Y.; Chen, J.; Hu, F.; Liu, J.; Li, M.; Zhao, L. M2 Macrophages as a Potential Target for Antiatherosclerosis Treatment. Neural Plast. 2019, 2019, e6724903. [Google Scholar] [CrossRef]
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The Role of Lipids and Lipoproteins in Atherosclerosis. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Bartosz, G. Druga Twarz Tlenu. Wolne Rodniki w Przyrodzie; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2019; ISBN 978-83-01-13847-9. [Google Scholar]
- Ignat, I.; Volf, I.; Popa, V.I. A Critical Review of Methods for Characterisation of Polyphenolic Compounds in Fruits and Vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, Food and Pharma. Current Knowledge and Directions for Future Research. Biochem. Pharm. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Riaz, S.; Sameen, A.; Naumovski, N.; Iqbal, M.W.; Rehman, A.; Mehany, T.; Zeng, X.-A.; Manzoor, M.F. The Disposition of Bioactive Compounds from Fruit Waste, Their Extraction, and Analysis Using Novel Technologies: A Review. Processes 2022, 10, 2014. [Google Scholar] [CrossRef]
- Gumul, D.; Korus, J.; Achremowicz, B. Wpływ Procesów Przetwórczych Na Aktywność Przeciwutleniającą Surowców Pochodzenia Roślinnego. ŻNTJ 2005, 4, 41–48. [Google Scholar]
- Vivarelli, S.; Costa, C.; Teodoro, M.; Giambò, F.; Tsatsakis, A.M.; Fenga, C. Polyphenols: A Route from Bioavailability to Bioactivity Addressing Potential Health Benefits to Tackle Human Chronic Diseases. Arch. Toxicol. 2023, 97, 3–38. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health Benefits of Polyphenols: A Concise Review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Caruso, G.; Godos, J.; Privitera, A.; Lanza, G.; Castellano, S.; Chillemi, A.; Bruni, O.; Ferri, R.; Caraci, F.; Grosso, G. Phenolic Acids and Prevention of Cognitive Decline: Polyphenols with a Neuroprotective Role in Cognitive Disorders and Alzheimer’s Disease. Nutrients 2022, 14, 819. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent Advances in Research on Lignans and Neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, N.Z.T.; Falcão, H.d.S.; Gomes, I.F.; Leite, T.J.d.A.; Lima, G.R.d.M.; Barbosa-Filho, J.M.; Tavares, J.F.; Silva, M.S.d.; Athayde-Filho, P.F.d.; Batista, L.M. Tannins, Peptic Ulcers and Related Mechanisms. Int. J. Mol. Sci. 2012, 13, 3203–3228. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source Plants, Chemistry, Biosynthesis, Pharmacology, Application and Problems Related to Their Clinical Application-A Comprehensive Review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef]
- Higbee, J.; Solverson, P.; Zhu, M.; Carbonero, F. The Emerging Role of Dark Berry Polyphenols in Human Health and Nutrition. Food Front. 2022, 3, 3–27. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic Biochemical Mechanisms behind the Health Benefits of Polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial Effects of Phenolic Compounds on Gut Microbiota and Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 3715. [Google Scholar] [CrossRef]
- Olszowy, M. What Is Responsible for Antioxidant Properties of Polyphenolic Compounds from Plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Noreen, S.; Khalid, W.; Mubarik, F.; Niazi, M.K.; Koraqi, H.; Ali, A.; Lima, C.M.G.; Alansari, W.S.; Eskandrani, A.A.; et al. Extraction of Bioactive Compounds from Different Vegetable Sprouts and Their Potential Role in the Formulation of Functional Foods against Various Disorders: A Literature-Based Review. Molecules 2022, 27, 7320. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Manzoor, M.F.; Ahmad, N.; Aadil, R.M.; Qin, H.; Siddique, R.; Riaz, S.; Ahmad, A.; Korma, S.A.; Khalid, W.; et al. The Burden of Cancer, Government Strategic Policies, and Challenges in Pakistan: A Comprehensive Review. Front Nutr. 2022, 9, 940514. [Google Scholar] [CrossRef]
- Stachowska, E. Żywienie w Zaburzeniach Mikrobioty Jelitowej; PZWL: Warszawa, Poland, 2021; ISBN 978-83-200-6328-8. [Google Scholar]
- Silva, R.F.M.; Pogačnik, L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef]
- Herrmann, K. On the Occurrence of Flavonol and Flavone Glycosides in Vegetables. Z. Leb.-Unters. Forsch. 1988, 186, 1–5. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Stuper-Szablewska, K.; Perkowski, J. Phenolic Acids in Cereal Grain: Occurrence, Biosynthesis, Metabolism and Role in Living Organisms. Crit Rev. Food Sci. Nutr. 2019, 59, 664–675. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, A.; Kasprzak, K.; Wójtowicz, A.; Oniszczuk, T.; Olech, M. The Impact of Processing Parameters on the Content of Phenolic Compounds in New Gluten-Free Precooked Buckwheat Pasta. Molecules 2019, 24, 1262. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. Chapter 26—The Beneficial Role of Rutin, A Naturally Occurring Flavonoid in Health Promotion and Disease Prevention: A Systematic Review and Update. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases, 2nd ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 457–479. ISBN 978-0-12-813820-5. [Google Scholar]
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus Phytochemical with Health-Promoting Properties. Biofactors 2017, 43, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, M.M.; Sharifi-Rad, J.; Herrera-Bravo, J.; Jara, E.L.; Salazar, L.A.; Kregiel, D.; Uprety, Y.; Akram, M.; Iqbal, M.; Martorell, M.; et al. Therapeutic Potential of Isoflavones with an Emphasis on Daidzein. Oxidative Med. Cell Longev. 2021, 2021, 6331630. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharm. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Roche, A.; Ross, E.; Walsh, N.; O’Donnell, K.; Williams, A.; Klapp, M.; Fullard, N.; Edelstein, S. Representative Literature on the Phytonutrients Category: Phenolic Acids. Crit. Rev. Food Sci. Nutr. 2017, 57, 1089–1096. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Li, C.-X.; Kakar, M.U.; Khan, M.S.; Wu, P.-F.; Amir, R.M.; Dai, D.-F.; Naveed, M.; Li, Q.-Y.; Saeed, M.; et al. Resveratrol (RV): A Pharmacological Review and Call for Further Research. Biomed. Pharm. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Ali, A.; Yu, L.; Kousar, S.; Khalid, W.; Maqbool, Z.; Aziz, A.; Arshad, M.S.; Aadil, R.M.; Trif, M.; Riaz, S.; et al. Crocin: Functional Characteristics, Extraction, Food Applications and Efficacy against Brain Related Disorders. Front Nutr. 2022, 9, 1009807. [Google Scholar] [CrossRef]
- Karam, J.; Bibiloni, M.D.M.; Tur, J.A. Polyphenol Estimated Intake and Dietary Sources among Older Adults from Mallorca Island. PLoS ONE 2018, 13, e0191573. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Gao, J.; Hao, R.; Yang, J.; Wei, J. Effects of Simulated Digestion on Black Chokeberry (Aronia Melanocarpa (Michx.) Elliot) Anthocyanins and Intestinal Flora. J. Food Sci. Technol. 2020, 58, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Mihaylova, D.; Desseva, I.; Stoyanova, M.; Petkova, N.; Terzyiska, M.; Lante, A. Impact of In Vitro Gastrointestinal Digestion on the Bioaccessibility of Phytochemical Compounds from Eight Fruit Juices. Molecules 2021, 26, 1187. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, T.; Widelska, G.; Oniszczuk, A.; Kasprzak, K.; Wójtowicz, A.; Olech, M.; Nowak, R.; Kulesza, K.W.; Jóźwiak, G.; Hajnos, M.W. Influence of Production Parameters on the Content of Polyphenolic Compounds in Extruded Porridge Enriched with Chokeberry Fruit (Aronia Melanocarpa (Michx.) Elliott). Open Chem. 2019, 17, 166–176. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Wang, L. Role and Mechanism of Gut Microbiota in Human Disease. Front. Cell. Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut Microbiota Influence Immunotherapy Responses: Mechanisms and Therapeutic Strategies. J. Hematol. Oncol. 2022, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.-X.; Goh, W.-R.; Wu, R.-N.; Yue, X.-Q.; Luo, X.; Khine, W.W.T.; Wu, J.-R.; Lee, Y.-K. Revisit Gut Microbiota and Its Impact on Human Health and Disease. J. Food Drug Anal. 2019, 27, 623–631. [Google Scholar] [CrossRef]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of Dietary Compounds, Especially Polyphenols, with the Intestinal Microbiota: A Review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Involvement of Gut Microbiota, Microbial Metabolites and Interaction with Polyphenol in Host Immunometabolism. Nutrients 2020, 12, 3054. [Google Scholar] [CrossRef]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]
- Naliyadhara, N.; Kumar, A.; Kumar Gangwar, S.; Nair Devanarayanan, T.; Hegde, M.; Alqahtani, M.S.; Abbas, M.; Sethi, G.; Kunnumakkara, A. Interplay of Dietary Antioxidants and Gut Microbiome in Human Health: What Has Been Learnt Thus Far? J. Funct. Foods 2023, 100, 105365. [Google Scholar] [CrossRef]
- Pathak, S.; Kesavan, P.; Banerjee, A.; Banerjee, A.; Sağdıçoğlu Celep, G.; Bissi, L.; Marotta, F. Metabolism of Dietary Polyphenols by Human Gut Microbiota and Their Health Benefits. In Polyphenols: Mechanisms of Action in Human Health and Disease; Academic Press: Cambridge, MA, USA, 2018; pp. 347–359. ISBN 978-0-12-813006-3. [Google Scholar]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Aura, A.-M. Microbial Metabolism of Dietary Phenolic Compounds in the Colon. Phytochem. Rev. 2008, 7, 407–429. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2022, 14, 137. [Google Scholar] [CrossRef]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.-S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.A.P.; Kulling, S.E. In Vivo and in Vitro Metabolism of Trans-Resveratrol by Human Gut Microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef]
- Catalkaya, G.; Venema, K.; Lucini, L.; Rocchetti, G.; Delmas, D.; Daglia, M.; De Filippis, A.; Xiao, H.; Quiles, J.L.; Xiao, J.; et al. Interaction of Dietary Polyphenols and Gut Microbiota: Microbial Metabolism of Polyphenols, Influence on the Gut Microbiota, and Implications on Host Health. Food Front. 2020, 1, 109–133. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Alminger, M.; Aura, A.-M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.C.; McDougall, G.J.; Requena, T.; et al. In Vitro Models for Studying Secondary Plant Metabolite Digestion and Bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef]
- Verediano, T.A.; Stampini Duarte Martino, H.; Dias Paes, M.C.; Tako, E. Effects of Anthocyanin on Intestinal Health: A Systematic Review. Nutrients 2021, 13, 1331. [Google Scholar] [CrossRef]
- Faria, A.; Fernandes, I.; Norberto, S.; Mateus, N.; Calhau, C. Interplay between Anthocyanins and Gut Microbiota. J. Agric. Food Chem. 2014, 62, 6898–6902. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Gancarz, M.; Kondracka, A.; Rusinek, R.; Oniszczuk, A. Curcumin and Weight Loss: Does It Work? Int. J. Mol. Sci. 2022, 23, 639. [Google Scholar] [CrossRef]
- Vinson, J.A.; Su, X.; Zubik, L.; Bose, P. Phenol Antioxidant Quantity and Quality in Foods: Fruits. J. Agric. Food Chem. 2001, 49, 5315–5321. [Google Scholar] [CrossRef]
- Ulrey, R.K.; Barksdale, S.M.; Zhou, W.; van Hoek, M.L. Cranberry Proanthocyanidins Have Anti-Biofilm Properties against Pseudomonas Aeruginosa. BMC Complement. Altern. Med. 2014, 14, 499. [Google Scholar] [CrossRef]
- Dias, R.; Pereira, C.B.; Pérez-Gregorio, R.; Mateus, N.; Freitas, V. Recent Advances on Dietary Polyphenol’s Potential Roles in Celiac Disease. Trends Food Sci. Technol. 2021, 107, 213–225. [Google Scholar] [CrossRef]
- Gowd, V.; Karim, N.; Shishir, M.R.I.; Xie, L.; Chen, W. Dietary Polyphenols to Combat the Metabolic Diseases via Altering Gut Microbiota. Trends Food Sci. Technol. 2019, 93, 81–93. [Google Scholar] [CrossRef]
- Buret, A.G.; Allain, T. Gut Microbiota Biofilms: From Regulatory Mechanisms to Therapeutic Targets. J. Exp. Med. 2023, 220, e20221743. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- Jaquet, M.; Rochat, I.; Moulin, J.; Cavin, C.; Bibiloni, R. Impact of Coffee Consumption on the Gut Microbiota: A Human Volunteer Study. Int. J. Food Microbiol. 2009, 130, 117–121. [Google Scholar] [CrossRef]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andrés-Lacueva, C.; Tinahones, F.J. Influence of Red Wine Polyphenols and Ethanol on the Gut Microbiota Ecology and Biochemical Biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef]
- Vetrani, C.; Maukonen, J.; Bozzetto, L.; Della Pepa, G.; Vitale, M.; Costabile, G.; Riccardi, G.; Rivellese, A.A.; Saarela, M.; Annuzzi, G. Diets Naturally Rich in Polyphenols and/or Long-Chain n-3 Polyunsaturated Fatty Acids Differently Affect Microbiota Composition in High-Cardiometabolic-Risk Individuals. Acta Diabetol. 2020, 57, 853–860. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A Polyphenol-Rich Cranberry Extract Protects from Diet-Induced Obesity, Insulin Resistance and Intestinal Inflammation in Association with Increased Akkermansia Spp. Population in the Gut Microbiota of Mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef]
- Lima, A.C.D.; Cecatti, C.; Fidélix, M.P.; Adorno, M.A.T.; Sakamoto, I.K.; Cesar, T.B.; Sivieri, K. Effect of Daily Consumption of Orange Juice on the Levels of Blood Glucose, Lipids, and Gut Microbiota Metabolites: Controlled Clinical Trials. J. Med. Food 2019, 22, 202–210. [Google Scholar] [CrossRef]
- Bae, E.A.; Han, M.J.; Kim, D.H. In Vitro Anti-Helicobacter Pylori Activity of Some Flavonoids and Their Metabolites. Planta Med. 1999, 65, 442–443. [Google Scholar] [CrossRef]
- Clavel, T.; Fallani, M.; Lepage, P.; Levenez, F.; Mathey, J.; Rochet, V.; Sérézat, M.; Sutren, M.; Henderson, G.; Bennetau-Pelissero, C.; et al. Isoflavones and Functional Foods Alter the Dominant Intestinal Microbiota in Postmenopausal Women. J. Nutr. 2005, 135, 2786–2792. [Google Scholar] [CrossRef]
- Potì, F.; Santi, D.; Spaggiari, G.; Zimetti, F.; Zanotti, I. Polyphenol Health Effects on Cardiovascular and Neurodegenerative Disorders: A Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 351. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W. Inflammation Revisited: Atherosclerosis in The Post-CANTOS Era. Eur. Cardiol. 2017, 12, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Iranshahy, M.; Iranshahi, M.; Abtahi, S.R.; Karimi, G. The Role of Nuclear Factor Erythroid 2-Related Factor 2 in Hepatoprotective Activity of Natural Products: A Review. Food Chem. Toxicol. 2018, 120, 261–276. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.; Kirkham, P. Regulation of Inflammation and Redox Signaling by Dietary Polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.; Vaccaro, O.; Masulli, M.; Bonora, E.; Del Prato, S.; Giorda, C.B.; Nicolucci, A.; Squatrito, S.; Auciello, S.; Babini, A.C.; et al. Polyphenol Intake and Cardiovascular Risk Factors in a Population with Type 2 Diabetes: The TOSCA.IT Study. Clin. Nutr. 2017, 36, 1686–1692. [Google Scholar] [CrossRef]
- Medina-Remón, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Estruch, R.; et al. Polyphenol Intake from a Mediterranean Diet Decreases Inflammatory Biomarkers Related to Atherosclerosis: A Substudy of the PREDIMED Trial. Br. J. Clin. Pharmacol. 2017, 83, 114–128. [Google Scholar] [CrossRef]
- Cullen, A.E.; Centner, A.M.; Deitado, R.; Fernandez, J.; Salazar, G. The Impact of Dietary Supplementation of Whole Foods and Polyphenols on Atherosclerosis. Nutrients 2020, 12, 2069. [Google Scholar] [CrossRef]
- Hirsova, P.; Kolouchova, G.; Dolezelova, E.; Cermanova, J.; Hyspler, R.; Kadova, Z.; Micuda, S. Epigallocatechin Gallate Enhances Biliary Cholesterol Secretion in Healthy Rats and Lowers Plasma and Liver Cholesterol in Ethinylestradiol-Treated Rats. Eur. J. Pharm. 2012, 691, 38–45. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nishizawa, M.; Inoue, N.; Hosoya, T.; Yoshida, M.; Ukawa, Y.; Sagesaka, Y.M.; Doi, T.; Nakayama, T.; Kumazawa, S.; et al. Epigallocatechin Gallate Decreases the Micellar Solubility of Cholesterol via Specific Interaction with Phosphatidylcholine. J. Agric. Food Chem. 2014, 62, 2881–2890. [Google Scholar] [CrossRef]
- Azorín-Ortuño, M.; Yáñez-Gascón, M.J.; González-Sarrías, A.; Larrosa, M.; Vallejo, F.; Pallarés, F.J.; Lucas, R.; Morales, J.C.; Tomás-Barberán, F.A.; García-Conesa, M.-T.; et al. Effects of Long-Term Consumption of Low Doses of Resveratrol on Diet-Induced Mild Hypercholesterolemia in Pigs: A Transcriptomic Approach to Disease Prevention. J. Nutr. Biochem. 2012, 23, 829–837. [Google Scholar] [CrossRef]
- Yasuda, A.; Natsume, M.; Osakabe, N.; Kawahata, K.; Koga, J. Cacao Polyphenols Influence the Regulation of Apolipoprotein in HepG2 and Caco2 Cells. J. Agric. Food Chem. 2011, 59, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Leikert, J.F.; Räthel, T.R.; Wohlfart, P.; Cheynier, V.; Vollmar, A.M.; Dirsch, V.M. Red Wine Polyphenols Enhance Endothelial Nitric Oxide Synthase Expression and Subsequent Nitric Oxide Release from Endothelial Cells. Circulation 2002, 106, 1614–1617. [Google Scholar] [CrossRef]
- Fisher, N.D.L.; Hughes, M.; Gerhard-Herman, M.; Hollenberg, N.K. Flavanol-Rich Cocoa Induces Nitric-Oxide-Dependent Vasodilation in Healthy Humans. J. Hypertens. 2003, 21, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Oak, M.-H.; Auger, C.; Belcastro, E.; Park, S.-H.; Lee, H.-H.; Schini-Kerth, V.B. Potential Mechanisms Underlying Cardiovascular Protection by Polyphenols: Role of the Endothelium. Free Radic. Biol. Med. 2018, 122, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Muñoz-González, I.; Cueva, C.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. A Survey of Modulation of Gut Microbiota by Dietary Polyphenols. BioMed Res. Int. 2015, 2015, e850902. [Google Scholar] [CrossRef]
- 2015–2020 Dietary Guidelines|Health.Gov. Available online: https://health.gov/our-work/nutrition-physical-activity/dietary-guidelines/previous-dietary-guidelines/2015 (accessed on 18 March 2023).
- Moss, J.W.E.; Williams, J.O.; Ramji, D.P. Nutraceuticals as Therapeutic Agents for Atherosclerosis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1864, 1562–1572. [Google Scholar] [CrossRef]
- Rassaf, T.; Rammos, C.; Hendgen-Cotta, U.B.; Heiss, C.; Kleophas, W.; Dellanna, F.; Floege, J.; Hetzel, G.R.; Kelm, M. Vasculoprotective Effects of Dietary Cocoa Flavanols in Patients on Hemodialysis: A Double–Blind, Randomized, Placebo–Controlled Trial. Clin. J. Am. Soc. Nephrol. CJASN 2016, 11, 108. [Google Scholar] [CrossRef]
- West, S.G.; McIntyre, M.D.; Piotrowski, M.J.; Poupin, N.; Miller, D.L.; Preston, A.G.; Wagner, P.; Groves, L.F.; Skulas-Ray, A.C. Effects of Dark Chocolate and Cocoa Consumption on Endothelial Function and Arterial Stiffness in Overweight Adults. Br. J. Nutr. 2014, 111, 653–661. [Google Scholar] [CrossRef]
- Ibero-Baraibar, I.; Abete, I.; Navas-Carretero, S.; Massis-Zaid, A.; Martinez, J.A.; Zulet, M.A. Oxidised LDL Levels Decreases after the Consumption of Ready-to-Eat Meals Supplemented with Cocoa Extract within a Hypocaloric Diet. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 416–422. [Google Scholar] [CrossRef]
- Baba, S.; Natsume, M.; Yasuda, A.; Nakamura, Y.; Tamura, T.; Osakabe, N.; Kanegae, M.; Kondo, K. Plasma LDL and HDL Cholesterol and Oxidized LDL Concentrations Are Altered in Normo- and Hypercholesterolemic Humans after Intake of Different Levels of Cocoa Powder1. J. Nutr. 2007, 137, 1436–1441. [Google Scholar] [CrossRef]
- Hsu, S.-P.; Wu, M.-S.; Yang, C.-C.; Huang, K.-C.; Liou, S.-Y.; Hsu, S.-M.; Chien, C.-T. Chronic Green Tea Extract Supplementation Reduces Hemodialysis-Enhanced Production of Hydrogen Peroxide and Hypochlorous Acid, Atherosclerotic Factors, and Proinflammatory Cytokines. Am. J. Clin. Nutr. 2007, 86, 1539–1547. [Google Scholar] [CrossRef]
- Oyama, J.-I.; Maeda, T.; Kouzuma, K.; Ochiai, R.; Tokimitsu, I.; Higuchi, Y.; Sugano, M.; Makino, N. Green Tea Catechins Improve Human Forearm Endothelial Dysfunction and Have Antiatherosclerotic Effects in Smokers. Circ. J. 2010, 74, 578–588. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse Association between Habitual Polyphenol Intake and Incidence of Cardiovascular Events in the PREDIMED Study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.; Marini, C.; et al. Cocoa Flavanol Consumption Improves Cognitive Function, Blood Pressure Control, and Metabolic Profile in Elderly Subjects: The Cocoa, Cognition, and Aging (CoCoA) Study--a Randomized Controlled Trial. Am. J. Clin. Nutr. 2015, 101, 538–548. [Google Scholar] [CrossRef]
- Sansone, R.; Rodriguez-Mateos, A.; Heuel, J.; Falk, D.; Schuler, D.; Wagstaff, R.; Kuhnle, G.G.C.; Spencer, J.P.E.; Schroeter, H.; Merx, M.W.; et al. Cocoa Flavanol Intake Improves Endothelial Function and Framingham Risk Score in Healthy Men and Women: A Randomised, Controlled, Double-Masked Trial: The Flaviola Health Study. BJN 2015, 114, 1246–1255. [Google Scholar] [CrossRef]
- Kurosawa, T.; Itoh, F.; Nozaki, A.; Nakano, Y.; Katsuda, S.; Osakabe, N.; Tsubone, H.; Kondo, K.; Itakura, H. Suppressive Effects of Cacao Liquor Polyphenols (CLP) on LDL Oxidation and the Development of Atherosclerosis in Kurosawa and Kusanagi-Hypercholesterolemic Rabbits. Atherosclerosis 2005, 179, 237–246. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.; van de Weijer, T.; Goossens, G.; Hoeks, J.; Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie Restriction-like Effects of 30 Days of Resveratrol Supplementation on Energy Metabolism and Metabolic Profile in Obese Humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Most, J.; Timmers, S.; Warnke, I.; Jocken, J.W.; van Boekschoten, M.; de Groot, P.; Bendik, I.; Schrauwen, P.; Goossens, G.H.; Blaak, E.E. Combined Epigallocatechin-3-Gallate and Resveratrol Supplementation for 12 Wk Increases Mitochondrial Capacity and Fat Oxidation, but Not Insulin Sensitivity, in Obese Humans: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2016, 104, 215–227. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Larrosa, M.; Yáñez-Gascón, M.J.; Dávalos, A.; Gil-Zamorano, J.; Gonzálvez, M.; García-Almagro, F.J.; Ruiz Ros, J.A.; Tomás-Barberán, F.A.; Espín, J.C.; et al. One-Year Supplementation with a Grape Extract Containing Resveratrol Modulates Inflammatory-Related MicroRNAs and Cytokines Expression in Peripheral Blood Mononuclear Cells of Type 2 Diabetes and Hypertensive Patients with Coronary Artery Disease. Pharmacol. Res. 2013, 72, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Huminiecki, L.; Atanasov, A.G.; Horbańczuk, J. Etiology of Atherosclerosis Informs Choice of Animal Models and Tissues for Initial Functional Genomic Studies of Resveratrol. Pharmacol. Res. 2020, 156, 104598. [Google Scholar] [CrossRef] [PubMed]
- Sanches-Silva, A.; Testai, L.; Nabavi, S.F.; Battino, M.; Pandima Devi, K.; Tejada, S.; Sureda, A.; Xu, S.; Yousefi, B.; Majidinia, M.; et al. Therapeutic Potential of Polyphenols in Cardiovascular Diseases: Regulation of MTOR Signaling Pathway. Pharmacol. Res. 2020, 152, 104626. [Google Scholar] [CrossRef]
- Kurdi, A.; Martinet, W.; De Meyer, G.R.Y. MTOR Inhibition and Cardiovascular Diseases: Dyslipidemia and Atherosclerosis. Transplantation 2018, 102, S44–S46. [Google Scholar] [CrossRef] [PubMed]
- Demidenko, Z.N.; Blagosklonny, M.V. At Concentrations That Inhibit MTOR, Resveratrol Suppresses Cellular Senescence. Cell Cycle 2009, 8, 1901–1904. [Google Scholar] [CrossRef]
- Song, J.; Huang, Y.; Zheng, W.; Yan, J.; Cheng, M.; Zhao, R.; Chen, L.; Hu, C.; Jia, W. Resveratrol Reduces Intracellular Reactive Oxygen Species Levels by Inducing Autophagy through the AMPK-MTOR Pathway. Front. Med. 2018, 12, 697–706. [Google Scholar] [CrossRef]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Majeed, M.; Sahebkar, A. Antioxidant and Anti-Inflammatory Effects of Curcuminoid-Piperine Combination in Subjects with Metabolic Syndrome: A Randomized Controlled Trial and an Updated Meta-Analysis. Clin. Nutr. 2015, 34, 1101–1108. [Google Scholar] [CrossRef]
- Sahebkar, A. A Systematic Review and Meta-Analysis of Randomized Controlled Trials Investigating the Effects of Curcumin on Blood Lipid Levels. Clin. Nutr. 2014, 33, 406–414. [Google Scholar] [CrossRef]
- Guo, S.; Long, M.; Li, X.; Zhu, S.; Zhang, M.; Yang, Z. Curcumin Activates Autophagy and Attenuates Oxidative Damage in EA.Hy926 Cells via the Akt/MTOR Pathway. Mol. Med. Rep. 2016, 13, 2187–2193. [Google Scholar] [CrossRef]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kürbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin Reduces Systolic Blood Pressure and Plasma Oxidised Low-Density Lipoprotein Concentrations in Overweight Subjects with a High-Cardiovascular Disease Risk Phenotype: A Double-Blinded, Placebo-Controlled Cross-over Study. Br J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef]
- Pratheeshkumar, P.; Budhraja, A.; Son, Y.-O.; Wang, X.; Zhang, Z.; Ding, S.; Wang, L.; Hitron, A.; Lee, J.-C.; Xu, M.; et al. Quercetin Inhibits Angiogenesis Mediated Human Prostate Tumor Growth by Targeting VEGFR- 2 Regulated AKT/MTOR/P70S6K Signaling Pathways. PLoS ONE 2012, 7, e47516. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, C.; Yao, P.; Gong, Z. Quercetin Alleviates High-Fat Diet-Induced Oxidized Low-Density Lipoprotein Accumulation in the Liver: Implication for Autophagy Regulation. BioMed Res. Int. 2015, 2015, e607531. [Google Scholar] [CrossRef]
- Kim, S.G.; Kim, J.-R.; Choi, H.C. Quercetin-Induced AMP-Activated Protein Kinase Activation Attenuates Vasoconstriction Through LKB1-AMPK Signaling Pathway. J. Med. Food 2018, 21, 146–153. [Google Scholar] [CrossRef]
- Shen, Y.; Ward, N.C.; Hodgson, J.M.; Puddey, I.B.; Wang, Y.; Zhang, D.; Maghzal, G.J.; Stocker, R.; Croft, K.D. Dietary Quercetin Attenuates Oxidant-Induced Endothelial Dysfunction and Atherosclerosis in Apolipoprotein E Knockout Mice Fed a High-Fat Diet: A Critical Role for Heme Oxygenase-1. Free Radic. Biol. Med. 2013, 65, 908–915. [Google Scholar] [CrossRef]
- Loke, W.M.; Proudfoot, J.; Hodgson, J.; Mckinley, A.; Hime, N.; Magat, M.; Stocker, R.; Croft, K. Specific Dietary Polyphenols Attenuate Atherosclerosis in Apolipoprotein E-Knockout Mice by Alleviating Inflammation and Endothelial Dysfunction. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Pieczynska, M.D.; Yang, Y.; Petrykowski, S.; Horbanczuk, O.K.; Atanasov, A.G.; Horbanczuk, J.O. Gut Microbiota and Its Metabolites in Atherosclerosis Development. Molecules 2020, 25, 594. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-C.; Ou, T.-T.; Chang, C.-H.; Chan, K.-C.; Wang, C.-J. Protocatechuic Acid Inhibits Oleic Acid-Induced Vascular Smooth Muscle Cell Proliferation through Activation of AMP-Activated Protein Kinase and Cell Cycle Arrest in G0/G1 Phase. J. Agric. Food Chem. 2015, 63, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Radtke, O.A.; Kiderlen, A.F.; Kayser, O.; Kolodziej, H. Gene Expression Profiles of Inducible Nitric Oxide Synthase and Cytokines in Leishmania major-Infected Macrophage-Like RAW 264.7 Cells Treated with Gallic Acid. Planta Med. 2004, 70, 924–928. [Google Scholar] [CrossRef]
- Ahuja, V.; Miura, K.; Vishnu, A.; Fujiyoshi, A.; Evans, R.; Zaid, M.; Miyagawa, N.; Hisamatsu, T.; Kadota, A.; Okamura, T.; et al. Significant Inverse Association of Equol-Producer Status with Coronary Artery Calcification but Not Dietary Isoflavones in Healthy Japanese Men. Br J. Nutr. 2017, 117, 260–266. [Google Scholar] [CrossRef]
- Sekikawa, A.; Ihara, M.; Lopez, O.; Kakuta, C.; Lopresti, B.; Higashiyama, A.; Aizenstein, H.; Chang, Y.-F.; Mathis, C.; Miyamoto, Y.; et al. Effect of S-Equol and Soy Isoflavones on Heart and Brain. Curr. Cardiol. Rev. 2019, 15, 114–135. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; López-Sabater, M.C.; Covas, M.I.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; et al. Polyphenol Intake and Mortality Risk: A Re-Analysis of the PREDIMED Trial. BMC Med. 2014, 12, 77. [Google Scholar] [CrossRef]
- Medina-Remón, A.; Tresserra-Rimbau, A.; Pons, A.; Tur, J.A.; Martorell, M.; Ros, E.; Buil-Cosiales, P.; Sacanella, E.; Covas, M.I.; Corella, D.; et al. Effects of Total Dietary Polyphenols on Plasma Nitric Oxide and Blood Pressure in a High Cardiovascular Risk Cohort. The PREDIMED Randomized Trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Tresserra-Rimbau, A.; Estruch, R.; Martínez-González, M.; Medina-Remón, A.; Castañer, O.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.M. Effects of Polyphenol, Measured by a Biomarker of Total Polyphenols in Urine, on Cardiovascular Risk Factors After a Long-Term Follow-Up in the PREDIMED Study. Oxidative Med. Cell. Longev. 2016, 2016, 2572606. [Google Scholar] [CrossRef]
- Jungeström, M.B.; Thompson, L.U.; Dabrosin, C. Flaxseed and Its Lignans Inhibit Estradiol-Induced Growth, Angiogenesis, and Secretion of Vascular Endothelial Growth Factor in Human Breast Cancer Xenografts in Vivo. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 1061–1067. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Pöhö, P.; Bozzetto, L.; Vetrani, C.; Patti, L.; Aura, A.-M.; Annuzzi, G.; Hyötyläinen, T.; Rivellese, A.A.; Orešič, M. Isoenergetic Diets Differing in Their N-3 Fatty Acid and Polyphenol Content Reflect Different Plasma and HDL-Fraction Lipidomic Profiles in Subjects at High Cardiovascular Risk. Mol. Nutr. Food Res. 2014, 58, 1873–1882. [Google Scholar] [CrossRef]
- Covas, M.-I.; Nyyssönen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.-J.F.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Bäumler, H.; et al. The Effect of Polyphenols in Olive Oil on Heart Disease Risk Factors. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef]
- Rosenblat, M.; Volkova, N.; Coleman, R.; Almagor, Y.; Aviram, M. Antiatherogenicity of Extra Virgin Olive Oil and Its Enrichment with Green Tea Polyphenols in the Atherosclerotic Apolipoprotein-E-Deficient Mice: Enhanced Macrophage Cholesterol Efflux. J. Nutr. Biochem. 2008, 19, 514–523. [Google Scholar] [CrossRef]
- Eilertsen, K.-E.; Mæhre, H.K.; Cludts, K.; Olsen, J.O.; Hoylaerts, M.F. Dietary Enrichment of Apolipoprotein E-Deficient Mice with Extra Virgin Olive Oil in Combination with Seal Oil Inhibits Atherogenesis. Lipids Health Dis. 2011, 10, 41. [Google Scholar] [CrossRef]
- Aviram, M.; Volkova, N.; Coleman, R.; Dreher, M.; Reddy, M.K.; Ferreira, D.; Rosenblat, M. Pomegranate Phenolics from the Peels, Arils, and Flowers Are Antiatherogenic: Studies in Vivo in Atherosclerotic Apolipoprotein E-Deficient (E0) Mice and in Vitro in Cultured Macrophages and Lipoproteins. J. Agric. Food Chem. 2008, 56, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Hayek, T.; Raz, A.; Coleman, R.; Dornfeld, L.; Vaya, J.; Aviram, M. Pomegranate Juice Supplementation to Atherosclerotic Mice Reduces Macrophage Lipid Peroxidation, Cellular Cholesterol Accumulation and Development of Atherosclerosis. J. Nutr. 2001, 131, 2082–2089. [Google Scholar] [CrossRef]
- Rosenblat, M.; Volkova, N.; Coleman, R.; Aviram, M. Pomegranate Byproduct Administration to Apolipoprotein E-Deficient Mice Attenuates Atherosclerosis Development as a Result of Decreased Macrophage Oxidative Stress and Reduced Cellular Uptake of Oxidized Low-Density Lipoprotein. J. Agric. Food Chem. 2006, 54, 1928–1935. [Google Scholar] [CrossRef]
- Peluzio, M.d.C.G.; Teixeira, T.F.S.; Oliveira, V.P.; Sabarense, C.M.; Dias, C.M.G.C.; Abranches, M.V.; Maldonado, I.R. dos S.C. Grape Extract and α-Tocopherol Effect in Cardiovascular Disease Model of Apo E -/- Mice. Acta Cir. Bras. 2011, 26, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Shema-Didi, L.; Kristal, B.; Sela, S.; Geron, R.; Ore, L. Does Pomegranate Intake Attenuate Cardiovascular Risk Factors in Hemodialysis Patients? Nutr. J. 2014, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-R.; Li, J.-Y.; Dong, X.-W.; Tan, Z.-J.; Wu, W.-Z.; Xie, Q.-M.; Yang, Y.-M. Apple Polyphenols Decrease Atherosclerosis and Hepatic Steatosis in ApoE−/− Mice through the ROS/MAPK/NF-ΚB Pathway. Nutrients 2015, 7, 7085–7105. [Google Scholar] [CrossRef] [PubMed]
- Auclair, S.; Silberberg, M.; Gueux, E.; Morand, C.; Mazur, A.; Milenkovic, D.; Scalbert, A. Apple Polyphenols and Fibers Attenuate Atherosclerosis in Apolipoprotein E-Deficient Mice. J. Agric. Food Chem. 2008, 56, 5558–5563. [Google Scholar] [CrossRef]
- Lin, W.; Liu, C.; Yang, H.; Wang, W.; Ling, W.; Wang, D. Chicory, a Typical Vegetable in Mediterranean Diet, Exerts a Therapeutic Role in Established Atherosclerosis in Apolipoprotein E-Deficient Mice. Mol. Nutr. Food Res. 2015, 59, 1803–1813. [Google Scholar] [CrossRef]
- Joo, H.K.; Choi, S.; Lee, Y.R.; Lee, E.O.; Park, M.S.; Park, K.B.; Kim, C.-S.; Lim, Y.P.; Park, J.-T.; Jeon, B.H. Anthocyanin-Rich Extract from Red Chinese Cabbage Alleviates Vascular Inflammation in Endothelial Cells and Apo E−/− Mice. Int. J. Mol. Sci. 2018, 19, 816. [Google Scholar] [CrossRef]
- Zhao, X.; Oduro, P.K.; Tong, W.; Wang, Y.; Gao, X.; Wang, Q. Therapeutic Potential of Natural Products against Atherosclerosis: Targeting on Gut Microbiota. Pharmacol. Res. 2021, 163, 105362. [Google Scholar] [CrossRef]
- Luo, Y.; Fang, J.-L.; Yuan, K.; Jin, S.-H.; Guo, Y. Ameliorative Effect of Purified Anthocyanin from Lycium Ruthenicum on Atherosclerosis in Rats through Synergistic Modulation of the Gut Microbiota and NF-ΚB/SREBP-2 Pathways. J. Funct. Foods 2019, 59, 223–233. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Li, S.; Koh, Y.-C.; Wu, J.-C.; Yang, M.-J.; Ho, C.-T.; Pan, M.-H. Oolong Tea Extract and Citrus Peel Polymethoxyflavones Reduce Transformation of L-Carnitine to Trimethylamine-N-Oxide and Decrease Vascular Inflammation in L-Carnitine Feeding Mice. J. Agric. Food Chem. 2019, 67, 7869–7879. [Google Scholar] [CrossRef]
- Yang, G.; Lin, C.-C.; Yang, Y.; Yuan, L.; Wang, P.; Wen, X.; Pan, M.-H.; Zhao, H.; Ho, C.-T.; Li, S. Nobiletin Prevents Trimethylamine Oxide-Induced Vascular Inflammation via Inhibition of the NF-ΚB/MAPK Pathways. J. Agric. Food Chem. 2019, 67, 6169–6176. [Google Scholar] [CrossRef]
- Wu, D.-N.; Guan, L.; Jiang, Y.-X.; Ma, S.-H.; Sun, Y.-N.; Lei, H.-T.; Yang, W.-F.; Wang, Q.-F. Microbiome and Metabonomics Study of Quercetin for the Treatment of Atherosclerosis. Cardiovasc. Diagn. Ther. 2019, 9, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Targeting the Gut Microbiota for Treating Colitis: Is FGF19 a Magic Bullet? EBioMedicine 2020, 55, 102754. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, K.; Lv, L.; Wu, S.; Guo, Z. Ferulic Acid Ameliorates Nonalcoholic Fatty Liver Disease and Modulates the Gut Microbiota Composition in High-Fat Diet Fed ApoE−/− Mice. Biomed. Pharmacother. 2019, 113, 108753. [Google Scholar] [CrossRef]
- Rom, O.; Korach-Rechtman, H.; Hayek, T.; Danin-Poleg, Y.; Bar, H.; Kashi, Y.; Aviram, M. Acrolein Increases Macrophage Atherogenicity in Association with Gut Microbiota Remodeling in Atherosclerotic Mice: Protective Role for the Polyphenol-Rich Pomegranate Juice. Arch. Toxicol. 2017, 91, 1709–1725. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Catry, E.; Taminiau, B.; Cani, P.D.; Bindels, L.B.; Daube, G.; Dessy, C.; Delzenne, N.M. Chitin–Glucan and Pomegranate Polyphenols Improve Endothelial Dysfunction. Sci. Rep. 2019, 9, 14150. [Google Scholar] [CrossRef]
- Chen, M.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.; Zhang, Q.; Mi, M. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 2016, 7, e02210–e02215. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.-L.; Zeng, B.-H.; Wang, W.; Li, G.-H.; Wu, F.; Wang, L.; Zhong, Q.-P.; Wei, H.; Fang, X. Impact of the Consumption of Tea Polyphenols on Early Atherosclerotic Lesion Formation and Intestinal Bifidobacteria in High-Fat-Fed ApoE−/− Mice. Front. Nutr. 2016, 3, 42. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, B.; Liu, Z.; Liao, Z.; Zhong, Q.; Gu, L.; Wei, H.; Fang, X. Green Tea Polyphenols Modulate Colonic Microbiota Diversity and Lipid Metabolism in High-Fat Diet Treated HFA Mice. J. Food Sci. 2018, 83, 864–873. [Google Scholar] [CrossRef]

| The Name of the Compound | Structure | Source of Occurrence |
|---|---|---|
| Quercetin (flavonoid) | 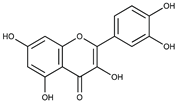 | Berries, cherries, grapes, apples, onions, tomatoes, kale and red vine [55]. |
| Rutin (flavonoid glycoside) | 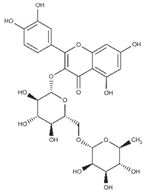 | Buckwheat [56] and asparagus [57] |
| Hesperetin (flavonoid) | 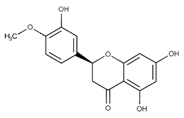 | Lime, lemon, sweet orange and tangelo [58] |
| Naringin (flavonoid) | 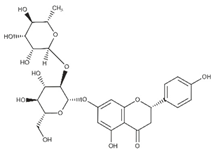 | Bergamot and sour orange and grapefruit [58] |
| Daidzein (Flavonoids) | 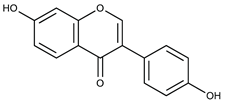 | Soy [59] |
| Cyanidin (flavonoid) | 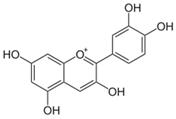 | Pomegranate, strawberry, raspberry and bilberry [34] |
| Protocatechuic Acid (non-flavonoid) | 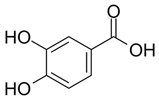 | Plums, gooseberries, grape, almonds ordinary, onion, bran and grain brown rice [60] |
| Ellagic acid (non-flavonoid) | 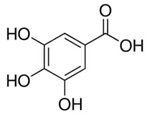 | White tea, blackberries and raspberries [61] |
| Gallic acid (non-flavonoid) | 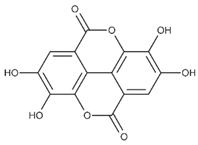 | Cranberries, strawberries, blueberries and blackberries [61] |
| Tannic acid (non-flavonoid) |  | Cranberries, persimmons, almonds, cocoa beans, grape seeds, parsley and peas [61] |
| Resveratrol (non-flavonoid) | 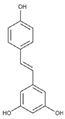 | Red grapes, mulberries, pomegranates, blueberries, pistachios and dark chocolate [62] |
| Curcumin (non-flavonoid) | 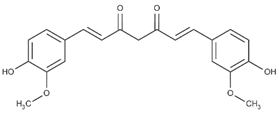 | Turmeric and zedoary rhizome [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziółkiewicz, A.; Kasprzak-Drozd, K.; Rusinek, R.; Markut-Miotła, E.; Oniszczuk, A. The Influence of Polyphenols on Atherosclerosis Development. Int. J. Mol. Sci. 2023, 24, 7146. https://doi.org/10.3390/ijms24087146
Ziółkiewicz A, Kasprzak-Drozd K, Rusinek R, Markut-Miotła E, Oniszczuk A. The Influence of Polyphenols on Atherosclerosis Development. International Journal of Molecular Sciences. 2023; 24(8):7146. https://doi.org/10.3390/ijms24087146
Chicago/Turabian StyleZiółkiewicz, Agnieszka, Kamila Kasprzak-Drozd, Robert Rusinek, Ewa Markut-Miotła, and Anna Oniszczuk. 2023. "The Influence of Polyphenols on Atherosclerosis Development" International Journal of Molecular Sciences 24, no. 8: 7146. https://doi.org/10.3390/ijms24087146
APA StyleZiółkiewicz, A., Kasprzak-Drozd, K., Rusinek, R., Markut-Miotła, E., & Oniszczuk, A. (2023). The Influence of Polyphenols on Atherosclerosis Development. International Journal of Molecular Sciences, 24(8), 7146. https://doi.org/10.3390/ijms24087146










