Recovery of W(VI) from Wolframite Ore Using New Synthetic Schiff Base Derivative
Abstract
1. Introduction
2. Result and Discussions
2.1. Characterizations of the Sorbent
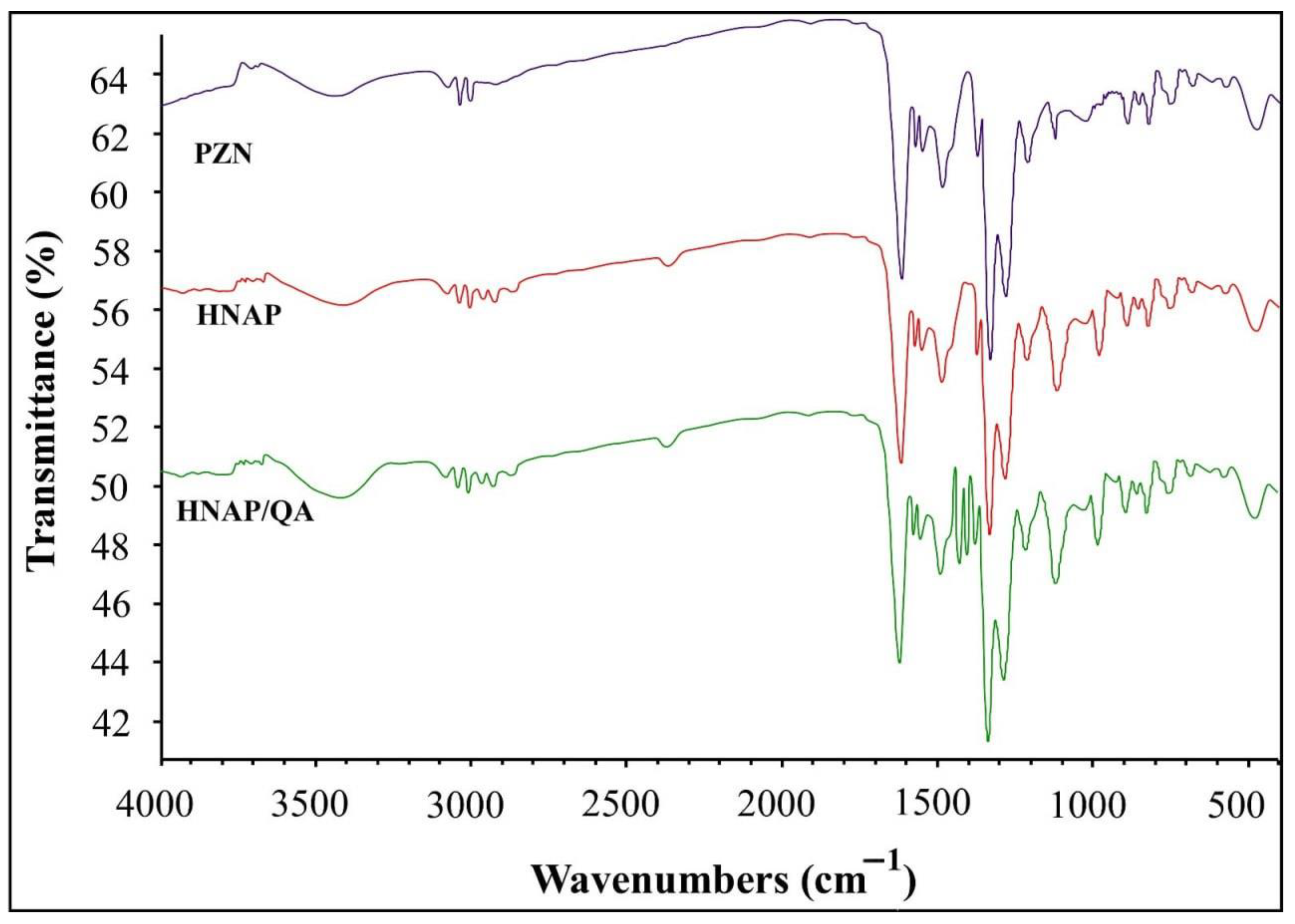
2.1.1. FTIR Analysis
2.1.2. BET Surface Analysis
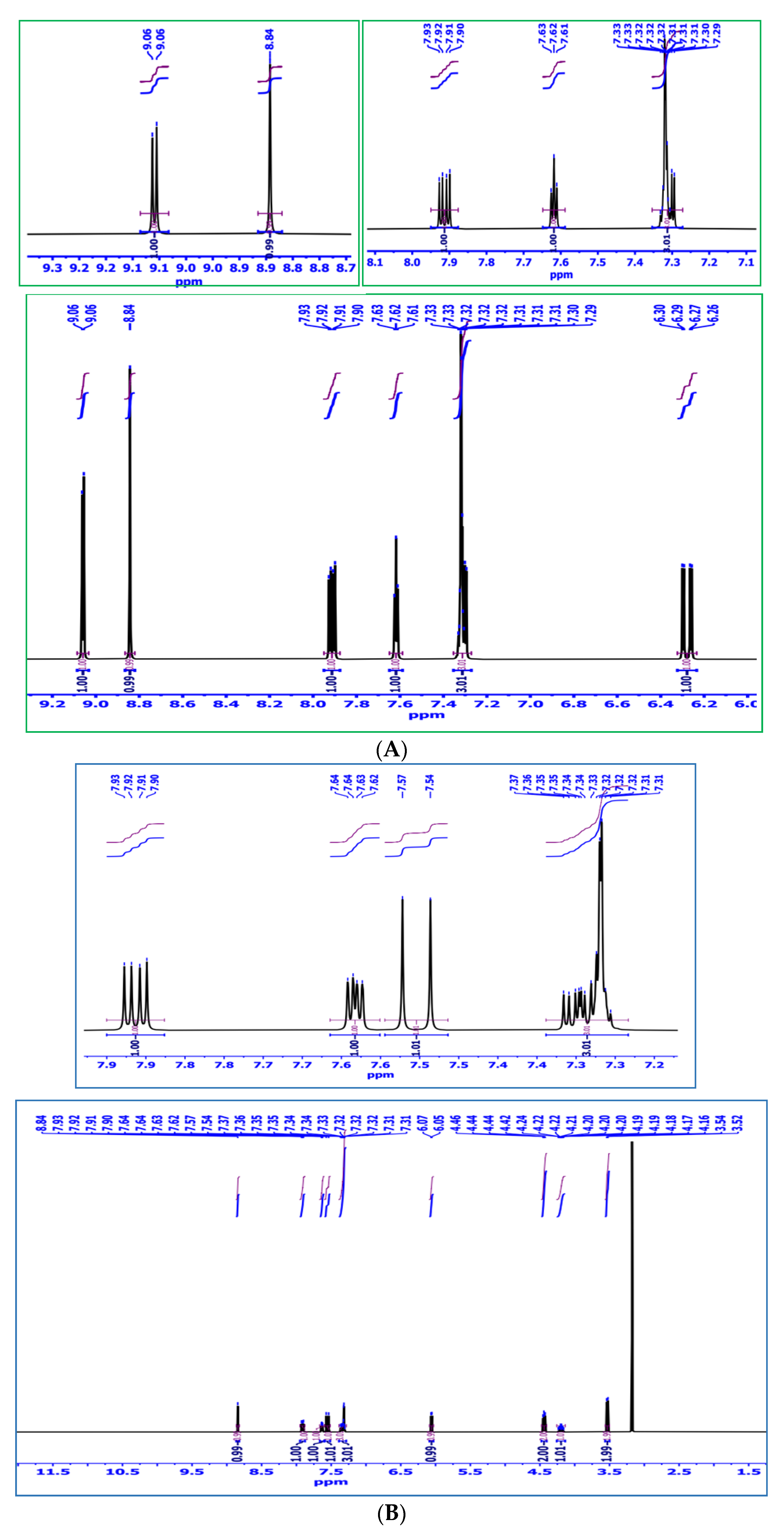
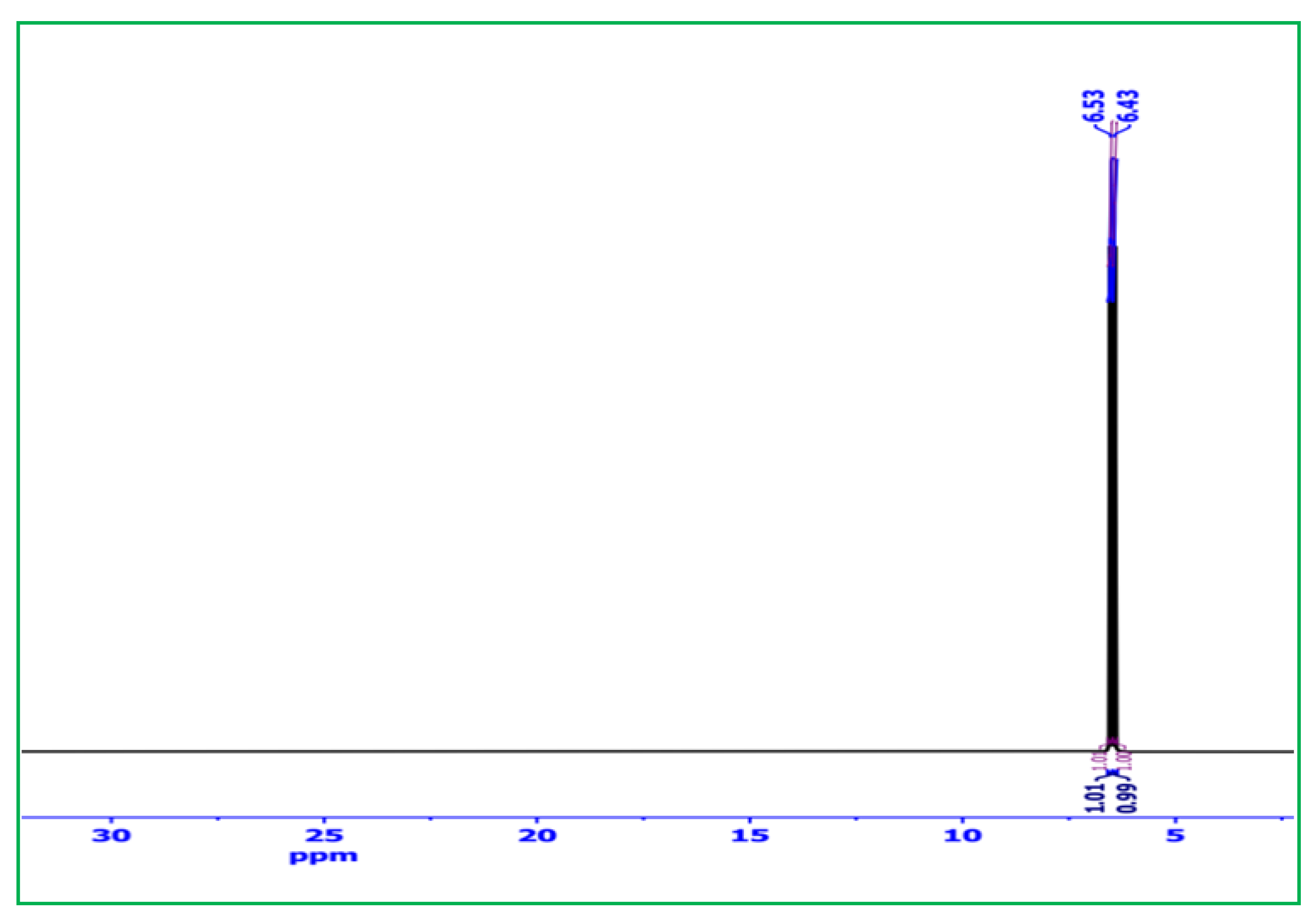
2.1.3. 1H-NMR Analysis
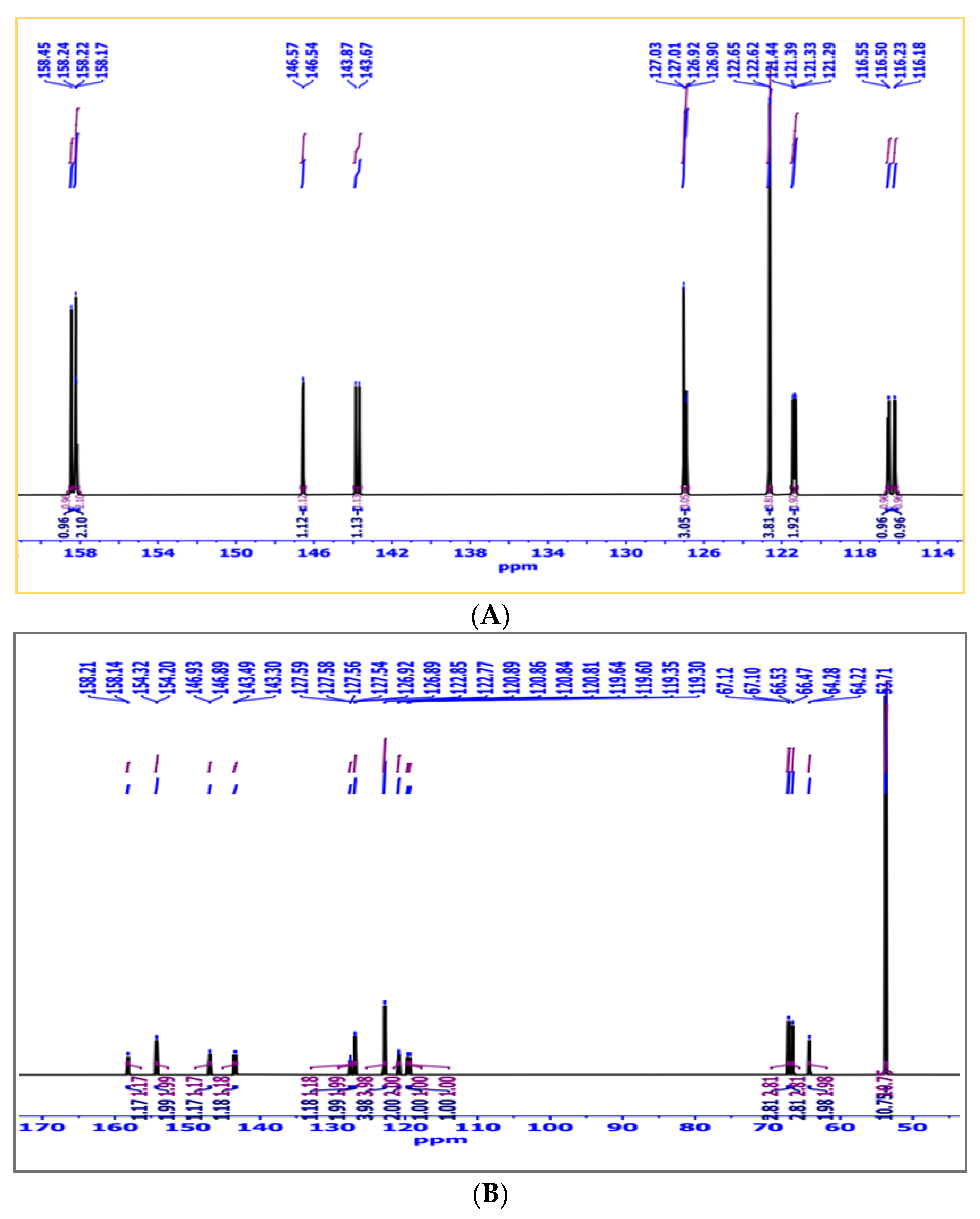
2.1.4. 13C-NMR Analysis
2.1.5. 31P-NMR Analysis
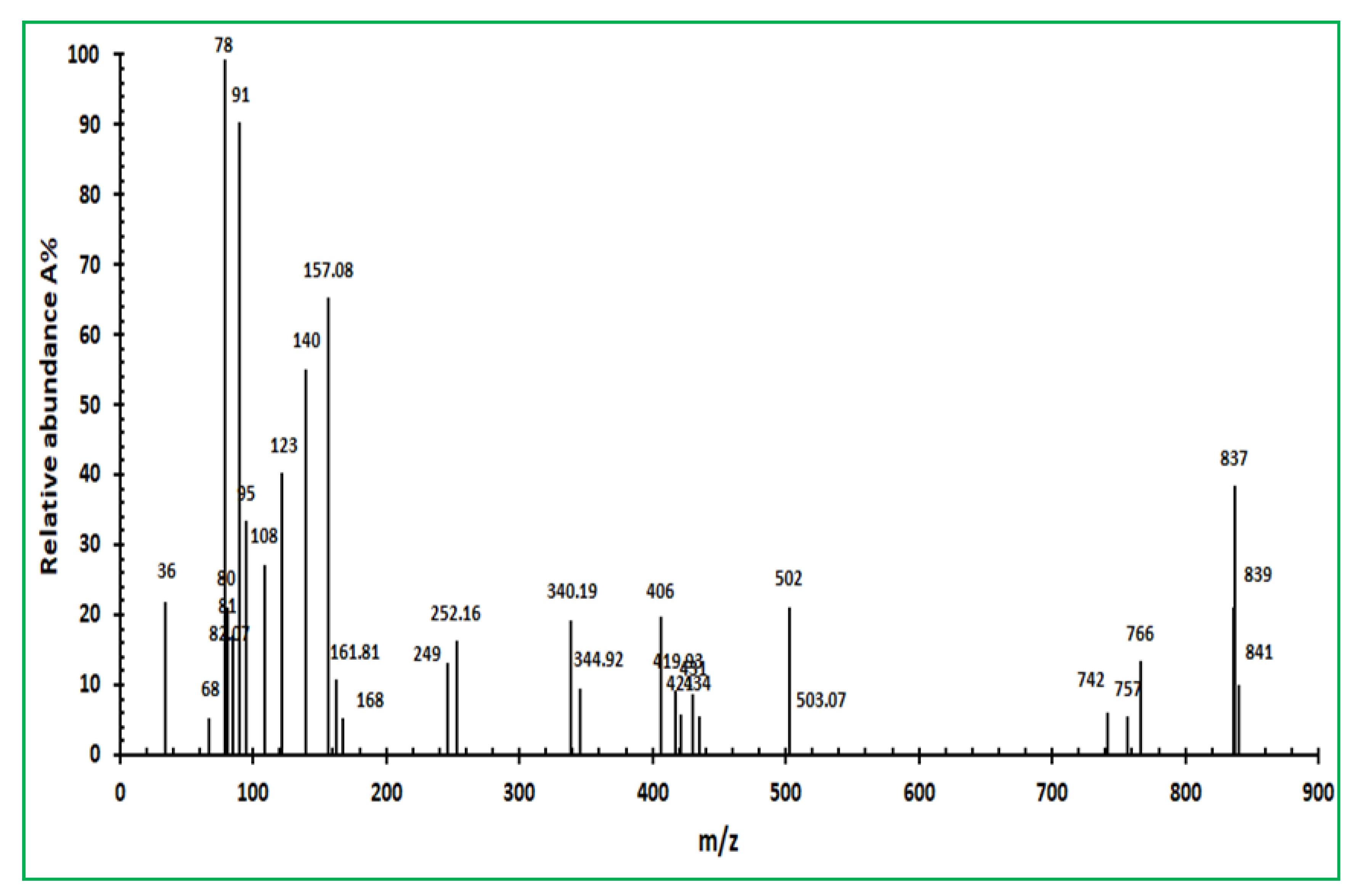
2.1.6. GC-MS Analysis
2.2. Sorption of W(VI) Ions
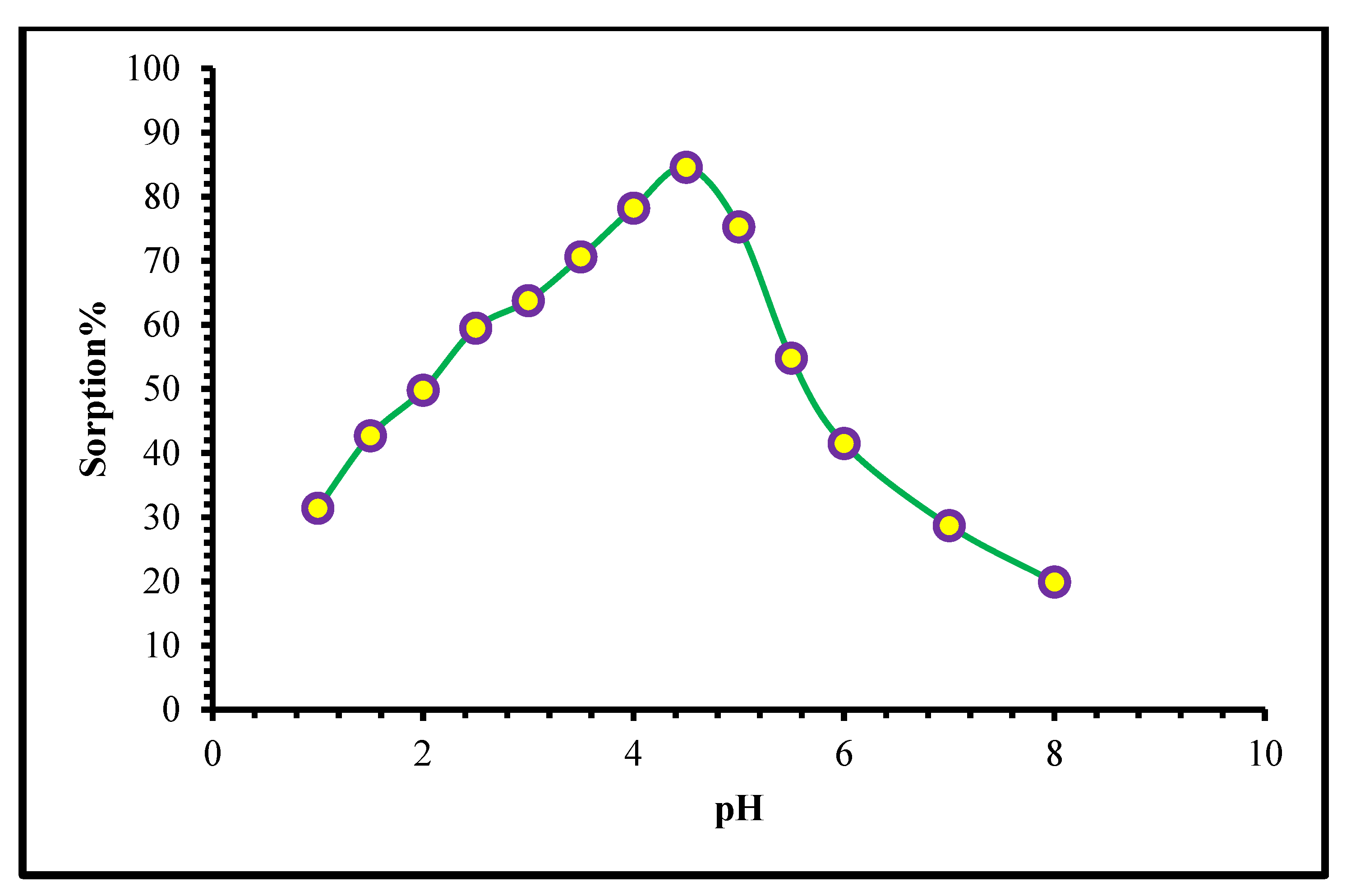
2.2.1. Influence of pH on W(VI) Ion Sorption
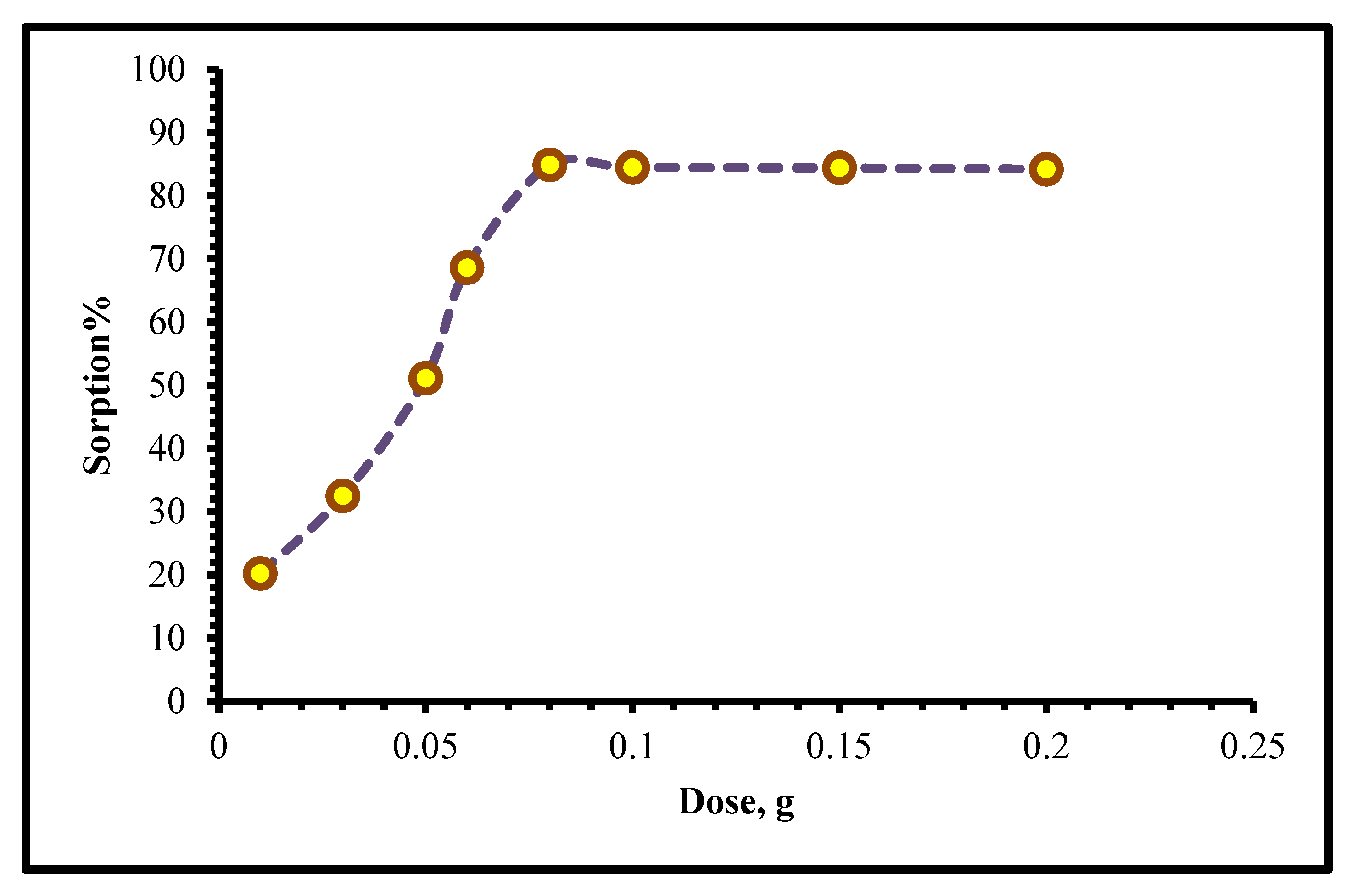
2.2.2. Effect of HNAP/QA Dose
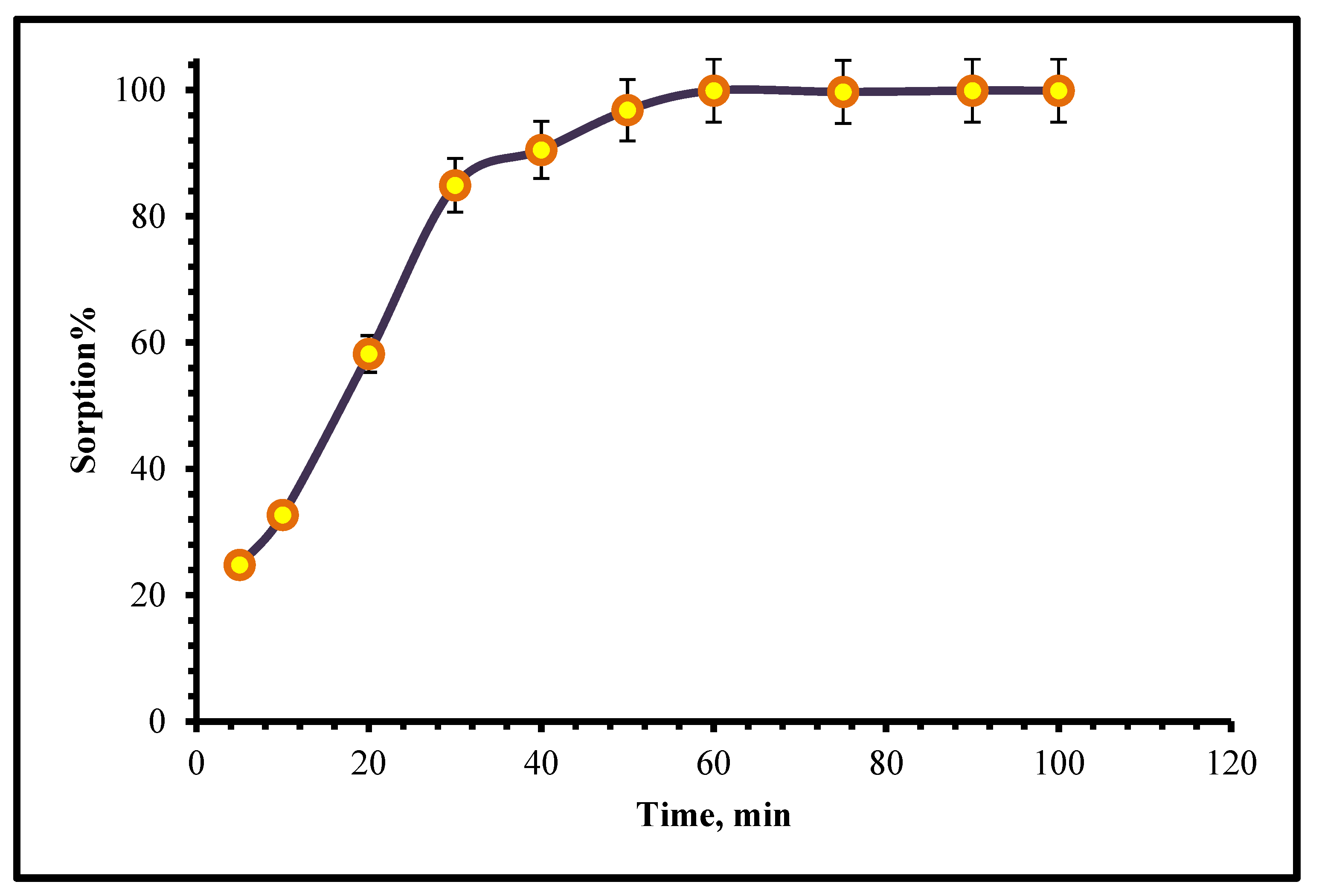
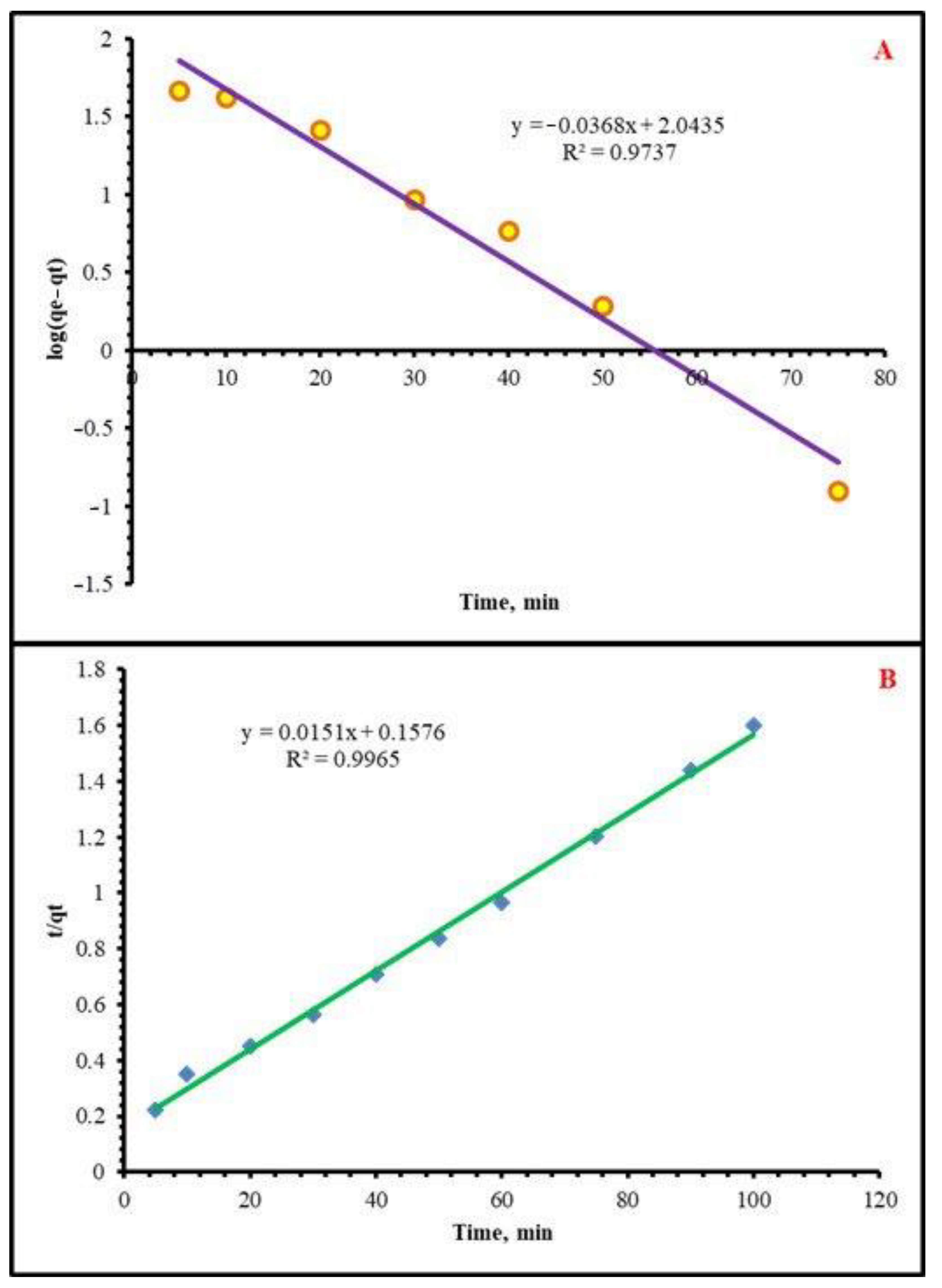
2.2.3. Kinetics Study
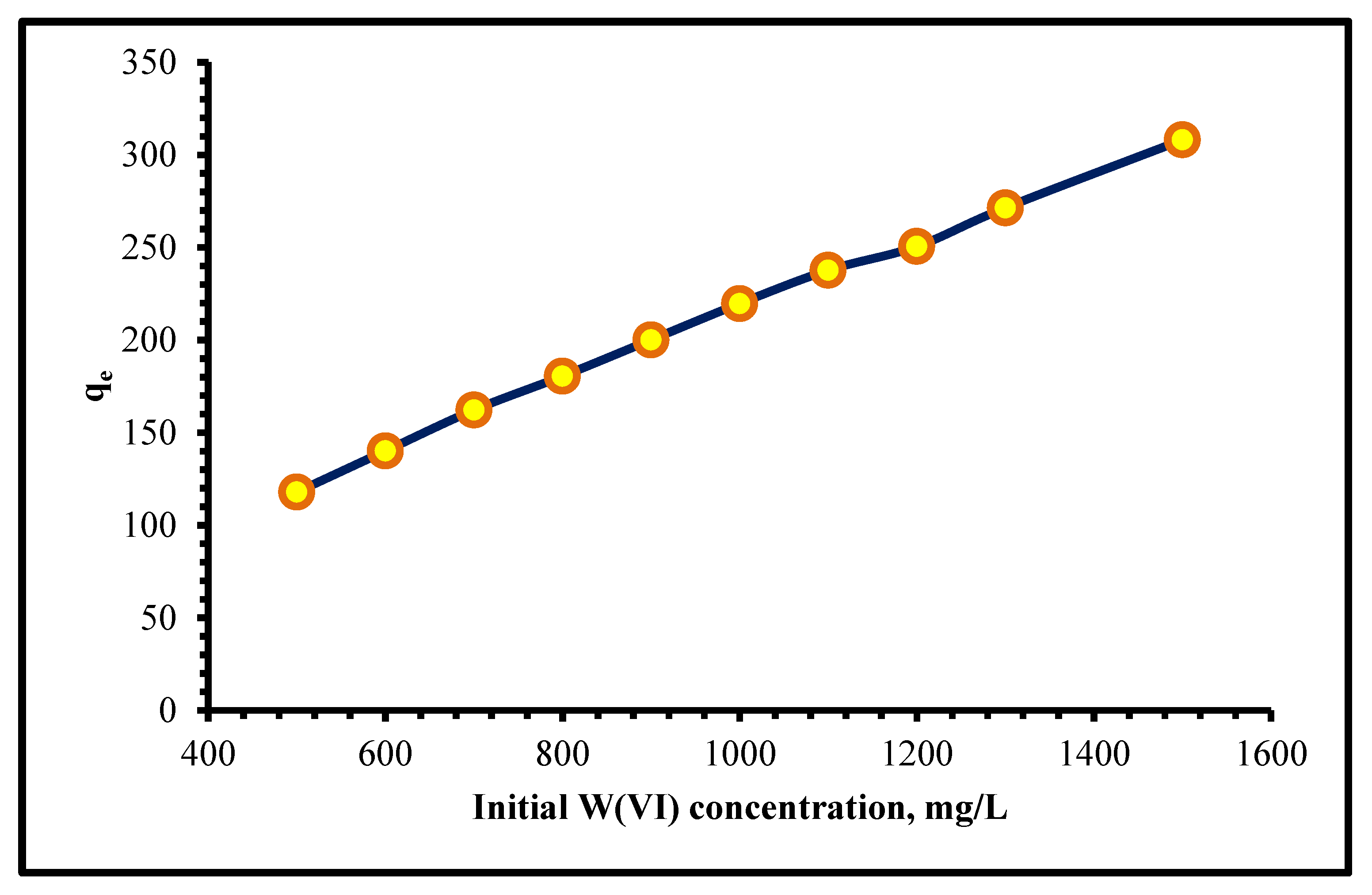
2.2.4. Effect of the Initial W(VI) Concentration
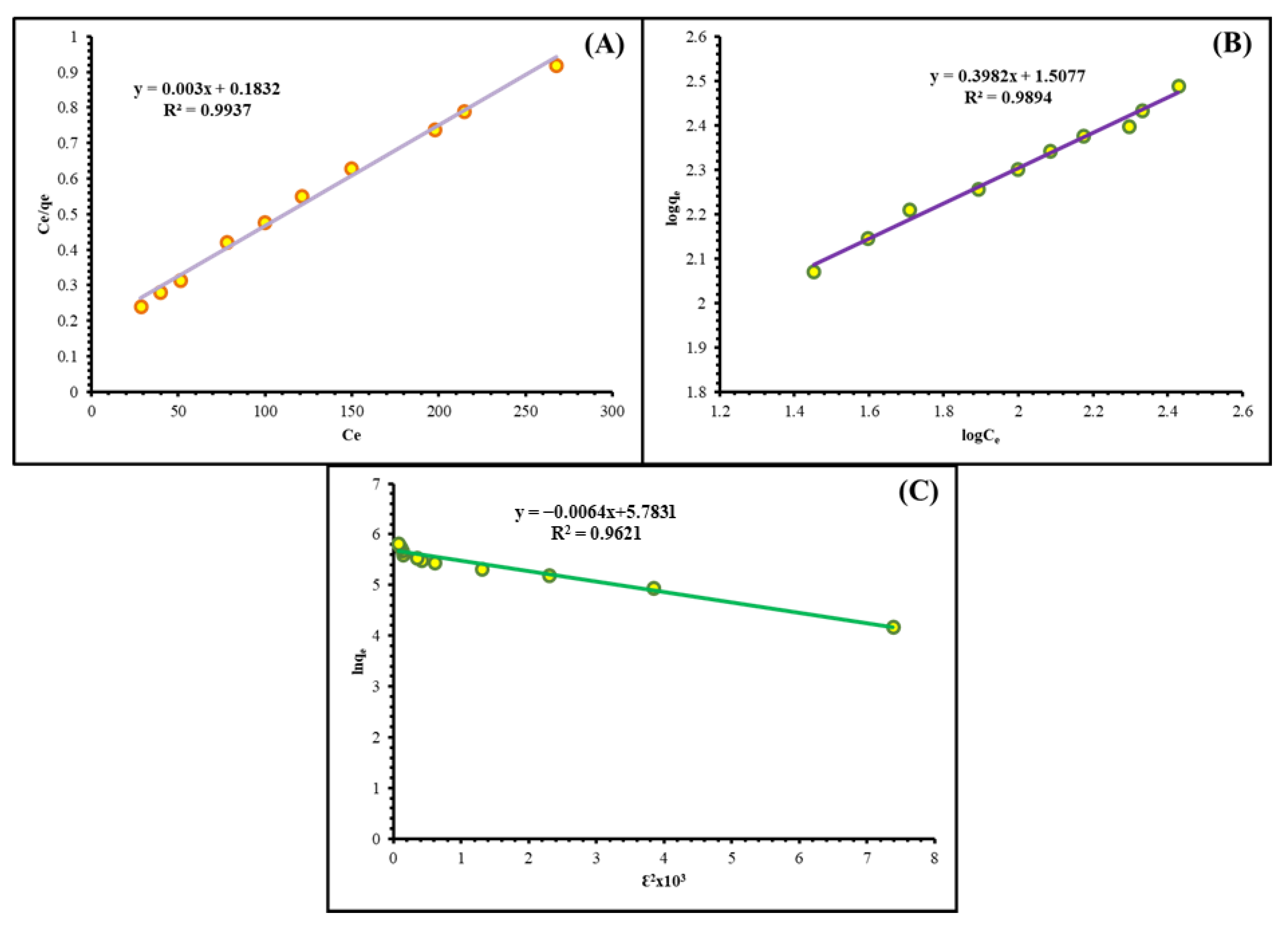
2.2.5. Isotherm Study
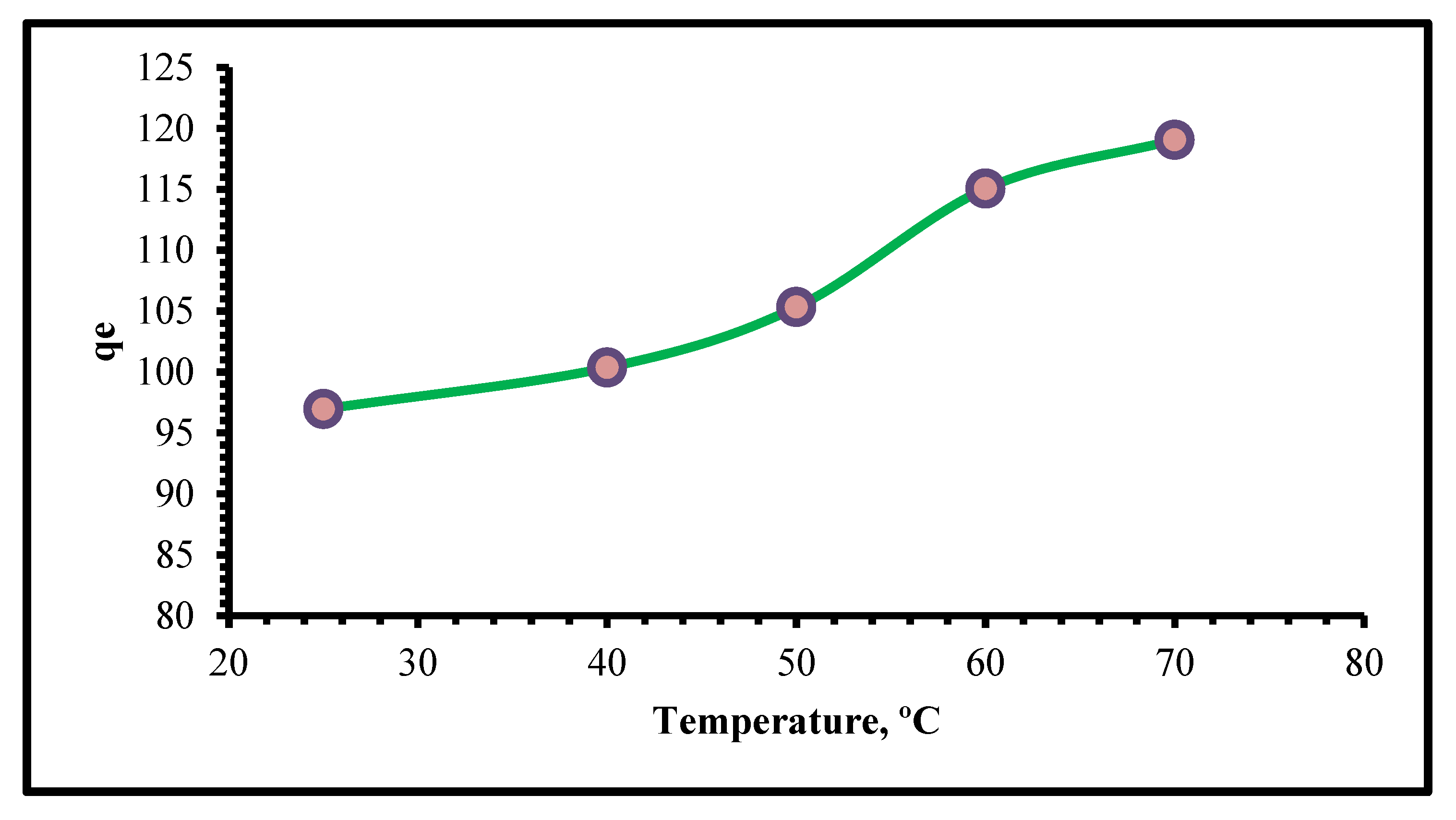
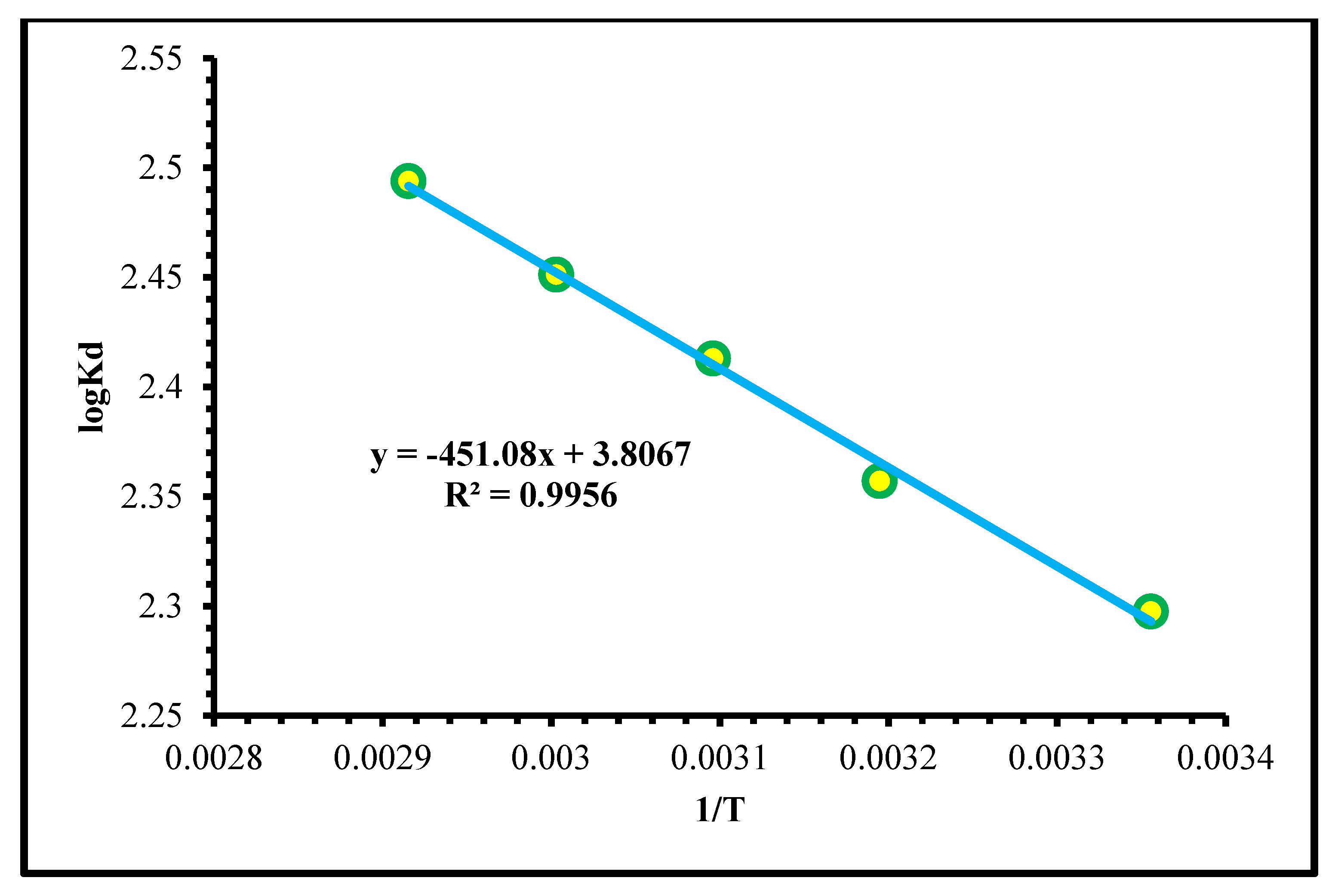
2.2.6. Thermodynamics Study
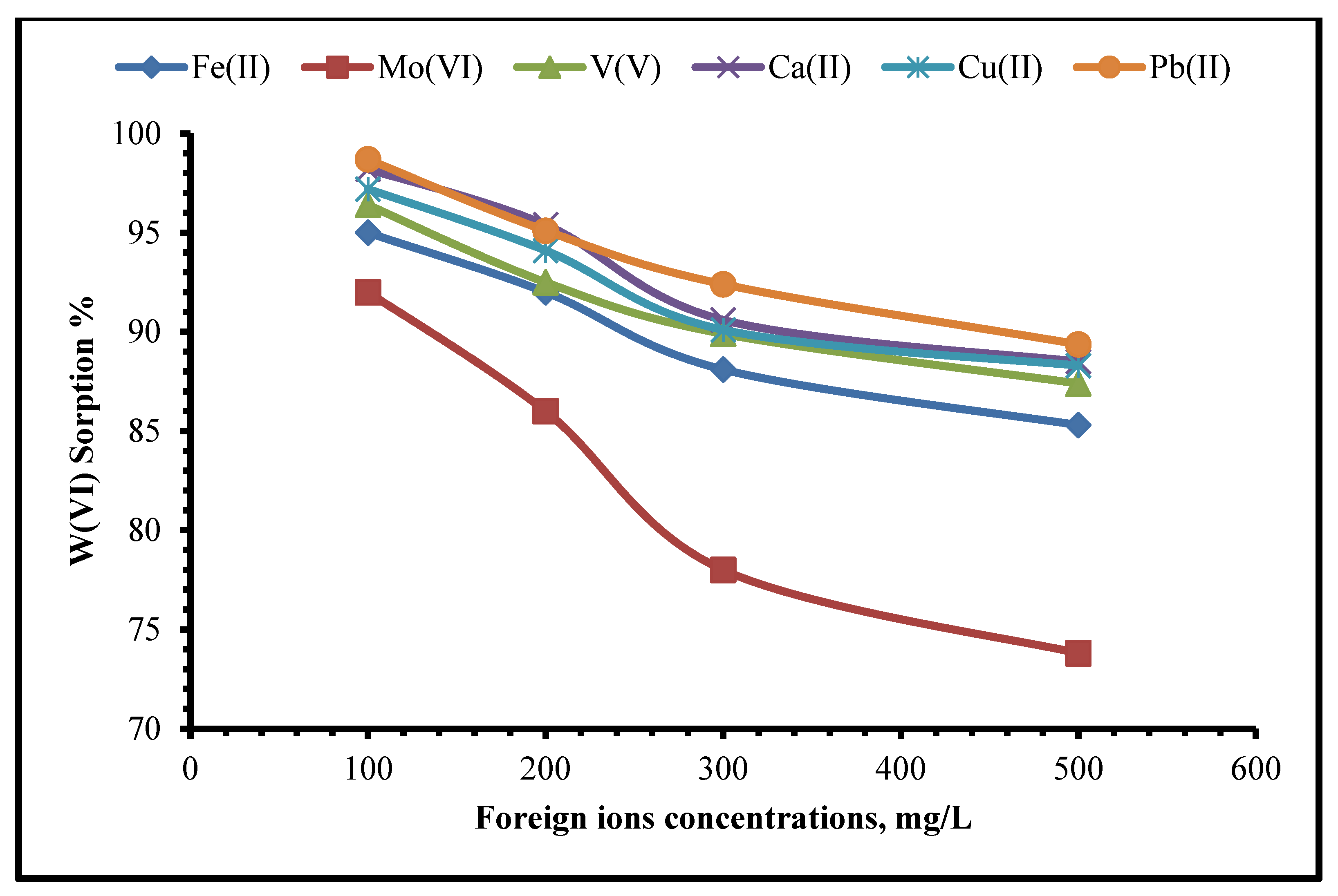
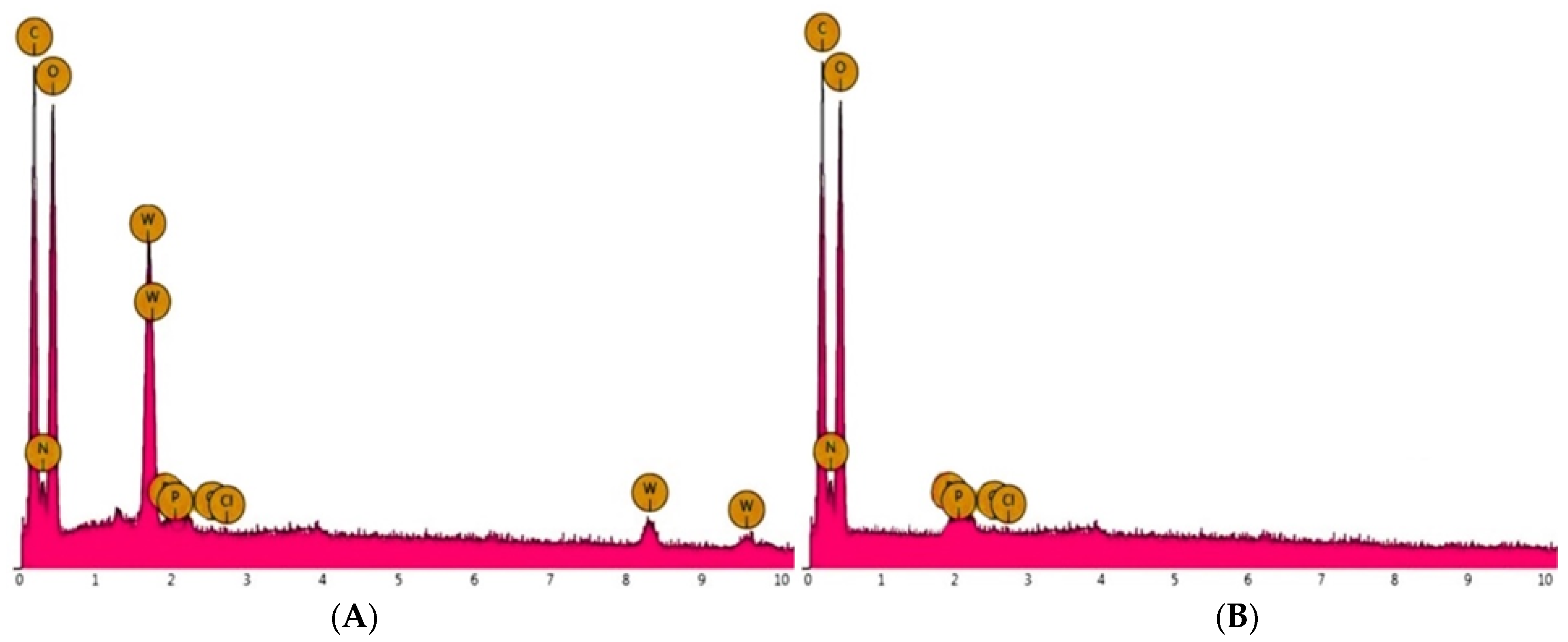
2.2.7. Effect of Foreign Metal Ions on Selectivity
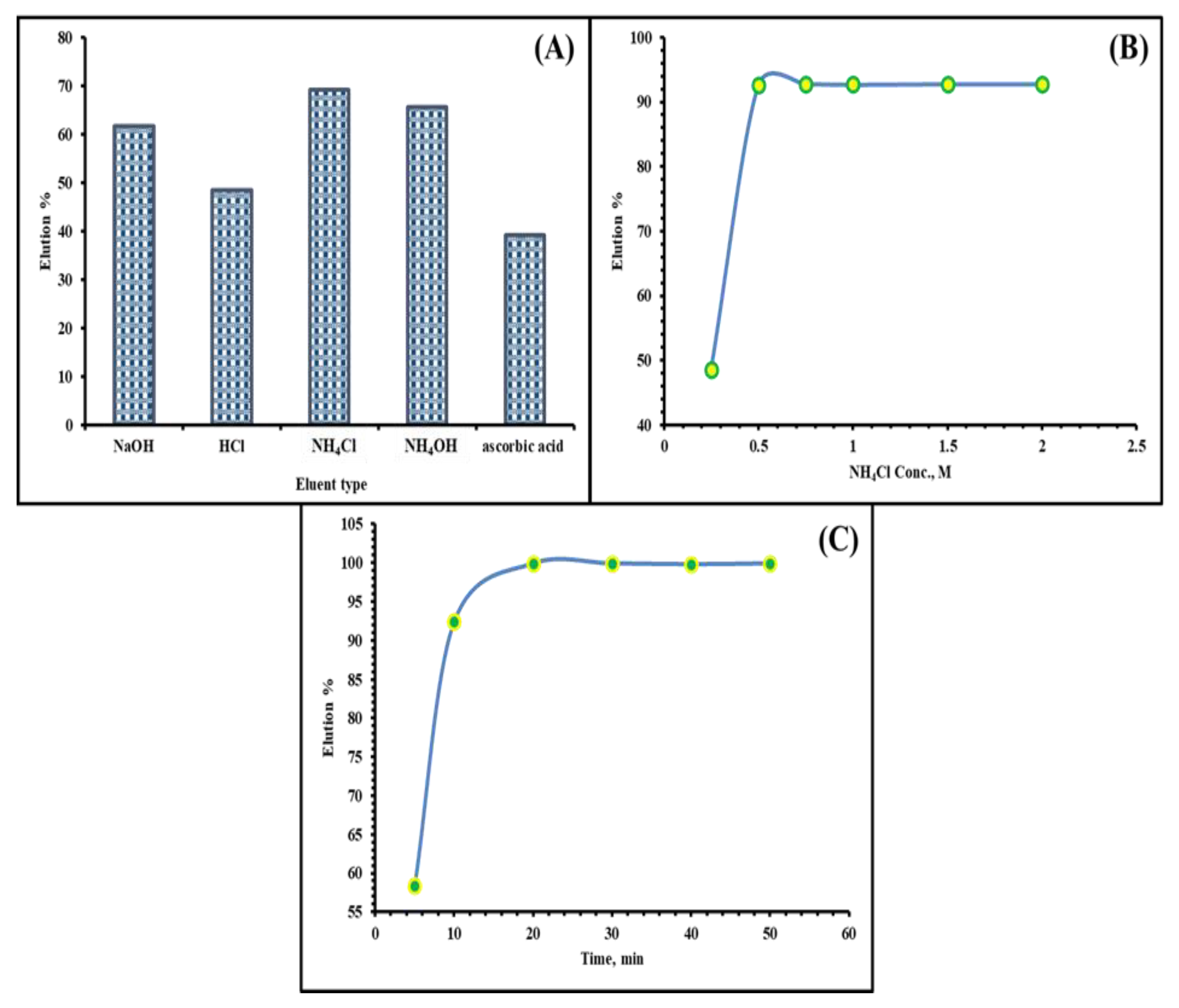
2.3. Desorption–Regeneration Study
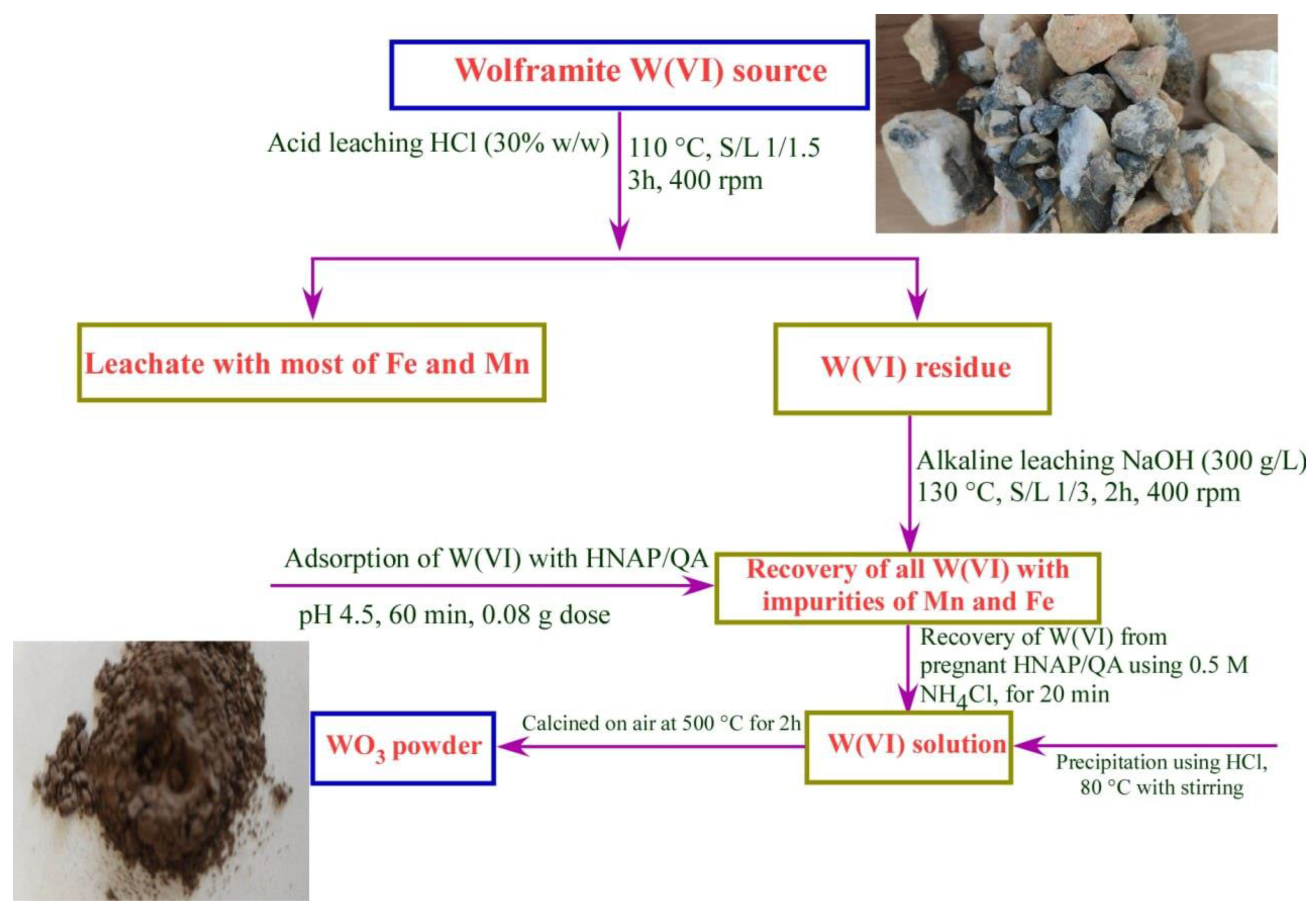
2.4. Recovery of W(VI) Ions from Wolframite Ore
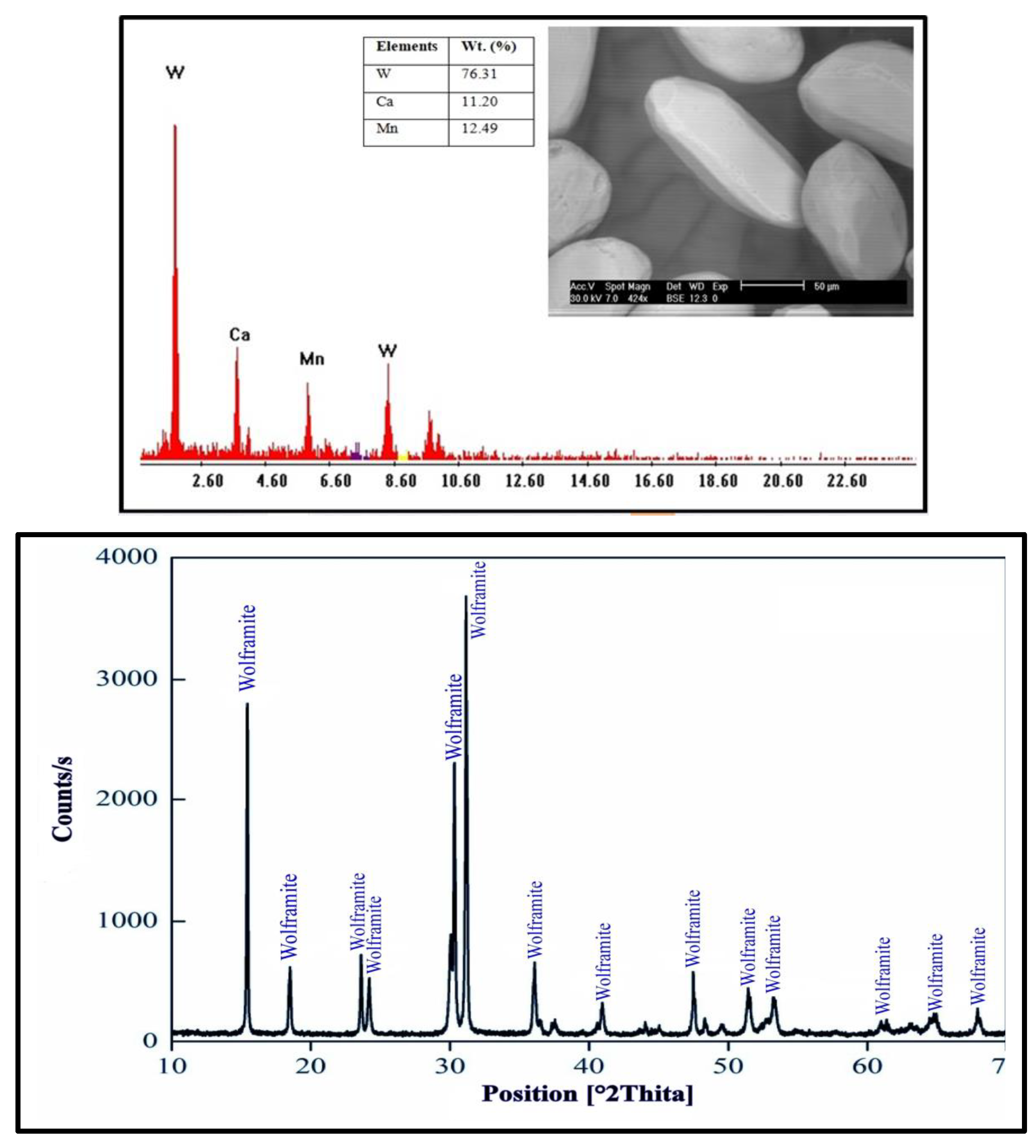
2.4.1. Pre-Concentration of Wolframite Ore
2.4.2. Characterizations of the Wolframite Ore
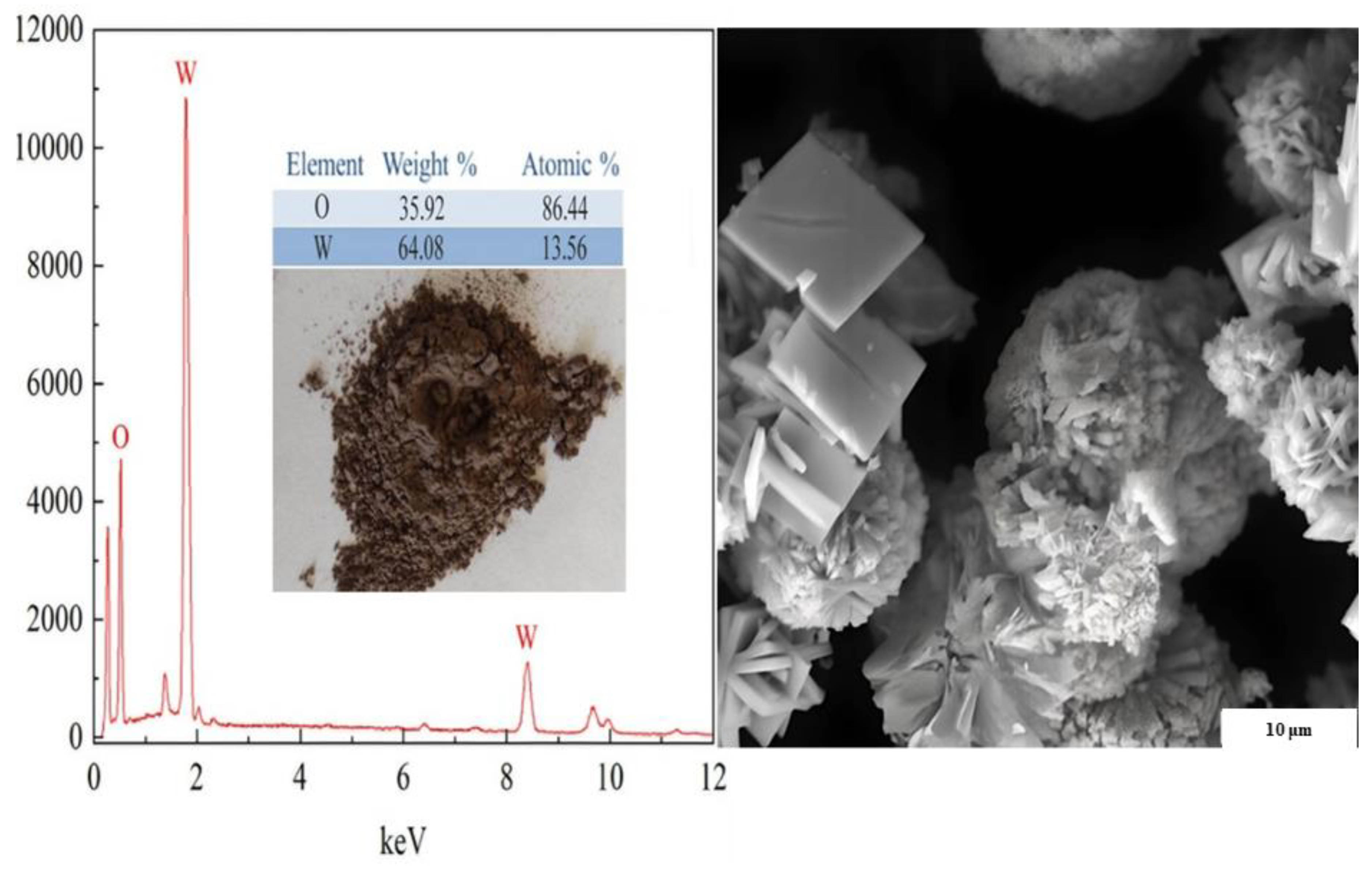
2.4.3. Extraction of Tungsten Oxide
3. Materials and Methods
3.1. Chemicals and Reagents
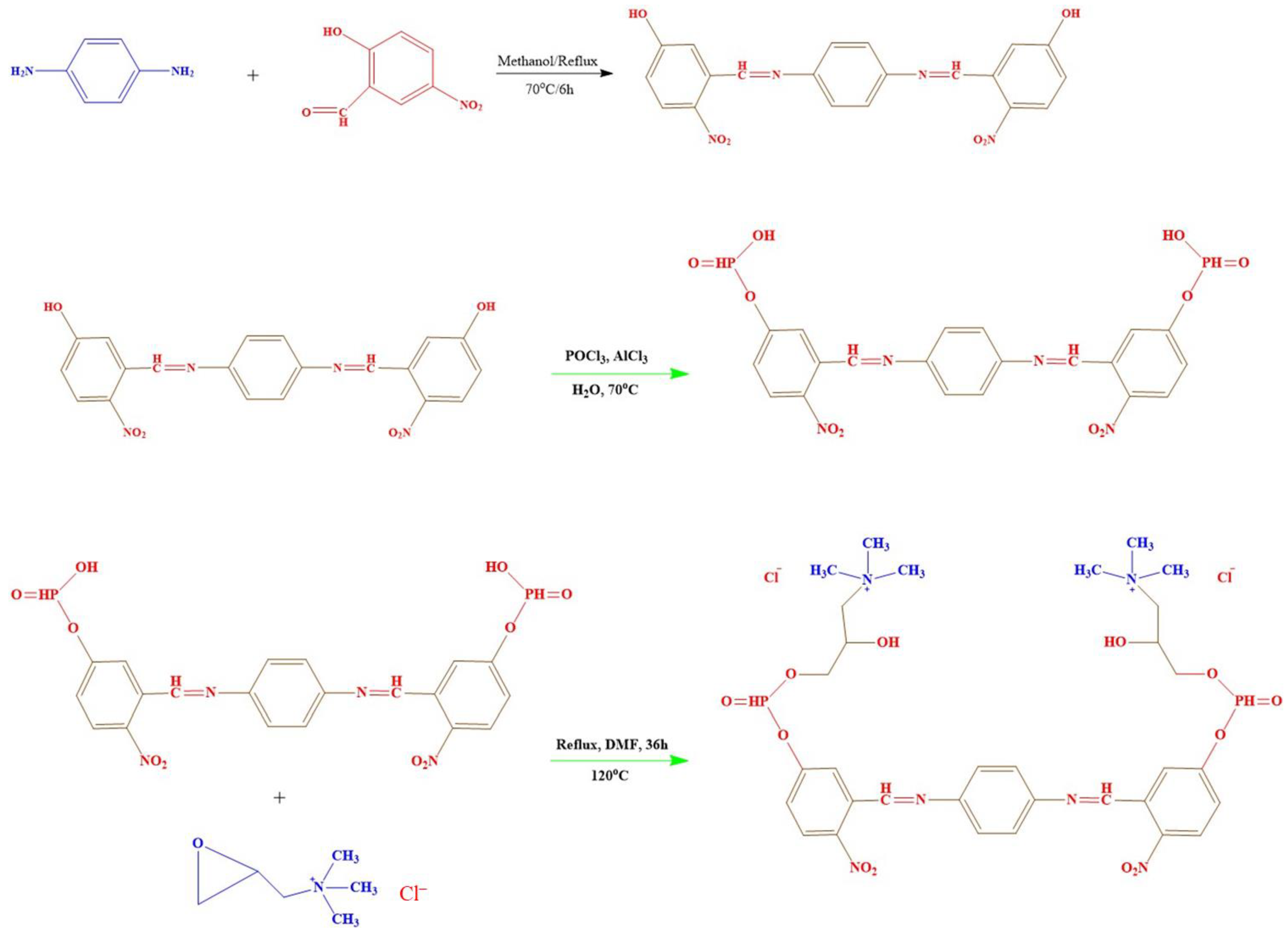
3.2. Preparation of the Adsorbent (HNAP/QA)
3.3. Instrumentation
3.4. Adsorption and Elution Experiments
3.5. Desorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Liu, H.; Nie, C.; Zhang, J.; Steenari, B.; Ekberg, C. Comprehensive treatments of tungsten slags in China: A critical review. J. Environ. Manag. 2020, 270, 110927. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Li, X.; Lindberg, D.; Taskinen, P. Tungsten extractive metallurgy: A review of processes and their challenges for sustainability. Miner. Eng. 2019, 142, 105934. [Google Scholar] [CrossRef]
- Datta, S.; Vero, S.E.; Hettiarachchi, G.M.; Johannesson, K. Tungsten contamination of soils and sediments: Current state of science. Curr. Pollut. Rep. 2017, 3, 55–64. [Google Scholar] [CrossRef]
- Koutsospyros, A.; Strigul, N.; Braida, W.; Christodoulatos, C. Tungsten: Environmental Pollution and Health Effects. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2011; pp. 418–426. [Google Scholar] [CrossRef]
- Ünal, Ü.; Somer, G. A new and very simple procedure for the differential pulse polarographic determination of ultra trace quantities of tungsten using catalytic hydrogen wave and application to tobacco sample. J. Electroanal. Chem. 2012, 687, 64–70. [Google Scholar] [CrossRef]
- Seiler, R.L.; Stollenwerk, K.G.; Garbarino, J.R. Factors controlling tungsten concentrations in ground water Carson Desert, Nevada. Appl. Geochem. 2005, 20, 423–441. [Google Scholar] [CrossRef]
- Bednar, A.; Mirecki, J.; Inouye, L.; Winfield, L.; Larson, S.; Ringelberg, D. The determination of tungsten, molybdenum, and phosphorus oxyanions by high performance liquid chromatography inductively coupled plasma mass spectrometery. Talanta 2007, 72, 1828–1832. [Google Scholar] [CrossRef]
- Li, S.; Deng, N.; Zheng, F.; Huang, Y. Spectrophotometric determination of tungsten (VI) enriched by nanometer-size titanium dioxide in water and sediment. Talanta 2003, 60, 1097–1104. [Google Scholar] [CrossRef]
- Bazel, Y.; Lešková, M.; Rečlo, M.; Šandrejová, J.; Simon, A.; Fizer, M.; Sidey, V. Structural and spectrophotometric characterization of 2-[4-(dimethylamino) styryl]-1-ethylquinolinium iodide as a reagent for sequential injection determination of tungsten. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 196, 398–405. [Google Scholar] [CrossRef]
- Chen, W.; Yang, W.Z.; Liu, X.Q.; Chen, X.M. Structural, dielectric and magnetic properties of Ba3SrLn2Fe2Nb8O30(Ln = La, Nd, Sm) filled tungsten bronze ceramics. J. Alloys Compd. 2016, 675, 311–316. [Google Scholar] [CrossRef]
- Chong, X.; Jiang, Y.; Zhou, R.; Feng, J. Stability, chemical bonding behavior, elastic properties and lattice thermal conductivity of molybdenum and tungsten borides under hydrostatic pressure. Ceram. Int. 2016, 42, 2117–2132. [Google Scholar] [CrossRef]
- Ravi, U.K.; Kumar, J.; Kumar, V.; Sankaranarayana, M.; Nageswara Rao, G.V.S.; Nandy, T.K. Effect of cyclic heat treatment and swaging on mechanical properties of the tungsten heavy alloys. Mater. Sci. Eng. A 2016, 656, 256–265. [Google Scholar] [CrossRef]
- Stanciu, V.I.; Vitry, V.; Delaunois, F. Tungsten carbide powder obtained by direct carburization of tungsten trioxide using mechanical alloying method. J. Alloys Compd. 2016, 659, 302–308. [Google Scholar] [CrossRef]
- Medici, S.; Costa, M.; Brocato, J.; Peana, M.; Oksuz, B.A.; Cartularo, L.; Vaughan, J.; Laulicht, F.; Wu, F.; Kluz, T.; et al. Tungsten-induced carcinogenesis in human bronchial epithelial cells. Toxicol. Appl. Pharmacol. 2015, 288, 33–39. [Google Scholar] [CrossRef]
- Chen, Y.; Huo, G.; Guo, X.; Wang, Q. Sustainable extraction of tungsten from the acid digestion product of tungsten concentrate by leaching-solvent extraction together with raffinate recycling. J. Clean. Prod. 2022, 375, 133924. [Google Scholar] [CrossRef]
- Huo, G.; Peng, C.; Song, Q.; Lu, X. Tungsten removal from molybdate solutions using ion exchange. Hydrometallurgy 2014, 147–148, 217–222. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, W.G.; Hwang, I.S.; Lee, J.Y.; Han, C. Recovery of tungsten from spent selective catalytic reduction catalysts by pressure leaching. J. Ind. Eng. Chem. 2015, 28, 73–77. [Google Scholar] [CrossRef]
- Qin, W.; Xi, X.; Zhang, L.; Nie, Z.; Liu, C.; Li, R. The effect of MOx (M = Zr, Ce, La, Y) additives on the electrochemical preparation of tungsten in eutectic Na2WO4–WO3 melt. J. Electroanal. Chem. 2023, 935, 117343. [Google Scholar] [CrossRef]
- Han, Z.; Golev, A.; Edraki, M. A Review of Tungsten Resources and Potential Extraction from Mine Waste. Minerals 2021, 11, 701. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, G.; Zeng, L.; Zhou, Q.; Li, Z.; Zhang, D.; Cao, Z.; Guan, W.; Li, Q.; Xiao, L. Study on removal of molybdenum from ammonium tungstate solutions using solvent extraction with quaternary ammonium salt extractant. Hydrometallurgy 2019, 186, 218–225. [Google Scholar] [CrossRef]
- Zhao, Z.; Cao, C.; Chen, X. Separation of macro amounts of tungsten and molybdenum by precipitation with ferrous salt. Trans. Nonferrous Met. Soc. 2011, 21, 2758–2763. [Google Scholar] [CrossRef]
- Cao, F.; Wang, W.; Wei, D.; Liu, W. Separation of tungsten and molybdenum with solvent extraction using functionalized ionic liquid tricaprylmethylammonium bis(2,4,4-trimethylpentyl) phosphinate. Int. J. Miner. Metall. Mater. 2021, 28, 1769–1776. [Google Scholar] [CrossRef]
- Xu, C.; Zhan, W.; Tang, X.; Mo, F.; Fu, L.; Lin, B. Self-healing chitosan/vanillin hydrogels based on Schiff-base bond/hydrogen bond hybrid linkages. Polym. Test. 2018, 66, 155–163. [Google Scholar] [CrossRef]
- Pandya, H.J.; Ganatra, K.J. Synthesis, characterization and biological evaluation of bis-bidentate Schiff base metal complexes. Inorg. Chem. Indian J. 2008, 3, 182–187. [Google Scholar]
- Li, L.; Sakr, A.K.; Schlöder, T.; Klein, S.; Beckers, H.; Kitsaras, M.-P.; Snelling, H.V.; Young, N.A.; Andrae, D.; Riedel, S. Searching for Monomeric Nickel Tetrafluoride: Unravelling Infrared Matrix Isolation Spectra of Higher Nickel Fluorides. Angew. Chem. Int. Ed. 2021, 60, 6391–6394. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wu, C.; Liu, Z.; Zhang, L.; Chen, L.; Wang, J.; Wang, X.; Yang, S.; Wang, S. Fabrication of a phosphorylated graphene oxide–chitosan composite for highly effective and selective capture of U(VI). Environ. Sci. Nano 2017, 4, 1876–1886. [Google Scholar] [CrossRef]
- Belfer, S.; Fainchtain, R.; Purinson, Y.; Kedem, O. Surface characterization by FTIR-ATR spectroscopy of polyethersulfone membranes-unmodified, modified and protein fouled. J. Membr. Sci. 2000, 172, 113–124. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. CHAPTER 10—Compounds Containing –NH2, –NHR, and –NR2 Groups. In The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Lin-Vien, D., Colthup, N.B., Fateley, W.G., Grasselli, J.G., Eds.; Academic Press: San Diego, CA, USA, 1991; pp. 155–178. [Google Scholar]
- Hamza, M.F.; Salih, K.A.M.; Zhou, K.; Wei, Y.; Abu Khoziem, H.A.; Alotaibi, S.H.; Guibal, E. Effect of bi-functionalization of algal/polyethyleneimine composite beads on the enhancement of tungstate sorption: Application to metal recovery from ore leachate. Sep. Purif. Technol. 2022, 290, 120893. [Google Scholar] [CrossRef]
- Sethuraman, P.R.; Leparulo, M.A.; Pope, M.T.; Zonnevijlle, F.; Brevard, C.; Lemerle, J. Heteropolypentatungstobisphosphonates —Cyclopentane-like pseudorotation of an oxometalate structure. J. Am. Chem. Soc. 1981, 103, 7665–7666. [Google Scholar] [CrossRef]
- Bajaber, M.A.; Ragab, A.H.; Sakr, A.K.; Atia, B.A.; Fathy, W.M.; Gado, M.A. Application of a new derivatives of traizole Schiff base on chromium recovery from its wastewater. Sep. Sci. Technol. 2023, 58, 737–758. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances, Zur theorie der sogenannten adsorption geloster stoffe. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Sakr, A.K.; Abdel Aal, M.M.; Abd El-Rahem, K.A.; Allam, E.M.; Abdel Dayem, S.M.; Elshehy, E.A.; Hanfi, M.Y.; Alqahtani, M.S.; Cheira, M.F. Characteristic Aspects of Uranium (VI) Adsorption Utilizing Nano-Silica/Chitosan from Wastewater Solution. Nanomaterials 2022, 12, 3866. [Google Scholar] [CrossRef] [PubMed]
- Negm, S.H.; Abd El-Hamid, A.A.M.; Gado, M.A.; El-Gendy, H.S. Selective uranium adsorption using modifed acrylamide resins. J. Radioanal. Nucl. Chem. 2018, 319, 327–337. [Google Scholar] [CrossRef]
- Washahy, A.R.; Sakr, A.K.; Gouda, A.A.; Atia, B.M.; Somaily, H.H.; Hanfi, M.Y.; Sayyed, M.I.; El Sheikh, R.; El-Sheikh, E.M.; Radwan, H.A.; et al. Selective Recovery of Cadmium, Cobalt, and Nickel from Spent Ni-Cd Batteries using Adogen® 464 and Mesoporous Silica Derivatives. Int. J. Mol. Sci. 2022, 23, 8677. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Ibrahium, H.A.; Atia, B.M.; Awwad, N.S.; Nayl, A.A.; Radwan, H.A.; Gado, M.A. Efficient preparation of phosphazene chitosan derivatives and its applications for the adsorption of molybdenum from spent hydrodesulfurization catalyst. J. Dispers. Sci. Technol. 2022, 43, 1–16. [Google Scholar] [CrossRef]
- Ruiping, L.; Chunye, L.; Xitao, L. Adsorption of tungstate on kaolinite: Adsorption models and kinetics. RSC Adv. 2006, 6, 19872–19877. [Google Scholar] [CrossRef]
- Alharbi, A.; Gouda, A.A.; Atia, B.M.; Gado, M.A.; Alluhaybi, A.A.; Alkabli, J. The Role of Modified Chelating Graphene Oxide for Vanadium Separation from Its Bearing Samples. Russ. J. Inorg. Chem. 2022, 67, 560–575. [Google Scholar] [CrossRef]
- Atia, B.M.; Sakr, A.K.; Gado, M.A.; El-Gendy, H.S.; Abdelazeem, N.M.; El-Sheikh, E.M.; Hanfi, M.Y.; Sayyed, M.I.; Al-Otaibi, J.S.; Cheira, M.F. Synthesis of a New Chelating Iminophosphorane Derivative (Phosphazene) for U (VI) Recovery. Polymers 2022, 14, 1687. [Google Scholar] [CrossRef]
- Atia, B.M.; Khawassek, Y.M.; Hussein, G.M.; Gado, M.A.; El-Sheify, M.A.; Cheira, M.F. One-pot synthesis of pyridine dicarboxamide derivative and its application for uranium separation from acidic medium. J. Environ. Chem. Eng. 2021, 9, 105726. [Google Scholar] [CrossRef]
- Atia, B.M.; Gado, M.A.; Cheira, M.F.; El-Gendy, H.S.; Yousef, M.A.; Hashem, M.D. Direct synthesis of a chelating carboxamide derivative and its application for thorium extraction from Abu Rusheid ore sample, South Eastern Desert, Egypt. Int. J. Environ. Anal. Chem. 2021, 101, 1–24. [Google Scholar] [CrossRef]
- Gado, M.; Rashad, M.; Kassab, W.; Badran, M. Highly Developed Surface Area Thiosemicarbazide Biochar Derived from Aloe Vera for Efficient Adsorption of Uranium. Radiochemistry 2021, 63, 353–363. [Google Scholar] [CrossRef]
- Akar, T.; Kaynak, Z.; Ulusoy, S.; Yuvaci, D.; Ozsari, G.; Akar, S.T. Enhanced Biosorption of Nickel (II) Ions by Silica-GelImmobilized Waste Biomass: Biosorption Characteristics in Batch and Dynamic Flow Mode. J. Hazard Mater. 2009, 163, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.H.; Chen, S.M. Biosorption of Azo Dyes from Aqueous Solution by Glutaraldehyde-Crosslinked Chitosans. J. Hazard Mater. 2009, 172, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.M. The Potential Theory of Adsorption of Gases and Vapors for Adsorbents with Energetically Nonuniform Surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Mittal, A.; Kaur, D.; Mittal, J. Batch and Bulk Removal of a Triarylmethane Dye, Fast Green FCF, from Wastewater by Adsorption over Waste Materials. J. Hazard Mater. 2009, 163, 568–577. [Google Scholar] [CrossRef]
- Chen, A.H.; Yang, C.Y.; Chen, C.Y.; Chen, C.Y.; Chen, C.W. The Chemically Crosslinked Metal-Complexed Chitosans for Comparative Adsorptions of Cu (II), Zn (II), Ni (II) and Pb (II) Ions in Aqueous Medium. J. Hazard Mater. 2009, 163, 1068–1075. [Google Scholar] [CrossRef]
- Kundu, S.; Gupta, A.K. Arsenic Adsorption onto Iron OxideCoated Cement (IOCC): Regression Analysis of Equilibrium Data with Several Isotherm Models and Their Optimization. Chem. Eng. J. 2006, 122, 93–106. [Google Scholar] [CrossRef]
- Hassanin, M.A.; Negm, S.H.; Youssef, M.A.; Sakr, A.K.; Mira, H.I.; Mohammaden, T.F.; Al-Otaibi, J.S.; Hanfi, M.Y.; Sayyed, M.I.; Cheira, M.F. Sustainable Remedy Waste to Generate SiO2 Functionalized on Graphene Oxide for Removal of U(VI) Ions. Sustainability 2022, 14, 2699. [Google Scholar] [CrossRef]
- Gado, M.; Atia, B. Adsorption of Thorium Ions Using Trioctylphosphine Oxide Impregnated Dowex 1 × 4 Powder. Radiochemistry 2019, 61, 168–176. [Google Scholar] [CrossRef]
- Weshahy, A.R.; Gouda, A.A.; Atia, B.M.; Sakr, A.K.; Al-Otaibi, J.S.; Almuqrin, A.; Hanfi, M.Y.; Sayyed, M.I.; El Sheikh, R.; Radwan, H.A.; et al. Efficient Recovery of Rare Earth Elements and Zinc from Spent Ni–Metal Hydride Batteries: Statistical Studies. Nanomaterials 2022, 12, 2305. [Google Scholar] [CrossRef] [PubMed]
- Borodina, E.; Karpov, S.I.; Selemenev, V.F.; Schwieger, W.; Maracke, S.; Fröba, M.; Rößner, F. Surface and texture properties of mesoporous silica materials modified by silicon-organic compounds containing quaternary amino groups for their application in base-catalyzed reactions. Microporous Mesoporous Mater. 2015, 203, 224–231. [Google Scholar] [CrossRef]
- Tarasova, N.P.; Smetannikov, Y.V.; Zanin, A.A. Radiation-chemical transformation of elemental phosphorus in the presence of ionic liquids. Dokl. Chem. 2013, 449, 111–113. [Google Scholar] [CrossRef]
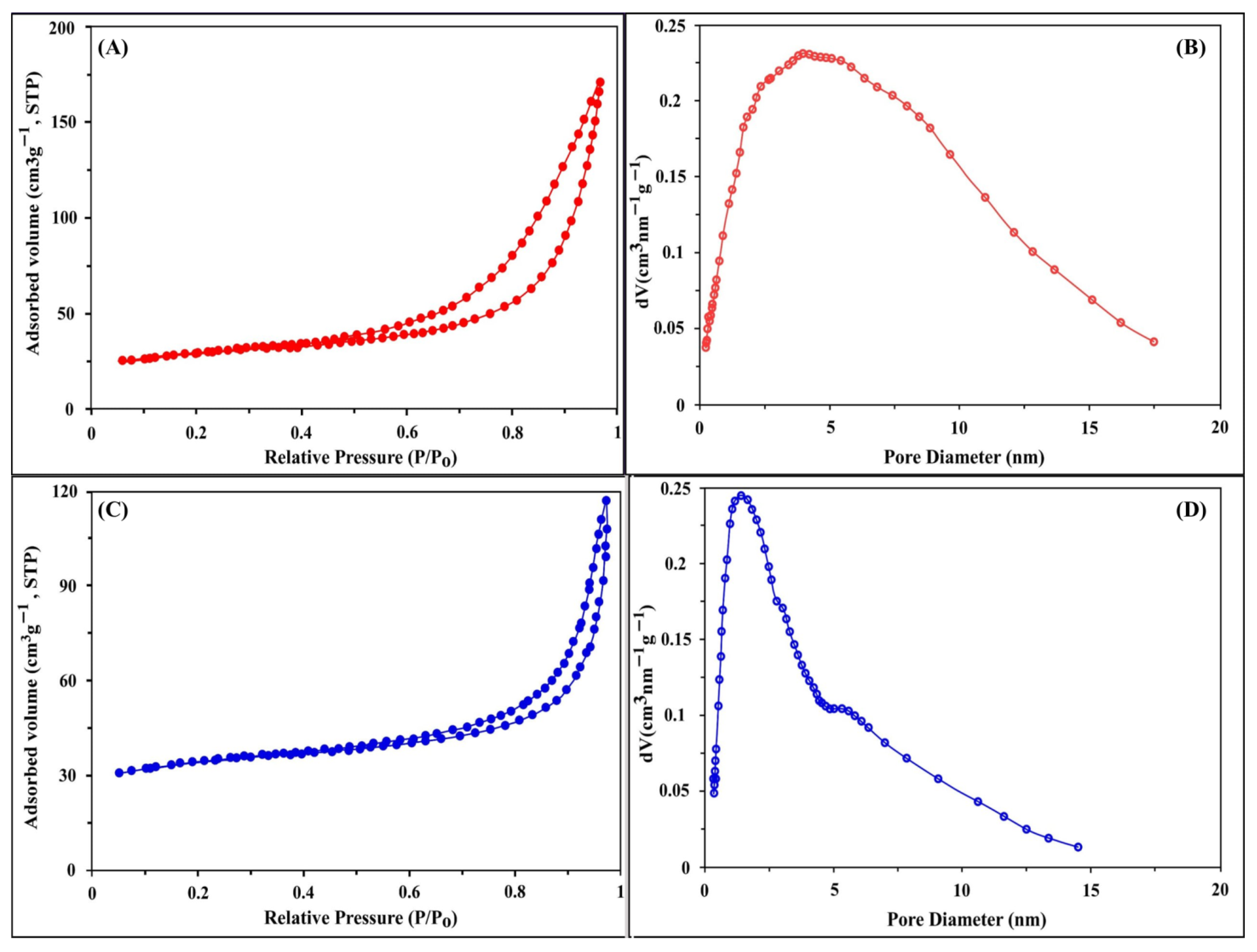

| Substances | SBET (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|
| HNAP | 101.6 | 0.205 | 2.8 |
| HNAP/QA | 178.4 | 0.288 | 1.78 |
| PFO Kinetic | PSO Kinetic | |||||
|---|---|---|---|---|---|---|
| K1 | qe(cal) | R2 | qe(exp) | K2 | qe(cal) | R2 |
| 0.11469 | 110.535 | 0.9737 | 62.44 | 0.00145 | 66.225 | 0.9965 |
| Models | Parameters | |
|---|---|---|
| Langmuir isotherm | Equation | y = 0.003x + 0.1832 |
| qmax (mg g−1) | 333.33 | |
| K1 | 0.0164 | |
| R² | 0.9937 | |
| Freundlich isotherm | Equation | y = 0.3982x + 1.5077 |
| Kf (mg g−1) | 32.189 | |
| 1/n (mg min g−1) | 0.3982 | |
| R² | 0.9894 | |
| D-R isotherm | Equation | y = −0.0064x + 5.7831 |
| qD (mg g−1) | 324.76 | |
| BD (Mol2 kJ−2) | 0.0064 | |
| E (kJ mol−1) | 8.8388 | |
| R² | 0.9621 | |
| Practical Capacity | qe(exp) (mg g−1) | 326.75 |
| ΔS° (J mol−1 K−1) | ΔH° (kJ mol−1) | ΔG° (kJ mol−1) | ||||
|---|---|---|---|---|---|---|
| 75.887 | 10.36 | 298 K | 313 K | 323 K | 333 K | 343 K |
| −21.89 | −22.81 | −23.54 | −24.27 | −25.0 | ||
| Element | Concentration (%) |
|---|---|
| WO3 | 77.13 |
| MnO | 8.99 |
| SiO2 | 0.58 |
| Al2O3 | 0.3 |
| Fe2O3 | 3.9 |
| SO3 | 0.49 |
| CaO | 7.57 |
| Others | 1.04 |
| Element | Concentration (%) |
|---|---|
| WO3 | 89.46 |
| MnO | 0.004 |
| SiO2 | 0.46 |
| Al2O3 | 0.3 |
| Fe2O3 | 0.002 |
| CaO | 7.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbshary, R.E.; Gouda, A.A.; El Sheikh, R.; Alqahtani, M.S.; Hanfi, M.Y.; Atia, B.M.; Sakr, A.K.; Gado, M.A. Recovery of W(VI) from Wolframite Ore Using New Synthetic Schiff Base Derivative. Int. J. Mol. Sci. 2023, 24, 7423. https://doi.org/10.3390/ijms24087423
Elbshary RE, Gouda AA, El Sheikh R, Alqahtani MS, Hanfi MY, Atia BM, Sakr AK, Gado MA. Recovery of W(VI) from Wolframite Ore Using New Synthetic Schiff Base Derivative. International Journal of Molecular Sciences. 2023; 24(8):7423. https://doi.org/10.3390/ijms24087423
Chicago/Turabian StyleElbshary, Rawan E., Ayman A. Gouda, Ragaa El Sheikh, Mohammed S. Alqahtani, Mohamed Y. Hanfi, Bahig M. Atia, Ahmed K. Sakr, and Mohamed A. Gado. 2023. "Recovery of W(VI) from Wolframite Ore Using New Synthetic Schiff Base Derivative" International Journal of Molecular Sciences 24, no. 8: 7423. https://doi.org/10.3390/ijms24087423
APA StyleElbshary, R. E., Gouda, A. A., El Sheikh, R., Alqahtani, M. S., Hanfi, M. Y., Atia, B. M., Sakr, A. K., & Gado, M. A. (2023). Recovery of W(VI) from Wolframite Ore Using New Synthetic Schiff Base Derivative. International Journal of Molecular Sciences, 24(8), 7423. https://doi.org/10.3390/ijms24087423








