CD44 Expression in Renal Tissue Is Associated with an Increase in Urinary Levels of Complement Components in Chronic Glomerulopathies
Abstract
1. Introduction
2. Results
2.1. Expression of CD44 by PEC
2.2. Expression of CD44 in the Mesangium
2.3. Expression of CD44 in the Interstitial Compartment
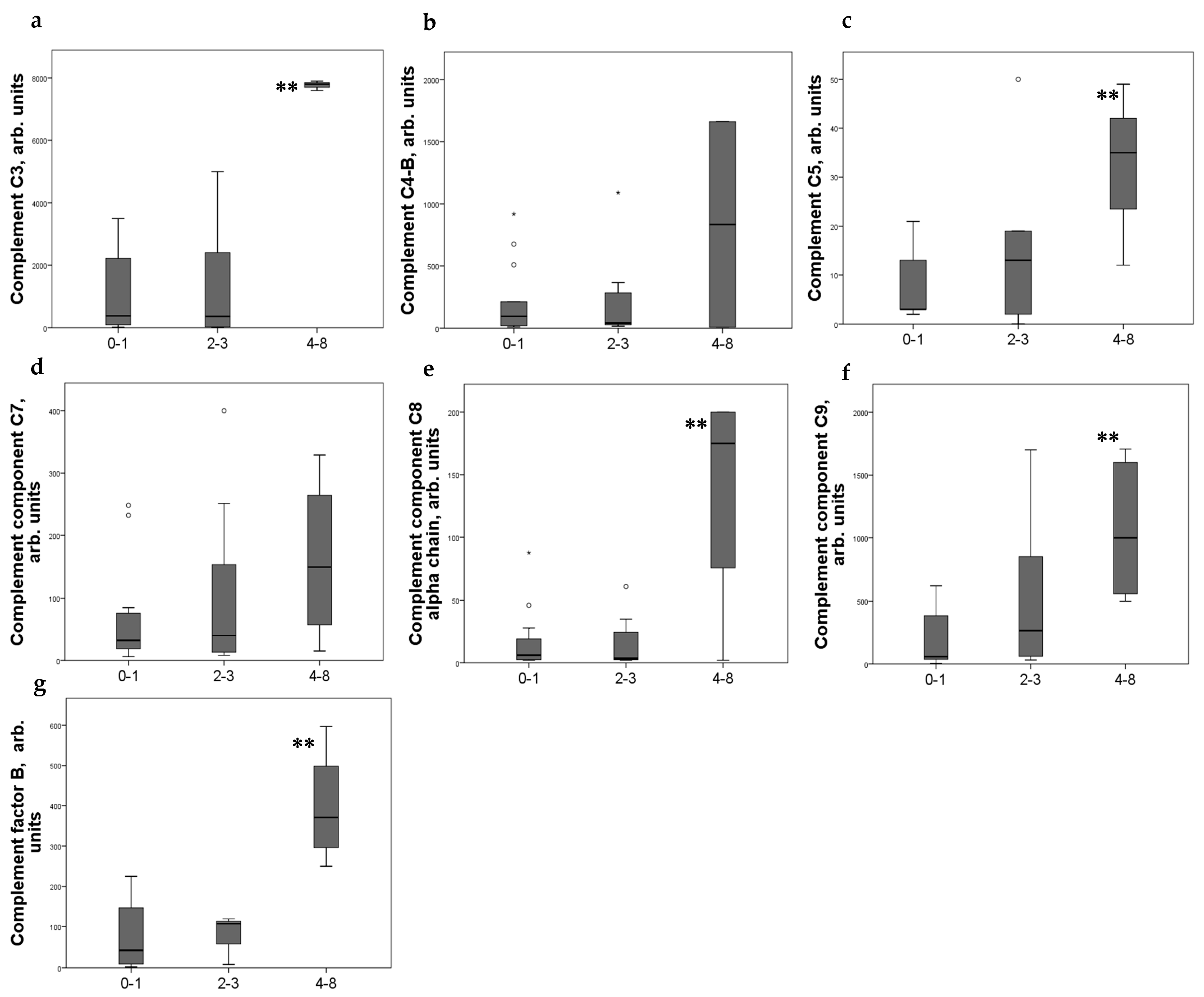
2.4. Relationship between Renal CD44 Expression and the Level of Complement Components in Urine
3. Discussion
4. Materials and Methods
4.1. Clinical Characteristics of the Patients
4.2. Quantitative Analysis of Complement Components in Urine by LC/MRM-MS
4.3. Histological Study
4.4. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodison, S.; Urquidi, V.; Tarin, D. CD44 cell adhesion molecules. Clin. Pathol. Mol. Pathol. 1999, 52, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, P.; Todd, L.; Shetye, S.; Monslow, J.; Puré, E. CD44-dependent inflammation, fibrogenesis, and collagenolysis regulates extracellular matrix remodeling and tensile strength during cutaneous wound healing. Matrix Biol. 2019, 75–76, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.R.; Racine, R.R.; Hennig, M.J.; Lokeshwar, V.B. The Role of CD44 in Disease Pathophysiology and Targeted Treatment. Front. Immunol. 2015, 6, 182. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Kitazawa, K.; Honda, H.; Sugisaki, T. Roles of and correlation between α-smooth muscle actin, CD44, hyalu-ronic acid and osteopontin in crescent formation in human glomerulonephritis. Clin. Nephrol. 2005, 64, 401–411. [Google Scholar] [CrossRef]
- Eymael, J.; Sharma, S.; Loeven, M.A.; Wetzels, J.F.; Mooren, F.; Florquin, S.; Deegens, J.K.; Willemsen, B.K.; Sharma, V.; van Kuppevelt, T.H.; et al. CD44 is required for the pathogenesis of experimental crescentic glomerulonephritis and collapsing focal segmental glomerulosclerosis. Kidney Int. 2017, 93, 626–642. [Google Scholar] [CrossRef]
- Choi, Y.W.; Kim, Y.G.; Song, M.-Y.; Moon, J.-Y.; Jeong, K.-H.; Lee, T.-W.; Ihm, C.-G.; Park, K.-S.; Lee, S.-H. Potential urine proteomics biomarkers for primary nephrotic syndrome. Clin. Proteom. 2017, 14, 18. [Google Scholar] [CrossRef]
- Nafar, M.; Kalantari, S.; Samavat, S.; Rezaei-Tavirani, M.; Rutishuser, D.; Zubarev, R.A. The Novel Diagnostic Biomarkers for Focal Segmental Glomerulosclerosis. Int. J. Nephrol. 2014, 2014, 574261. [Google Scholar] [CrossRef]
- Smeets, B.; Kuppe, C.; Sicking, E.-M.; Fuss, A.; Jirak, P.; van Kuppevelt, T.H.; Endlich, K.; Wetzels, J.F.; Gröne, H.-J.; Floege, J.; et al. Parietal Epithelial Cells Participate in the Formation of Sclerotic Lesions in Focal Segmental Glomerulosclerosis. J. Am. Soc. Nephrol. 2011, 22, 1262–1274. [Google Scholar] [CrossRef]
- Hayashi, A.; Okamoto, T.; Yamazaki, T.; Sato, Y.; Takahashi, T.; Ariga, T. CD44-Positive Glomerular Parietal Epithelial Cells in a Mouse Model of Calcineurin Inhibitors-Induced Nephrotoxicity. Nephron 2019, 142, 71–81. [Google Scholar] [CrossRef]
- Miesen, L.; Bándi, P.; Willemsen, B.; Mooren, F.; Strieder, T.; Boldrini, E.; Drenic, V.; Eymael, J.; Wetzels, R.; Lotz, J.; et al. Parietal epithelial cells maintain the epithelial cell continuum forming Bowman’s space in focal segmental glomerulosclerosis. Dis. Model. Mech. 2022, 15, dmm046342. [Google Scholar] [CrossRef]
- Khalili, M.; Bonnefoy, A.; Genest, D.S.; Quadri, J.; Rioux, J.-P.; Troyanov, S. Clinical Use of Complement, Inflammation, and Fibrosis Biomarkers in Autoimmune Glomerulonephritis. Kidney Int. Rep. 2020, 5, 1690–1699. [Google Scholar] [CrossRef]
- Genest, D.S.; Bonnefoy, A.; Khalili, M.; Merlen, C.; Genest, G.; Lapeyraque, A.-L.; Patey, N.; Smail, N.; Royal, V.; Troyanov, S. Comparison of Complement Pathway Activation in Autoimmune Glomerulonephritis. Kidney Int. Rep. 2022, 7, 1027–1036. [Google Scholar] [CrossRef]
- Huang, J.; Cui, Z.; Gu, Q.-H.; Zhang, Y.-M.; Qu, Z.; Wang, X.; Wang, F.; Cheng, X.-Y.; Meng, L.-Q.; Liu, G.; et al. Complement activation profile of patients with primary focal segmental glomerulosclerosis. PLoS ONE 2020, 15, e0234934. [Google Scholar] [CrossRef]
- Thurman, J.M.; Wong, M.; Renner, B.; Frazer-Abel, A.; Giclas, P.C.; Joy, M.S.; Jalal, D.; Radeva, M.K.; Gassman, J.; Gipson, D.S.; et al. Complement Activation in Patients with Focal Segmental Glomerulosclerosis. PLoS ONE 2015, 10, e0136558. [Google Scholar] [CrossRef]
- Chebotareva, N.V.; Vinogradov, A.; Brzhozovskiy, A.G.; Kashirina, D.N.; Indeykina, M.I.; Bugrova, A.E.; Lebedeva, M.; Moiseev, S.; Nikolaev, E.N.; Kononikhin, A.S. Potential Urine Proteomic Biomarkers for Focal Segmental Glomerulosclerosis and Minimal Change Disease. Int. J. Mol. Sci. 2022, 23, 12607. [Google Scholar] [CrossRef]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef]
- Huebener, P.; Abou-Khamis, T.; Zymek, P.; Bujak, M.; Ying, X.; Chatila, K.; Haudek, S.; Thakker, G.; Frangogiannis, N.G. CD44 Is Critically Involved in Infarct Healing by Regulating the Inflammatory and Fibrotic Response. J. Immunol. 2008, 180, 2625–2633. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, D.; Liang, J.; Meltzer, E.B.; Gray, A.; Miura, R.; Wogensen, L.; Yamaguchi, Y.; Noble, P.W. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J. Exp. Med. 2011, 208, 1459–1471. [Google Scholar] [CrossRef]
- Roeder, S.S.; Barnes, T.J.; Lee, J.S.; Kato, I.; Eng, D.G.; Kaverina, N.V.; Sunseri, M.W.; Daniel, C.; Amann, K.; Pippin, J.W.; et al. Activated ERK1/2 increases CD44 in glomerular parietal epithelial cells leading to matrix expansion. Kidney Int. 2016, 91, 896–913. [Google Scholar] [CrossRef]
- Chan, G.C.; Eng, D.G.; Miner, J.H.; Alpers, C.E.; Hudkins, K.L.; Chang, A.; Pippin, J.W.; Shankland, S.J. Differential expression of parietal epithelial cell and podocyte extracellular matrix proteins in focal segmental glomerulosclerosis and diabetic nephropathy. Am. J. Physiol. Physiol. 2019, 317, F1680–F1694. [Google Scholar] [CrossRef]
- Jun, Z.; Hill, P.A.; Lan, H.Y.; Foti, R.; Mu, W.; Atkins, R.C.; Nikolic-Paterson, D.J. CD44 and hyaluronan expression in the development of experimental crescentic glomerulone-phritis. Clin. Exp. Immunol. 1997, 108, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Wüthrich, R.P. The proinflammatory role of hyaluronan–CD44 interactions in renal injury. Nephrol. Dial. Transplant. 1999, 14, 2554–2556. [Google Scholar] [CrossRef] [PubMed]
- Hamatani, H.; Eng, D.G.; Hiromura, K.; Pippin, J.W.; Shankland, S.J. CD44 impacts glomerular parietal epithelial cell changes in the aged mouse kidney. Physiol. Rep. 2020, 8, e14487. [Google Scholar] [CrossRef] [PubMed]
- Nikolic-Paterson, D.J.; Jun, Z.; Tesch, G.H.; Lan, H.Y.; Foti, R.; Atkins, R.C. De novo CD44 expression by proliferating mesangial cells in rat anti-Thy-1 nephritis. J. Am. Soc. Nephrol. 1996, 7, 1006–1014. [Google Scholar] [CrossRef]
- Morita, Y.; Ikeguchi, H.; Nakamura, J.; Hotta, N.; Yuzawa, Y.; Matsuo, S. Complement Activation Products in the Urine from Proteinuric Patients. J. Am. Soc. Nephrol. 2000, 11, 700–707. [Google Scholar] [CrossRef]
- Alexopoulos, E.; Stangou, M.; Papagianni, A.; Pantzaki, A.; Papadimitriou, M. Factors influencing the course and the response to treatment in primary focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 2000, 15, 1348–1356. [Google Scholar] [CrossRef]
- Han, R.; Hu, S.; Qin, W.; Shi, J.; Zeng, C.; Bao, H.; Liu, Z. Upregulated long noncoding RNA LOC105375913 induces tubulointerstitial fibrosis in focal segmental glomerulosclerosis. Sci. Rep. 2019, 9, 716. [Google Scholar] [CrossRef]
- Mikecz, K.; Brennan, F.R.; Kim, J.H.; Glant, T.T. Anti-CD44 treatment abrogates tissue oedema and leukocyte infiltration in murine arthritis. Nat. Med. 1995, 1, 558–563. [Google Scholar] [CrossRef]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar]


| CD44 Expression Score | FSGS (n = 29) | MCD (n = 10) | MN (n = 10) | IgA Nephropathy (n = 11) | p (Two-Tailed Fisher Test) |
|---|---|---|---|---|---|
| Mesangium | n (%) | ||||
| 0–1 | 2 (7) | 1 (10) | 2 (20) | 1 (9) | FSGS/IgAN vs. MCD/MN p < 0.05 |
| 2–3 | 7 (24) | 7 (70) | 5 (50) | 1 (9) | |
| 4–8 | 15 (52) | 2 (20) | 3 (30) | 9 (82) | |
| 9–12 | 5 (17) | 0 | 0 | 0 | |
| PEC | n (%) | ||||
| 0–1 | 9 (31) | 7 (70) | 8 (80) | 3 (27) | FSGS/IgAN vs. MCD/MN p < 0.05 |
| 2–3 | 10 (34.5) | 3 (30) | 2 (20) | 6 (55) | |
| 4–8 | 10 (34.5) | 0 | 0 | 2 (18) | |
| 9–12 | 0 | 0 | 0 | 0 | |
| Podocytes | n (%) | ||||
| 0–1 | 6 (21) | 3 (30) | 3 (30) | 1 (9) | p > 0.05 |
| 2–3 | 4 (14) | 2 (20) | 3 (30) | 3 (27) | |
| 4–8 | 12 (41) | 5 (50) | 4 (40) | 6 (55) | |
| 9–12 | 7 (24) | 0 | 0 | 1 (9) | |
| Interstitial | n (%) | ||||
| 0–1 | 0 | 0 | 0 | 0 | p > 0.05 |
| 2–3 | 0 | 0 | 0 | 0 | |
| 4–8 | 9 (31) | 4 (40) | 3 (30) | 1 (9) | |
| 9–12 | 20 (69) | 6 (60) | 7 (70) | 10 (91) | |
| CD44 PEC | CD44 MC | CD44 Podocytes | CD44 Interstitial Cells | |
|---|---|---|---|---|
| 24-h proteinuria p | 0.065 0.625 | 0.092 0.484 | 0.023 0.362 | 0.017 0.900 |
| Haematuria p | 0.030 0.849 | 0.344 0.022 * | 0.291 0.055 | 0.068 0.660 |
| eGFR CKD-EPI p | −0.162 0.217 | −0.169 0.192 | −0.193 0.136 | −0.167 0.199 |
| % of globally sclerotic glomeruli p | 0.258 0.065 | 0.273 0.048 * | 0.145 0.301 | 0.233 0.094 |
| Tubulointerstitial fibrosis (TIF) p | 0.476 0.001 * | 0.062 0.660 | −0.141 0.313 | 0.270 0.047 * |
| C2 | C3 | C4b | C5 | C7 | C8alpha | C9 | CFB | CFI | |
|---|---|---|---|---|---|---|---|---|---|
| Proteinuria g/24 h p | 0.585 0.088 | 0.613 0.001 * | 0.655 0.001 * | 0.600 0.001 * | 0.580 0.001 * | 0.603 0.001 * | 0.551 0.001 * | 0.627 0.001 * | 0.260 0.203 |
| Creatinine, mkmol/L p | 0.556 0.015 * | 0.459 0.004 * | 0.518 0.001 * | 0.440 0.019 * | 0.524 0.001 * | 0.494 0.001 * | 0.554 0.001 * | 0.464 0.020 * | 0.235 0.203 |
| GFR, mL/min/1.73 m2 p | −0.404 0.088 | −0.324 0.050 * | −0.409 0.004 * | −0.288 0.137 | −0.430 0.002 * | −0.371 0.009 * | −0.518 0.003 * | −0.363 0.074 | −0.297 0.105 |
| Percent of globally sclerotic glomeruli p | 0.592 0.043 * | 0.155 0.439 | 0.204 0.256 | 0.332 0.165 | 0.393 0.022 * | 0.238 0.175 | 0.458 0.037 * | 0.391 0.134 | 0.444 0.034 * |
| Tubulointerstitial fibrosis, score p | 0.289 0.389 | 0.392 0.048 * | 0.480 0.006 * | 0.432 0.074 | 0.419 0.017 * | 0.607 0.001 * | 0.436 0.055 | 0.346 0.206 | 0.550 0.008 * |
| Parameters | FSGS (n = 29) | MCD (n = 10) | MN (n = 10) | IgA Nephropathy (n = 11) |
|---|---|---|---|---|
| Age, years | 35 (30.0; 55.0) | 31.0 (25.3; 39.5) | 46.0 (40.5; 51.0) | 34.0 (28.0; 41.0) |
| Gender (male), n (%) | 15 (51.7) | 1 (10.0) | 8 (80) | 6 (54.5) |
| Arterial hypetension, n (%) | 21 (72.4) | 6 (60.0) | 8 (80) | 8 (72.7) |
| Proteinuria, g/24 h | 3.92 (2.10; 5.20) | 3.05 (1.70; 7.99) | 3.9 (2.75; 5.83) | 2.54 (2.02; 3.04) |
| Serum albumin, g/L | 31.70 (23.00; 38.40) | 30.15 (21.6; 37.82) | 28.00 (25.8; 31.00) | 35.8 (32.05; 38.75) |
| Serum protein, g/L | 58.4 (47.70; 64.90) | 52.35 (43.73; 61.08) | 50.75 (45.55; 55.75) | 63.3 (58.4; 66.95) |
| Nephrotic syndrome, n (%) | 16 (55.2) | 6 (10.0) | 8 (72.7) | 3 (27.3) |
| Creatinine, mkmol/L | 96.8 (73.37; 162.66) | 87.75 (76.08; 101.98) | 86.30 (78.05; 97.45) | 116 (86.65; 114.00) |
| eGFR CKD-EPI, mL/min/1.73 m2 | 82.00 (46.00; 101.00) | 77.50 (64.00; 89.94) | 89.00 (77.5; 97.9) | 68.00 (47.0; 76.00) |
| eGFR < 60 mL/min/1.73 m2, n (%) | 11 (37.9) | 2 (10.0) | 1 (10) | 5 (45.5) |
| Steroid-resistant NS, n (%) | 11 (37.9) | 2 (20.0) | 0 (0) | 1 (0.09) |
| Positive Cells (PP) | Intensity of Staining (IS) | IRS (PP × IS) |
|---|---|---|
| 0 | 0 | 0–1 = negative |
| <10% (=1) | 1 (weak) | 2–3 = weak |
| 10–50% (=2) | 2 (moderate) | 4–8 = moderate |
| 51–80% (=3) | 3 (strong) | 9–12 = strong |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chebotareva, N.; Vinogradov, A.; Tsoy, L.; Varshavskiy, V.; Stoljarevich, E.; Bugrova, A.; Lerner, Y.; Krasnova, T.; Biryukova, E.; Kononikhin, A.S. CD44 Expression in Renal Tissue Is Associated with an Increase in Urinary Levels of Complement Components in Chronic Glomerulopathies. Int. J. Mol. Sci. 2023, 24, 7190. https://doi.org/10.3390/ijms24087190
Chebotareva N, Vinogradov A, Tsoy L, Varshavskiy V, Stoljarevich E, Bugrova A, Lerner Y, Krasnova T, Biryukova E, Kononikhin AS. CD44 Expression in Renal Tissue Is Associated with an Increase in Urinary Levels of Complement Components in Chronic Glomerulopathies. International Journal of Molecular Sciences. 2023; 24(8):7190. https://doi.org/10.3390/ijms24087190
Chicago/Turabian StyleChebotareva, Natalia, Anatoliy Vinogradov, Larisa Tsoy, Vladimir Varshavskiy, Ekaterina Stoljarevich, Anna Bugrova, Yulia Lerner, Tatyana Krasnova, Evgeniya Biryukova, and Alexey S. Kononikhin. 2023. "CD44 Expression in Renal Tissue Is Associated with an Increase in Urinary Levels of Complement Components in Chronic Glomerulopathies" International Journal of Molecular Sciences 24, no. 8: 7190. https://doi.org/10.3390/ijms24087190
APA StyleChebotareva, N., Vinogradov, A., Tsoy, L., Varshavskiy, V., Stoljarevich, E., Bugrova, A., Lerner, Y., Krasnova, T., Biryukova, E., & Kononikhin, A. S. (2023). CD44 Expression in Renal Tissue Is Associated with an Increase in Urinary Levels of Complement Components in Chronic Glomerulopathies. International Journal of Molecular Sciences, 24(8), 7190. https://doi.org/10.3390/ijms24087190






