Polystyrene Microplastics Induce Oxidative Stress in Mouse Hepatocytes in Relation to Their Size
Abstract
1. Introduction
2. Results
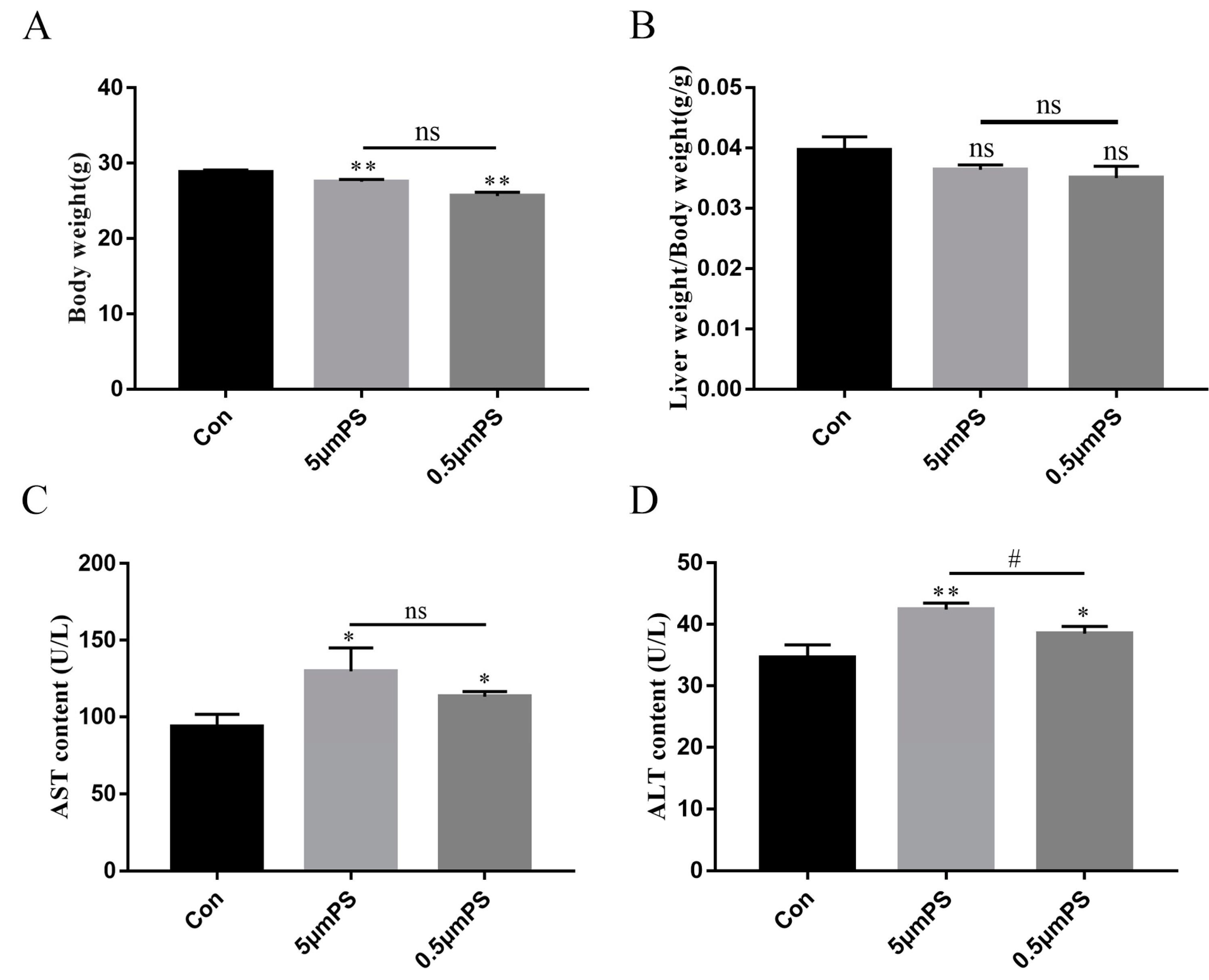
2.1. Effect of PS-MPs Exposure on Body Weight and Liver Coefficients
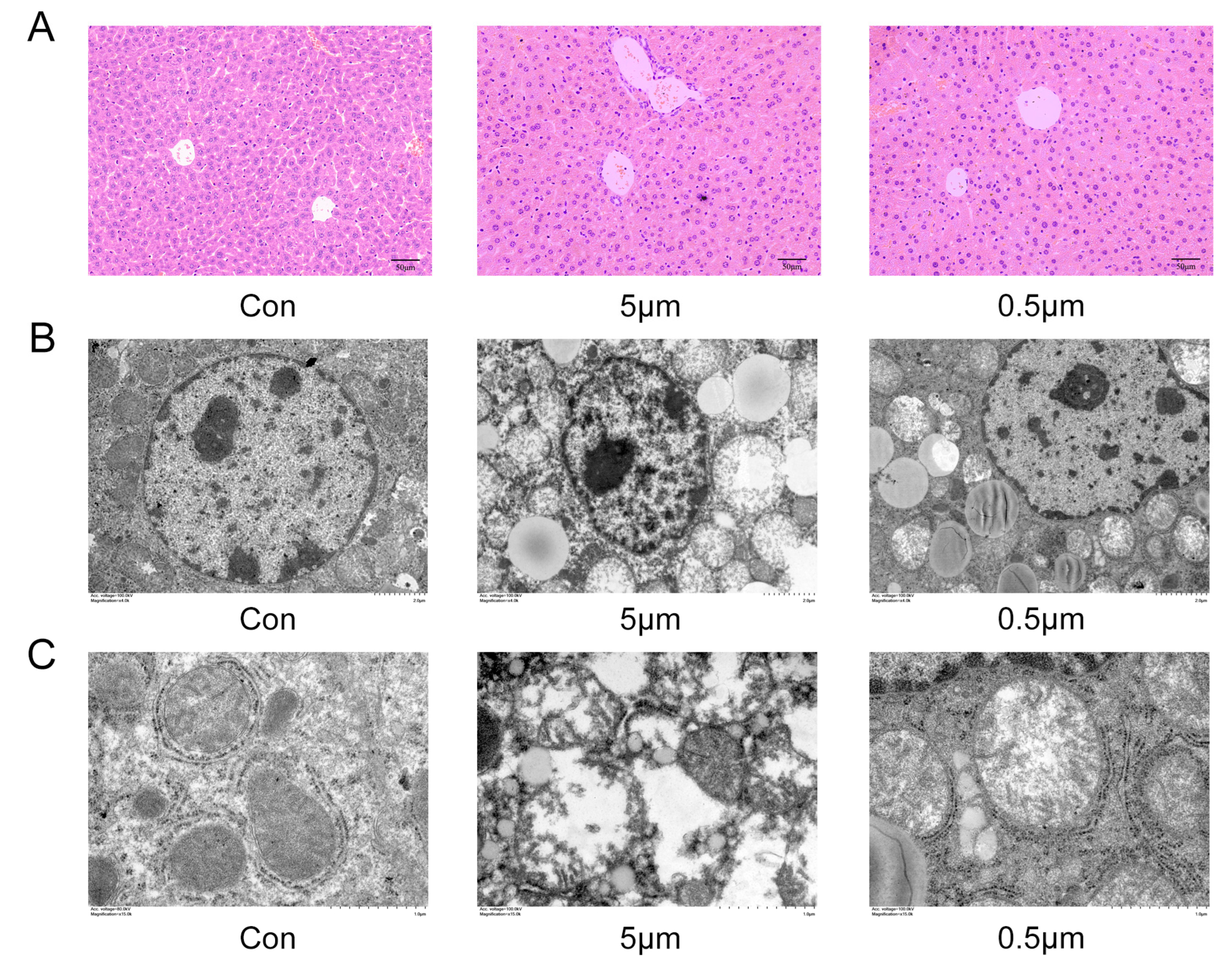
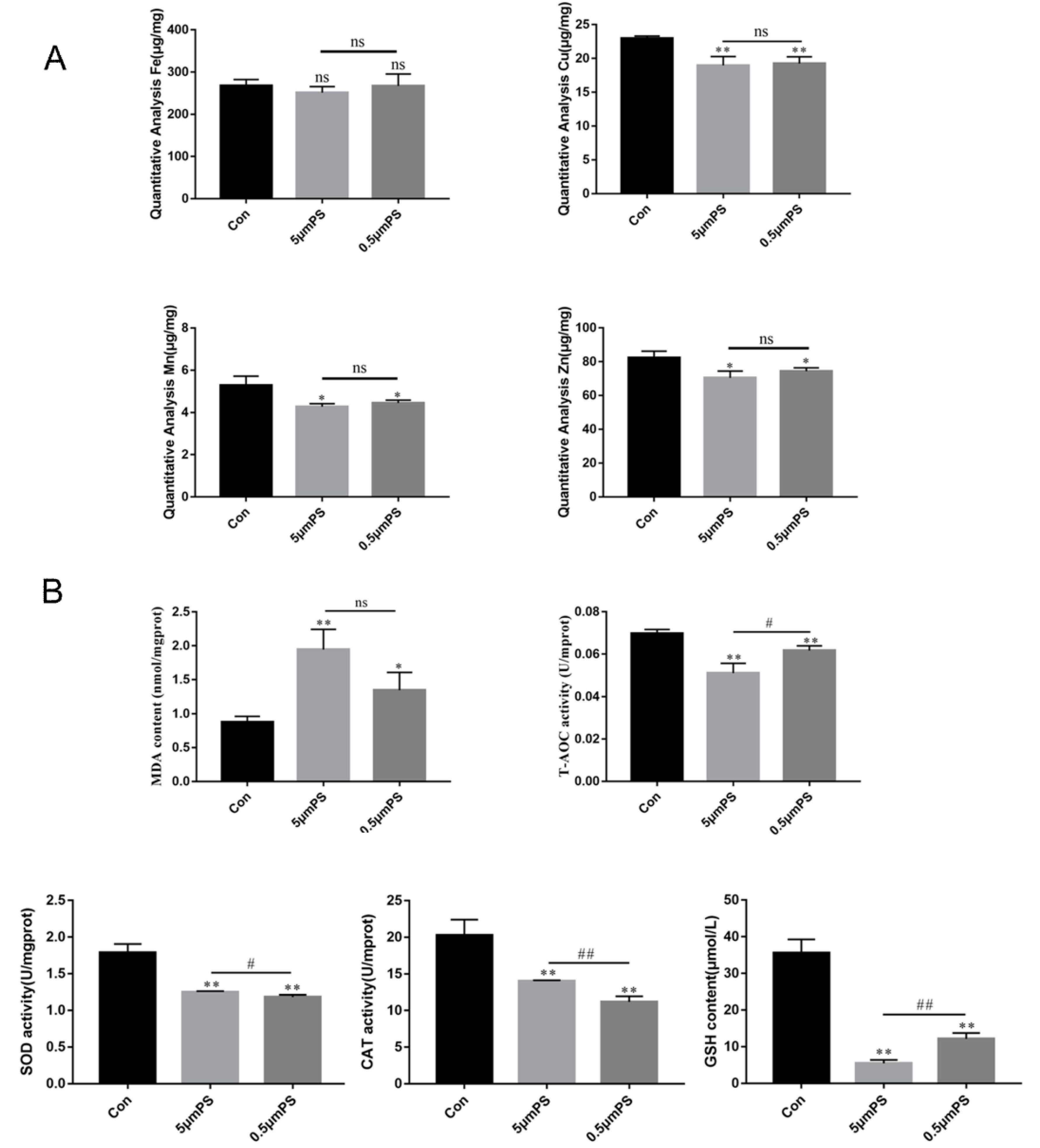
2.2. PS-MP-Induced Liver Injury
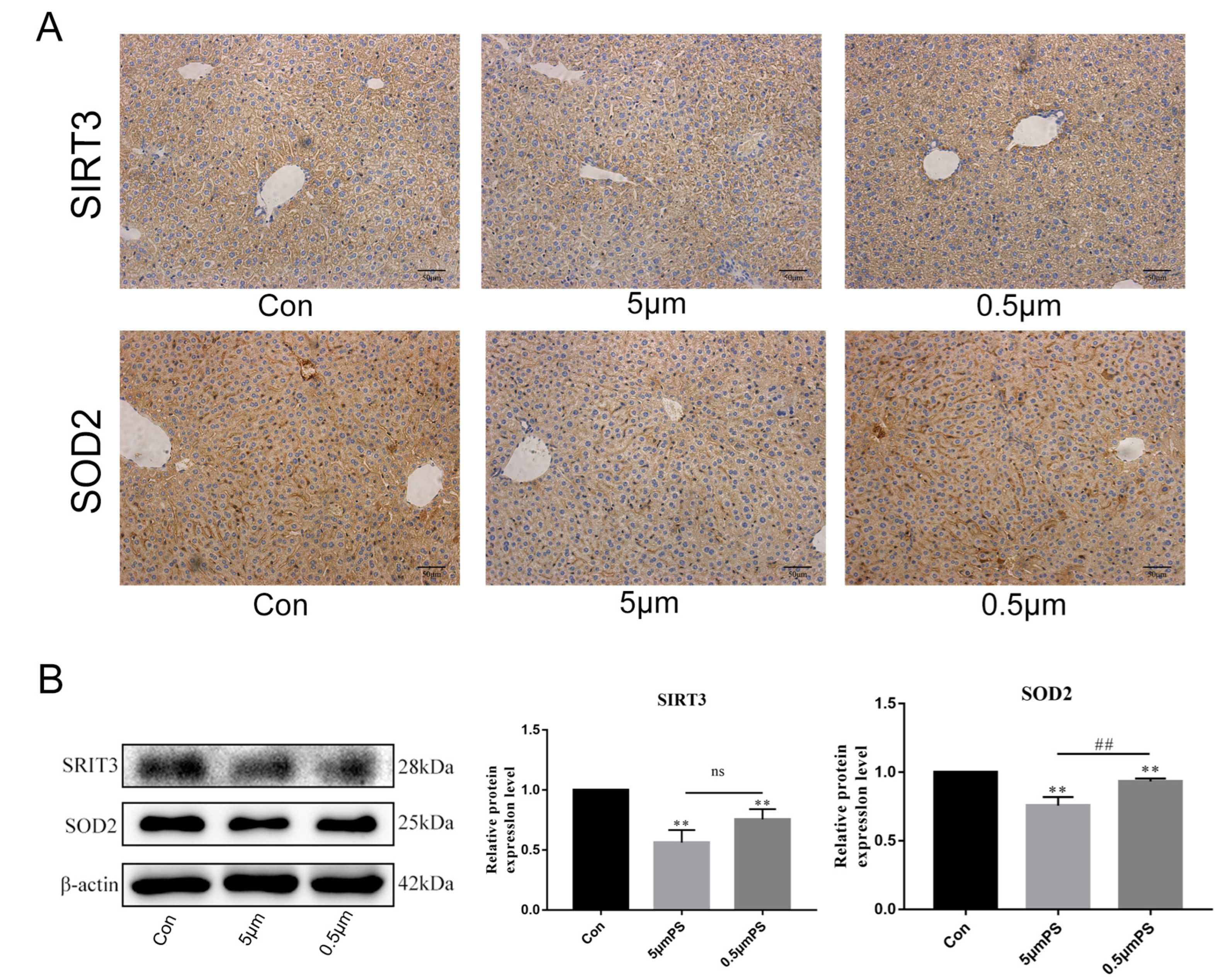
2.3. Effect of PS-MPs Exposure on Oxidative-Stress-Related Proteins SIRT3 and SOD2
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment
4.2. Hematoxylin and Eosin (H&E) and Immunohistological Staining
4.3. Transmission Electron Microscopy (TEM)
4.4. Detection of Oxidative Stress-Related Indexes
4.5. Determination of Trace Elements by Atomic Absorption Spectrometry
4.6. Western Blotting
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T.; et al. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef] [PubMed]
- Mężyńska, M.; Brzóska, M.M.; Rogalska, J.; Piłat-Marcinkiewicz, B. Extract from Aronia melanocarpa L. Berries Prevents Cadmium-Induced Oxidative Stress in the Liver: A Study in A Rat Model of Low-Level and Moderate Lifetime Human Exposure to this Toxic Metal. Nutrients 2018, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.; Elangovan, P.; Pari, L. Protective Role of Tetrahydrocurcumin: An Active Polyphenolic Curcuminoid on Cadmium-InducedOxidative Damage in Rats. Appl. Biochem. Biotechnol. 2017, 183, 51–69. [Google Scholar] [CrossRef]
- Zheng, W.; Song, Z.; Li, S.; Hu, M.; Shaukat, H.; Qin, H. Protective Effects of Sesamol against Liver Oxidative Stress and Inflammation in High-Fat Diet-Induced Hepatic Steatosis. Nutrients 2021, 13, 4484. [Google Scholar] [CrossRef] [PubMed]
- Sadasivam, N.; Kim, Y.J.; Radhakrishnan, K.; Kim, D.K. Oxidative Stress, Genomic Integrity, and Liver Diseases. Molecules 2022, 27, 3159. [Google Scholar] [CrossRef]
- Zhao, C.; Liang, W.; Yang, Z.; Chen, Z.; Du, Z.; Gong, S. SIRT3-mediated deacetylation protects inner hair cell synapses in a H2O2-induced oxidative stress model in vitro. Exp. Cell Res. 2022, 418, 113280. [Google Scholar] [CrossRef]
- Sun, J.; Yu, F.; Wang, T.; Bian, J.; Liu, Z.; Zou, H. The role of DRP1- PINK1-Parkin-mediated mitophagy in early cadmium-induced liver damage. Toxicology 2022, 466, 153082. [Google Scholar] [CrossRef]
- Li, J.; Xian, L.; Zheng, R.; Wang, Y.; Wan, X.; Liu, Y. Corrigendum to “Canthaxanthin shows anti-liver aging and anti-liver fibrosis effects by down-regulating inflammation and oxidative stress in vivo and in vitro” [Int. Immunopharmacol. 110 (2022) 108942]. Int. Immunopharmacol. 2022, 112, 109189. [Google Scholar] [CrossRef]
- Lewandowski, Ł.; Kepinska, M.; Milnerowicz, H. Inhibition of copper-zinc superoxide dismutase activity by selected environmental xenobiotics. Environ. Toxicol. Pharmacol. 2018, 58, 105–113. [Google Scholar] [CrossRef]
- Holley, A.K.; Dhar, S.K.; Xu, Y.; St Clair, D.K. Manganese superoxide dismutase: Beyond life and death. Amino Acids 2012, 42, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Dikalova, A.E.; Itani, H.A.; Nazarewicz, R.R.; McMaster, W.G.; Flynn, C.R.; Uzhachenko, R.; Fessel, J.P.; Gamboa, J.L.; Harrison, D.G.; Dikalov, S.I. Sirt3 Impairment and SOD2 Hyperacetylation in Vascular Oxidative Stress and Hypertension. Circ. Res. 2017, 121, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Winnik, S.; Auwerx, J.; Sinclair, D.A.; Matter, C.M. Protective effects of sirtuins in cardiovascular diseases: From bench to bedside. Eur. Heart J. 2015, 36, 3404–3412. [Google Scholar] [CrossRef]
- Cheng, W.; Li, X.; Zhou, Y.; Yu, H.; Xie, Y.; Guo, H.; Wang, H.; Li, Y.; Feng, Y.; Wang, Y. Polystyrene microplastics induce hepatotoxicity and disrupt lipid metabolism in the liver organoids. Sci. Total Environ. 2022, 806, 150328. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef]
- Qiang, L.; Cheng, J. Exposure to polystyrene microplastics impairs gonads of zebrafish (Danio rerio). Chemosphere 2021, 263, 128161. [Google Scholar] [CrossRef]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef]
- Chen, H.; Hua, X.; Li, H.; Wang, C.; Dang, Y.; Ding, P.; Yu, Y. Transgenerational neurotoxicity of polystyrene microplastics induced by oxidative stress in Caenorhabditis elegans. Chemosphere 2021, 272, 129642. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Liang, B.; Zhong, Y.; Huang, Y.; Lin, X.; Liu, J.; Lin, L.; Hu, M.; Jiang, J.; Dai, M.; Wang, B.; et al. Underestimated health risks: Polystyrene micro- and nanoplastics jointly induce intestinal barrier dysfunction by ROS-mediated epithelial cell apoptosis. Part. Fibre Toxicol. 2021, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Lee, Y.H.; Hsu, Y.H.; Chiu, I.J.; Huang, C.C.; Huang, C.C.; Chia, Z.C.; Lee, C.P.; Lin, Y.F.; Chiu, H.W. The Kidney-Related Effects of Polystyrene Microplastics on Human Kidney Proximal Tubular Epithelial Cells HK-2 and Male C57BL/6 Mice. Environ. Health Perspect. 2021, 129, 57003. [Google Scholar] [CrossRef] [PubMed]
- Goodman, K.E.; Hua, T.; Sang, Q.A. Effects of Polystyrene Microplastics on Human Kidney and Liver Cell Morphology, Cellular Proliferation, and Metabolism. ACS Omega 2022, 7, 34136–34153. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Chen, Q.; Li, J.; Li, B.; Liang, W.; Su, L.; Shi, H. Distribution and translocation of micro- and nanoplastics in fish. Crit. Rev. Toxicol. 2021, 51, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, J.A.; Choi, C.Y. Oxidative Stress and Apoptosis in Goldfish (Carassius auratus) Caused by Exposure to Different Concentrations of Micro-polystyrene. Ocean. Polar Res. 2021, 43, 141–148. [Google Scholar]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Xu, D.; Li, J.; Wang, Z.; Ding, Y.; Wang, X.; Li, X.; Xu, N.; Mai, K.; Ai, Q. Dietary polystyrene nanoplastics exposure alters liver lipid metabolism and muscle nutritional quality in carnivorous marine fish large yellow croaker (Larimichthys crocea). J. Hazard. Mater. 2021, 419, 126454. [Google Scholar] [CrossRef]
- Auguet, T.; Bertran, L.; Barrientos-Riosalido, A.; Fabregat, B.; Villar, B.; Aguilar, C.; Sabench, F. Are Ingested or Inhaled Microplastics Involved in Nonalcoholic Fatty Liver Disease? Int. J. Environ. Res. Public Health 2022, 19, 13495. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Wang, F.; Liu, T.; Wang, Z. Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice. J. Hazard. Mater. 2021, 405, 124028. [Google Scholar] [CrossRef]
- Green, R.M.; Flamm, S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology 2002, 123, 1367–1384. [Google Scholar] [CrossRef]
- Mu, Y.; Sun, J.; Li, Z.; Zhang, W.; Liu, Z.; Li, C.; Peng, C.; Cui, G.; Shao, H.; Du, Z. Activation of pyroptosis and ferroptosis is involved in the hepatotoxicity induced by polystyrene microplastics in mice. Chemosphere 2022, 291, 132944. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Duan, X.; Feng, X.; Wang, T.; Wang, P.; Ding, M.; Wang, W.; Zhou, X.; Yao, W.; et al. The interaction effects of coke oven emissions exposure and metabolic enzyme Gene variants on total antioxidant capacity of workers. Environ. Toxicol. Pharmacol. 2019, 70, 103197. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Yu, H.; Peng, J.; Cao, X.; Wang, Y.; Zhang, Z.; Xu, Y.; Qi, W. Effects of microplastics and glyphosate on growth rate, morphological plasticity, photosynthesis, and oxidative stress in the aquatic species Salvinia cucullata. Environ. Pollut. 2021, 279, 116900. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, C.; Zhou, J.; Shen, M.; Wang, X.; Fu, Z.; Jin, Y. Effects of polystyrene microplastics on the composition of the microbiome and metabolism in larval zebrafish. Chemosphere 2019, 217, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jian, S.; Zhang, S.; Wu, D.; Wang, J.; Gao, M.; Sheng, J.; Hong, Y. Enrichment of polystyrene microplastics induces histological damage, oxidative stress, Keap1-Nrf2 signaling pathway-related gene expression in loach juveniles (Paramisgurnus dabryanus). Ecotoxicol. Environ. Saf. 2022, 237, 113540. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, J.; Wang, W.; Gonzalez-Gil, G.; Vrouwenvelder, J.S.; Li, Z. Effects of nano- and microplastics on kidney: Physicochemical properties, bioaccumulation, oxidative stress and immunoreaction. Chemosphere 2022, 288, 132631. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Huang, H. Effect of Microplastics on Antioxidant Enzyme System in Juvenile Red Crucian Carp. Environ. Sci. Technol. 2019, 42, 23–27. [Google Scholar] [CrossRef]
- Prokic, M.D.; Radovanovic, T.B.; Gavric, J.P.; Faggio, C. Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. Trac-Trends Anal. Chem. 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Morigi, M.; Perico, L.; Benigni, A. Sirtuins in Renal Health and Disease. J. Am. Soc. Nephrol. 2018, 29, 1799–1809. [Google Scholar] [CrossRef]
- Tao, R.; Coleman, M.C.; Pennington, J.D.; Ozden, O.; Park, S.H.; Jiang, H.; Kim, H.S.; Flynn, C.R.; Hill, S.; Hayes McDonald, W.; et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol. Cell 2010, 40, 893–904. [Google Scholar] [CrossRef]
- Gao, J.; Feng, Z.; Wang, X.; Zeng, M.; Liu, J.; Han, S.; Xu, J.; Chen, L.; Cao, K.; Long, J.; et al. SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Differ. 2018, 25, 229–240. [Google Scholar] [CrossRef]
- Jin, Z.H.; Wang, S.F.; Liao, W. Zoledronic acid accelerates osteogenesis of bone marrow mesenchymal stem cells by attenuating oxidative stress via the SIRT3/SOD2 pathway and thus alleviates osteoporosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2095–2101. [Google Scholar] [CrossRef]
- Pi, H.; Xu, S.; Reiter, R.J.; Guo, P.; Zhang, L.; Li, Y.; Li, M.; Cao, Z.; Tian, L.; Xie, J.; et al. SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy 2015, 11, 1037–1051. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, X.; Shao, X.; Wang, H.; Liu, X.; Ke, X.; Xiong, C.; Wei, L.; Zou, H. tert-Butylhydroquinone Treatment Alleviates Contrast-Induced Nephropathy in Rats by Activating the Nrf2/Sirt3/SOD2 Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 4657651. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, Y.; Shen, J.; Yu, B.; Bai, J.; Lin, J.; Guo, X.; Sun, H.; Chen, Z.; Yang, H.; et al. Melatonin Increases Bone Mass around the Prostheses of OVX Rats by Ameliorating Mitochondrial Oxidative Stress via the SIRT3/SOD2 Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 4019619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Ge, X.; Zhang, F. Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid hemorrhage mice. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 109, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Yang, Y.; Zhou, Y.; Maharana, C.; Lu, D.; Peng, W.; Liu, Y.; Wan, R.; Marosi, K.; Misiak, M.; et al. Mitochondrial SIRT3 Mediates Adaptive Responses of Neurons to Exercise and Metabolic and Excitatory Challenges. Cell Metab. 2016, 23, 128–142. [Google Scholar] [CrossRef]
- Tao, R.; Vassilopoulos, A.; Parisiadou, L.; Yan, Y.; Gius, D. Regulation of MnSOD enzymatic activity by Sirt3 connects the mitochondrial acetylome signaling networks to aging and carcinogenesis. Antioxid. Redox Signal. 2014, 20, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Shengchen, W.; Jing, L.; Yujie, Y.; Yue, W.; Shiwen, X. Polystyrene microplastics-induced ROS overproduction disrupts the skeletal muscle regeneration by converting myoblasts into adipocytes. J. Hazard. Mater. 2021, 417, 125962. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, H.; Qu, H.; Bian, Y.; Sun, J.; Wang, T.; Ma, Y.; Yuan, Y.; Gu, J.; Bian, J.; Liu, Z. Polystyrene Microplastics Induce Oxidative Stress in Mouse Hepatocytes in Relation to Their Size. Int. J. Mol. Sci. 2023, 24, 7382. https://doi.org/10.3390/ijms24087382
Zou H, Qu H, Bian Y, Sun J, Wang T, Ma Y, Yuan Y, Gu J, Bian J, Liu Z. Polystyrene Microplastics Induce Oxidative Stress in Mouse Hepatocytes in Relation to Their Size. International Journal of Molecular Sciences. 2023; 24(8):7382. https://doi.org/10.3390/ijms24087382
Chicago/Turabian StyleZou, Hui, Huayi Qu, Yusheng Bian, Jian Sun, Tao Wang, Yonggang Ma, Yan Yuan, Jianhong Gu, Jianchun Bian, and Zongping Liu. 2023. "Polystyrene Microplastics Induce Oxidative Stress in Mouse Hepatocytes in Relation to Their Size" International Journal of Molecular Sciences 24, no. 8: 7382. https://doi.org/10.3390/ijms24087382
APA StyleZou, H., Qu, H., Bian, Y., Sun, J., Wang, T., Ma, Y., Yuan, Y., Gu, J., Bian, J., & Liu, Z. (2023). Polystyrene Microplastics Induce Oxidative Stress in Mouse Hepatocytes in Relation to Their Size. International Journal of Molecular Sciences, 24(8), 7382. https://doi.org/10.3390/ijms24087382








