Integrated Analysis of Transcriptome Expression Profiles Reveals miRNA-326–NKX3.2-Regulated Porcine Chondrocyte Differentiation
Abstract
1. Introduction
2. Results
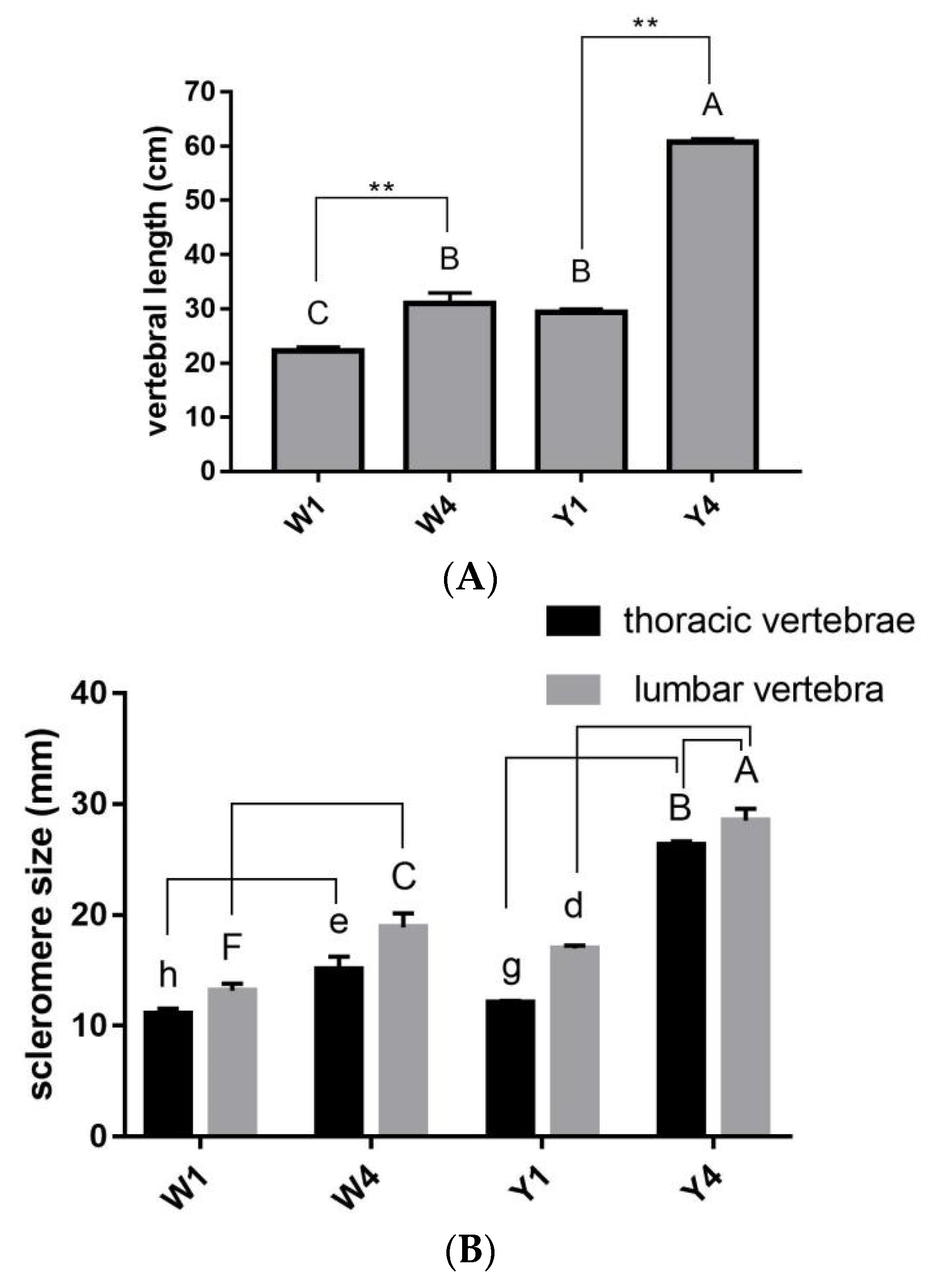
2.1. Vertebral Development in Pigs
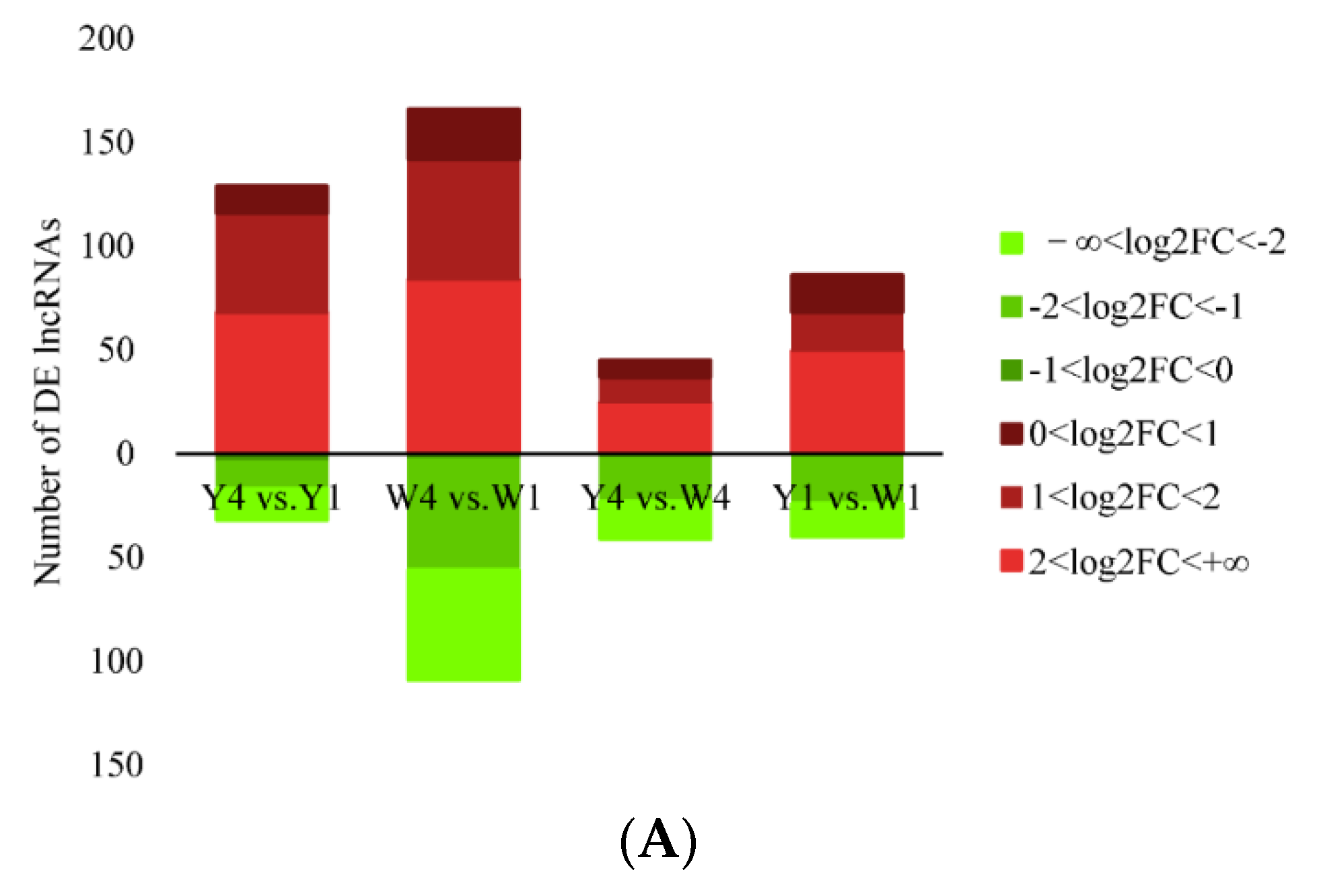
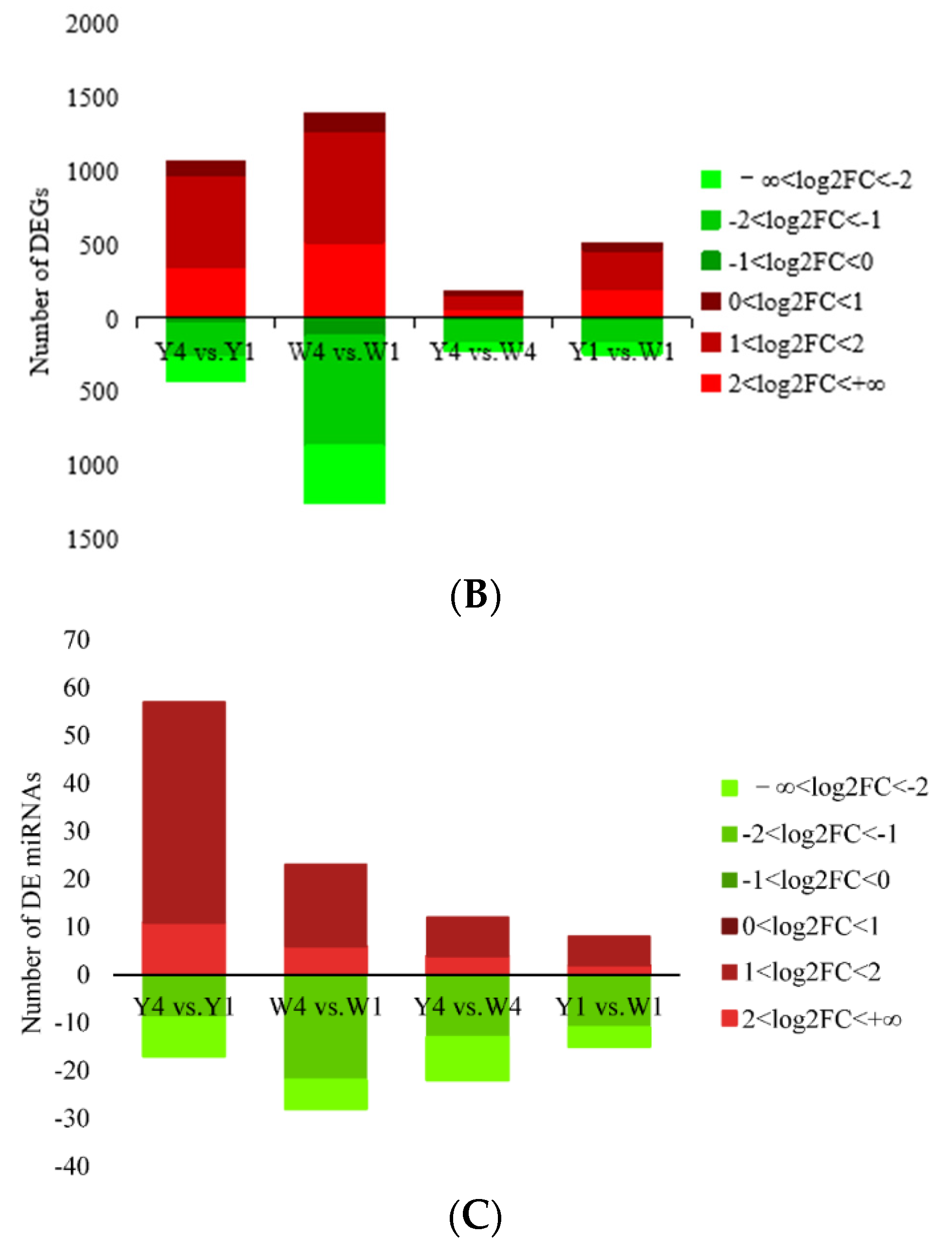
2.2. RNA-Seq Analysis Identifies DETs in the TIC
2.3. Functional Analysis of DETs
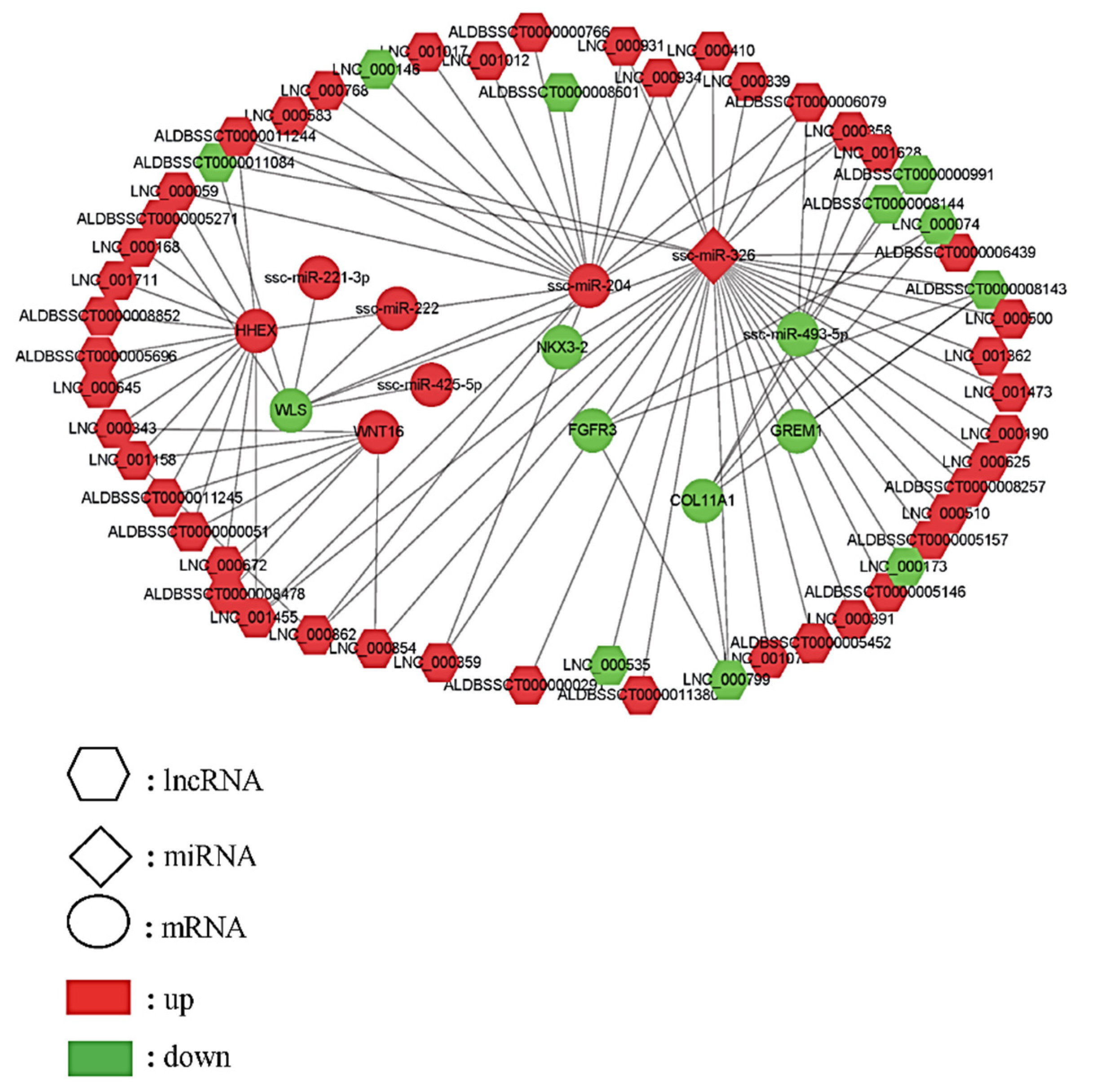
2.4. LncRNA, miRNA, and Gene Interaction Network Construction
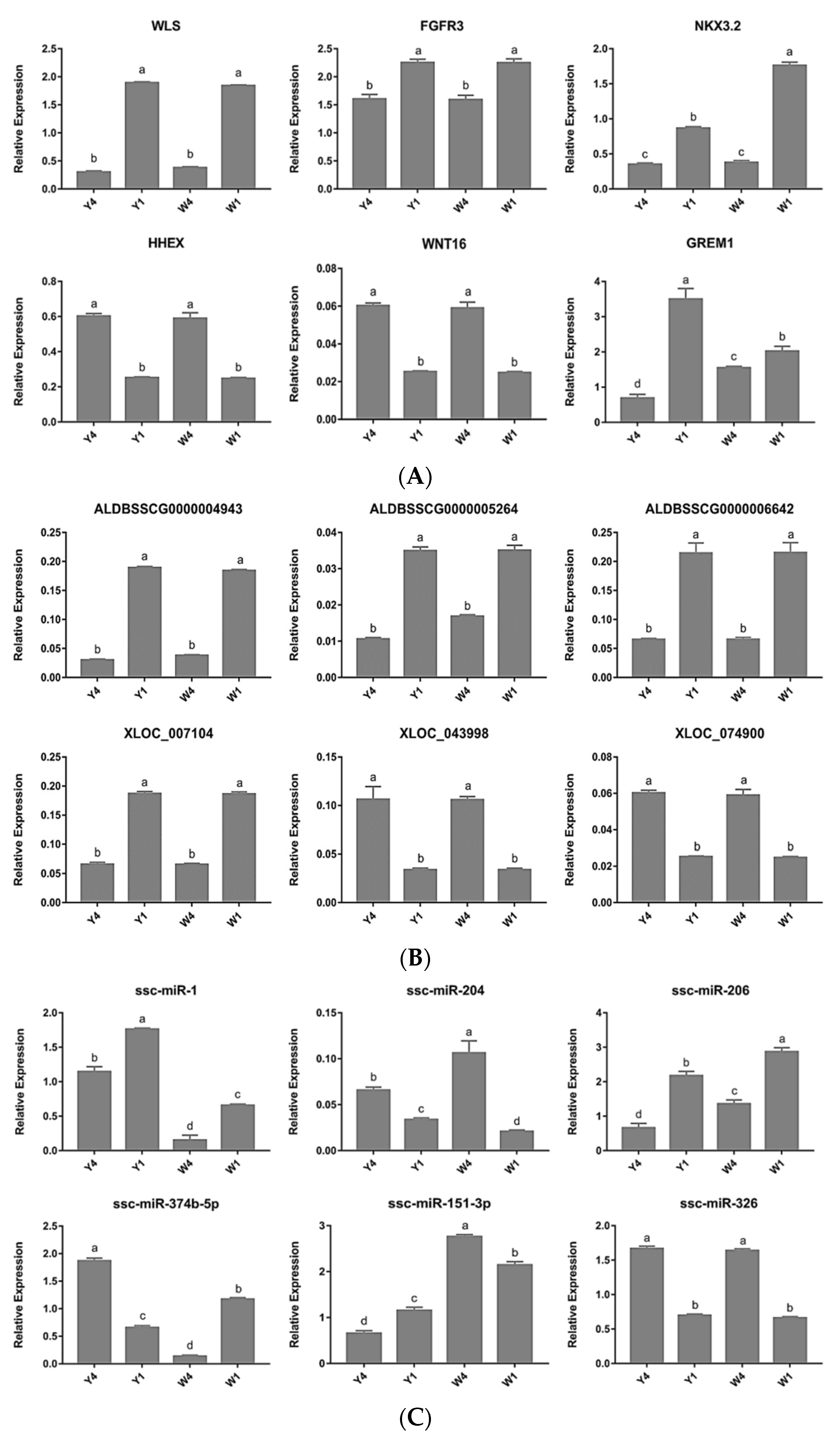
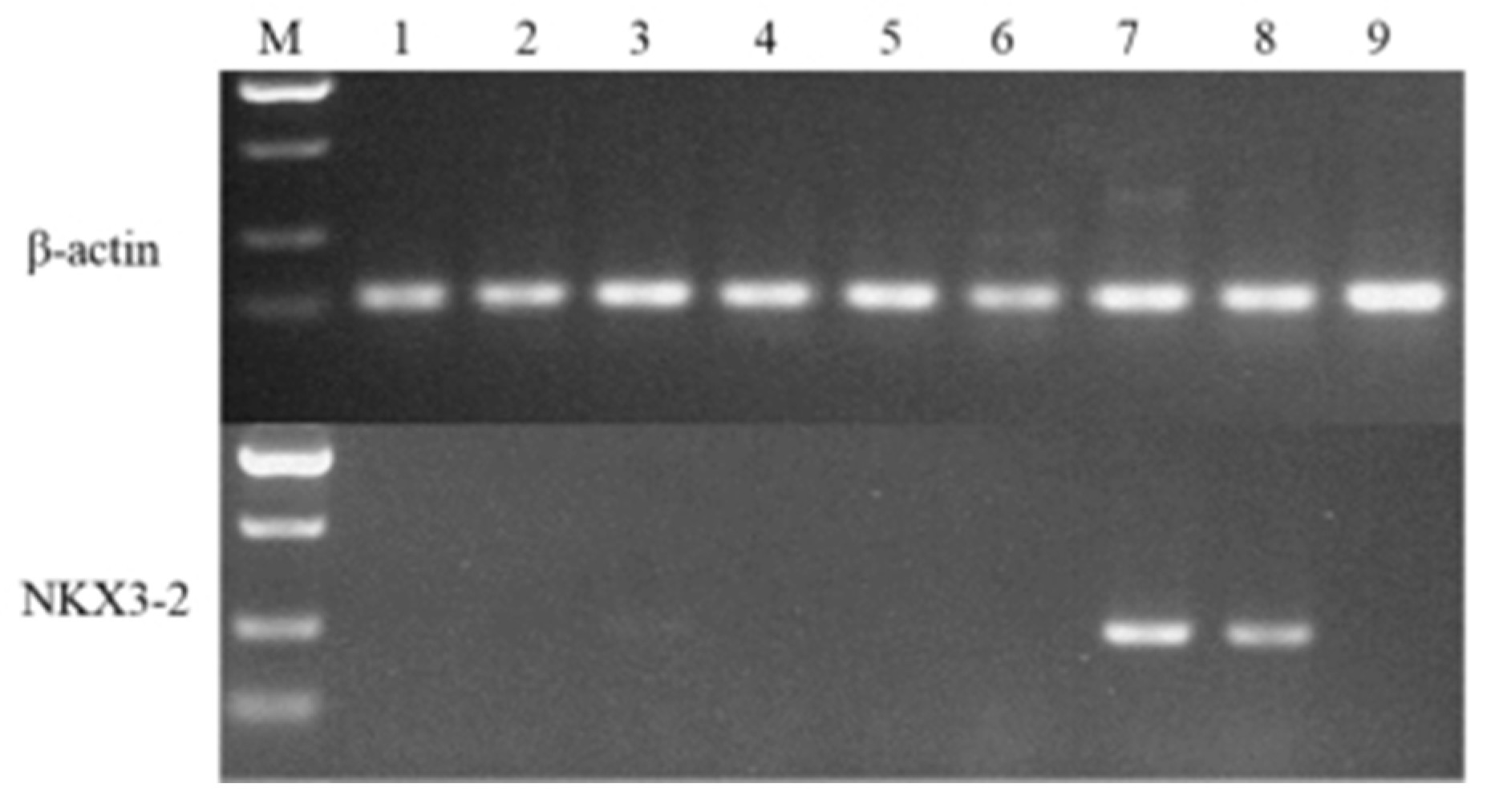
2.5. Quantitative PCR Validation of Differentially Expressed Genes
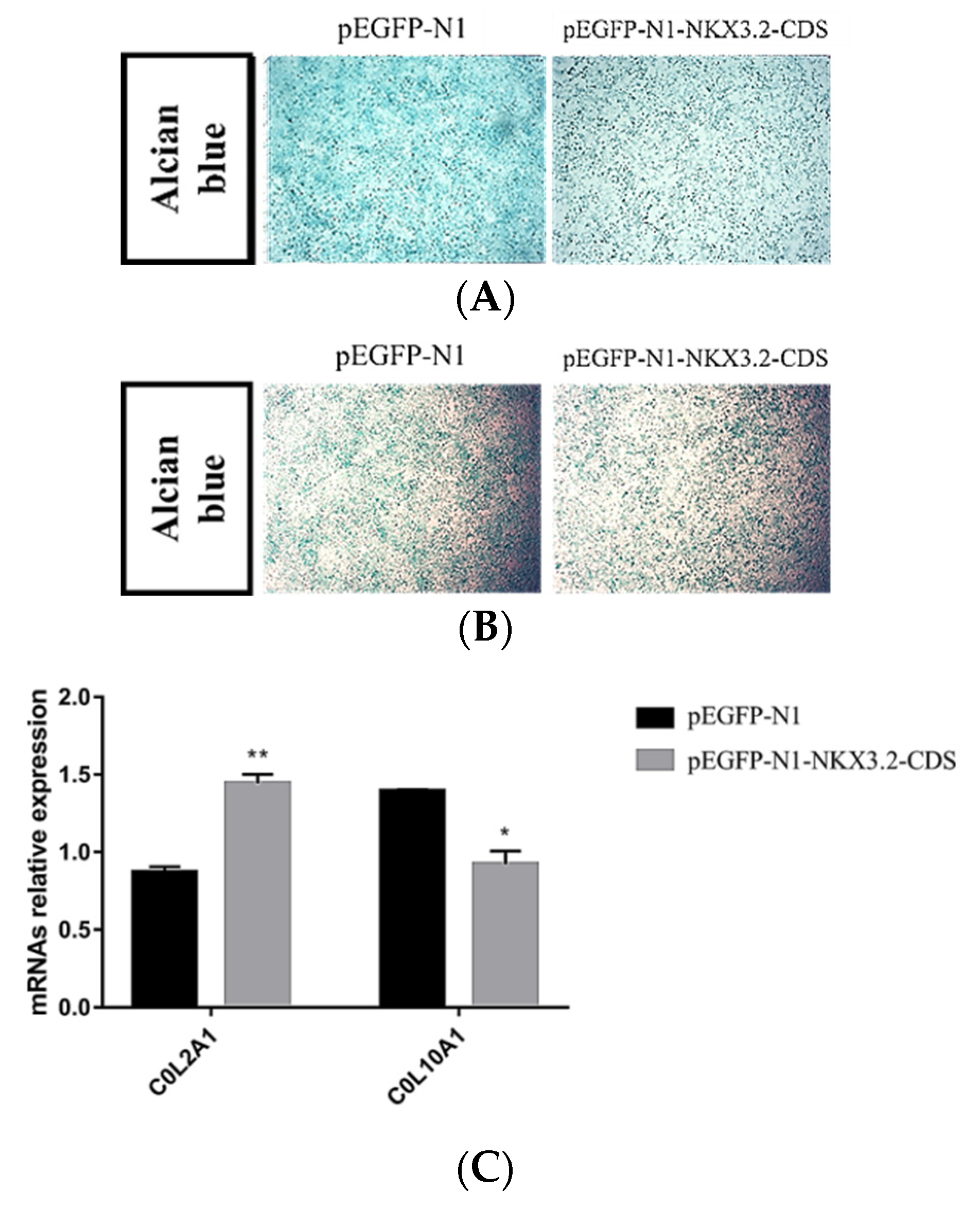
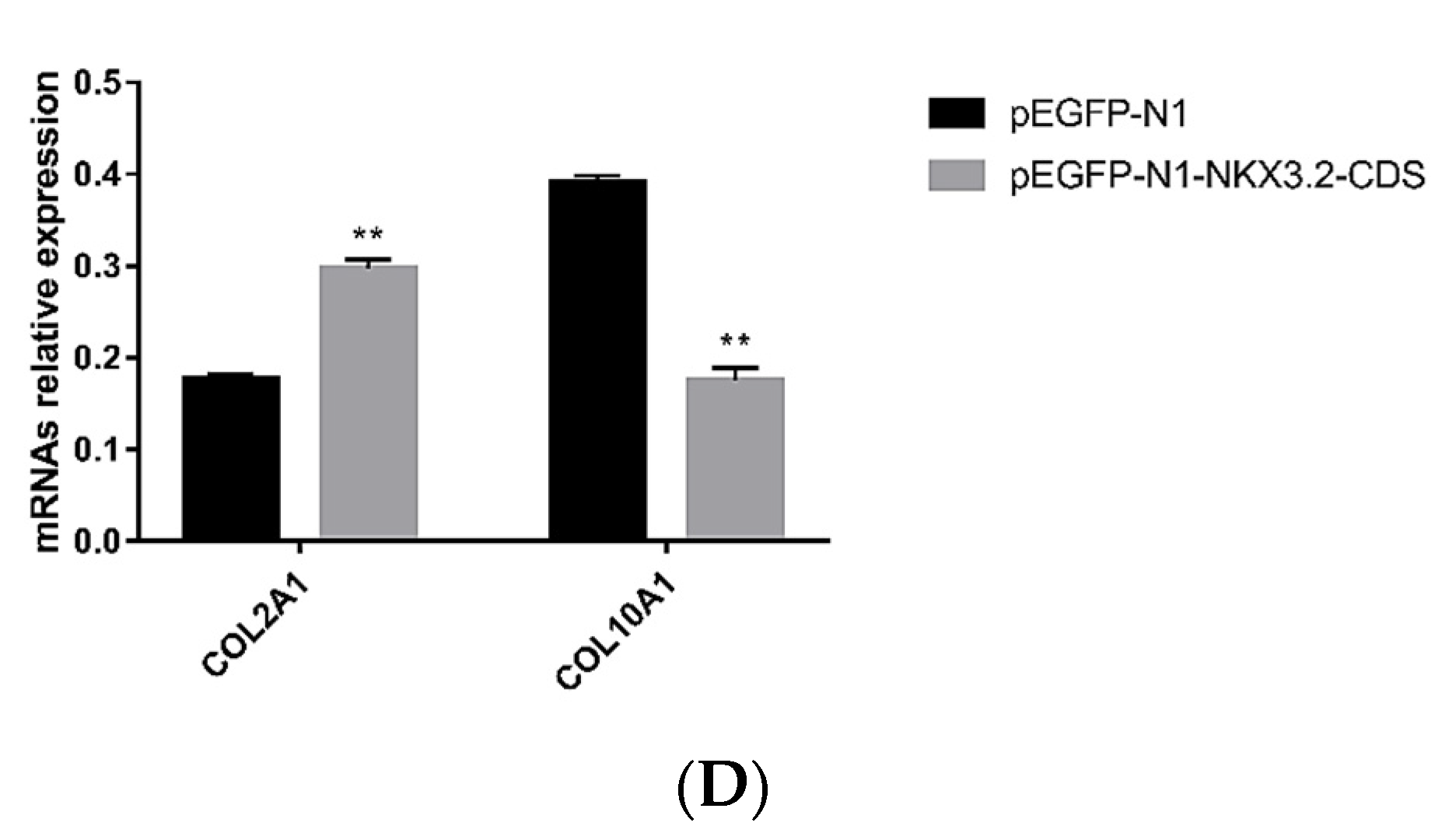
2.6. NKX3.2 Inhibits Chondrocyte Differentiation
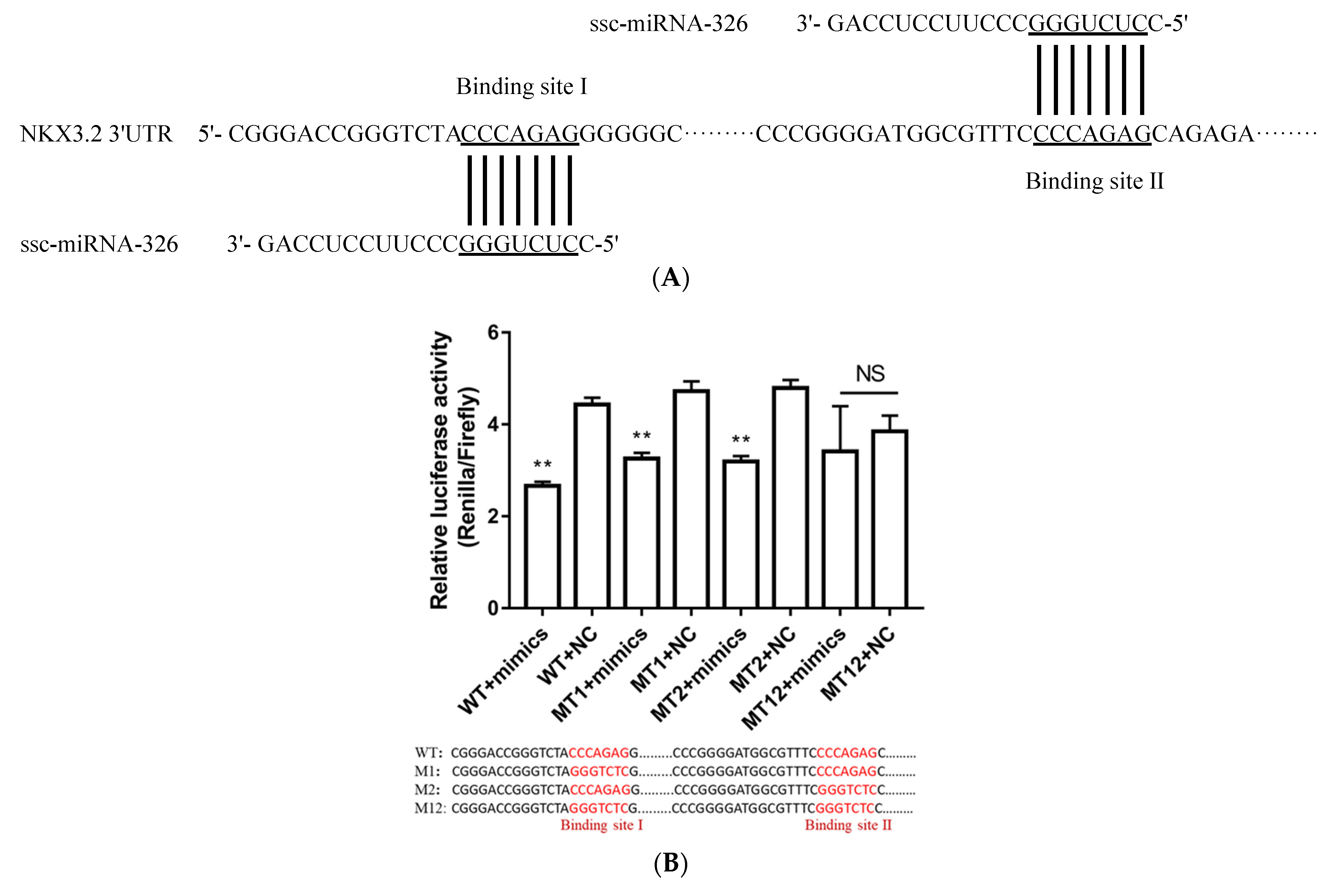
2.7. NKX3.2 Was the Direct Target of miRNA-326
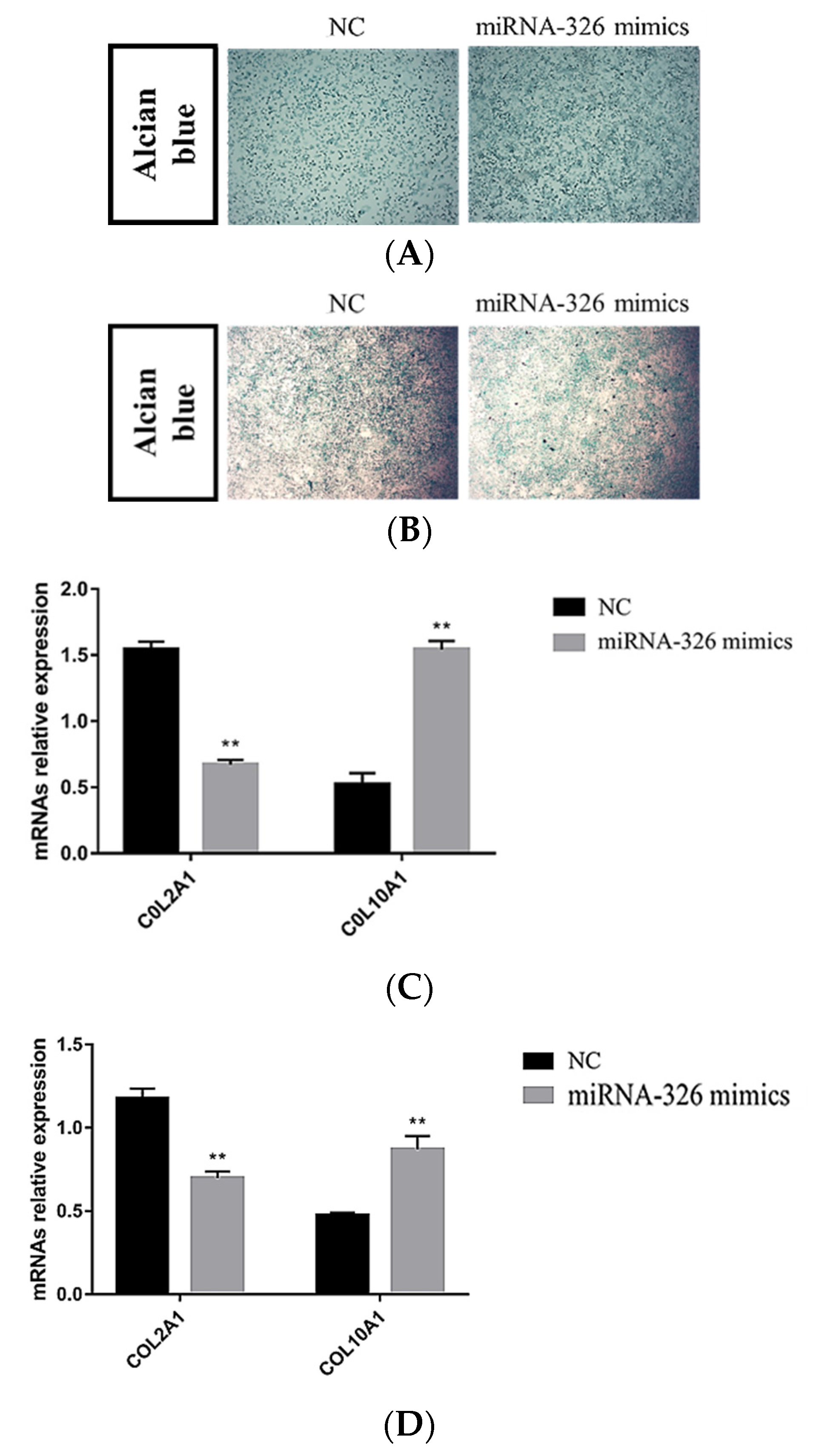
2.8. miRNA-326 Promotes Chondrocyte Differentiation
3. Discussion
4. Materials and Methods
4.1. Animal Sample Collection and RNA Isolated
4.2. Library Preparation for lncRNA Sequencing and Data Analysis
4.3. Library Preparation for microRNA Sequencing and Data Analysis
4.4. GO and KEGG Enrichment Analysis
4.5. Construction of lncRNA, miRNA, and mRNA Regulatory Networks
4.6. Cell Isolation, Culture, and Histological Analysis
4.7. Plasmid Construction and Dual-Luciferase Reporter Assay
4.8. Measurement of Gene Expression
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wake, D.B. Comparative Osteology and Evolution of the Lungless Salamanders, Family Plethodontidae; University of Southern California: Los Angeles, CA, USA, 1964. [Google Scholar]
- Lindsey, C.C. Pleomerism, the widespread tendency among related fish species for vertebral number to be correlated with maximum body length. J. Fish. Board Can. 1975, 32, 2453–2469. [Google Scholar] [CrossRef]
- Ward, A.B.; Brainerd, E.L. Evolution of axial patterning in elongate fishes. Biol. J. Linn. Soc. 2007, 90, 97–116. [Google Scholar] [CrossRef]
- Woltering, J.M. From lizard to snake; Behind the evolution of an extreme body plan. Curr. Genom. 2012, 13, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Reece, J.S.; Mehta, R.S. Evolutionary history of elongation and maximum body length in moray eels (Anguilliformes: Muraenidae). Biol. J. Linn. Soc. 2013, 109, 861–875. [Google Scholar] [CrossRef]
- Narita, Y.; Kuratani, S. Evolution of the vertebral formulae in mammals: A perspective on developmental constraints. J. Exp. Zool. B Mol. Dev. Evol. 2005, 304, 91–106. [Google Scholar] [CrossRef]
- Mikawa, S.; Hayashi, T.; Nii, M.; Shimanuki, S.; Morozumi, T.; Awata, T. Two quantitative trait loci on Sus scrofa chromosomes 1 and 7 affecting the number of vertebrae. J. Anim. Sci. 2005, 83, 2247–2254. [Google Scholar] [CrossRef]
- Mikawa, S.; Morozumi, T.; Shimanuki, S.; Hayashi, T.; Uenishi, H.; Domukai, M.; Okumura, N.; Awata, T. Fine mapping of a swine quantitative trait locus for number of vertebrae and analysis of an orphan nuclear receptor, germ cell nuclear factor (NR6A1). Genome Res. 2007, 17, 586–593. [Google Scholar] [CrossRef]
- Mikawa, S.; Sato, S.; Nii, M.; Morozumi, T.; Yoshioka, G.; Imaeda, N.; Yamaguchi, T.; Hayashi, T. Identification of a second gene associated with variation in vertebral number in domestic pigs. BMC Genet. 2011, 12, 5. [Google Scholar] [CrossRef]
- Ren, D.R.; Ren, J.; Ruan, G.F.; Guo, Y.M.; Wu, L.H.; Yang, G.C.; Zhou, L.H.; Li, L.; Zhang, Z.Y.; Huang, L.S. Mapping and fine mapping of quantitative trait loci for the number of vertebrae in a W hite D uroc× C hinese E rhualian intercross resource population. Anim. Genet. 2012, 43, 545–551. [Google Scholar] [CrossRef]
- Rubin, C.J.; Megens, H.J.; Martinez, B.A.; Maqbool, K.; Sayyab, S.; Schwochow, D.; Wang, C.; Carlborg, O.; Jern, P.; Jorgensen, C.B.; et al. Strong signatures of selection in the domestic pig genome. Proc. Natl. Acad. Sci. USA 2012, 109, 19529–19536. [Google Scholar] [CrossRef]
- Rohrer, G.A.; Nonneman, D.J.; Wiedmann, R.T.; Schneider, J.F. A study of vertebra number in pigs confirms the association of vertnin and reveals additional QTL. BMC Genet. 2015, 16, 129. [Google Scholar] [CrossRef]
- Berendsen, A.D.; Olsen, B.R. Bone development. Bone 2015, 80, 14–18. [Google Scholar] [CrossRef]
- Akiyama, H.; Chaboissier, M.C.; Martin, J.F.; Schedl, A.; de Crombrugghe, B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002, 16, 2813–2828. [Google Scholar] [CrossRef]
- Javed, A.; Chen, H.; Ghori, F.Y. Genetic and transcriptional control of bone formation. Oral Maxillofac. Surg. Clin. 2010, 22, 283–293. [Google Scholar] [CrossRef]
- Rainbow, R.S.; Won, H.K.; Zeng, L. The role of Nkx3.2 in chondrogenesis. Front. Biol. 2014, 9, 376–381. [Google Scholar] [CrossRef]
- Takei, Y.; Minamizaki, T.; Yoshiko, Y. Functional diversity of fibroblast growth factors in bone formation. Int. J. Endocrinol. 2015, 2015, 729352. [Google Scholar] [CrossRef]
- Beederman, M.; Lamplot, J.D.; Nan, G.; Wang, J.; Liu, X.; Yin, L.; Li, R.; Shui, W.; Zhang, H.; Kim, S.H.; et al. BMP signaling in mesenchymal stem cell differentiation and bone formation. J. Biomed. Sci. Eng. 2013, 6, 32–52. [Google Scholar] [CrossRef]
- Zhong, Z.; Ethen, N.J.; Williams, B.O. WNT signaling in bone development and homeostasis. Wiley Interdiscip. Rev. Dev. Biol. 2014, 3, 489–500. [Google Scholar] [CrossRef]
- Mead, T.J.; Yutzey, K.E. Notch pathway regulation of chondrocyte differentiation and proliferation during appendicular and axial skeleton development. Proc. Natl. Acad. Sci. USA 2009, 106, 14420–14425. [Google Scholar] [CrossRef]
- Papaioannou, G. MiRNAs in bone development. Curr. Genom. 2015, 16, 427–434. [Google Scholar] [CrossRef]
- Fang, S.; Deng, Y.; Gu, P.; Fan, X. MicroRNAs regulate bone development and regeneration. Int. J. Mol. Sci. 2015, 16, 8227–8253. [Google Scholar] [CrossRef] [PubMed]
- McGlinn, E.; Yekta, S.; Mansfield, J.H.; Soutschek, J.; Bartel, D.P.; Tabin, C.J. In ovo application of antagomiRs indicates a role for miR-196 in patterning the chick axial skeleton through Hox gene regulation. Proc. Natl. Acad. Sci. USA 2009, 106, 18610–18615. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Inloes, J.B.; Katagiri, T.; Kobayashi, T. Chondrocyte-specific microRNA-140 regulates endochondral bone development and targets Dnpep to modulate bone morphogenetic protein signaling. Mol. Cell. Biol. 2011, 31, 3019–3028. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Huang, Z.; Liu, N.; Su, R.; Xie, G.; Zhong, B.; Zhang, K.; Wang, S.; Hu, X.; Zhang, J.; et al. MicroRNA-140-5p impairs zebrafish embryonic bone development via targeting BMP-2. FEBS Lett. 2016, 590, 1438–1446. [Google Scholar] [CrossRef]
- Novikova, I.V.; Hennelly, S.P.; Tung, C.S.; Sanbonmatsu, K.Y. Rise of the RNA machines: Exploring the structure of long non-coding RNAs. J. Mol. Biol. 2013, 425, 3731–3746. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Pan, J.; Feng, X.; Duan, P.; Yin, X.; Xu, Y.; Wang, X.; Zou, S. Insights into the roles of lncRNAs in skeletal and dental diseases. Cell Biosci. 2018, 8, 8. [Google Scholar] [CrossRef]
- Ponjavic, J.; Oliver, P.L.; Lunter, G.; Ponting, C.P. Genomic and transcriptional co-localization of protein-coding and long non-coding RNA pairs in the developing brain. PLoS Genet. 2009, 5, e1000617. [Google Scholar] [CrossRef]
- Pennisi, E. Long noncoding RNAs may alter chromosome’s 3D structure. Science 2013, 340, 910. [Google Scholar] [CrossRef]
- Seila, A.C.; Calabrese, J.M.; Levine, S.S.; Yeo, G.W.; Rahl, P.B.; Flynn, R.A.; Young, R.A.; Sharp, P.A. Divergent transcription from active promoters. Science 2008, 322, 1849–1851. [Google Scholar] [CrossRef]
- Preker, P.; Nielsen, J.; Kammler, S.; Lykke-Andersen, S.; Christensen, M.S.; Mapendano, C.K.; Schierup, M.H.; Jensen, T.H. RNA exosome depletion reveals transcription upstream of active human promoters. Science 2008, 322, 1851–1854. [Google Scholar] [CrossRef]
- Hubaud, A.; Pourquie, O. Signalling dynamics in vertebrate segmentation. Nat. Rev. Mol. Cell Biol. 2014, 15, 709–721. [Google Scholar] [CrossRef]
- Feng, S.; Du, X.; Wang, C.; Ye, D.; Ma, G.; Zhao, S.; Wang, H.; Liu, X. Identification of mRNAs related to tibial cartilage development of yorkshire piglets. BioMed Res. Int. 2019, 2019, 2365416. [Google Scholar] [CrossRef]
- Kawato, Y.; Hirao, M.; Ebina, K.; Tamai, N.; Shi, K.; Hashimoto, J.; Yoshikawa, H.; Myoui, A. Nkx3.2-induced suppression of Runx2 is a crucial mediator of hypoxia-dependent maintenance of chondrocyte phenotypes. Biochem. Biophys. Res. Commun. 2011, 416, 205–210. [Google Scholar] [CrossRef]
- Caron, M.M.; Emans, P.J.; Cremers, A.; Surtel, D.A.; Coolsen, M.M.; van Rhijn, L.W.; Welting, T.J. Hypertrophic differentiation during chondrogenic differentiation of progenitor cells is stimulated by BMP-2 but suppressed by BMP-7. Osteoarthr. Cartil. 2013, 21, 604–613. [Google Scholar] [CrossRef]
- Castro, J.P.; Yancoskie, M.N.; Marchini, M.; Belohlavy, S.; Hiramatsu, L.; Kucka, M.; Beluch, W.H.; Naumann, R.; Skuplik, I.; Cobb, J.; et al. An integrative genomic analysis of the Longshanks selection experiment for longer limbs in mice. eLife 2019, 88, e42014. [Google Scholar] [CrossRef]
- Smeeton, J.; Natarajan, N.; Naveen, K.A.; Miyashita, T.; Baddam, P.; Fabian, P.; Graf, D.; Crump, J.G. Zebrafish model for spondylo-megaepiphyseal-metaphyseal dysplasia reveals post-embryonic roles of Nkx3.2 in the skeleton. Development 2021, 148, dev193409. [Google Scholar] [CrossRef]
- Waldmann, L.; Leyhr, J.; Zhang, H.; Ohman-Magi, C.; Allalou, A.; Haitina, T. The broad role of Nkx3.2 in the development of the zebrafish axial skeleton. PLoS ONE 2021, 16, e0255953. [Google Scholar] [CrossRef]
- Simsek-Kiper, P.O.; Kosukcu, C.; Akgun-Dogan, O.; Gocmen, R.; Utine, G.E.; Soyer, T.; Korkmaz-Toygar, A.; Nishimura, G.; Alikasifoglu, M.; Boduroglu, K. A novel NKX3-2 mutation associated with perinatal lethal phenotype of spondylo-megaepiphyseal-metaphyseal dysplasia in a neonate. Eur. J. Med. Genet. 2019, 62, 21–26. [Google Scholar] [CrossRef]
- Jeong, D.U.; Choi, J.Y.; Kim, D.W. Cartilage-Specific and Cre-Dependent nkx3.2 overexpression in vivo causes skeletal dwarfism by delaying cartilage hypertrophy. J. Cell. Physiol. 2017, 232, 78–90. [Google Scholar] [CrossRef]
- Zhong, Z.A.; Zahatnansky, J.; Snider, J.; Van Wieren, E.; Diegel, C.R.; Williams, B.O. Wntless spatially regulates bone development through beta-catenin-dependent and independent mechanisms. Dev. Dyn. 2015, 244, 1347–1355. [Google Scholar] [CrossRef]
- Lu, C.; Wan, Y.; Cao, J.; Zhu, X.; Yu, J.; Zhou, R.; Yao, Y.; Zhang, L.; Zhao, H.; Li, H.; et al. Wnt-mediated reciprocal regulation between cartilage and bone development during endochondral ossification. Bone 2013, 53, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Lawson, L.Y.; Brodt, M.D.; Migotsky, N.; Chermside-Scabbo, C.J.; Palaniappan, R.; Silva, M.J. Osteoblast-Specific Wnt Secretion Is Required for Skeletal Homeostasis and Loading-Induced Bone Formation in Adult Mice. J. Bone Miner. Res. 2022, 37, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E.; Parker, K.; Zanotti, S. Gremlin1 is required for skeletal development and postnatal skeletal homeostasis. J. Cell. Physiol. 2012, 227, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Lancman, J.J.; Hasso, S.M.; Suzuki, T.; Kherdjemil, Y.; Kmita, M.; Ferris, A.; Dong, P.; Ros, M.A.; Fallon, J.F. Downregulation of Grem1 expression in the distal limb mesoderm is a necessary precondition for phalanx development. Dev. Dyn. 2022, 251, 1439–1455. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Jin, S.; Johnson, J.; Xuan, H.; Lu, D.; Li, J.; Zhai, L.; Li, X.; Zhao, Y. TRIM28 secures skeletal stem cell fate during skeletogenesis by silencing neural gene expression and repressing GREM1/AKT/mTOR signaling axis. Cell Rep. 2023, 42, 112012. [Google Scholar] [CrossRef]
- Colvin, J.S.; Bohne, B.A.; Harding, G.W.; McEwen, D.G.; Ornitz, D.M. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat. Genet. 1996, 12, 390–397. [Google Scholar] [CrossRef]
- Deng, C.; Wynshaw-Boris, A.; Zhou, F.; Kuo, A.; Leder, P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell 1996, 84, 911–921. [Google Scholar] [CrossRef]
- Wen, X.; Li, X.; Tang, Y.; Tang, J.; Zhou, S.; Xie, Y.; Guo, J.; Yang, J.; Du, X.; Su, N.; et al. Chondrocyte FGFR3 regulates bone mass by inhibiting osteogenesis. J. Biol. Chem. 2016, 291, 24912–24921. [Google Scholar] [CrossRef]
- Kawashima, I.; Matsushita, M.; Mishima, K.; Kamiya, Y.; Osawa, Y.; Ohkawara, B.; Ohno, K.; Kitoh, H.; Imagama, S. Activated FGFR3 suppresses bone regeneration and bone mineralization in an ovariectomized mouse model. BMC Musculoskelet. Disord. 2023, 24, 200. [Google Scholar] [CrossRef]
- Kim, H.Y.; Ko, J.M. Clinical management and emerging therapies of FGFR3-related skeletal dysplasia in childhood. Ann. Pediatr. Endocrinol. Metab. 2022, 27, 90–97. [Google Scholar] [CrossRef]
- Morimoto, R.; Yamamoto, A.; Akimoto, Y.; Obinata, A. Homeoprotein Hex is expressed in mouse developing chondrocytes. J. Biochem. 2011, 150, 61–71. [Google Scholar] [CrossRef]
- Watanabe, H.; Okada, H.; Hirose, J.; Omata, Y.; Matsumoto, T.; Matsumoto, M.; Nakamura, M.; Saito, T.; Miyamoto, T.; Tanaka, S. Transcription Factor Hematopoietically Expressed Homeobox Protein (Hhex) Negatively Regulates Osteoclast Differentiation by Controlling Cyclin-Dependent Kinase Inhibitors. JBMR Plus 2022, 6, e10608. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, Q.; Xue, M.; Wang, Y.; Yang, X.; Chan, S.; Tang, Q.; Wang, F.; Sun, R.; Chao, Z.; et al. Novel Haplotype in the HHEX Gene Promoter Associated with Body Length in Pigs. Genes 2023, 14, 511. [Google Scholar] [CrossRef]
- Li, Y.; Lacerda, D.A.; Warman, M.L.; Beier, D.R.; Yoshioka, H.; Ninomiya, Y.; Oxford, J.T.; Morris, N.P.; Andrikopoulos, K.; Ramirez, F.; et al. A fibrillar collagen gene, Col11a1, is essential for skeletal morphogenesis. Cell 1995, 80, 423–430. [Google Scholar] [CrossRef]
- Hufnagel, S.B.; Weaver, K.N.; Hufnagel, R.B.; Bader, P.I.; Schorry, E.K.; Hopkin, R.J. A novel dominant COL11A1 mutation resulting in a severe skeletal dysplasia. Am. J. Med Genet. Part A 2014, 164, 2607–2612. [Google Scholar] [CrossRef]
- Mirtavoos Mahyari, H.; Ajami, S.; Mehrtash, A.; Marashiyan, S.M.; Bahreini, F.; Sheikhy, K.; Ghanbari, S.; Ardeshirdavani, A. Clinical whole-exome sequencing analysis reveals a novel missense COL11A1 mutation resulting in an 18-week Iranian male aborted fetus with Fibrochondrogenesis 1: A case report. Clin. Case Rep. 2022, 10, e6574. [Google Scholar] [CrossRef]
- Moverare-Skrtic, S.; Henning, P.; Liu, X.; Nagano, K.; Saito, H.; Borjesson, A.E.; Sjogren, K.; Windahl, S.H.; Farman, H.; Kindlund, B.; et al. Osteoblast-derived WNT16 represses osteoclastogenesis and prevents cortical bone fragility fractures. Nat. Med. 2014, 20, 1279–1288. [Google Scholar] [CrossRef]
- Qu, X.; Liao, M.; Liu, W.; Cai, Y.; Yi, Q.; Long, J.; Tan, L.; Deng, Y.; Deng, H.; Chen, X. Loss of Wnt16 Leads to Skeletal Deformities and Downregulation of Bone Developmental Pathway in Zebrafish. Int. J. Mol. Sci. 2021, 22, 6673. [Google Scholar] [CrossRef]
- Hassan, M.Q.; Tye, C.E.; Stein, G.S.; Lian, J.B. Non-coding RNAs: Epigenetic regulators of bone development and homeostasis. Bone 2015, 81, 746–756. [Google Scholar] [CrossRef]
- Luo, Z.; Li, Y.; Liu, X.; Luo, M.; Xu, L.; Luo, Y.; Xiao, B.; Yang, H. Systems biology of myasthenia gravis, integration of aberrant lncRNA and mRNA expression changes. BMC Med. Genom. 2015, 8, 13. [Google Scholar] [CrossRef]
- Jiang, Z.; Cushing, L.; Ai, X.; Lu, J. MiR-326 is downstream of Sonic hedgehog signaling and regulates the expression of Gli2 and smoothened. Am. J. Respir. Cell Mol. Biol. 2014, 51, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Kuroda, J.; Kawakami, K.; Wada, H. Epidermal regulation of bone morphogenesis through the development and regeneration of osteoblasts in the zebrafish scale. Dev. Biol. 2018, 437, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, X.; Chao, Z.; Wang, K.; Wang, J.; Tang, Q.; Luo, Y.; Zheng, J.; Tan, S.; Fang, M. Transcriptomic analysis of coding genes and Non-Coding RNAs reveals complex regulatory networks underlying the black back and white belly coat phenotype in chinese wuzhishan pigs. Genes 2019, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Guttman, M.; Garber, M.; Levin, J.Z.; Donaghey, J.; Robinson, J.; Adiconis, X.; Fan, L.; Koziol, M.J.; Gnirke, A.; Nusbaum, C.; et al. Ab initio reconstruction of cell type-specific transcriptomes in mouse reveals the conserved multi-exonic structure of lincRNAs. Nat. Biotechnol. 2010, 28, 503–510. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, Y.; Ye, Z.Q.; Liu, X.Q.; Zhao, S.Q.; Wei, L.; Gao, G. CPC: Assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007, 35, W345–W349. [Google Scholar] [CrossRef]
- Punta, M.; Coggill, P.C.; Eberhardt, R.Y.; Mistry, J.; Tate, J.; Boursnell, C.; Pang, N.; Forslund, K.; Ceric, G.; Clements, J.; et al. The Pfam protein families database. Nucleic Acids Res. 2011, 40, D290–D301. [Google Scholar] [CrossRef]
- Lin, M.F.; Jungreis, I.; Kellis, M. PhyloCSF: A comparative genomics method to distinguish protein coding and non-coding regions. Bioinformatics 2011, 27, i275–i282. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, Y.; Lynch, K.H.; Dennis, J.J.; Wishart, D.S. PHAST: A fast phage search tool. Nucleic Acids Res. 2011, 39, W347–W352. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, J.; Li, Z.; Li, X.; Hu, X.; Huang, Y.; Zhao, X.; Liang, C.; Wang, Y.; Sun, L.; et al. Integrated profiling of microRNAs and mRNAs: MicroRNAs located on Xq27.3 associate with clear cell renal cell carcinoma. PLoS ONE 2010, 5, e15224. [Google Scholar] [CrossRef]
- Smoot, M.E.; Ono, K.; Ruscheinski, J.; Wang, P.L.; Ideker, T. Cytoscape 2.8: New features for data integration and network visualization. Bioinformatics 2011, 27, 431–432. [Google Scholar] [CrossRef]
- Dao, D.Y.; Jonason, J.H.; Zhang, Y.; Hsu, W.; Chen, D.; Hilton, M.J.; O’Keefe, R.J. Cartilage-specific beta-catenin signaling regulates chondrocyte maturation, generation of ossification centers, and perichondrial bone formation during skeletal development. J. Bone Miner. Res. 2012, 27, 1680–1694. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Zhang, B.; Lu, Y.; Yang, Y.; Ban, D.; Zhang, H. Single nucleotide polymorphism scanning and expression of the FRZB gene in pig populations. Gene 2014, 543, 198–203. [Google Scholar] [CrossRef]
| Sample Name | Raw Reads | Clean Reads | Error Rate (%) | Q20 (%) | Q30 (%) | % of Mapped Reads | Uniquely Mapped Reads | Reads Mapped to mRNA |
|---|---|---|---|---|---|---|---|---|
| W11 | 113,505,514 | 11,044,2434 | 0.01 | 97.92 | 94.59 | 79.28 | 72,844,716 | 21,521,718 (55.61%) |
| W12 | 106,805,890 | 103,934,400 | 0.01 | 97.54 | 93.7 | 79.25 | 69,206,807 | 22,967,413 (62.93%) |
| W13 | 108,723,412 | 105,856,996 | 0.01 | 97.79 | 94.32 | 79.78 | 71,235,208 | 23,608,836 (62.78%) |
| W41 | 89,009,068 | 86,255,042 | 0.02 | 97.26 | 93.11 | 78.16 | 59,558,565 | 20,819,812 (66.35%) |
| W42 | 94,091,046 | 91,527,780 | 0.01 | 97.69 | 94.05 | 78.16 | 60,244,736 | 21,465,873 (67.78%) |
| W43 | 103,048,596 | 100,034,036 | 0.01 | 97.39 | 93.48 | 76.30 | 68,714,104 | 23,544,258 (64.73%) |
| Y11 | 98,092,402 | 95,272,188 | 0.02 | 97.35 | 93.27 | 81.80 | 68,345,799 | 22,528,226 (63.61%) |
| Y12 | 99,505,204 | 96,391,826 | 0.02 | 97.01 | 92.59 | 80.71 | 69,699,510 | 23,019,609 (62.46%) |
| Y13 | 102,313,346 | 99,334,904 | 0.02 | 97.18 | 92.93 | 80.24 | 70,930,309 | 22,432,338 (60.06%) |
| Y41 | 103,154,682 | 100,089,328 | 0.01 | 97.41 | 93.42 | 81.12 | 71,909,642 | 24,156,222 (65.58%) |
| Y42 | 95,680,318 | 93,170,738 | 0.01 | 97.65 | 93.98 | 80.75 | 66,502,651 | 20,678,419 (59.13%) |
| Y43 | 101,495,130 | 98,308,836 | 0.02 | 97.31 | 93.18 | 79.44 | 68,162,985 | 22,480,597 (64.02%) |
| GO Term | p-Value | Genes |
|---|---|---|
| Bone remodeling | 6.22 × 10−7 | WNT16, RASSF2, RAB3D, PTK2B, RAC3, ADAM8, MEPE, UBASH3B, DCSTAMP, SYK, GREM1, INPP5D, PTGER4, BGLAP, CEACAM1 |
| Bone resorption | 6.45 × 10−5 | RAB3D, PTK2B, RAC3, ADAM8, UBASH3B, SYK, INPP5D, PTGER4, BGLAP |
| Bone maturation | 0.000233 | LTF, FGFR3, GREM1, THBS3, RFLNA, RYR1 |
| Bone development | 0.000411 | TYROBP, LTF, FGFR3, PTPRCAP, IMPAD1, PTPN6, CSGALNACT1, FLI1, MATN1, GREM1, THBS3, AKAP13, HAS2, PTGER4, RFLNA, RYR1, BGLAP |
| Bone mineralization | 0.001976 | LTF, FGFR3, PTK2B, SRGN, RSPO2, MEPE, MATN1, PKDCC, GREM1, RFLNA, BGLAP |
| Bone morphogenesis | 0.031658 | LTF, FGFR3, IMPAD1, CSGALNACT1, MATN1, THBS3, HAS2 |
| Cartilage development | 0.003859 | SCIN, FGFR3, IMPAD1, NKX3-2, MAPK14, SOX5, CNMD, CSGALNACT1, RSPO2, MATN1, PKDCC, GREM1, THBS3, CYTL1, RFLNA |
| Skeletal system development | 0.000388 | SCIN, TYROBP, LTF, FGFR3, PTPRCAP, RASSF2, IMPAD1, NKX3-2, PTPN6, MAPK14, MDFI, SLC35D1, MATN3, SOX5, CNMD, HAPLN3, CSGALNACT1, RSPO2, FLI1, MEPE, EXTL1, MATN1, PKDCC, HHIP, GREM1, THBS3, AKAP13, CYTL1, HAS2, PTGER4, SOX11, RFLNA, RYR1, COL11A1, BGLAP |
| Axis specification | 0.05477 | COBL, MDFI, WLS, STIL, FOXA2, HHEX, BASP1 |
| Axis elongation | 0.693058 | PTK7 |
| Chondrocyte differentiation | 0.001366 | SCIN, FGFR3, IMPAD1, NKX3-2, MAPK14, SOX5, MATN1, PKDCC, GREM1, CYTL1, RFLNA |
| Regulation of osteoblast proliferation | 0.06172 | LTF, GREM1, HPSE |
| Osteoblast differentiation | 0.344098 | LTF, RASSF2, RSPO2, NOCT, IL6R, PTGER4, ID4, SOX11, BGLAP |
| Osteoclast differentiation | 0.005422 | TYROBP, LTF, RASSF2, MAPK14, UBASH3B, DCSTAMPINPP5D, LILRB4, BGLAP |
| Genes | Transcript ID | Gene ID/Name | Y4_vs_Y1_ log2FC | W4_vs_W1_ log2FC | p-Adjust |
|---|---|---|---|---|---|
| lncRNAs | ALDBSSCT0000008143 | ALDBSSCG0000004943 | −1.7 | −1.8 | 0.0022 |
| LNC_001455 | XLOC_074900 | 3.7 | 3.9 | 0.0032 | |
| LNC_000862 | XLOC_043998 | 2.7 | 4.1 | 0.0012 | |
| LNC_000173 | XLOC_007104 | −3.6 | −5.7 | 0.0092 | |
| ALDBSSCT0000010956 | ALDBSSCG0000006642 | −4.6 | −3.3 | 0.0446 | |
| ALDBSSCT0000008644 | ALDBSSCG0000005264 | −4.7 | −3.7 | 0.0119 | |
| mRNAs | ENSSSCG00000024594 | NKX3.2 | −1.7 | −2.3 | 0.0012 |
| ENSSSCG00000004808 | GREM1 | −1.5 | −1.8 | 0.0206 | |
| ENSSSCG00000003795 | WLS | −1.2 | −1.2 | 0.0022 | |
| ENSSSCG00000030827 | FGFR3 | −1.9 | −1.2 | 0.0056 | |
| ENSSSCG00000010472 | HHEX | 1.1 | 1.8 | 0.0302 | |
| ENSSSCG00000016617 | WNT16 | 3.6 | 4.5 | 0.0012 | |
| microRNAs | ssc-miR−1 | −2.1 | −2.0 | 0.0000 | |
| ssc-miR-204 | 1.7 | 1.3 | 0.0012 | ||
| ssc-miR-206 | −2.0 | −2.9 | 0.0000 | ||
| ssc-miR-326 | 2.6 | 3.4 | 0.0098 | ||
| ssc-miR-151-3p | −1.5 | 1.3 | 0.0000 | ||
| ssc-miR-374b-5p | 1.4 | −1.6 | 0.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Luo, Y.; Chao, Z.; Zhang, J.; Liu, X.; Tang, Q.; Wang, K.; Tan, S.; Fang, M. Integrated Analysis of Transcriptome Expression Profiles Reveals miRNA-326–NKX3.2-Regulated Porcine Chondrocyte Differentiation. Int. J. Mol. Sci. 2023, 24, 7257. https://doi.org/10.3390/ijms24087257
Xu Q, Luo Y, Chao Z, Zhang J, Liu X, Tang Q, Wang K, Tan S, Fang M. Integrated Analysis of Transcriptome Expression Profiles Reveals miRNA-326–NKX3.2-Regulated Porcine Chondrocyte Differentiation. International Journal of Molecular Sciences. 2023; 24(8):7257. https://doi.org/10.3390/ijms24087257
Chicago/Turabian StyleXu, Qiao, Yabiao Luo, Zhe Chao, Jibin Zhang, Ximing Liu, Qiguo Tang, Kejun Wang, Shuyi Tan, and Meiying Fang. 2023. "Integrated Analysis of Transcriptome Expression Profiles Reveals miRNA-326–NKX3.2-Regulated Porcine Chondrocyte Differentiation" International Journal of Molecular Sciences 24, no. 8: 7257. https://doi.org/10.3390/ijms24087257
APA StyleXu, Q., Luo, Y., Chao, Z., Zhang, J., Liu, X., Tang, Q., Wang, K., Tan, S., & Fang, M. (2023). Integrated Analysis of Transcriptome Expression Profiles Reveals miRNA-326–NKX3.2-Regulated Porcine Chondrocyte Differentiation. International Journal of Molecular Sciences, 24(8), 7257. https://doi.org/10.3390/ijms24087257







