The Overlap of Kidney Failure in Extrapulmonary Sarcoidosis in Children—Case Report and Review of Literature
Abstract
1. Introduction
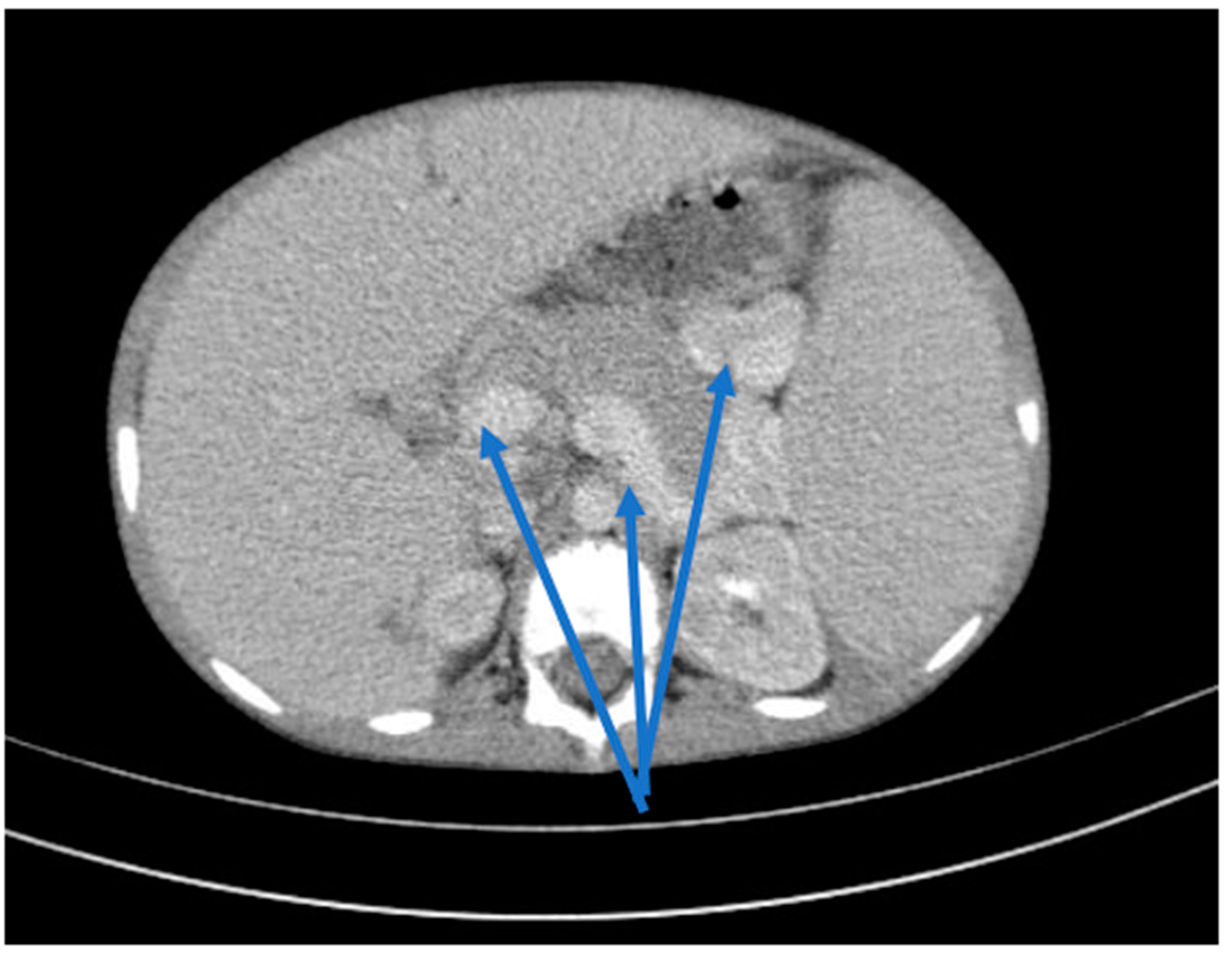
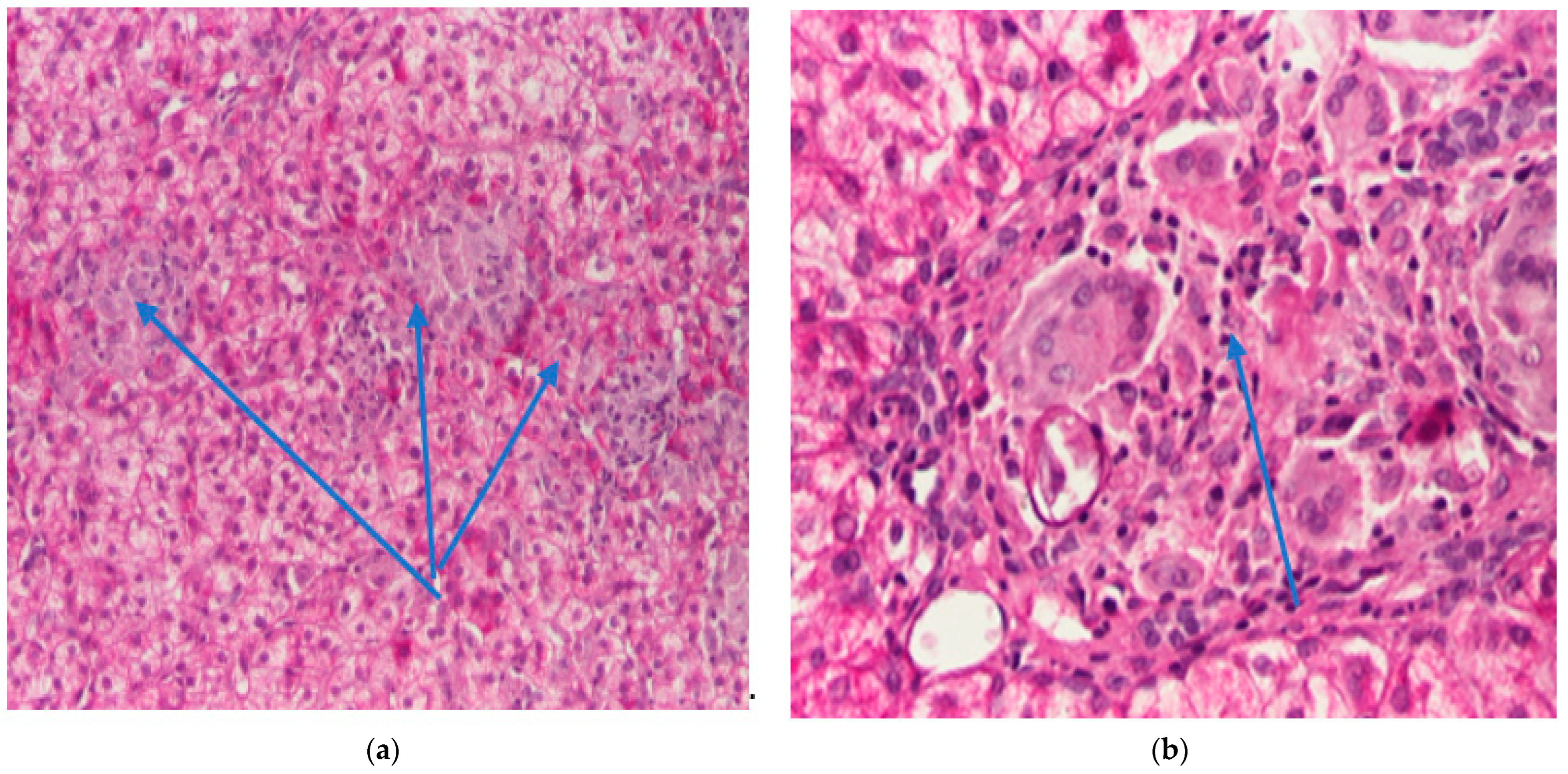
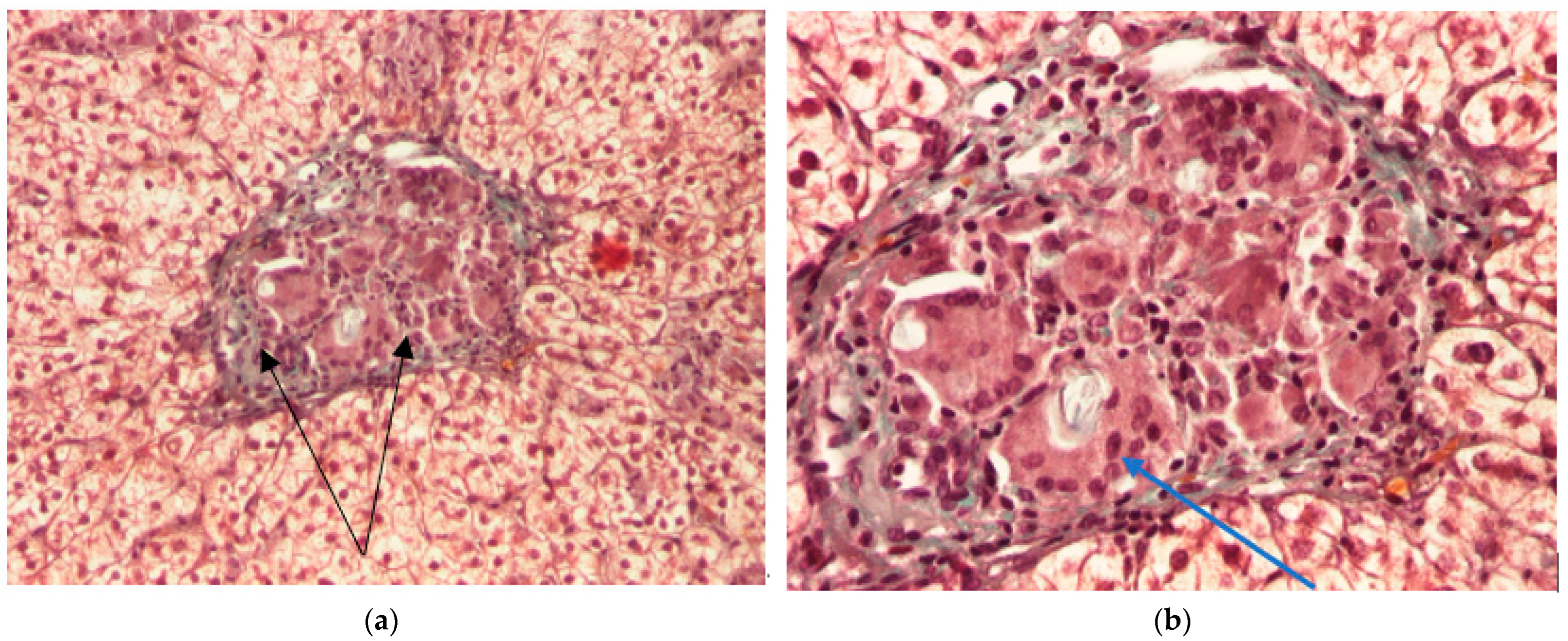
2. Clinical Case
3. Discussions
3.1. Pediatric Sarcoidosis—Epidemiology
3.2. Sarcoidosis—Etiopathology
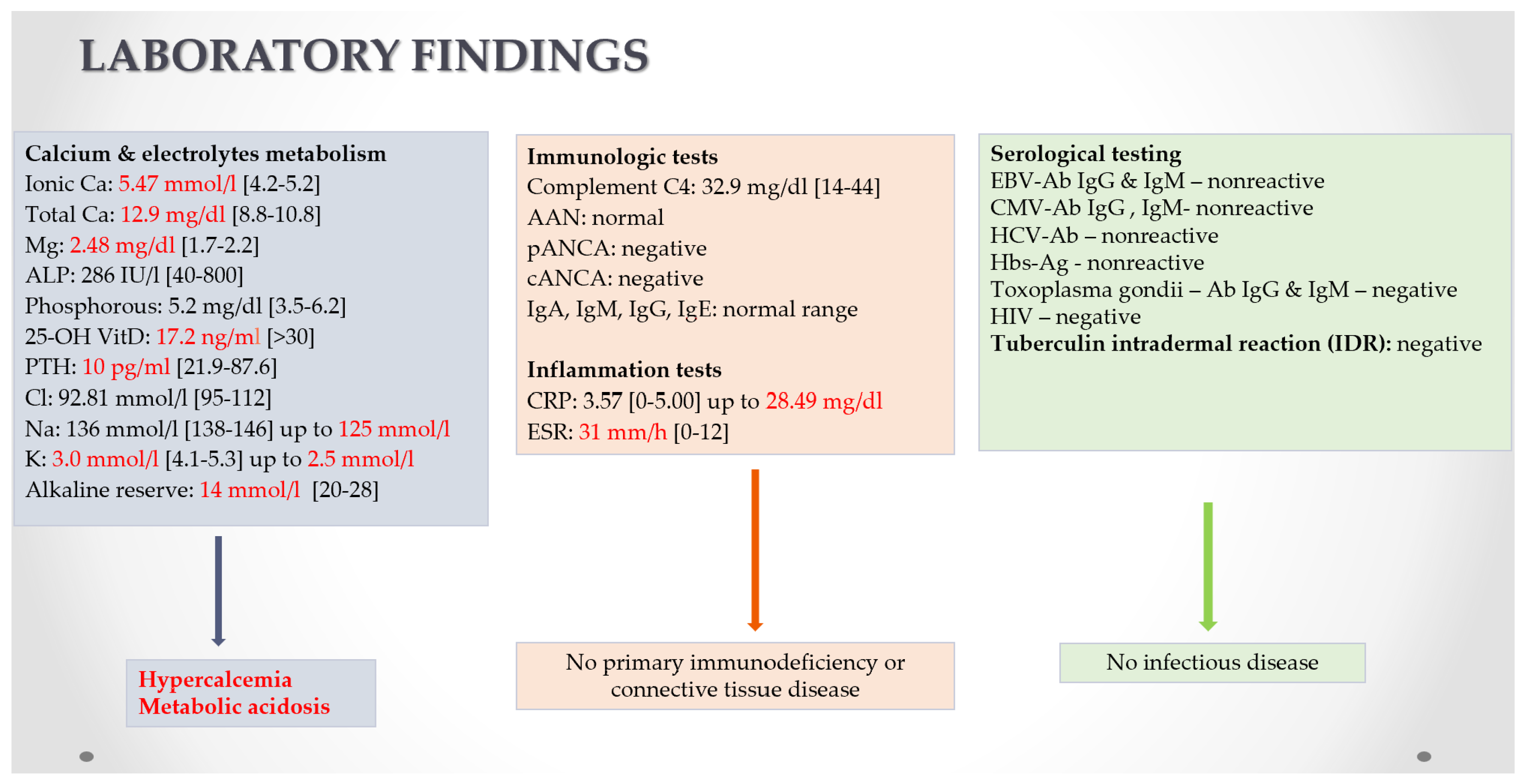
3.3. Sarcoidosis—Laboratory Diagnosis
3.4. Pediatric Sarcoidosis—Clinical Findings
3.5. Pediatric Sarcoidosis—Renal Involvement
3.6. Pediatric Extrapulmonary Sarcoidosis—Treatment
3.7. Pediatric Extrapulmonary Sarcoidosis—Evolution
3.8. Pediatric Extrapulmonary Sarcoidosis—Prognosis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Semenzato, G. ACCESS: A Case Control Etiologic Study of Sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2005, 22, 83–86. [Google Scholar]
- Lhote, R.; Annesi-Maesano, I.; Nunes, H.; Launay, D.; Borie, R.; Sacré, K.; Schleinitz, N.; Hamidou, M.; Mahevas, M.; Devilliers, H.; et al. Clinical phenotypes of extrapulmonary sarcoidosis: An analysis of a French, multi-ethnic, multicentre cohort. Eur. Respir. J. 2021, 57, 2001160. [Google Scholar] [CrossRef]
- Gedalia, A.; Khan, T.A.; Shetty, A.; Dimitriades, V.; Espinoza, L.R. Childhood sarcoidosis: Louisiana experience. Clin. Rheumatol. 2016, 35, 1879–1884. [Google Scholar] [CrossRef]
- Hoffmann, A.L.; Milman, N.; Byg, K.-E. Childhood sarcoidosis in Denmark 1979–1994: Incidence, clinical features and laboratory results at presentation in 48 children. Acta Paediatr. 2004, 93, 30–36. [Google Scholar] [CrossRef]
- Nathan, N.; Marcelo, P.; Houdouin, V.; Epaud, R.; de Blic, J.; Valeyre, D.; Houzel, A.; Busson, P.-F.; Corvol, H.; Deschildre, A.; et al. Lung sarcoidosis in children: Update on disease expression and management. Thorax 2015, 70, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.; Bhardwaj, M. New sarcoidosis guidelines: Are we near to perfection? Lung India 2022, 39, 217–219. [Google Scholar] [CrossRef]
- Levin, A.M.; Iannuzzi, M.C.; Montgomery, C.G.; Trudeau, S.; Datta, I.; McKeigue, P.; Fischer, A.; Nebel, A.; Rybicki, B.A. Association of ANXA11 genetic variation with sarcoidosis in African Americans and European Americans. Genes Immun. 2013, 14, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Gerke, A.K.; Judson, M.A.; Cozier, Y.C.; Culver, D.A.; Koth, L.L. Disease Burden and Variability in Sarcoidosis. Ann. Am. Thorac. Soc. 2017, 14, S421–S428. [Google Scholar] [CrossRef]
- Spagnolo, P.; Rossi, G.; Trisolini, R.; Sverzellati, N.; Baughman, R.P.; Wells, A.U. Pulmonary sarcoidosis. Lancet Respir. Med. 2018, 6, 389–402. [Google Scholar] [CrossRef]
- Nathan, N.; Sileo, C.; Calender, A.; Pacheco, Y.; Rosental, P.A.; Cavalin, C.; Macchi, O.; Valeyre, D.; Clement, A.; Silicosis Research Group. Paediatric sarcoidosis. Paediatr. Respir. Rev. 2019, 29, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Nathan, N.; Montagne, M.E.; Macchi, O.; Rosental, P.A.; Chauveau, S.; Jeny, F.; Sesé, L.; Abou Taam, R.; Brocvielle, M.; Brouard, J.; et al. Exposure to in-organic particles in paediatric sarcoidosis: The PEDIASARC study. Thorax 2022, 77, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Chiu, B.; Chan, J.; Das, S.; Alshamma, Z.; Sergi, C. Pediatric Sarcoidosis: A Review with Emphasis on Early Onset and High-Risk Sarcoidosis and Diagnostic Challenges. Diagnostics 2019, 9, 160. [Google Scholar] [CrossRef]
- Lee, S.M.; Choi, H.; Lim, S.; Shin, J.; Kang, J.-M.; Ahn, J.G. Pediatric Sarcoidosis Misdiagnosed as Hepatosplenic Abscesses: A Case Report and Review. J. Rheum. Dis. 2022, 29, 181–186. [Google Scholar] [CrossRef]
- Heinle, R.; Chang, C. Diagnostic criteria for sarcoidosis. Autoimmun. Rev. 2014, 13, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.D.; Wouters, C.H. Pediatric sarcoidosis. In Textbook of Pediatric Rheumatology, 8th ed.; Petty, R.E., Laxer, R.M., Lindsley, C.B., Wedderburn, L.R., Mellins, E.D., Fuhlbrigge, R.C., Eds.; Elsevier: Philadelphia, PA, USA, 2021; pp. 559–566.e2. [Google Scholar]
- Thomas, P.D.; Hunninghake, G.W. Current concepts of the pathogenesis of sarcoidosis. Am. Rev. Respir. Dis. 1987, 135, 747–760. [Google Scholar] [PubMed]
- Agostini, C.; Adami, F.; Semenzato, G. New pathogenetic insights into the sarcoid granuloma. Curr. Opin. Rheumatol. 2000, 12, 71–76. [Google Scholar] [CrossRef]
- Hunninghake, G.W.; Garrett, K.C.; Richerson, H.B.; Fantone, J.C.; Ward, P.A.; Rennard, S.I.; Bitterman, P.B.; Crystal, R.G. Pathogenesis of the granulomatous lung diseases. Am. Rev. Respir. Dis. 1984, 130, 476–496. [Google Scholar] [PubMed]
- Rybicki, B.A.; Iannuzzi, M.C.; Frederick, M.M.; Thompson, B.W.; Rossman, M.D.; Bresnitz, E.A.; Terrin, M.L.; Moller, D.R.; Barnard, J.; Baughman, R.P.; et al. Familial aggregation of sarcoidosis. A case control etiologic study of sar-coidosis (ACCESS). Am. J. Respir. Crit. Care Med. 2001, 164, 2085. [Google Scholar] [CrossRef]
- Beijer, E.; Kraaijvanger, R.; Roodenburg, C.; Grutters, J.C.; Meek, B.; Veltkamp, M. Simultaneous testing of immunological sensitization to multiple antigens in sarcoidosis reveals an association with inorganic antigens specifically related to a fibrotic phenotype. Clin. Exp. Immunol. 2021, 203, 115–124. [Google Scholar] [CrossRef]
- Grunewald, J.; Kaiser, Y.; Ostadkarampour, M.; Rivera, N.V.; Vezzi, F.; Lötstedt, B.; Olsen, R.A.; Sylwan, L.; Lundin, S.; Käller, M.; et al. T-cell receptor-HLA-DRB1 associations suggest specific antigens in pul-monary sarcoidosis. Eur. Respir. J. 2016, 47, 898. [Google Scholar] [CrossRef]
- Eberhardt, C.; Thillai, M.; Parker, R.; Siddiqui, N.; Potiphar, L.; Goldin, R.; Timms, J.F.; Wells, A.U.; Kon, O.M.; Wickremasinghe, M.; et al. Proteomic Analysis of Kveim Reagent Identifies Targets of Cellular Immunity in Sarcoidosis. PLoS ONE 2017, 12, e0170285. [Google Scholar] [CrossRef]
- Chen, E.S.; Moller, D.R. Etiologies of Sarcoidosis. Clin. Rev. Allergy Immunol. 2015, 49, 6. [Google Scholar] [CrossRef] [PubMed]
- de Brouwer, B.; Veltkamp, M.; Wauters, C.A.; Grutters, J.C.; Janssen, R. Propionibacterium acnes isolated from lymph nodes of patients with sar-coidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2015, 14, 271–274. [Google Scholar]
- Fang, C.; Huang, H.; Xu, Z. Immunological Evidence for the Role of Mycobacteria in Sarcoidosis: A Meta-Analysis. PLoS ONE 2016, 11, e0154716. [Google Scholar] [CrossRef]
- Saboor, S.; McFadden, J.; Johnson, N. Detection of mycobacterial DNA in sarcoidosis and tuberculosis with polymerase chain reaction. Lancet 1992, 339, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.; Greinert, U.; Kirsten, D.; Rüsch-Gerdes, S.; Schlüter, C.; Duchrow, M.; Galle, J.; Magnussen, H.; Schlaak, M.; Flad, H.D.; et al. Assessment of mycobacterial DNA in cells and tissues of mycobacterial and sarcoid lesions. Am. J. Respir. Crit. Care Med. 1996, 153, 375–380. [Google Scholar] [CrossRef]
- Fritz, D.; Ferwerda, B.; Brouwer, M.C.; van de Beek, D. Whole genome sequencing identifies variants associated with sarcoidosis in a family with a high prevalence of sarcoidosis. Clin. Rheumatol. 2021, 40, 3735–3743. [Google Scholar] [CrossRef]
- Chen, E.S.; Moller, D.R. Sarcoidosis—Scientific progress and clinical challenges. Nat. Rev. Rheumatol. 2011, 7, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Rybicki, B.A. Granuloma genes in sarcoidosis: What is new? Curr. Opin. Pulm. Med. 2015, 21, 510. [Google Scholar] [CrossRef]
- Maliarik, M.J.; Rybicki, B.A.; Malvitz, E.; Sheffer, R.G.; Major, M.; Popovich, J.; Iannuzzi, M.C. Angiotensin-converting Enzyme Gene Polymorphism and Risk of Sarcoidosis. Am. J. Respir. Crit. Care Med. 1998, 158, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Nautiyal, A.; Kalkanis, A.; Judson, M.A. Drug-Induced Sarcoidosis-Like Reactions. Chest 2018, 154, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Alsarhan, A.; Habeeb, S.M.; Popatia, R.; Fathalla, B. Biomarkers in Juvenile Systemic Sarcoidosis: A Case Report and Literature Review. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Gwadera, Ł.; Białas, A.J.; Iwański, M.A.; Górski, P.; Piotrowski, W.J. Sarcoidosis and calcium homeostasis disturbances-Do we know where we stand? Chron. Respir. Dis. 2019, 16, 1479973119878713. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.K.; Gedalia, A. Childhood sarcoidosis: A rare but fascinating disorder. Pediatr. Rheumatol. 2008, 6, 16. [Google Scholar] [CrossRef]
- Eurelings, L.E.M.; Miedema, J.R.; Dalm, V.A.S.H.; van Daele, P.L.A.; van Hagen, P.M.; van Laar, J.A.M.; Dik, W.A. Sensitivity and specicity of serum soluble interleukin-2 receptor for diagnosing sarcoidosis in a population of patients suspected of sarcoidosis. PLoS ONE 2019, 14, e0223897. [Google Scholar] [CrossRef]
- Gundlach, E.; Hoffmann, M.M.; Prasse, A.; Heinzelmann, S.; Ness, T. Interleukin-2 Receptor and Angiotensin-Converting Enzyme as Markers for Ocular Sarcoidosis. PLoS ONE 2016, 11, e0147258. [Google Scholar] [CrossRef]
- Thi Hong Nguyen, C.; Kambe, N.; Kishimoto, I.; Ueda-Hayakawa, I.; Okamoto, H. Serum soluble interleukin2 receptor level is more sensitive than angiotensin-converting enzyme or lysozyme for diagnosis of sarcoidosis and may be a marker of multiple organ involvement. J. Dermatol. 2017, 44, 789–797. [Google Scholar] [CrossRef]
- Chauveau, S.; Jeny, F.; Montagne, M.-E.; Taam, R.A.; Houdouin, V.; Meinzer, U.; Delacourt, C.; Epaud, R.; Aubart, F.C.; Chapelon-Abric, C.; et al. Child–Adult Transition in Sarcoidosis: A Series of 52 Patients. J. Clin. Med. 2020, 9, 2097. [Google Scholar] [CrossRef]
- Al-Kofahi, K.; Korsten, P.; Ascoli, C.; Virupannavar, S.; Mirsaeidi, M.; Chang, I.; Qaqish, N.; Saketkoo, L.A.; Baughman, R.P.; Sweiss, N.J. Management of extrapulmonary sarcoidosis: Challenges and solutions. Ther. Clin. Risk Manag. 2016, 12, 1623–1634. [Google Scholar] [CrossRef]
- Rossi, G.; Ziol, M.; Roulot, D.; Valeyre, D.; Mahévas, M. Hepatic Sarcoidosis: Current Concepts and Treatments. Semin. Respir. Crit. Care Med. 2020, 41, 652–658. [Google Scholar] [CrossRef]
- Tadros, M.; Forouhar, F.; Wu, G.Y. Hepatic sarcoidosis. J. Clin. Transl. Hepatol. 2013, 1, 87–93. [Google Scholar]
- Mañá, J.; Rubio-Rivas, M.; Villalba, N.; Marcoval, J.; Iriarte, A.; Molina-Molina, M.; Llatjos, R.; García, O.; Martínez-Yélamos, S.; Vicens-Zygmunt, V.; et al. Multidisciplinary approach and long-term follow-up in a series of 640 consecutive patients with sarcoidosis: Cohort study of a 40-year clinical experience at a tertiary referral center in Barcelona, Spain. Medicine 2017, 96, e7595. [Google Scholar] [CrossRef]
- Rosé, C.D.; Doyle, T.M.; McIlvain-Simpson, G.; Coffman, J.E.; Rosenbaum, J.T.; Davey, M.P.; Martin, T.M. Blau syndrome mutation of card15/nod2 in sporadic early onset granulomatous arthritis. J. Rheumatol. 2005, 32, 373–375. [Google Scholar]
- Correia, F.A.S.C.; Marchini, G.S.; Torricelli, F.C.; Danilovic, A.; Vicentini, F.C.; Srougi, M.; Nahas, W.C.; Mazzucchi, E. Renal manifestations of sarcoidosis: From accurate diagnosis to specific treatment. Int. Braz. J. Urol. 2020, 46, 15–25. [Google Scholar] [CrossRef]
- Stârcea, M.; Gavrilovici, C.; Munteanu, M.; Miron, I. Focal segmental glomerulosclerosis in children complicated by posterior reversible encephalopathy syndrome. J. Int. Med. Res. 2018, 46, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Berliner, A.R.; Haas, M.; Choi, M.J. Sarcoidosis: The nephrologist’s perspective. Am. J. Kidney Dis. 2006, 48, 856–870. [Google Scholar] [CrossRef]
- Casella, F.J.; Allon, M. The kidney in sarcoidosis. J. Am. Soc. Nephrol. 1993, 3, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Darabi, K.; Torres, G.; Chewaproug, D. Nephrolithiasis as primary symptom in sarcoidosis. Scand. J. Urol. Nephrol. 2005, 39, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Muther, R.S.; McCarron, D.A.; Bennett, W.M. Renal manifestations of sarcoidosis. Arch. Intern. Med. 1981, 141, 643–645. [Google Scholar] [CrossRef]
- Bergner, R.; Hoffmann, M.; Waldherr, R.; Uppenkamp, M. Frequency of kidney disease in chronic sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2003, 20, 126–132. [Google Scholar]
- Mahévas, M.; Lescure, F.X.; Boffa, J.J.; Delastour, V.; Belenfant, X.; Chapelon, C.; Cordonnier, C.; Makdassi, R.; Piette, J.C.; Naccache, J.M.; et al. Renal sarcoidosis: Clinical, laboratory, and histologic presentation and outcome in 47 patients. Medicine 2009, 88, 98. [Google Scholar] [CrossRef] [PubMed]
- Klaus, R.; Jansson, A.F.; Griese, M.; Seeman, T.; Amann, K.; Lange-Sperandio, B. Case Report: Pediatric Renal Sarcoidosis and Prognostic Factors in Reviewed Cases. Front. Pediatr. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Coutant, R.; Leroy, B.; Niaudet, P.; Loirat, C.; Dommergues, J.P.; André, J.L.; Baculard, A.; Bensman, A. Renal granulomatous sarcoidosis in childhood: A report of 11 cases and a review of the literature. Eur. J. Pediatr. 1999, 158, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Fink, C.W.; Cimaz, R. Early onset sarcoidosis: Not a benign disease. J. Rheumatol. 1997, 24, 174–177. [Google Scholar]
- Dimitriades, C.; Shetty, A.K.; Vehaskari, M.; Craver, R.D.; Gedalia, A. Membranous nephropathy associated with childhood sar-coidosis. Pediatr. Nephrol. 1999, 13, 444–447. [Google Scholar] [CrossRef]
- Thumfart, J.; Müller, D.; Rudolph, B.; Zimmering, M.; Querfeld, U.; Haffner, D. Isolated sarcoid granulomatous interstitial nephritis responding to infliximab therapy. Am. J. Kidney Dis. 2005, 45, 411–414. [Google Scholar] [CrossRef]
- Moudgil, A.; Przygodzki, R.M.; Kher, K.K. Successful steroid-sparing treatment of renal limited sarcoidosis with mycophenolate mofetil. Pediatr. Nephrol. 2006, 21, 281–285. [Google Scholar] [CrossRef]
- Rheault, M.N.; Manivel, J.C.; Levine, S.C.; Sinaiko, A.R. Sarcoidosis presenting with hearing loss and granulomatous interstitial nephritis in an adolescent. Pediatr. Nephrol. 2006, 21, 1323–1326. [Google Scholar] [CrossRef]
- Hobbs, D.J.; Barletta, G.-M.; Chung, J.Y.; Bunchman, T.E. Isolated sarcoid granulomatous interstitial nephritis in pediatrics: A case report and review of literature. Clin. Nephrol. 2009, 72, 410–413. [Google Scholar]
- Ito, S.; Harada, T.; Nakamura, T.; Imagawa, T.; Nagahama, K.; Sasaki, T.; Fujinaga, S.; Aihara, Y.; Yokota, S. Mizoribine for renal sarcoidosis: Effective steroid tapering and prevention of recurrence. Pediatr. Nephrol. 2009, 24, 411–414. [Google Scholar] [CrossRef]
- Vargas, F.; Gedalia, A.; Craver, R.D.; Vehaskari, V.M. Recurrence of granulomatous interstitial nephritis in transplanted kidney. Pediatr. Transplant. 2010, 14, e54–e57. [Google Scholar] [CrossRef]
- Roslinah, M.; Muzaliha, N.M.; Anusiah, S.; Raja, O.R.; Juliana, J.; Jalil, A.F.; Ismail, S. Ocular and renal sarcoidosis in an asian teenager: A case report. Int. J. Ophthalmol. 2010, 10, 1659–1661. [Google Scholar]
- Pepple, K.L.; Lam, D.L.; Finn, L.S.; Van Gelder, R. Urinary beta2-microglobulin testing in pediatric uveitis: A case report of a 9-year-old boy with renal and ocular sarcoidosis. Case Rep. Ophthalmol. 2015, 6, 101–105. [Google Scholar] [CrossRef]
- Downie, M.L.; Mulder, J.; Schneider, R.; Lim, L.; Tehrani, N.; Wasserman, J.; Fuchs, S.; John, R.; Noone, D.G.; Hebert, D. A curious case of growth failure and hypercalcemia: Questions. Pediatr. Nephrol. 2017, 33, 991–993. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, H.; Zhang, T.; Xu, H.; Shen, J.; Feng, J.; Sun, L. Acute kidney injury as a rare manifestation of pediatric sarcoidosis: A case report and systematic literature review. Clin. Chim. Acta 2018, 489, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Löffler, C.; Löffler, U.; Tuleweit, A.; Waldherr, R.; Uppenkamp, M.; Bergner, R. Renal sarcoidosis: Epidemiological and follow-up data in a cohort of 27 patients. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2014, 31, 306–315. [Google Scholar]
- Papanikolaou, I.C.; Antonakis, E.; Pandi, A. State-of-the-Art Treatments for Sarcoidosis. Methodist DeBakey Cardiovasc. J. 2022, 18, 94–105. [Google Scholar] [CrossRef]
- James, W.E.; Baughman, R. Treatment of sarcoidosis: Grading the evidence. Expert Rev. Clin. Pharmacol. 2018, 11, 677–687. [Google Scholar] [CrossRef]
- Mărginean, C.O.; Meliţ, L.E.; Grigorescu, G.; Puiac, C.; Simu, I. Hypercalcemia, an Important Puzzle Piece in Uncommon Onset Pediatric Sarcoidosis—A Case Report and a Review of the Literature. Front. Pediatr. 2020, 8, 497. [Google Scholar] [CrossRef]
- Milman, N.; Hoffmann, A.L. Childhood sarcoidosis: Long-term follow-up. Eur. Respir. J. 2008, 31, 592–598. [Google Scholar] [CrossRef]
- Arcana, R.I.; Crișan-Dabija, R.; Cernomaz, A.T.; Buculei, I.; Burlacu, A.; Zabară, M.L.; Trofor, A.C. The Risk of Sarcoidosis Misdiagnosis and the Harmful Effect of Corticosteroids When the Disease Picture Is Incomplete. Biomedicines 2023, 11, 175. [Google Scholar] [CrossRef]
- Valeyre, D.; Jeny, F.; Rotenberg, C.; Bouvry, D.; Uzunhan, Y.; Sève, P.; Nunes, H.; Bernaudin, J.-F. How to Tackle the Diagnosis and Treatment in the Diverse Scenarios of Extrapulmonary Sarcoidosis. Adv. Ther. 2021, 38, 4605–4627. [Google Scholar] [CrossRef]
- Terasaki, F.; Azuma, A.; Anzai, T.; Ishizaka, N.; Ishida, Y.; Isobe, M.; Inomata, T.; Ishibashi-Ueda, H.; Eishi, Y.; Kitakaze, M.; et al. JCS 2016 Guideline on Diagnosis and Treatment of Cardiac Sarcoidosis—Digest Version—. Circ. J. 2019, 83, 2329–2388. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Rajakariar, R.; Ashman, N.; Raftery, M.; Brown, H.; Martin, J.E. Infliximab as long-term maintenance in steroid-resistant and recurrent sarcoidosis in a renal transplant with central nervous system involvement. Clin. Kidney J. 2012, 5, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Drent, M.; Jessurun, N.T.; Wijnen, P.A.; Bekers, O.; Bast, A. Drug-induced comorbidities in patients with sarcoidosis. Curr. Opin. Pulm. Med. 2022, 28, 468–477. [Google Scholar] [CrossRef]
- Baughman, R.P.; Valeyre, D.; Korsten, P.; Mathioudakis, A.G.; Wuyts, W.A.; Wells, A.; Rottoli, P.; Nunes, H.; Lower, E.E.; Judson, M.A.; et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur. Respir. J. 2021, 58, 2004079. [Google Scholar] [CrossRef] [PubMed]
- Hilderson, I.; Van Laecke, S.; Wauters, A.; Donck, J. Treatment of renal sarcoidosis: Is there a guideline? Overview of the different treatment options. Nephrol. Dial. Transplant. 2014, 29, 1841–1847. [Google Scholar] [CrossRef]
- Valeyre, D.; Prasse, A.; Nunes, H.; Uzunhan, Y.; Brillet, P.Y.; Müller Quernheim, J. Sarcoidosis. Lancet 2014, 383, 1155–1167. [Google Scholar] [CrossRef]
- Aouizerate, J.; Matignon, M.; Kamar, N.; Thervet, E.; Randoux, C.; Moulin, B.; Raffray, L.; Buchler, M.; Villar, E.; Mahevas, M.; et al. Renal transplantation in patients with sarcoidosis: A French multicenter study. Clin. J. Am. Soc. Nephrol. 2010, 5, 2101–2108. [Google Scholar] [CrossRef]
- Mann, D.; Fyfe, B.; Osband, A.; Lebowitz, J.; Laskow, D.; Jones, J.; Mann, R. Sarcoidosis Within a Renal Allograft: A Case Report and Review of the Literature. Transplant. Proc. 2013, 45, 838–841. [Google Scholar] [CrossRef]
- Calatroni, M.; Moroni, G.; Reggiani, F.; Ponticelli, C. Renal sarcoidosis. J. Nephrol. 2023, 36, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Herrera, R.R.; Rodríguez, L.C.; Maruri, C.M.; de Uña Flores, A.; Morales, G.G.; Santos, E.B. Late-onset childhood sarcoidosis with multiorgan involvement. Report of two cases and review of the literature. Rev. Latin. Infect. Pediatr. 2022, 35, 30–45. [Google Scholar]
- McKeever, A.; Cox, A.; Garnett, M.; Cunniffe, N.G. Hydrocephalus as the first presenting symptom of neurosarcoidosis in two patients: A diagnosis more forthcoming in the context of systemic disease. BMJ Case Rep. 2019, 12, e229903. [Google Scholar] [CrossRef]
- Aa, J.S.; Toftedal, P.; Schultz, J.D.; Fast, S. Severe laryngeal sarcoidosis in a child managed by intralesional steroid, debulking, and methotrexate. Acta Oto-Laryngol. Case Rep. 2022, 7, 74–77. [Google Scholar] [CrossRef]
- Kobak, S. Catch the rainbow: Prognostic factor of sarcoidosis. Lung India 2020, 37, 425–432. [Google Scholar] [CrossRef]
- Jamilloux, Y.; Valeyre, D.; Lortholary, O.; Bernard, C.; Kerever, S.; Lelievre, L.; Neel, A.; Broussolle, C.; Seve, P. The spectrum of opportunistic diseases com-plicating sarcoidosis. Autoimmun. Rev. 2015, 14, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Reich, J.M.; Mullooly, J.P.; Johnson, R.E. Linkage Analysis of Malignancy-Associated Sarcoidosis. Chest 1995, 107, 605–613. [Google Scholar] [CrossRef] [PubMed]


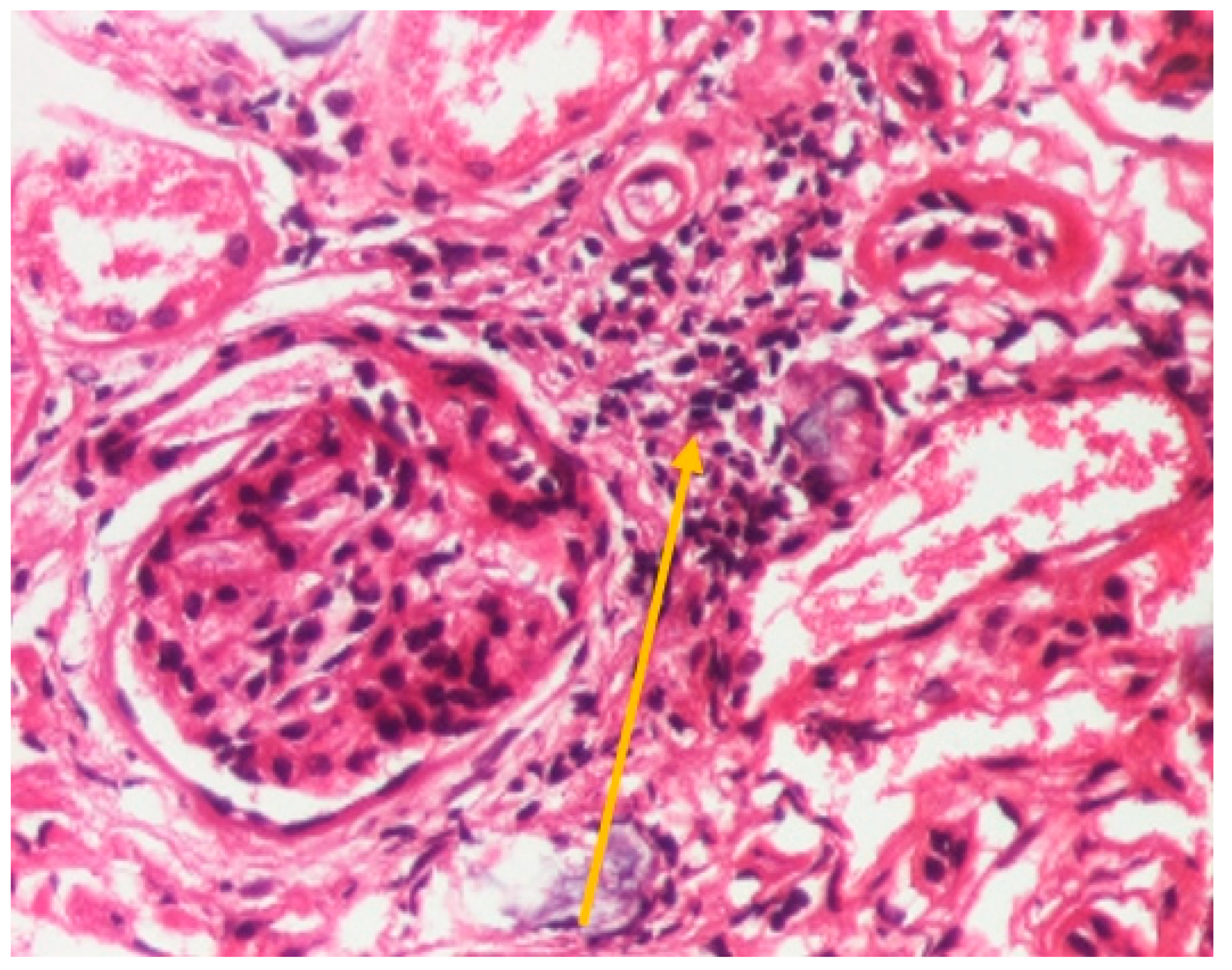
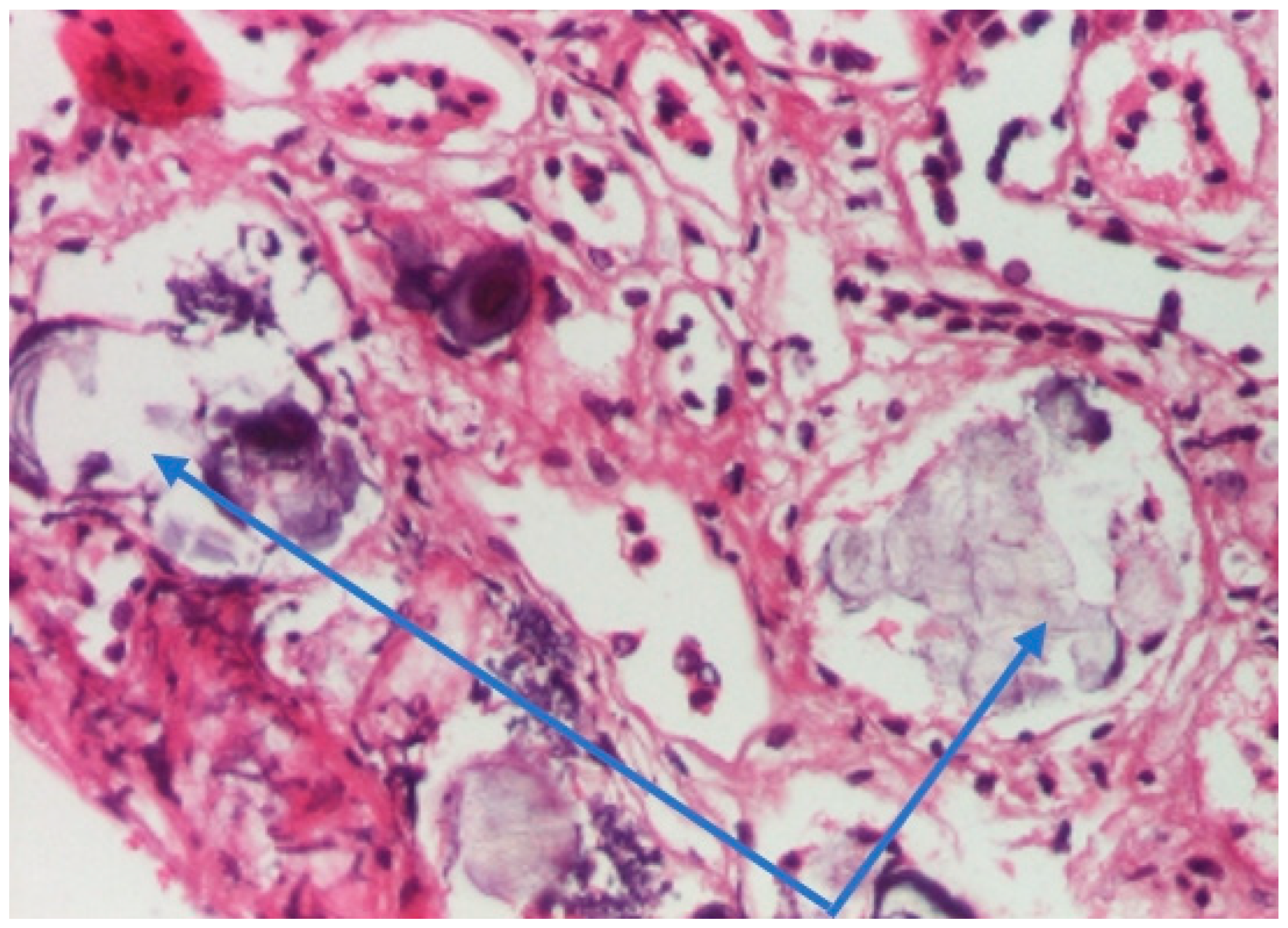

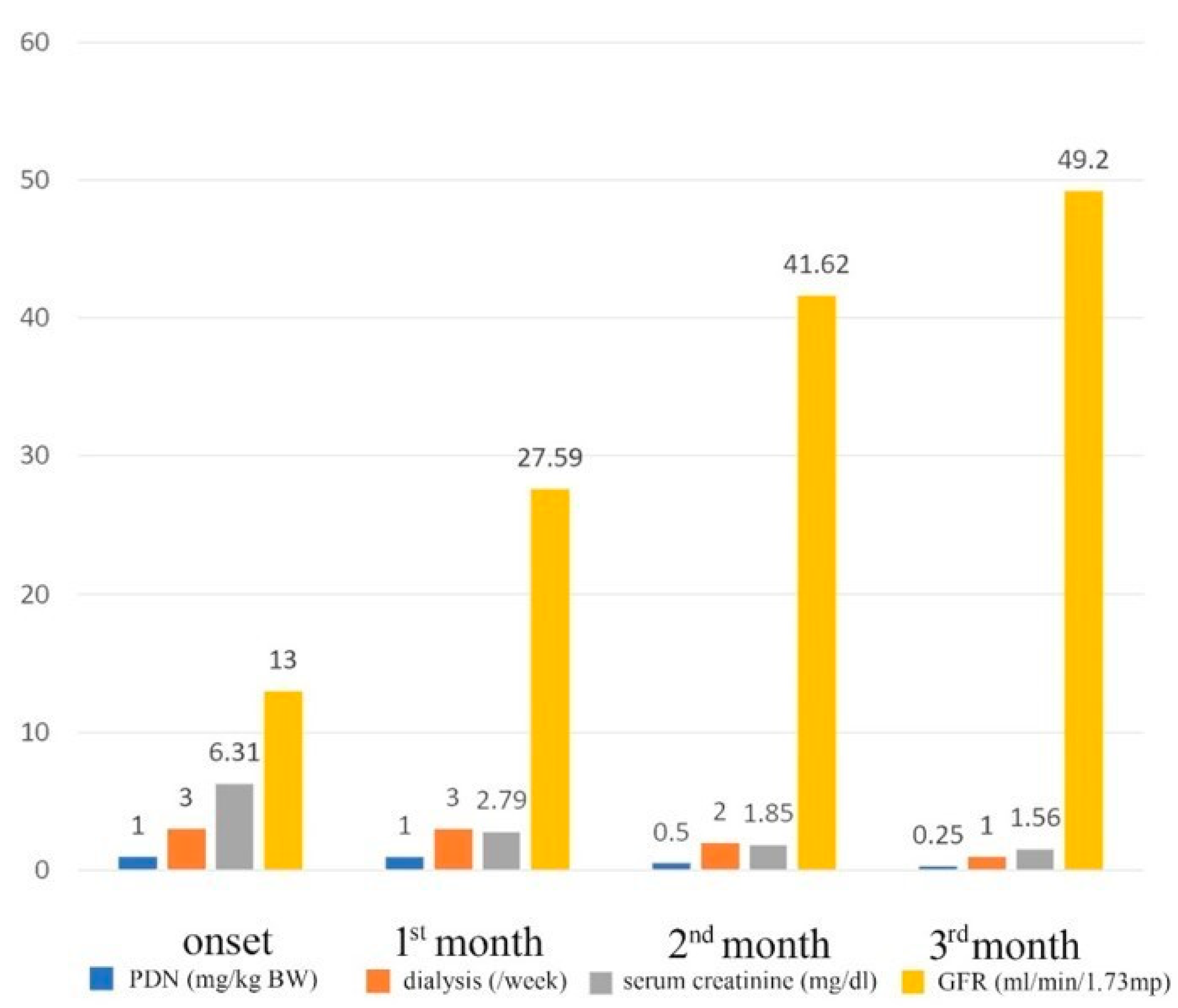
| Onset | After 3 Months | |
|---|---|---|
| General symptoms | Fatigue, HSM | Cushing syndrome, no HSM |
| Laboratory | ||
| TGP | 71 U/L | 34 U/L |
| TGO | 86 U/L | 25 U/L |
| Alkaline phosphatase (ALP) | 286 U/L | 213 U/L |
| GGT | 163 U/L | 54 U/L |
| CRP | 28.49 mg/dL | 4 mg/dL |
| ESR | 61 mm/1 h | 10 mm/1 h |
| ACE | 105 U/L | 20 U/L |
| Calcium | 12.9 mg/dL | 9.8 mg/dL |
| Nephrological | ||
| Serum creatinine | 6.31 mg/dL | 1.56 mg/dL |
| eGFR (Schwartz’s pediatric equation) | 13 (mL/min/1.73 m2) before dialysis initiation | 49.2 mL/min/1.73 m2 dialysis discontinuation |
| Hematuria | +++ | + |
| Proteinuria/24 h | 848 mg/day | 627 mg/day |
| Leukocyturia | ++ | + |
| Urinary Calcium/creatinine ratio | 0.7 | 0.21 |
| Urinary 24 h calcium | 5.19 mg/kg/day | 2.9 mg/kg/day |
| B2M | 437 mcg/L | 325 mcg/L |
| Hemodialysis | 3 times/week | Dialysis discontinuation |
| Hepatic histology | Granulomatous aspect | Not repeated |
| Ophthalmology | Band keratopathy | Not repeated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mocanu, A.; Bogos, R.A.; Trandafir, L.M.; Cojocaru, E.; Ioniuc, I.; Alecsa, M.; Lupu, V.V.; Miron, L.; Lazaruc, T.I.; Lupu, A.; et al. The Overlap of Kidney Failure in Extrapulmonary Sarcoidosis in Children—Case Report and Review of Literature. Int. J. Mol. Sci. 2023, 24, 7327. https://doi.org/10.3390/ijms24087327
Mocanu A, Bogos RA, Trandafir LM, Cojocaru E, Ioniuc I, Alecsa M, Lupu VV, Miron L, Lazaruc TI, Lupu A, et al. The Overlap of Kidney Failure in Extrapulmonary Sarcoidosis in Children—Case Report and Review of Literature. International Journal of Molecular Sciences. 2023; 24(8):7327. https://doi.org/10.3390/ijms24087327
Chicago/Turabian StyleMocanu, Adriana, Roxana Alexandra Bogos, Laura Mihaela Trandafir, Elena Cojocaru, Ileana Ioniuc, Mirabela Alecsa, Vasile Valeriu Lupu, Lucian Miron, Tudor Ilie Lazaruc, Ancuta Lupu, and et al. 2023. "The Overlap of Kidney Failure in Extrapulmonary Sarcoidosis in Children—Case Report and Review of Literature" International Journal of Molecular Sciences 24, no. 8: 7327. https://doi.org/10.3390/ijms24087327
APA StyleMocanu, A., Bogos, R. A., Trandafir, L. M., Cojocaru, E., Ioniuc, I., Alecsa, M., Lupu, V. V., Miron, L., Lazaruc, T. I., Lupu, A., Miron, I. C., & Starcea, I. M. (2023). The Overlap of Kidney Failure in Extrapulmonary Sarcoidosis in Children—Case Report and Review of Literature. International Journal of Molecular Sciences, 24(8), 7327. https://doi.org/10.3390/ijms24087327






