ABCG2 in Acute Myeloid Leukemia: Old and New Perspectives
Abstract
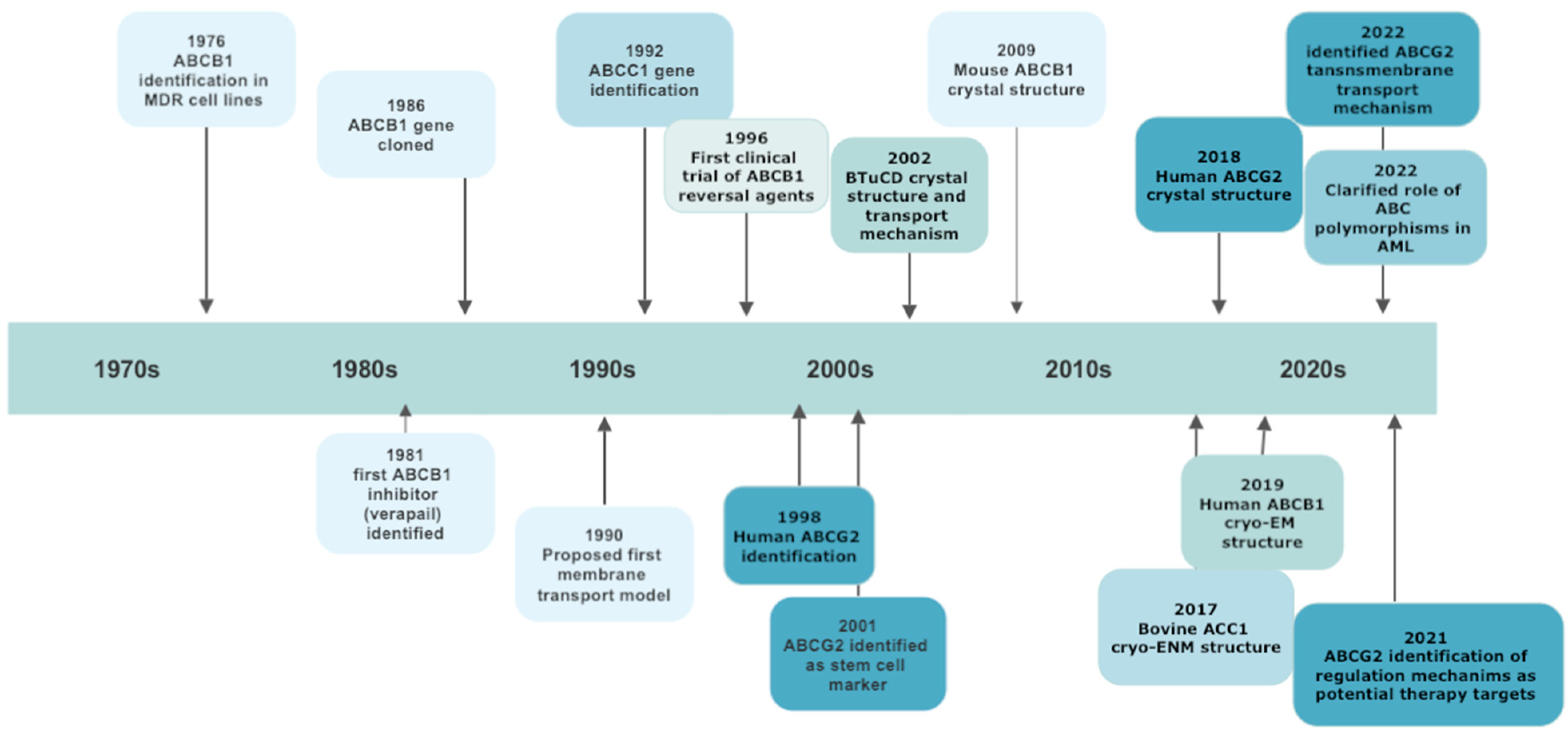
1. Introduction
2. ABCG Subfamily
2.1. ABCG1
2.2. ABCG4
2.3. ABCG5 and ABCG8
2.4. ABCG2
2.4.1. Physiologic ABCG2 Tissue Expression
Placenta
Blood–Brain Barrier (BBB)
Mammary Gland
Testis
Gastrointestinal Tract
Kidney
Liver and Biliary Tract
Hematopoietic Stem Cells
2.5. ABCG2 Substrates
3. Expression and Clinical Significance of ABCG2 in AML
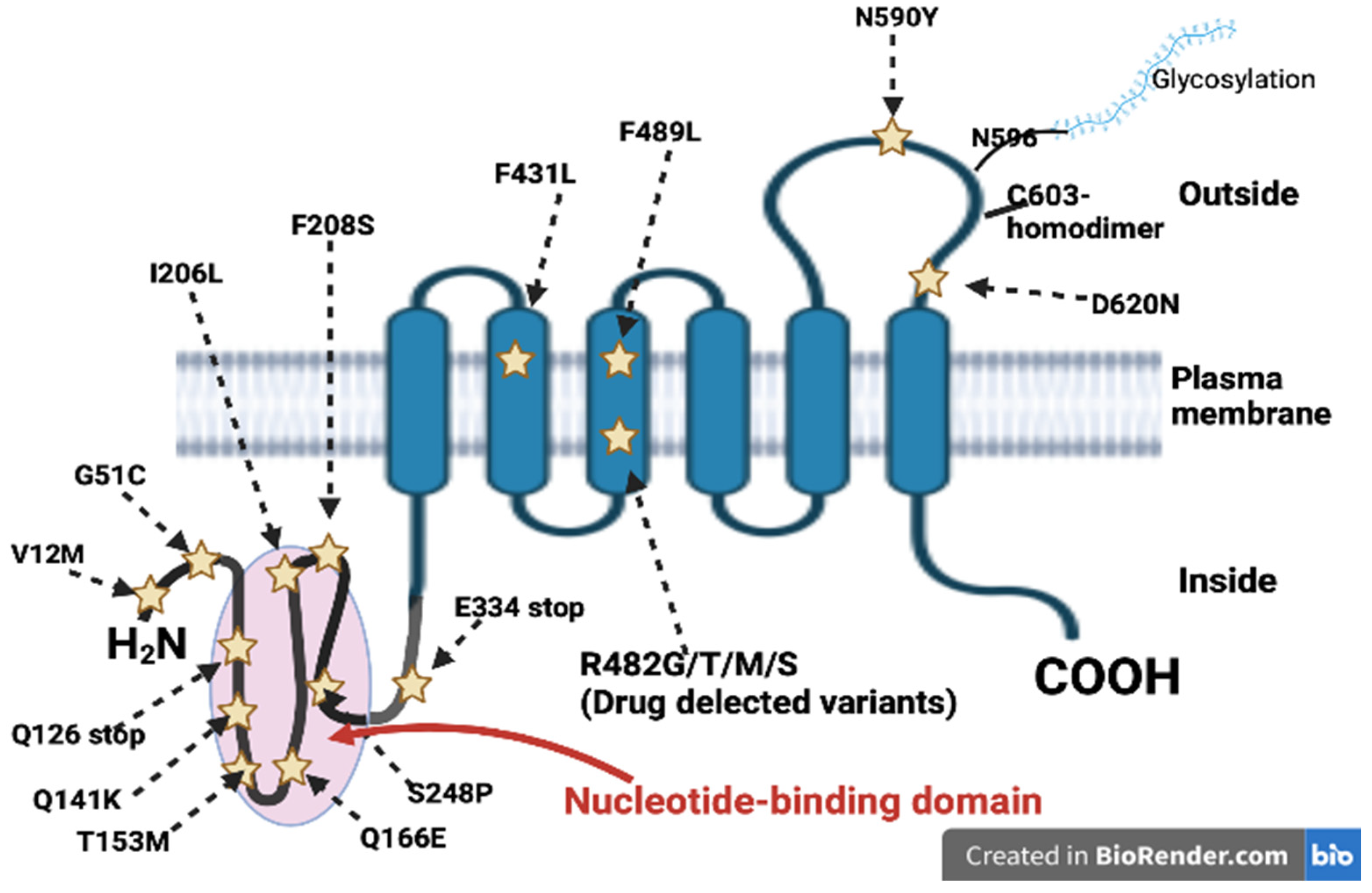
4. ABCG2 Polymorphisms in AML
5. ABCG2 and Extracellular Vesicles
6. ABCG2 Inhibition
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huntly, B.J.; Gilliland, D.G. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat. Rev. Cancer 2005, 5, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Jan, M.; Snyder, T.M.; Corces-Zimmerman, M.R.; Vyas, P.; Weissman, I.L.; Quake, S.R.; Majeti, R. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci. Transl. Med. 2012, 4, 149ra118. [Google Scholar] [CrossRef] [PubMed]
- Juliusson, G.; Antunovic, P.; Derolf, A.; Lehmann, S.; Mollgard, L.; Stockelberg, D.; Tidefelt, U.; Wahlin, A.; Hoglund, M. Age and acute myeloid leukemia: Real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 2009, 113, 4179–4187. [Google Scholar] [CrossRef] [PubMed]
- Morton, L.M.; Dores, G.M.; Schonfeld, S.J.; Linet, M.S.; Sigel, B.S.; Lam, C.J.K.; Tucker, M.A.; Curtis, R.E. Association of Chemotherapy for Solid Tumors With Development of Therapy-Related Myelodysplastic Syndrome or Acute Myeloid Leukemia in the Modern Era. JAMA Oncol. 2019, 5, 318–325. [Google Scholar] [CrossRef]
- Sasaki, K.; Ravandi, F.; Kadia, T.M.; DiNardo, C.D.; Short, N.J.; Borthakur, G.; Jabbour, E.; Kantarjian, H.M. De novo acute myeloid leukemia: A population-based study of outcome in the United States based on the Surveillance, Epidemiology, and End Results (SEER) database, 1980 to 2017. Cancer 2021, 127, 2049–2061. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Short, N.J.; Fathi, A.T.; Marcucci, G.; Ravandi, F.; Tallman, M.; Wang, E.S.; Wei, A.H. Acute Myeloid Leukemia: Historical Perspective and Progress in Research and Therapy Over 5 Decades. Clin. Lymphoma Myeloma Leuk. 2021, 21, 580–597. [Google Scholar] [CrossRef]
- Dohner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Shimony, S.; Stahl, M.; Stone, R.M. Acute myeloid leukemia: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2023, 98, 502–526. [Google Scholar] [CrossRef]
- Assaraf, Y.G.; Brozovic, A.; Goncalves, A.C.; Jurkovicova, D.; Line, A.; Machuqueiro, M.; Saponara, S.; Sarmento-Ribeiro, A.B.; Xavier, C.P.R.; Vasconcelos, M.H. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist. Updat 2019, 46, 100645. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Lavi, O.; Hall, M.D.; Gillet, J.P. Toward a Better Understanding of the Complexity of Cancer Drug Resistance. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 85–102. [Google Scholar] [CrossRef]
- Biedler, J.L.; Riehm, H. Cellular resistance to actinomycin D in Chinese hamster cells in vitro: Cross-resistance, radioautographic, and cytogenetic studies. Cancer Res. 1970, 30, 1174–1184. [Google Scholar] [PubMed]
- Dano, K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1973, 323, 466–483. [Google Scholar] [CrossRef]
- Juliano, R.L.; Ling, V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1976, 455, 152–162. [Google Scholar] [CrossRef]
- Gros, P.; Croop, J.; Roninson, I.; Varshavsky, A.; Housman, D.E. Isolation and characterization of DNA sequences amplified in multidrug-resistant hamster cells. Proc. Natl. Acad. Sci. USA 1986, 83, 337–341. [Google Scholar] [CrossRef]
- Roninson, I.B.; Chin, J.E.; Choi, K.G.; Gros, P.; Housman, D.E.; Fojo, A.; Shen, D.W.; Gottesman, M.M.; Pastan, I. Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proc. Natl. Acad. Sci. USA 1986, 83, 4538–4542. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Ling, V. The molecular basis of multidrug resistance in cancer: The early years of P-glycoprotein research. FEBS Lett. 2006, 580, 998–1009. [Google Scholar] [CrossRef]
- Theodoulou, F.L.; Kerr, I.D. ABC transporter research: Going strong 40 years on. Biochem. Soc. Trans. 2015, 43, 1033–1040. [Google Scholar] [CrossRef]
- Higgins, C.F. ABC transporters: From microorganisms to man. Annu. Rev. Cell Biol. 1992, 8, 67–113. [Google Scholar] [CrossRef]
- Dean, M.; Annilo, T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu. Rev. Genomics. Hum. Genet. 2005, 6, 123–142. [Google Scholar] [CrossRef]
- Patel, H.; Wu, Z.X.; Chen, Y.; Bo, L.; Chen, Z.S. Drug resistance: From bacteria to cancer. Mol. Biomed. 2021, 2, 27. [Google Scholar] [CrossRef]
- Dean, M.; Hamon, Y.; Chimini, G. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 2001, 42, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.I.; Williams, R.T.; Henderson, M.J.; Norris, M.D.; Haber, M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist. Updat. 2016, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, C.; Sun, Y.; Song, W.; Lin, J.; Li, J.; Guan, X. p62/mTOR/LXRalpha pathway inhibits cholesterol efflux mediated by ABCA1 and ABCG1 during autophagy blockage. Biochem. Biophys. Res. Commun. 2019, 514, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Sag, D.; Cekic, C.; Wu, R.; Linden, J.; Hedrick, C.C. The cholesterol transporter ABCG1 links cholesterol homeostasis and tumour immunity. Nat. Commun. 2015, 6, 6354. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Gebre, A.K.; Parks, J.S.; Hedrick, C.C. ATP-binding cassette transporter G1 negatively regulates thymocyte and peripheral lymphocyte proliferation. J. Immunol. 2010, 184, 173–183. [Google Scholar] [CrossRef]
- Wang, F.; Beck-Garcia, K.; Zorzin, C.; Schamel, W.W.; Davis, M.M. Inhibition of T cell receptor signaling by cholesterol sulfate, a naturally occurring derivative of membrane cholesterol. Nat. Immunol. 2016, 17, 844–850. [Google Scholar] [CrossRef]
- Goossens, P.; Rodriguez-Vita, J.; Etzerodt, A.; Masse, M.; Rastoin, O.; Gouirand, V.; Ulas, T.; Papantonopoulou, O.; Van Eck, M.; Auphan-Anezin, N.; et al. Membrane Cholesterol Efflux Drives Tumor-Associated Macrophage Reprogramming and Tumor Progression. Cell Metab. 2019, 29, 1376–1389.e1374. [Google Scholar] [CrossRef]
- Tian, C.; Huang, D.; Yu, Y.; Zhang, J.; Fang, Q.; Xie, C. ABCG1 as a potential oncogene in lung cancer. Exp. Ther. Med. 2017, 13, 3189–3194. [Google Scholar] [CrossRef]
- Roundhill, E.A.; Jabri, S.; Burchill, S.A. ABCG1 and Pgp identify drug resistant, self-renewing osteosarcoma cells. Cancer Lett. 2019, 453, 142–157. [Google Scholar] [CrossRef]
- Pan, H.; Zheng, Y.; Pan, Q.; Chen, H.; Chen, F.; Wu, J.; Di, D. Expression of LXR-beta, ABCA1 and ABCG1 in human triple-negative breast cancer tissues. Oncol. Rep. 2019, 42, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Sano, O.; Tsujita, M.; Shimizu, Y.; Kato, R.; Kobayashi, A.; Kioka, N.; Remaley, A.T.; Michikawa, M.; Ueda, K.; Matsuo, M. ABCG1 and ABCG4 Suppress gamma-Secretase Activity and Amyloid beta Production. PLoS ONE 2016, 11, e0155400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Wang, X.; Zhou, Y.; Xu, H.; Wang, J.; Huang, L.; Tian, Y.; Cheng, Q. The expression of ABCG4, V-ATPase and clinic significance of their correlation with NSCLC. Zhongguo Fei Ai Za Zhi 2008, 11, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mitsche, M.A.; Lutjohann, D.; Cohen, J.C.; Xie, X.S.; Hobbs, H.H. Relative roles of ABCG5/ABCG8 in liver and intestine. J. Lipid Res. 2015, 56, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Bastida, J.M.; Benito, R.; Gonzalez-Porras, J.R.; Rivera, J. ABCG5 and ABCG8 gene variations associated with sitosterolemia and platelet dysfunction. Platelets 2021, 32, 573–577. [Google Scholar] [CrossRef]
- Tada, H.; Nohara, A.; Inazu, A.; Sakuma, N.; Mabuchi, H.; Kawashiri, M.A. Sitosterolemia, Hypercholesterolemia, and Coronary Artery Disease. J. Atheroscler. Thromb. 2018, 25, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Liu, M.; Portincasa, P.; Wang, D.Q. Recent Advances in the Critical Role of the Sterol Efflux Transporters ABCG5/G8 in Health and Disease. Adv. Exp. Med. Biol. 2020, 1276, 105–136. [Google Scholar] [CrossRef]
- Chen, Y.N.; Mickley, L.A.; Schwartz, A.M.; Acton, E.M.; Hwang, J.L.; Fojo, A.T. Characterization of adriamycin-resistant human breast cancer cells which display overexpression of a novel resistance-related membrane protein. J. Biol. Chem. 1990, 265, 10073–10080. [Google Scholar] [CrossRef]
- Lee, J.S.; Scala, S.; Matsumoto, Y.; Dickstein, B.; Robey, R.; Zhan, Z.; Altenberg, G.; Bates, S.E. Reduced drug accumulation and multidrug resistance in human breast cancer cells without associated P-glycoprotein or MRP overexpression. J. Cell. Biochem. 1997, 65, 513–526. [Google Scholar] [CrossRef]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670. [Google Scholar] [CrossRef]
- Annilo, T.; Chen, Z.Q.; Shulenin, S.; Costantino, J.; Thomas, L.; Lou, H.; Stefanov, S.; Dean, M. Evolution of the vertebrate ABC gene family: Analysis of gene birth and death. Genomics 2006, 88, 1–11. [Google Scholar] [CrossRef]
- Mickley, L.; Jain, P.; Miyake, K.; Schriml, L.M.; Rao, K.; Fojo, T.; Bates, S.; Dean, M. An ATP-binding cassette gene (ABCG3) closely related to the multidrug transporter ABCG2 (MXR/ABCP) has an unusual ATP-binding domain. Mamm. Genome 2001, 12, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Dell, K.J.; Hassel, B.; Doyle, L.A.; Ross, D.D. Promoter characterization and genomic organization of the human breast cancer resistance protein (ATP-binding cassette transporter G2) gene. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 2001, 1520, 234–241. [Google Scholar] [CrossRef]
- Knutsen, T.; Rao, V.K.; Ried, T.; Mickley, L.; Schneider, E.; Miyake, K.; Ghadimi, B.M.; Padilla-Nash, H.; Pack, S.; Greenberger, L.; et al. Amplification of 4q21-q22 and the MXR gene in independently derived mitoxantrone-resistant cell lines. Genes Chromosomes Cancer 2000, 27, 110–116. [Google Scholar] [CrossRef]
- Natarajan, K.; Xie, Y.; Baer, M.R.; Ross, D.D. Role of breast cancer resistance protein (BCRP/ABCG2) in cancer drug resistance. Biochem. Pharmacol. 2012, 83, 1084–1103. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, P.; Ross, D.D.; Nakanishi, T.; Bailey-Dell, K.; Zhou, S.; Mercer, K.E.; Sarkadi, B.; Sorrentino, B.P.; Schuetz, J.D. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J. Biol. Chem. 2004, 279, 24218–24225. [Google Scholar] [CrossRef]
- Ee, P.L.; Kamalakaran, S.; Tonetti, D.; He, X.; Ross, D.D.; Beck, W.T. Identification of a novel estrogen response element in the breast cancer resistance protein (ABCG2) gene. Cancer Res. 2004, 64, 1247–1251. [Google Scholar] [CrossRef]
- Wang, H.; Lee, E.W.; Zhou, L.; Leung, P.C.; Ross, D.D.; Unadkat, J.D.; Mao, Q. Progesterone receptor (PR) isoforms PRA and PRB differentially regulate expression of the breast cancer resistance protein in human placental choriocarcinoma BeWo cells. Mol. Pharmacol. 2008, 73, 845–854. [Google Scholar] [CrossRef]
- To, K.K.; Robey, R.; Zhan, Z.; Bangiolo, L.; Bates, S.E. Upregulation of ABCG2 by romidepsin via the aryl hydrocarbon receptor pathway. Mol. Cancer Res. 2011, 9, 516–527. [Google Scholar] [CrossRef]
- Szatmari, I.; Vamosi, G.; Brazda, P.; Balint, B.L.; Benko, S.; Szeles, L.; Jeney, V.; Ozvegy-Laczka, C.; Szanto, A.; Barta, E.; et al. Peroxisome proliferator-activated receptor gamma-regulated ABCG2 expression confers cytoprotection to human dendritic cells. J. Biol. Chem. 2006, 281, 23812–23823. [Google Scholar] [CrossRef]
- Honorat, M.; Mesnier, A.; Di Pietro, A.; Lin, V.; Cohen, P.; Dumontet, C.; Payen, L. Dexamethasone down-regulates ABCG2 expression levels in breast cancer cells. Biochem. Biophys. Res. Commun. 2008, 375, 308–314. [Google Scholar] [CrossRef]
- Turner, J.G.; Gump, J.L.; Zhang, C.; Cook, J.M.; Marchion, D.; Hazlehurst, L.; Munster, P.; Schell, M.J.; Dalton, W.S.; Sullivan, D.M. ABCG2 expression, function, and promoter methylation in human multiple myeloma. Blood 2006, 108, 3881–3889. [Google Scholar] [CrossRef]
- To, K.K.; Zhan, Z.; Litman, T.; Bates, S.E. Regulation of ABCG2 expression at the 3′ untranslated region of its mRNA through modulation of transcript stability and protein translation by a putative microRNA in the S1 colon cancer cell line. Mol. Cell. Biol. 2008, 28, 5147–5161. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.; Robey, R.W.; Knutsen, T.; Zhan, Z.; Ried, T.; Bates, S.E. Escape from hsa-miR-519c enables drug-resistant cells to maintain high expression of ABCG2. Mol. Cancer Ther. 2009, 8, 2959–2968. [Google Scholar] [CrossRef]
- Wang, F.; Xue, X.; Wei, J.; An, Y.; Yao, J.; Cai, H.; Wu, J.; Dai, C.; Qian, Z.; Xu, Z.; et al. hsa-miR-520h downregulates ABCG2 in pancreatic cancer cells to inhibit migration, invasion, and side populations. Br. J. Cancer 2010, 103, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.Z.; Morris, M.E.; Yu, A.M. MicroRNA-328 negatively regulates the expression of breast cancer resistance protein (BCRP/ABCG2) in human cancer cells. Mol. Pharmacol. 2009, 75, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Takada, T.; Suzuki, H.; Gotoh, Y.; Sugiyama, Y. Regulation of the cell surface expression of human BCRP/ABCG2 by the phosphorylation state of Akt in polarized cells. Drug Metab. Dispos. 2005, 33, 905–909. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, W.; Chen, Y.; Feng, M.; Ouyang, Y.; Yu, Y.; He, Z. Up-regulation of breast cancer resistance protein plays a role in HER2-mediated chemoresistance through PI3K/Akt and nuclear factor-kappa B signaling pathways in MCF7 breast cancer cells. Acta Biochim. Biophys. Sin. 2011, 43, 647–653. [Google Scholar] [CrossRef]
- Polgar, O.; Robey, R.W.; Bates, S.E. ABCG2: Structure, function and role in drug response. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1–15. [Google Scholar] [CrossRef]
- Sarkadi, B.; Homolya, L.; Hegedus, T. The ABCG2/BCRP transporter and its variants-from structure to pathology. FEBS Lett. 2020, 594, 4012–4034. [Google Scholar] [CrossRef]
- McDevitt, C.A.; Collins, R.F.; Conway, M.; Modok, S.; Storm, J.; Kerr, I.D.; Ford, R.C.; Callaghan, R. Purification and 3D structural analysis of oligomeric human multidrug transporter ABCG2. Structure 2006, 14, 1623–1632. [Google Scholar] [CrossRef]
- Robey, R.W.; To, K.K.; Polgar, O.; Dohse, M.; Fetsch, P.; Dean, M.; Bates, S.E. ABCG2: A perspective. Adv. Drug Deliv. Rev. 2009, 61, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Ross, D.D. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 2003, 22, 7340–7358. [Google Scholar] [CrossRef]
- Robey, R.W.; Polgar, O.; Deeken, J.; To, K.W.; Bates, S.E. ABCG2: Determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007, 26, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Unadkat, J.D.; Mao, Q. Breast cancer resistance protein 1 limits fetal distribution of nitrofurantoin in the pregnant mouse. Drug Metab. Dispos. 2007, 35, 2154–2158. [Google Scholar] [CrossRef]
- Gedeon, C.; Anger, G.; Piquette-Miller, M.; Koren, G. Breast cancer resistance protein: Mediating the trans-placental transfer of glyburide across the human placenta. Placenta 2008, 29, 39–43. [Google Scholar] [CrossRef]
- Myllynen, P.; Kummu, M.; Kangas, T.; Ilves, M.; Immonen, E.; Rysa, J.; Pirila, R.; Lastumaki, A.; Vahakangas, K.H. ABCG2/BCRP decreases the transfer of a food-born chemical carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in perfused term human placenta. Toxicol. Appl. Pharmacol. 2008, 232, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Cooray, H.C.; Blackmore, C.G.; Maskell, L.; Barrand, M.A. Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport 2002, 13, 2059–2063. [Google Scholar] [CrossRef]
- Hori, S.; Ohtsuki, S.; Tachikawa, M.; Kimura, N.; Kondo, T.; Watanabe, M.; Nakashima, E.; Terasaki, T. Functional expression of rat ABCG2 on the luminal side of brain capillaries and its enhancement by astrocyte-derived soluble factor(s). J. Neurochem. 2004, 90, 526–536. [Google Scholar] [CrossRef]
- Jonker, J.W.; Merino, G.; Musters, S.; van Herwaarden, A.E.; Bolscher, E.; Wagenaar, E.; Mesman, E.; Dale, T.C.; Schinkel, A.H. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat. Med. 2005, 11, 127–129. [Google Scholar] [CrossRef]
- Merino, G.; Jonker, J.W.; Wagenaar, E.; van Herwaarden, A.E.; Schinkel, A.H. The breast cancer resistance protein (BCRP/ABCG2) affects pharmacokinetics, hepatobiliary excretion, and milk secretion of the antibiotic nitrofurantoin. Mol. Pharmacol. 2005, 67, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- van Herwaarden, A.E.; Wagenaar, E.; Merino, G.; Jonker, J.W.; Rosing, H.; Beijnen, J.H.; Schinkel, A.H. Multidrug transporter ABCG2/breast cancer resistance protein secretes riboflavin (vitamin B2) into milk. Mol. Cell. Biol. 2007, 27, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Fetsch, P.A.; Abati, A.; Litman, T.; Morisaki, K.; Honjo, Y.; Mittal, K.; Bates, S.E. Localization of the ABCG2 mitoxantrone resistance-associated protein in normal tissues. Cancer Lett. 2006, 235, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Bart, J.; Hollema, H.; Groen, H.J.; de Vries, E.G.; Hendrikse, N.H.; Sleijfer, D.T.; Wegman, T.D.; Vaalburg, W.; van der Graaf, W.T. The distribution of drug-efflux pumps, P-gp, BCRP, MRP1 and MRP2, in the normal blood-testis barrier and in primary testicular tumours. Eur. J. Cancer 2004, 40, 2064–2070. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, H.; Hruz, P.; Zimmermann, C.; Beglinger, C.; Drewe, J. Distribution of breast cancer resistance protein (BCRP/ABCG2) mRNA expression along the human GI tract. Biochem. Pharmacol. 2005, 70, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, S.; de Vries, N.A.; Buckle, T.; Bolijn, M.J.; van Eijndhoven, M.A.; Beijnen, J.H.; Mazzanti, R.; van Tellingen, O.; Schellens, J.H. Effect of the ATP-binding cassette drug transporters ABCB1, ABCG2, and ABCC2 on erlotinib hydrochloride (Tarceva) disposition in in vitro and in vivo pharmacokinetic studies employing Bcrp1-/-/Mdr1a/1b-/- (triple-knockout) and wild-type mice. Mol. Cancer Ther. 2008, 7, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Huls, M.; Brown, C.D.; Windass, A.S.; Sayer, R.; van den Heuvel, J.J.; Heemskerk, S.; Russel, F.G.; Masereeuw, R. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 2008, 73, 220–225. [Google Scholar] [CrossRef]
- Mizuno, N.; Takahashi, T.; Kusuhara, H.; Schuetz, J.D.; Niwa, T.; Sugiyama, Y. Evaluation of the role of breast cancer resistance protein (BCRP/ABCG2) and multidrug resistance-associated protein 4 (MRP4/ABCC4) in the urinary excretion of sulfate and glucuronide metabolites of edaravone (MCI-186; 3-methyl-1-phenyl-2-pyrazolin-5-one). Drug Metab. Dispos. 2007, 35, 2045–2052. [Google Scholar] [CrossRef]
- Vander Borght, S.; Libbrecht, L.; Katoonizadeh, A.; van Pelt, J.; Cassiman, D.; Nevens, F.; Van Lommel, A.; Petersen, B.E.; Fevery, J.; Jansen, P.L.; et al. Breast cancer resistance protein (BCRP/ABCG2) is expressed by progenitor cells/reactive ductules and hepatocytes and its expression pattern is influenced by disease etiology and species type: Possible functional consequences. J. Histochem. Cytochem. 2006, 54, 1051–1059. [Google Scholar] [CrossRef]
- Aust, S.; Obrist, P.; Jaeger, W.; Klimpfinger, M.; Tucek, G.; Wrba, F.; Penner, E.; Thalhammer, T. Subcellular localization of the ABCG2 transporter in normal and malignant human gallbladder epithelium. Lab. Invest. 2004, 84, 1024–1036. [Google Scholar] [CrossRef]
- Goodell, M.A.; Brose, K.; Paradis, G.; Conner, A.S.; Mulligan, R.C. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 1996, 183, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Morris, J.J.; Barnes, Y.; Lan, L.; Schuetz, J.D.; Sorrentino, B.P. Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 12339–12344. [Google Scholar] [CrossRef] [PubMed]
- Scharenberg, C.W.; Harkey, M.A.; Torok-Storb, B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood 2002, 99, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Schuetz, J.D.; Bunting, K.D.; Colapietro, A.M.; Sampath, J.; Morris, J.J.; Lagutina, I.; Grosveld, G.C.; Osawa, M.; Nakauchi, H.; et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 2001, 7, 1028–1034. [Google Scholar] [CrossRef]
- Jonker, J.W.; Buitelaar, M.; Wagenaar, E.; Van Der Valk, M.A.; Scheffer, G.L.; Scheper, R.J.; Plosch, T.; Kuipers, F.; Elferink, R.P.; Rosing, H.; et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc. Natl. Acad. Sci. USA 2002, 99, 15649–15654. [Google Scholar] [CrossRef]
- Gyongy, Z.; Mocsar, G.; Hegedus, E.; Stockner, T.; Ritter, Z.; Homolya, L.; Schamberger, A.; Orban, T.I.; Remenyik, J.; Szakacs, G.; et al. Nucleotide binding is the critical regulator of ABCG2 conformational transitions. Elife 2023, 12, e83976. [Google Scholar] [CrossRef]
- Yu, Q.; Ni, D.; Kowal, J.; Manolaridis, I.; Jackson, S.M.; Stahlberg, H.; Locher, K.P. Structures of ABCG2 under turnover conditions reveal a key step in the drug transport mechanism. Nat. Commun. 2021, 12, 4376. [Google Scholar] [CrossRef]
- Jackson, S.M.; Manolaridis, I.; Kowal, J.; Zechner, M.; Taylor, N.M.I.; Bause, M.; Bauer, S.; Bartholomaeus, R.; Bernhardt, G.; Koenig, B.; et al. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat. Struct. Mol. Biol. 2018, 25, 333–340. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, T.; Zhang, L.; Zhu, P.; Deng, M.; Huang, C.; Hu, T.; Jiang, L.; Li, J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016, 370, 153–164. [Google Scholar] [CrossRef]
- Sargent, J.M.; Williamson, C.J.; Maliepaard, M.; Elgie, A.W.; Scheper, R.J.; Taylor, C.G. Breast cancer resistance protein expression and resistance to daunorubicin in blast cells from patients with acute myeloid leukaemia. Br. J. Haematol. 2001, 115, 257–262. [Google Scholar] [CrossRef]
- van der Kolk, D.M.; Vellenga, E.; Scheffer, G.L.; Muller, M.; Bates, S.E.; Scheper, R.J.; de Vries, E.G. Expression and activity of breast cancer resistance protein (BCRP) in de novo and relapsed acute myeloid leukemia. Blood 2002, 99, 3763–3770. [Google Scholar] [CrossRef] [PubMed]
- Abbott, B.L.; Colapietro, A.M.; Barnes, Y.; Marini, F.; Andreeff, M.; Sorrentino, B.P. Low levels of ABCG2 expression in adult AML blast samples. Blood 2002, 100, 4594–4601. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel-Eibrink, M.M.; Wiemer, E.A.; Prins, A.; Meijerink, J.P.; Vossebeld, P.J.; van der Holt, B.; Pieters, R.; Sonneveld, P. Increased expression of the breast cancer resistance protein (BCRP) in relapsed or refractory acute myeloid leukemia (AML). Leukemia 2002, 16, 833–839. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel-Eibrink, M.M.; van der Holt, B.; Burnett, A.K.; Knauf, W.U.; Fey, M.F.; Verhoef, G.E.; Vellenga, E.; Ossenkoppele, G.J.; Lowenberg, B.; Sonneveld, P. CD34-related coexpression of MDR1 and BCRP indicates a clinically resistant phenotype in patients with acute myeloid leukemia (AML) of older age. Ann. Hematol. 2007, 86, 329–337. [Google Scholar] [CrossRef]
- Liu, B.; Li, L.J.; Gong, X.; Zhang, W.; Zhang, H.; Zhao, L. Co-expression of ATP binding cassette transporters is associated with poor prognosis in acute myeloid leukemia. Oncol. Lett. 2018, 15, 6671–6677. [Google Scholar] [CrossRef]
- Marzac, C.; Garrido, E.; Tang, R.; Fava, F.; Hirsch, P.; De Benedictis, C.; Corre, E.; Lapusan, S.; Lallemand, J.Y.; Marie, J.P.; et al. ATP Binding Cassette transporters associated with chemoresistance: Transcriptional profiling in extreme cohorts and their prognostic impact in a cohort of 281 acute myeloid leukemia patients. Haematologica 2011, 96, 1293–1301. [Google Scholar] [CrossRef]
- van der Pol, M.A.; Broxterman, H.J.; Pater, J.M.; Feller, N.; van der Maas, M.; Weijers, G.W.; Scheffer, G.L.; Allen, J.D.; Scheper, R.J.; van Loevezijn, A.; et al. Function of the ABC transporters, P-glycoprotein, multidrug resistance protein and breast cancer resistance protein, in minimal residual disease in acute myeloid leukemia. Haematologica 2003, 88, 134–147. [Google Scholar]
- Galimberti, S.; Guerrini, F.; Palumbo, G.A.; Consoli, U.; Fazzi, R.; Morabito, F.; Santini, V.; Petrini, M. Evaluation of BCRP and MDR-1 co-expression by quantitative molecular assessment in AML patients. Leuk. Res. 2004, 28, 367–372. [Google Scholar] [CrossRef]
- Benderra, Z.; Faussat, A.M.; Sayada, L.; Perrot, J.Y.; Tang, R.; Chaoui, D.; Morjani, H.; Marzac, C.; Marie, J.P.; Legrand, O. MRP3, BCRP, and P-glycoprotein activities are prognostic factors in adult acute myeloid leukemia. Clin. Cancer Res. 2005, 11, 7764–7772. [Google Scholar] [CrossRef]
- Ho, M.M.; Hogge, D.E.; Ling, V. MDR1 and BCRP1 expression in leukemic progenitors correlates with chemotherapy response in acute myeloid leukemia. Exp. Hematol. 2008, 36, 433–442. [Google Scholar] [CrossRef]
- Uggla, B.; Stahl, E.; Wagsater, D.; Paul, C.; Karlsson, M.G.; Sirsjo, A.; Tidefelt, U. BCRP mRNA expression v. clinical outcome in 40 adult AML patients. Leuk. Res. 2005, 29, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Damiani, D.; Tiribelli, M.; Michelutti, A.; Geromin, A.; Cavallin, M.; Fabbro, D.; Pianta, A.; Malagola, M.; Damante, G.; Russo, D.; et al. Fludarabine-based induction therapy does not overcome the negative effect of ABCG2 (BCRP) over-expression in adult acute myeloid leukemia patients. Leuk. Res. 2010, 34, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Damiani, D.; Tiribelli, M.; Geromin, A.; Cerno, M.; Zanini, F.; Michelutti, A.; Fanin, R. ABCG2, Cytogenetics, and Age Predict Relapse after Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia in Complete Remission. Biol. Blood Marrow Transpl. 2016, 22, 1621–1626. [Google Scholar] [CrossRef]
- Steinbach, D.; Sell, W.; Voigt, A.; Hermann, J.; Zintl, F.; Sauerbrey, A. BCRP gene expression is associated with a poor response to remission induction therapy in childhood acute myeloid leukemia. Leukemia 2002, 16, 1443–1447. [Google Scholar] [CrossRef] [PubMed]
- Bartholomae, S.; Gruhn, B.; Debatin, K.M.; Zimmermann, M.; Creutzig, U.; Reinhardt, D.; Steinbach, D. Coexpression of Multiple ABC-Transporters is Strongly Associated with Treatment Response in Childhood Acute Myeloid Leukemia. Pediatr. Blood Cancer 2016, 63, 242–247. [Google Scholar] [CrossRef]
- Sharom, F.J. ABC multidrug transporters: Structure, function and role in chemoresistance. Pharmacogenomics 2008, 9, 105–127. [Google Scholar] [CrossRef]
- Honjo, Y.; Hrycyna, C.A.; Yan, Q.W.; Medina-Perez, W.Y.; Robey, R.W.; van de Laar, A.; Litman, T.; Dean, M.; Bates, S.E. Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res. 2001, 61, 6635–6639. [Google Scholar]
- Ejendal, K.F.; Diop, N.K.; Schweiger, L.C.; Hrycyna, C.A. The nature of amino acid 482 of human ABCG2 affects substrate transport and ATP hydrolysis but not substrate binding. Protein Sci. 2006, 15, 1597–1607. [Google Scholar] [CrossRef]
- Cai, X.; Bikadi, Z.; Ni, Z.; Lee, E.W.; Wang, H.; Rosenberg, M.F.; Mao, Q. Role of basic residues within or near the predicted transmembrane helix 2 of the human breast cancer resistance protein in drug transport. J. Pharmacol. Exp. Ther. 2010, 333, 670–681. [Google Scholar] [CrossRef]
- Miwa, M.; Tsukahara, S.; Ishikawa, E.; Asada, S.; Imai, Y.; Sugimoto, Y. Single amino acid substitutions in the transmembrane domains of breast cancer resistance protein (BCRP) alter cross resistance patterns in transfectants. Int. J. Cancer 2003, 107, 757–763. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Nakagawa, H.; Tamura, A.; Koshiba, S.; Hoshijima, K.; Komada, M.; Ishikawa, T. Intramolecular disulfide bond is a critical check point determining degradative fates of ATP-binding cassette (ABC) transporter ABCG2 protein. J. Biol. Chem. 2007, 282, 27841–27846. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Miao, L.; Qin, L.; Xiang, Y.; Zhang, X.; Peng, H.; Mailamuguli; Sun, Y.; Yao, H. A meta-analysis of the associations between the Q141K and Q126X ABCG2 gene variants and gout risk. Int. J. Clin. Exp. Pathol. 2015, 8, 9812–9823. [Google Scholar] [PubMed]
- Zhang, L.; Spencer, K.L.; Voruganti, V.S.; Jorgensen, N.W.; Fornage, M.; Best, L.G.; Brown-Gentry, K.D.; Cole, S.A.; Crawford, D.C.; Deelman, E.; et al. Association of functional polymorphism rs2231142 (Q141K) in the ABCG2 gene with serum uric acid and gout in 4 US populations: The PAGE Study. Am. J. Epidemiol. 2013, 177, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.L.; Wallace, M.C.; Phipps-Green, A.J.; Topless, R.; Drake, J.M.; Tan, P.; Dalbeth, N.; Merriman, T.R.; Stamp, L.K. ABCG2 loss-of-function polymorphism predicts poor response to allopurinol in patients with gout. Pharm. J. 2017, 17, 201–203. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, Y.; Zhang, X.; Gu, X.; Wang, H.; Luo, X.; Zhang, J.; Zou, H.; Guan, M. Functional polymorphisms of the ABCG2 gene are associated with gout disease in the Chinese Han male population. Int. J. Mol. Sci. 2014, 15, 9149–9159. [Google Scholar] [CrossRef] [PubMed]
- Feher, A.; Juhasz, A.; Laszlo, A.; Pakaski, M.; Kalman, J.; Janka, Z. Association between the ABCG2 C421A polymorphism and Alzheimer’s disease. Neurosci. Lett. 2013, 550, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Manautou, J.E.; Rasmussen, T.P.; Zhong, X.B. Development of precision medicine approaches based on inter-individual variability of BCRP/ABCG2. Acta Pharm. Sin. B 2019, 9, 659–674. [Google Scholar] [CrossRef]
- Varatharajan, S.; Panetta, J.C.; Abraham, A.; Karathedath, S.; Mohanan, E.; Lakshmi, K.M.; Arthur, N.; Srivastava, V.M.; Nemani, S.; George, B.; et al. Population pharmacokinetics of Daunorubicin in adult patients with acute myeloid leukemia. Cancer Chemother. Pharmacol. 2016, 78, 1051–1058. [Google Scholar] [CrossRef]
- Hampras, S.S.; Sucheston, L.; Weiss, J.; Baer, M.R.; Zirpoli, G.; Singh, P.K.; Wetzler, M.; Chennamaneni, R.; Blanco, J.G.; Ford, L.; et al. Genetic polymorphisms of ATP-binding cassette (ABC) proteins, overall survival and drug toxicity in patients with Acute Myeloid Leukemia. Int. J. Mol. Epidemiol. Genet. 2010, 1, 201–207. [Google Scholar]
- Wang, F.; Liang, Y.J.; Wu, X.P.; Chen, L.M.; To, K.K.; Dai, C.L.; Yan, Y.Y.; Wang, Y.S.; Tong, X.Z.; Fu, L.W. Prognostic value of the multidrug resistance transporter ABCG2 gene polymorphisms in Chinese patients with de novo acute leukaemia. Eur. J. Cancer 2011, 47, 1990–1999. [Google Scholar] [CrossRef]
- Megias-Vericat, J.E.; Montesinos, P.; Herrero, M.J.; Moscardo, F.; Boso, V.; Rojas, L.; Martinez-Cuadron, D.; Hervas, D.; Boluda, B.; Garcia-Robles, A.; et al. Impact of ABC single nucleotide polymorphisms upon the efficacy and toxicity of induction chemotherapy in acute myeloid leukemia. Leuk. Lymphoma 2017, 58, 1197–1206. [Google Scholar] [CrossRef]
- Muller, P.; Asher, N.; Heled, M.; Cohen, S.B.; Risch, A.; Rund, D. Polymorphisms in transporter and phase II metabolism genes as potential modifiers of the predisposition to and treatment outcome of de novo acute myeloid leukemia in Israeli ethnic groups. Leuk. Res. 2008, 32, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Tiribelli, M.; Fabbro, D.; Franzoni, A.; Fanin, R.; Damante, G.; Damiani, D. Q141K polymorphism of ABCG2 protein is associated with poor prognosis in adult acute myeloid leukemia treated with idarubicin-based chemotherapy. Haematologica 2013, 98, e28–e29. [Google Scholar] [CrossRef] [PubMed]
- Jabalee, J.; Towle, R.; Garnis, C. The Role of Extracellular Vesicles in Cancer: Cargo, Function, and Therapeutic Implications. Cells 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Safaei, R.; Larson, B.J.; Cheng, T.C.; Gibson, M.A.; Otani, S.; Naerdemann, W.; Howell, S.B. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol. Cancer Ther. 2005, 4, 1595–1604. [Google Scholar] [CrossRef]
- Goler-Baron, V.; Assaraf, Y.G. Structure and function of ABCG2-rich extracellular vesicles mediating multidrug resistance. PLoS ONE 2011, 6, e16007. [Google Scholar] [CrossRef]
- Samuel, P.; Fabbri, M.; Carter, D.R.F. Mechanisms of Drug Resistance in Cancer: The Role of Extracellular Vesicles. Proteomics 2017, 17, 23–24. [Google Scholar] [CrossRef]
- Goler-Baron, V.; Assaraf, Y.G. Overcoming multidrug resistance via photodestruction of ABCG2-rich extracellular vesicles sequestering photosensitive chemotherapeutics. PLoS ONE 2012, 7, e35487. [Google Scholar] [CrossRef]
- Distefano, M.; Scambia, G.; Ferlini, C.; Gaggini, C.; De Vincenzo, R.; Riva, A.; Bombardelli, E.; Ojima, I.; Fattorossi, A.; Panici, P.B.; et al. Anti-proliferative activity of a new class of taxanes (14beta-hydroxy-10-deacetylbaccatin III derivatives) on multidrug-resistance-positive human cancer cells. Int. J. Cancer 1997, 72, 844–850. [Google Scholar] [CrossRef]
- Shionoya, M.; Jimbo, T.; Kitagawa, M.; Soga, T.; Tohgo, A. DJ-927, a novel oral taxane, overcomes P-glycoprotein-mediated multidrug resistance in vitro and in vivo. Cancer Sci. 2003, 94, 459–466. [Google Scholar] [CrossRef]
- Glavinas, H.; Krajcsi, P.; Cserepes, J.; Sarkadi, B. The role of ABC transporters in drug resistance, metabolism and toxicity. Curr. Drug Deliv. 2004, 1, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Szakacs, G.; Hall, M.D.; Gottesman, M.M.; Boumendjel, A.; Kachadourian, R.; Day, B.J.; Baubichon-Cortay, H.; Di Pietro, A. Targeting the Achilles heel of multidrug-resistant cancer by exploiting the fitness cost of resistance. Chem. Rev. 2014, 114, 5753–5774. [Google Scholar] [CrossRef] [PubMed]
- Rabindran, S.K.; He, H.; Singh, M.; Brown, E.; Collins, K.I.; Annable, T.; Greenberger, L.M. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Res. 1998, 58, 5850–5858. [Google Scholar] [PubMed]
- Zattoni, I.F.; Delabio, L.C.; Dutra, J.P.; Kita, D.H.; Scheiffer, G.; Hembecker, M.; Pereira, G.D.S.; Moure, V.R.; Valdameri, G. Targeting breast cancer resistance protein (BCRP/ABCG2): Functional inhibitors and expression modulators. Eur. J. Med. Chem. 2022, 237, 114346. [Google Scholar] [CrossRef] [PubMed]
- Moinul, M.; Amin, S.A.; Jha, T.; Gayen, S. Updated chemical scaffolds of ABCG2 inhibitors and their structure-inhibition relationships for future development. Eur. J. Med. Chem. 2022, 241, 114628. [Google Scholar] [CrossRef] [PubMed]
- Guragossian, N.; Belhani, B.; Moreno, A.; Nunes, M.T.; Gonzalez-Lobato, L.; Marminon, C.; Berthier, L.; Rocio Andrade Pires, A.D.; Ozvegy-Laczka, C.; Sarkadi, B.; et al. Uncompetitive nanomolar dimeric indenoindole inhibitors of the human breast cancer resistance pump ABCG2. Eur. J. Med. Chem. 2021, 211, 113017. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Kathawala, R.J.; Chufan, E.E.; Patel, A.; Ambudkar, S.V.; Chen, Z.S.; Chen, X. Tivozanib reverses multidrug resistance mediated by ABCB1 (P-glycoprotein) and ABCG2 (BCRP). Future Oncol. 2014, 10, 1827–1841. [Google Scholar] [CrossRef]
- Sen, R.; Natarajan, K.; Bhullar, J.; Shukla, S.; Fang, H.B.; Cai, L.; Chen, Z.S.; Ambudkar, S.V.; Baer, M.R. The novel BCR-ABL and FLT3 inhibitor ponatinib is a potent inhibitor of the MDR-associated ATP-binding cassette transporter ABCG2. Mol. Cancer Ther. 2012, 11, 2033–2044. [Google Scholar] [CrossRef]
- Elsby, R.; Martin, P.; Surry, D.; Sharma, P.; Fenner, K. Solitary Inhibition of the Breast Cancer Resistance Protein Efflux Transporter Results in a Clinically Significant Drug-Drug Interaction with Rosuvastatin by Causing up to a 2-Fold Increase in Statin Exposure. Drug Metab. Dispos. 2016, 44, 398–408. [Google Scholar] [CrossRef]
- Miyata, H.; Takada, T.; Toyoda, Y.; Matsuo, H.; Ichida, K.; Suzuki, H. Identification of Febuxostat as a New Strong ABCG2 Inhibitor: Potential Applications and Risks in Clinical Situations. Front. Pharmacol. 2016, 7, 518. [Google Scholar] [CrossRef]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport--an update. AAPS J. 2015, 17, 65–82. [Google Scholar] [CrossRef] [PubMed]
- de Bruin, M.; Miyake, K.; Litman, T.; Robey, R.; Bates, S.E. Reversal of resistance by GF120918 in cell lines expressing the ABC half-transporter, MXR. Cancer Lett. 1999, 146, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Kuhnle, M.; Egger, M.; Muller, C.; Mahringer, A.; Bernhardt, G.; Fricker, G.; Konig, B.; Buschauer, A. Potent and selective inhibitors of breast cancer resistance protein (ABCG2) derived from the p-glycoprotein (ABCB1) modulator tariquidar. J. Med. Chem. 2009, 52, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Sim, H.M.; Lee, C.Y.; Ee, P.L.; Go, M.L. Dimethoxyaurones: Potent inhibitors of ABCG2 (breast cancer resistance protein). Eur. J. Pharm. Sci. 2008, 35, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wong, I.L.; Jiang, T.; Wang, S.W.; Liu, T.; Wen, B.J.; Chow, L.M.; Wan Sheng, B. Synthesis of methylated quercetin derivatives and their reversal activities on P-gp- and BCRP-mediated multidrug resistance tumour cells. Eur. J. Med. Chem. 2012, 54, 413–422. [Google Scholar] [CrossRef]
- Kita, D.H.; Guragossian, N.; Zattoni, I.F.; Moure, V.R.; Rego, F.G.M.; Lusvarghi, S.; Moulenat, T.; Belhani, B.; Picheth, G.; Bouacida, S.; et al. Mechanistic basis of breast cancer resistance protein inhibition by new indeno[1,2-b]indoles. Sci. Rep. 2021, 11, 1788. [Google Scholar] [CrossRef]
- Limtrakul, P.; Chearwae, W.; Shukla, S.; Phisalphong, C.; Ambudkar, S.V. Modulation of function of three ABC drug transporters, P-glycoprotein (ABCB1), mitoxantrone resistance protein (ABCG2) and multidrug resistance protein 1 (ABCC1) by tetrahydrocurcumin, a major metabolite of curcumin. Mol. Cell. Biochem. 2007, 296, 85–95. [Google Scholar] [CrossRef]
- Sorf, A.; Sucha, S.; Morell, A.; Novotna, E.; Staud, F.; Zavrelova, A.; Visek, B.; Wsol, V.; Ceckova, M. Targeting Pharmacokinetic Drug Resistance in Acute Myeloid Leukemia Cells with CDK4/6 Inhibitors. Cancers 2020, 12, 1596. [Google Scholar] [CrossRef]
- Sabet, Z.; Vagiannis, D.; Budagaga, Y.; Zhang, Y.; Novotna, E.; Hanke, I.; Rozkos, T.; Hofman, J. Talazoparib Does Not Interact with ABCB1 Transporter or Cytochrome P450s, but Modulates Multidrug Resistance Mediated by ABCC1 and ABCG2: An in Vitro and Ex Vivo Study. Int. J. Mol. Sci. 2022, 23, 14338. [Google Scholar] [CrossRef]
- Wang, J.Q.; Li, J.Y.; Teng, Q.X.; Lei, Z.N.; Ji, N.; Cui, Q.; Zeng, L.; Pan, Y.; Yang, D.H.; Chen, Z.S. Venetoclax, a BCL-2 Inhibitor, Enhances the Efficacy of Chemotherapeutic Agents in Wild-Type ABCG2-Overexpression-Mediated MDR Cancer Cells. Cancers 2020, 12, 466. [Google Scholar] [CrossRef]
- Kukal, S.; Guin, D.; Rawat, C.; Bora, S.; Mishra, M.K.; Sharma, P.; Paul, P.R.; Kanojia, N.; Grewal, G.K.; Kukreti, S.; et al. Multidrug efflux transporter ABCG2: Expression and regulation. Cell. Mol. Life Sci. 2021, 78, 6887–6939. [Google Scholar] [CrossRef] [PubMed]
| Chemotherapy Drugs |
|---|
| Daunorubicin, Doxorubicin, Idarubicin, Epirubicin, Etoposide, Gefitinib, Imatinib, Irinotecan, Mitoxantrone, Methotrexate, SN-38, Teniposide, Topotecan |
| Non-chemotherapy agents |
| Antibiotics: Ciprofloxacin, Ofloxacin, Norfloxacin, Erythromycin, Nitrofurantoin |
| Antivirals: Zidovudine, Lamivudine, Delavirdine, Lopinavir |
| Antihypertensive: Reserpine |
| Calcium channel blockers: Nicardipine, Azidopine, Nitrendipene, Dipyridamole |
| HMG-CoA reductase inhibitors: Rosuvastin, Cerivastatin, Pravastatin |
| Carcinogens: Aflatoxin B, 2-amino-1 methyl-6-phenyl-[4,5-b]imidazolpyridine (PhIP), 2-amino-3,8-dimethylimnidazo [4,5-f]quinoxaline (MelQx), 2-amini-3-methylimidazol[4,5-f]quinoline (IQ), 3-amini-1,4-dimethyl-5H-pyridol[4,3-b]indole (Trp-P-1) Others: Sulfasalazine, Cimetidine, Riboflavin, Vitamin K3, Glyburide, d-Luciferin, Quercetin |
| SNP | Author (Refs) | Disease Status | n | Ethnicity | Age (Range) | Chemotherapy | Outcome |
|---|---|---|---|---|---|---|---|
| G34A rs2231137 | Hampras, 2010 [119] | De novo (75%) Secondary (25%) | 261 | Caucasian (86%) Others (14%) USA | 61.5 (20–85) | ANT + AraC | - OS: GG↓OS (p = 0.05) (SCT censored) - Toxicity: AA/AG↑risk of toxicity grade ≥ 3 |
| Wang, 2011 [120] | De novo + ALL | 141 | Asian | 32 (5–70) | AraC/Dauno/mitox | - CR: trend to↑CR (p = 0.053) - OS: GG↑OS (p < 0.001) - Haplotype GG (rs2231137) with CA(rs2231142) and CT (rs22331149), ↓DFS, OS (p < 0.001) | |
| Megías-Vericat, 2017 [121] | De novo | 225 | Caucasian | 52.5 (16–78) | AraC/Ida | - CR, DDI: no influence - Toxicity: no influence | |
| C421A rs2231142 | Müller, 2008 [122] | De novo | 139 | Jews (61.2%) Arabs (38.8%) | 46.3 (15–86) | AraC/Ant ± Fluda ± Mit | OS (SCT censored): no influence |
| Hampras, 2010 [119,122] | De novo (75%) Secondary (25%) | 261 | Caucasian (86%) Others (14%) USA | 61.5 (20–85) | ANT + AraC | - OS: no influence (SCT censored); Unadjusted HR: AA↓OS - Toxicity: no influence | |
| Wang, 2011 [120] | De novo + ALL | 141 | Asian | 32 (5–70) | AraC/Dauno/Mit | - CR: no influence - OS:CC↑OS (p < 0.05, only univariate analysis). DFS: no influence * - Haplotype GG (rs2231137) with CA(rs2231142) and CT (rs22331149)↓DFS, OS (p < 0.001) | |
| Tiribelli, 2013 [123] | De novo | 125 | Caucasian (Italy) | 59.2 (20–84) | AraC/IDA/Fluda ± Etop | - 3yOS:CC + low ABCG2↑OS (p = 0.02) - 3yDFS: CC + low ABCG2↑DFS (p = 0.04) | |
| Megías-Vericat, 2017 [121] | De novo | 225 | Caucasian | 52.5 (16–78) | AraC/Ida | - CR, DDI: no influence - Toxicity: CA↑cardiac (p = 0.004),↑lung (p = 0.038) | |
| Ile619Ile (C>T) | Wang, 2011 [120] | De novo + ALL | 141 | Asian | 32 (5–70) | AraC/Dauno/mitox | - CR, OS, DFS: no influence * |
| rs2231149 (C>T) | Wang, 2011 [120] | De novo + ALL | 141 | Asian | 32 (5–70) | AraC/Dauno/mitox | - CR: no influence - OS: CC↑OS (p = 0.01; lost in multivariate analysis) - DFS: CC↑DSF (p < 0.05; lost in multivariate analysis) * - Haplotype GG (rs2231137) with CA(rs2231142) and CT (rs22331149), ↓DFS, OS (p < 0.001) |
| rs2231162 | Wang, 2011 [120] | De novo + ALL | 141 | Asian | 32 (5–70) | AraC/Dauno/mitox | CR, OS, DFS: no influence * |
| rs2231164 | Wang, 2011 [120] | De novo + ALL | 141 | Asian | 32 (5–70) | AraC/Dauno/mitox | CR, OS, DFS: no influence * |
| ABCG2 + SLC | |||||||
| ABCG2 rs2231142(C>A) SLC22A16 rs714368(A>C) | Megías-Vericat, 2017 [121] | De novo | 225 | Caucasian | 52.5 (16–78) | AraC/Ida | - CR, DDI: no influence - Toxicity:genotype AC + AA:↑cardiac (p = 0.033) |
| Structural Class Title 1 | Compound | IC50 (μM) |
|---|---|---|
| Chalcones | Indolylphenylproenone | 0.27 |
| Chromones | Chromone4a | 0.086 |
| Chromone31 | 0.046 | |
| Diketopiperazines | Ko143 (FTC analog) | 0.01 |
| Flavonois | Flavone | 2.8 |
| 6-prenulchrysin | 0.29 | |
| Flavonoid dimer | 1 | |
| Hedgehog pathway inhibitors | Vismodegib | 1.4 |
| Immunosuppressants | Sirolimus | 1.9 |
| Non-purine xanthine oxidase inhibitors | Febuxostat | 0.027 |
| Topiroxostat | 0.18 | |
| ABCB1 inhibitors | Tariquidar | 0.9 |
| Tariquidar derivative 6 | 0.06 | |
| Indenoindole-type derivatives | Indeno[1,2-b]indole | 0.21 |
| 9-hydroxyindeno[1,2-b]indole | 0.21 | |
| Indeno[1,2-b]indole homodimer | 0.024 | |
| Tariquidar-related triazoles | IR-MB19 | 0.14 |
| UR-MB108 | 0.079 | |
| Thrombopoietin receptor | Eltrombopag | 3.1 |
| Tyrosine kinase inhibitors | Alectinib | 1.5 |
| Bosutinib | 2 | |
| Dasatinib | 2 | |
| Erlotinib | 0.13 | |
| Fostamatinib | 0.05 | |
| Gefitinb | 0.5 | |
| Ponatinib | 0.04 | |
| Vandetanib | 0.2 | |
| Tivozanib | 0.07 | |
| Imatinib | 1 |
| Transcriptional Regulation | Post-Translational Regulation |
|---|---|
| Dexamethasone Genistein Resveratrol Gefitinib LY294002 (PI3K inhibitor) Glasdegib Vadadustat SP600125 (JNK inhibitor) Telatinib | Imatinib Nilotinib Dasatinib Sorafenib Gefitinib LY294002 (PI3K inhibitor) PPAR-γ agonists (telmisartan) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damiani, D.; Tiribelli, M. ABCG2 in Acute Myeloid Leukemia: Old and New Perspectives. Int. J. Mol. Sci. 2023, 24, 7147. https://doi.org/10.3390/ijms24087147
Damiani D, Tiribelli M. ABCG2 in Acute Myeloid Leukemia: Old and New Perspectives. International Journal of Molecular Sciences. 2023; 24(8):7147. https://doi.org/10.3390/ijms24087147
Chicago/Turabian StyleDamiani, Daniela, and Mario Tiribelli. 2023. "ABCG2 in Acute Myeloid Leukemia: Old and New Perspectives" International Journal of Molecular Sciences 24, no. 8: 7147. https://doi.org/10.3390/ijms24087147
APA StyleDamiani, D., & Tiribelli, M. (2023). ABCG2 in Acute Myeloid Leukemia: Old and New Perspectives. International Journal of Molecular Sciences, 24(8), 7147. https://doi.org/10.3390/ijms24087147






