Anti-Skin Inflammatory and Anti-Oxidative Effects of the Neoflavonoid Latifolin Isolated from Dalbergia odorifera in HaCaT and BJ-5ta Cells
Abstract
1. Introduction
2. Results
2.1. Effects of Latifolin on Secretion of IL-6, IL-8, MDC, and RANTES in TNF-α/IFN-γ-Treated HaCaT Cells
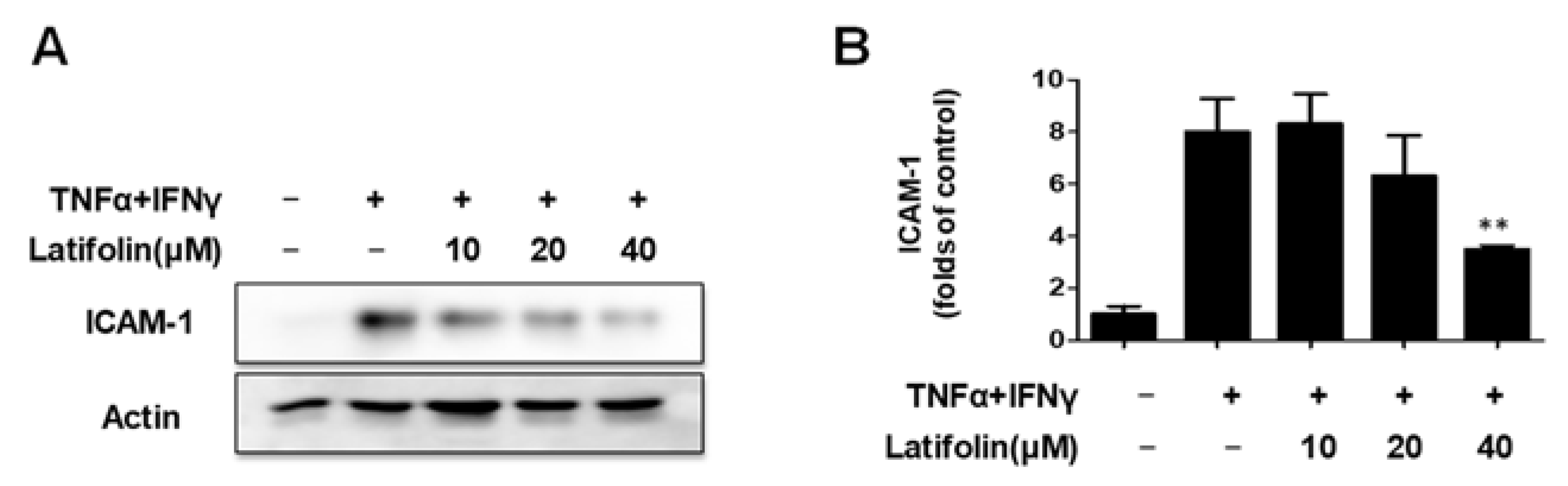
2.2. Effects of Latifolin on TNF-α/IFN-γ-Treated ICAM-1 Expression
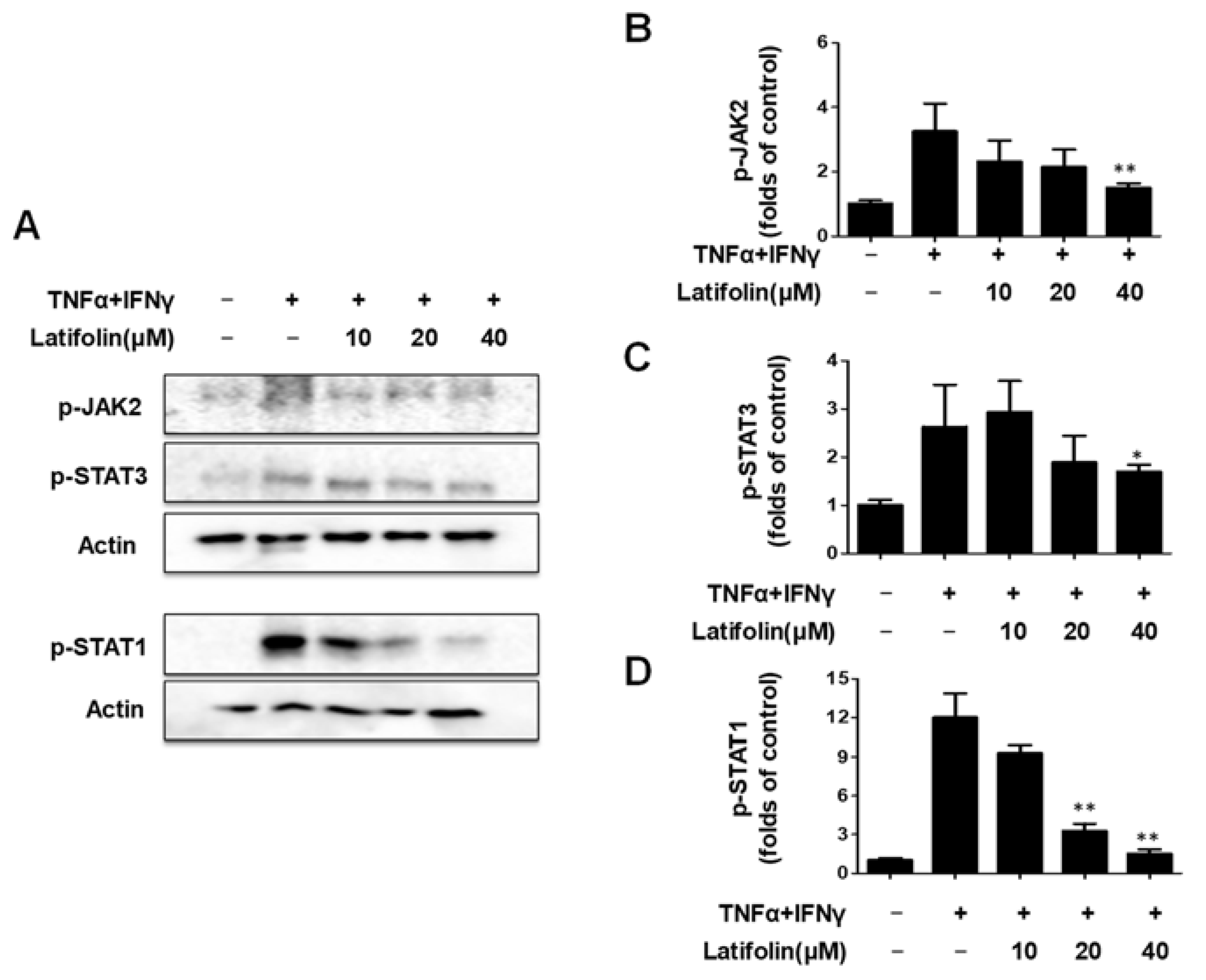
2.3. Effects of Latifolin on JAK2/STAT1(3) Pathway in HaCaT Cells
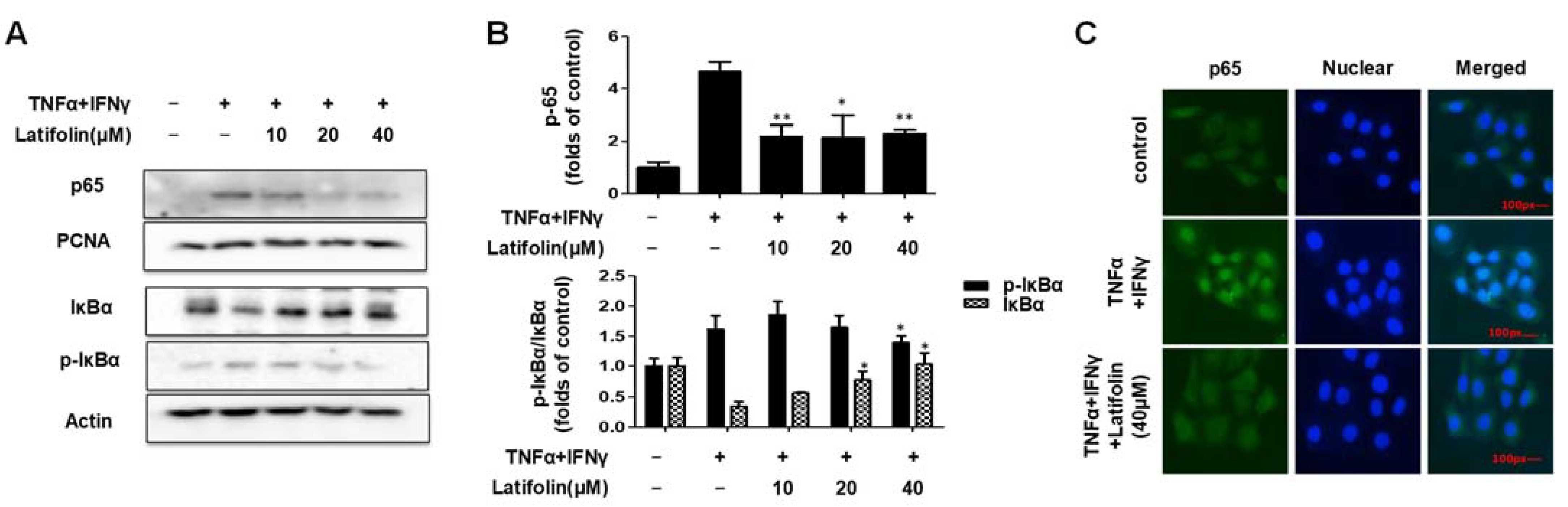
2.4. Effects of Latifolin on NF-κB Signaling Pathways in HaCaT Cells
2.5. Effects of Latifolin on Cell Viability in t-BHP-Induced BJ-5ta Cells
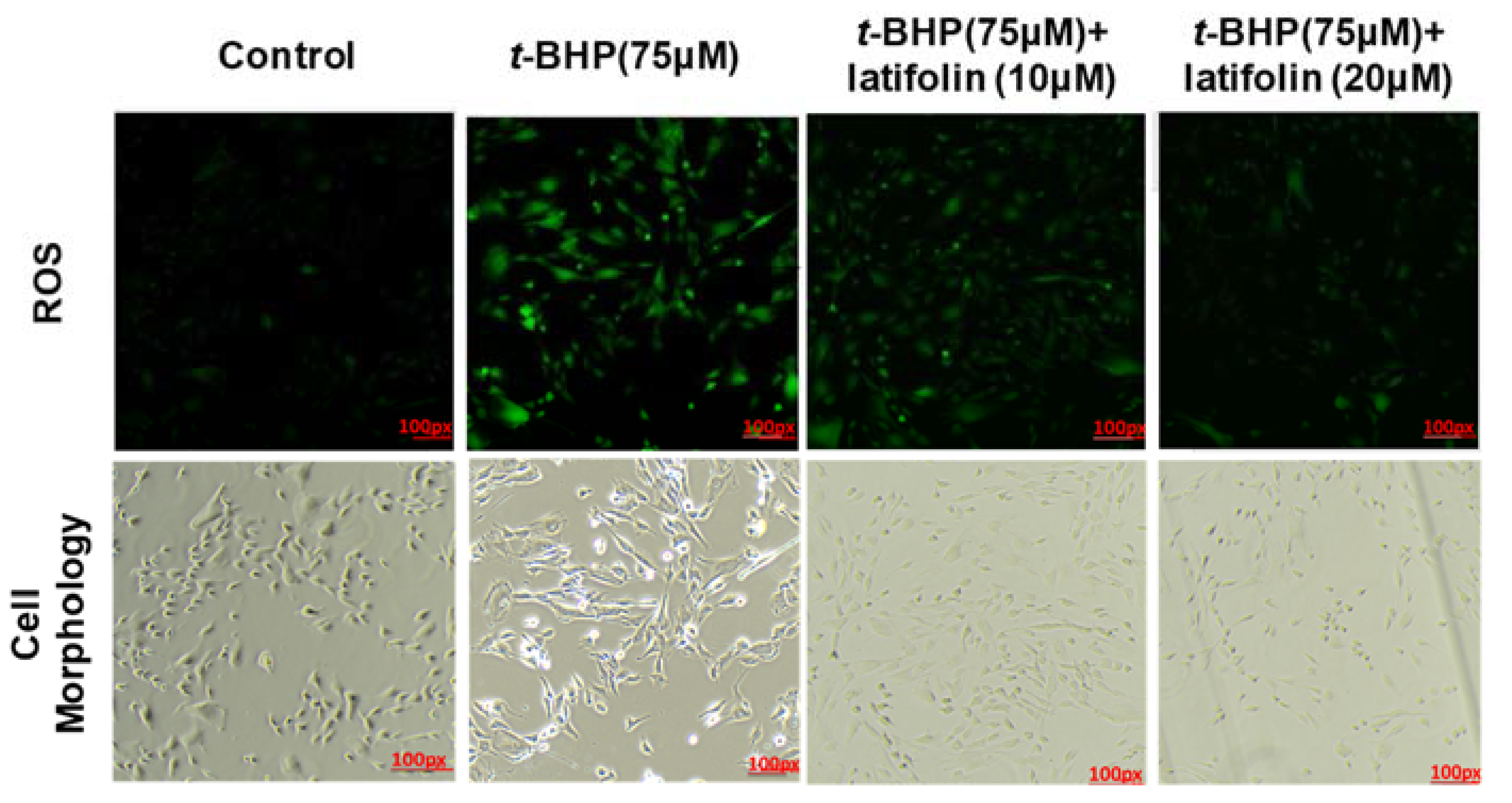
2.6. Effects of Latifolin on ROS Production Induced by t-BHP in BJ-5ta Cells
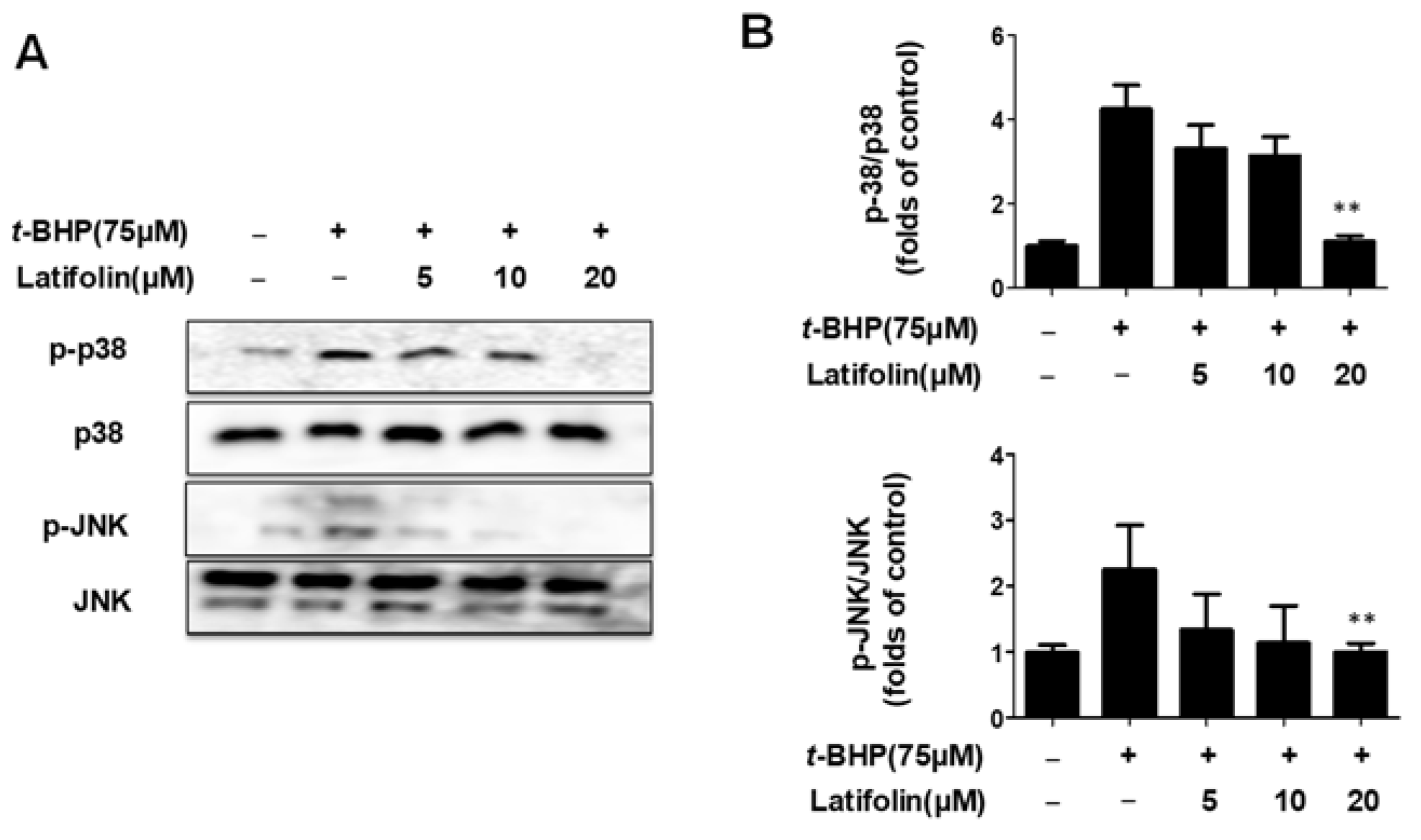
2.7. Effects of Latifolin on Mitogen-Activated Protein Kinase (MAPK) Signaling Pathways in BJ-5ta Cells
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Isolation of Latifolin
4.2. The Structure Identification of Latifolin
4.3. Cell Culture and Reagents
4.4. MTT Assay
4.5. IL-6, IL-8, MDC, and RANTES Detection in Cell Supernatant
4.6. Extraction of Total, Nuclear, and Cytosolic Protein
4.7. Western Blot Analysis
4.8. Immunofluorescence Analysis
4.9. ROS Staining
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, P.; Aral, M.; Kurutaș, E.B.; Kİreççİ, E.; Çelİk, M. Serum Levels of IL-8, Tnf-α and IL-6 in Children with Atopic Dermatitis. J. Curr. Pediatr. 2012, 10, 50–54. [Google Scholar]
- Chang, T.M.; Tsen, J.H.; Yen, H.; Yang, T.Y.; Huang, H.C. Extract from Periostracum cicadae inhibits oxidative stress and inflammation induced by ultraviolet B irradiation on HaCaT keratinocytes. Evid.-Based Complement. Altern. Med. ECAM 2017, 2017, 8325049. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Chan, P.M.; Kanagasabapathy, G.; Tan, Y.S.; Sabaratnam, V.; Kuppusamy, U.R. Amauroderma rugosum (Blume & T. Nees) Torrend: Nutritional composition and antioxidant and potential anti-inflammatory properties. Evid.-Based Complement. Alternat. Med. 2013, 2013, 304713. [Google Scholar]
- Hanson, K.M.; Clegg, R.M. Observation and Quantification of Ultraviolet-induced Reactive Oxygen Species in Ex Vivo Human Skin. Photochem. Photobiol. 2002, 76, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.W.; Kupper T, S. T cells and the skin: From protective immunity to inflammatory skin disorders. Nat. Rev. Immunol. 2019, 19, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Albanesi, C.; Scarponi, C.; Giustizieri, M.L.; Girolomoni, G. Keratinocytes in inflammatory skin diseases. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef]
- Mo, Q.; Fu, H.; Zhao, D.; Zhang, J.; Wang, C.; Wang, D.; Li, M. Protective Effects of Mogroside V on Oxidative Stress Induced by H2O2 in Skin Fibroblasts. Drug Des. Dev. Ther. 2021, 15, 4901–4909. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P. The antioxidant benefits of oral carotenoids for protecting the skin against photoaging. In Nutritional Cosmetics; William Andrew: Norwich, NY, USA, 2009; pp. 185–198. [Google Scholar]
- Pinnell, S.R. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J. Am. Acad. Dermatol. 2003, 48, 1–19. [Google Scholar] [CrossRef]
- Tabassum, N.; Hamdani, M. Plants used to treat skin diseases. Pharmacogn. Rev. 2014, 8, 52–60. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Ninh The, S. A Review on the Medicinal Plant Dalbergia odorifera Species: Phytochemistry and Biological Activity. Evid. Based Complement. Altern. Med. 2017, 2017, 7142370. [Google Scholar] [CrossRef]
- Lai, X.X.; Zhang, N.; Chen, L.Y. Latifolin protects against myocardial infarction by alleviating myocardial inflammatory via the HIF-1α/NF-κB/IL-6 pathway. Pharm. Biol. 2020, 58, 1156–1166. [Google Scholar] [CrossRef]
- Yun, H.M.; Park, J.E.; Lee, J.Y.; Park, K.R. Latifolin, a Natural Flavonoid, Isolated from the Heartwood of Dalbergia odorifera Induces Bioactivities through Apoptosis, Autophagy, and Necroptosis in Human Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 13629. [Google Scholar] [CrossRef]
- Lee, D.S.; Kim, K.S.; Ko, W.; Li, B.; Keo, S.; Jeong, G.S.; Kim, Y.C. The Neoflavonoid Latifolin Isolated from MeOH Extract of Dalbergia odorifera Attenuates Inflammatory Responses by Inhibiting NF-κB Activation via Nrf2-Mediated Heme Oxygenase-1 Expression. Phytother. Res. 2014, 288, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q. Inhibitory effects of isoliquiritin on an atopic dermatitis model through the CD177/JAK2/STAT pathway in vitro and in vivo. Ann. Transl. Med. 2022, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Gaynor, R.B. Role of the NF-kB pathway in the pathogenesis of human disease states. Curr. Mol. Med. 2001, 1, 287–296. [Google Scholar] [CrossRef]
- Kopp, E.B.; Ghosh, S. NF-kB and Rel proteins in innate immunity. Adv. Immunol. 1995, 58, 1–27. [Google Scholar] [PubMed]
- Tripathi, P.; Aggarwal, A. NF-kB transcription factor: A key player in the generation of immune response. Curr. Sci. 2006, 90, 519–531. [Google Scholar]
- Maiese, K.; Chong, Z.Z.; Hou, J.; Shang, Y.C. Oxidative stress: Biomarkers and novel therapeutic pathways. Exp. Gerontol. 2010, 45, 21–234. [Google Scholar] [CrossRef]
- Ki, Y.W.; Park, J.H.; Lee, J.E.; Shin, I.C.; Koh, H.C. JNK and p38 MAPK regulate oxidative stress and the inflammatory response in chlorpyrifos-induced apoptosis. Toxicol. Lett. 2013, 218, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Murata, S.; Kaneko, S.; Morita, E. Interleukin-8 Levels in the Stratum Corneum as a Biomarker for Monitoring Therapeutic Effect in Atopic Dermatitis Patients. Int. Arch. Allergy Immunol. 2021, 182, 592–606. [Google Scholar] [CrossRef]
- Albanesi, C.; Cavani, A.; Girolomoni, G. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: Synergistic or antagonist effects with IFN-gamma and TNF-alpha. J. Immunol. 1999, 162, 494–502. [Google Scholar] [CrossRef]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The JAK/STAT Signaling Pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Liu, Z.; Dong, L.; Cheong, S.H.; Lee, D.S. Lycopus maackianus Makino MeOH Extract Exhibits Antioxidant and Anti-Neuroinflammatory Effects in Neuronal Cells and Zebrafish Model. Antioxidants 2022, 11, 690. [Google Scholar] [CrossRef]
- Ko, W.; Lee, H.; Kim, N.; Jo, H.G.; Woo, E.R.; Lee, K.; Han, Y.S.; Park, S.R.; Ahn, G.; Cheong, S.H.; et al. The Anti-Oxidative and Anti-Neuroinflammatory Effects of Sargassum horneri by Heme Oxygenase-1 Induction in BV2 and HT22 Cells. Antioxidants 2021, 10, 859. [Google Scholar] [CrossRef]
- Annibaldi, A.; Meier, P. Checkpoints in TNF-Induced Cell Death: Implications in Inflammation and Cancer. Trends Mol. Med. 2018, 24, 49–65. [Google Scholar] [CrossRef]
- Watt, F.M.; Fujiwara, H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb Perspect Biol. 2011, 34, a005124. [Google Scholar] [CrossRef]
- Xu, W.; Jia, S.; Xie, P.; Zhong, A.; Galiano, R.D.; Mustoe, T.A.; Hong, S.J. The expression of proinflammatory genes in epidermal keratinocytes is regulated by hydration status. J. Investig. Dermatol. 2014, 134, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Finley, J. Potential cell culture models for antioxidant research. J. Agric. Food Chem. 2005, 53, 4311–4314. [Google Scholar] [CrossRef] [PubMed]
- Rubinfeld, H.; Seger, R. The ERK Cascade: A Prototype of MAPK Signaling. Mol. Biotechnol. 2005, 31, 151–174. [Google Scholar] [CrossRef]
- Zaid, N.A.; Sekar, M.; Bonam, S.R.; Gan, S.H.; Lum, P.T.; Begum, M.Y.; Rani, N.N.I.M.; Vaijanathappa, J.; Wu, Y.S.; Subramaniyan, V. Promising Natural Products in New Drug Design, Development, and Therapy for Skin Disorders: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. Drug Des. Dev. Ther. 2022, 16, 23–66. [Google Scholar] [CrossRef]
- Welsch, K.; Holstein, J.; Laurence, A.; Ghoreschi, K. Targeting JAK/STAT signalling in inflammatory skin diseases with small molecule inhibitors. Eur. J. Immunol. 2017, 47, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Calautti, E.; Avalle, L.; Poli, V. Psoriasis: A STAT3-Centric View. Int. J. Mol. Sci. 2018, 19, 171. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Berlett, B.S. Reactive Oxygen-Mediated Protein Oxidation in Aging and Disease. Drug Metab. Rev. 1998, 30, 225–243. [Google Scholar] [CrossRef]
- Chen, C.C.; Chiang, A.N.; Liu, H.N.E. Gb-761 prevents ultraviolet B-induced photoaging via inactivation of mitogen-activated protein kinases and proinflammatory cytokine expression. J. Dermatol. Sci. 2014, 75, 55–62. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Lee, H.; Liu, Z.; Lee, D.-S. Anti-Skin Inflammatory and Anti-Oxidative Effects of the Neoflavonoid Latifolin Isolated from Dalbergia odorifera in HaCaT and BJ-5ta Cells. Int. J. Mol. Sci. 2023, 24, 7371. https://doi.org/10.3390/ijms24087371
Dong L, Lee H, Liu Z, Lee D-S. Anti-Skin Inflammatory and Anti-Oxidative Effects of the Neoflavonoid Latifolin Isolated from Dalbergia odorifera in HaCaT and BJ-5ta Cells. International Journal of Molecular Sciences. 2023; 24(8):7371. https://doi.org/10.3390/ijms24087371
Chicago/Turabian StyleDong, Linsha, Hwan Lee, Zhiming Liu, and Dong-Sung Lee. 2023. "Anti-Skin Inflammatory and Anti-Oxidative Effects of the Neoflavonoid Latifolin Isolated from Dalbergia odorifera in HaCaT and BJ-5ta Cells" International Journal of Molecular Sciences 24, no. 8: 7371. https://doi.org/10.3390/ijms24087371
APA StyleDong, L., Lee, H., Liu, Z., & Lee, D.-S. (2023). Anti-Skin Inflammatory and Anti-Oxidative Effects of the Neoflavonoid Latifolin Isolated from Dalbergia odorifera in HaCaT and BJ-5ta Cells. International Journal of Molecular Sciences, 24(8), 7371. https://doi.org/10.3390/ijms24087371





