Multiple Nucleocapsid Structural Forms of Shrimp White Spot Syndrome Virus Suggests a Novel Viral Morphogenetic Pathway
Abstract
1. Introduction
2. Results and Discussion
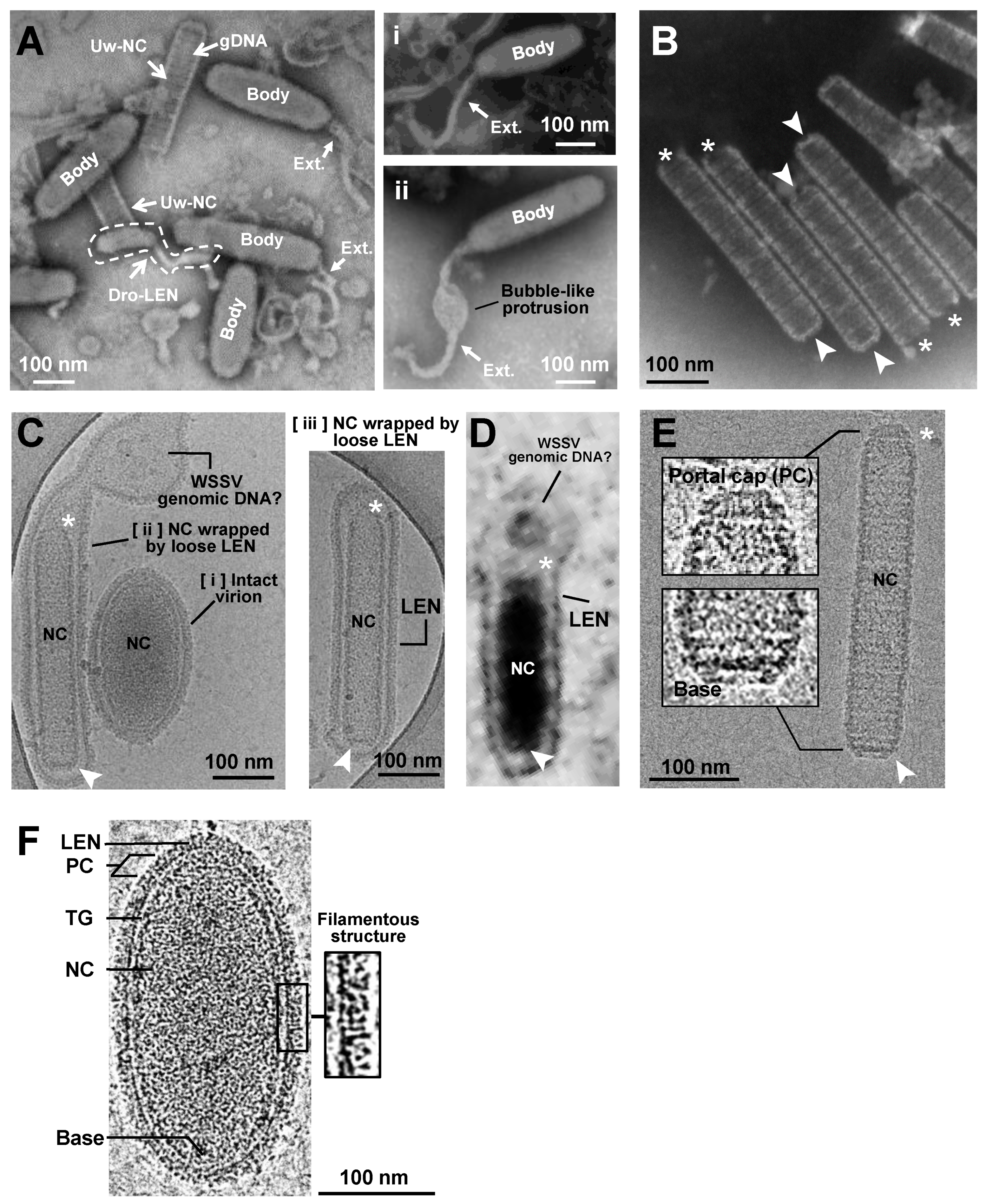
2.1. TEM and Cryo-EM Analysis Showed Differential Shapes of Enveloped WSSV Virions and Nucleocapsids
2.2. Three-Dimensional Volume Reconstruction of WSSV Nucleocapsid by Cryo-Electron Tomography (Cryo-ET)
2.3. Proposed WSSV Genomic DNA Translocation through the Cap End of WSSV Nucleocapsids
2.4. Cryo-EM Map of the Middle Part of the WSSV Nucleocapsid (M-NC) Revealed a Unique Architecture with C14 Symmetry
2.5. WSSV VP664 Is the Main Component of the 14 NUs of WSSV Nucleocapsid
2.6. Proposed Model for WSSV Morphogenesis
3. Materials and Methods
3.1. Preparation of WSSV Inoculum
3.2. Purification of WSSV Virions and Nucleocapsids
3.3. Cryo-EM Sample Vitrification
3.4. Data Acquisition
3.5. Image Processing
3.6. Ultrastructural Observations by Transmission Electron Microscopy
3.7. Localization of WSSV Major Nucleocapsid Protein VP664 on the Nucleocapsid Rings by Immunoelectron Microscopy (IEM)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dey, B.K.; Dugassa, G.H.; Hinzano, S.M.; Bossier, P. Causative agent, diagnosis and management of white spot disease in shrimp: A review. Rev. Aquac. 2020, 12, 822–865. [Google Scholar] [CrossRef]
- Chou, H.Y.; Huang, C.Y.; Wang, C.H.; Chiang, H.C.; Lo, C.F. Pathogenicity of a baculovirus infection causing White Spot Syndrome in cultured Penaeid shrimp in Taiwan. Dis. Aquat. Organ. 1995, 23, 165–173. [Google Scholar] [CrossRef]
- Yang, F.; He, J.; Lin, X.; Li, Q.; Pan, D.; Zhang, X.; Xu, X. Complete genome sequence of shrimp white spot bacilliform virus. J. Virol. 2001, 75, 11811–11820. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Wang, H.C.; Huang, C.J.; Peng, S.E.; Chen, Y.G.; Lin, S.J.; Chen, W.Y.; Dai, C.F.; Yu, H.T.; Wang, C.H.; et al. Transcriptional analysis of the DNA polymerase gene of shrimp white spot syndrome virus (WSSV). Virology 2002, 301, 136–147. [Google Scholar] [CrossRef]
- Wang, H.C.; Hirono, I.; Maningas, M.B.B.; Somboonwiwat, K.; Stentiford, G. ICTV Report Consortium. ICTV Virus Taxonomy Profile: Nimaviridae. J. Gen. Virol. 2019, 100, 1053–1054. [Google Scholar] [CrossRef]
- Durand, S.; Lightner, D.V.; Redman, R.M.; Bonami, J.R. Ultrastructure and morphogenesis of white spot syndrome baculovirus (WSSV). Dis. Aquat. Organ. 1997, 29, 205–211. [Google Scholar] [CrossRef]
- Lu, C.P.; Zhu, S.; Guo, F.S.; Wu, S.Y. Electron microscopic observation on a non-occluded baculo-like virus in shrimps. Arch. Virol. 1997, 142, 2073–2078. [Google Scholar] [CrossRef]
- Tsai, J.M.; Wang, H.C.; Leu, J.H.; Wang, A.H.; Zhuang, Y.; Walker, P.J.; Kou, G.H.; Lo, C.F. Identification of the nucleocapsid, tegument, and envelope proteins of the shrimp white spot syndrome virus virion. J. Virol. 2006, 80, 3021–3029. [Google Scholar] [CrossRef]
- Tsai, J.M.; Wang, H.C.; Leu, J.H.; Hsiao, H.H.; Wang, A.H.; Kou, G.H.; Lo, C.F. Genomic and proteomic analysis of thirty-nine structural proteins of shrimp white spot syndrome virus. J. Virol. 2004, 78, 11360–11370. [Google Scholar] [CrossRef]
- Xie, X.; Xu, L.; Yang, F. Proteomic analysis of the major envelope and nucleocapsid proteins of white spot syndrome virus. J. Virol. 2006, 80, 10615–10623. [Google Scholar] [CrossRef]
- Sritunyalucksana, K.; Wannapapho, W.; Lo, C.F.; Flegel, T.W. PmRab7 is a VP28-binding protein involved in white spot syndrome virus infection in shrimp. J. Virol. 2006, 80, 10734–10742. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Zhu, C.; Shi, D.; He, P.; Jia, R. Advances in the study of tegument protein VP26 in white spot syndrome virus. Aquac. Fish. 2020, 6, 448–454. [Google Scholar] [CrossRef]
- Wang, C.H.; Lo, C.F.; Leu, J.H.; Chou, C.M.; Yeh, P.Y.; Chou, H.Y.; Tung, M.C.; Chang, C.F.; Su, M.S.; Kou, G.H. Purification and genomic analysis of baculovirus associated with white spot syndrome (WSBV) of Penaeus monodon. Dis. Aquat. Organ. 1995, 23, 239–242. [Google Scholar] [CrossRef]
- Amano, Y.; Diaz, C.L.; Melena, C.J. Fine structure analysis of white spot syndrome virus of shrimp. Brazilian J. Vet. Pathol. 2011, 4, 214–218. [Google Scholar]
- Leu, J.H.; Tsai, J.M.; Wang, H.C.; Wang, A.H.-J.; Wang, C.H.; Kou, G.H.; Lo, C.F. The unique stacked rings in the nucleocapsid of the WSSV virion are formed by the major structural protein VP664, the largest viral structural protein ever found. J. Virol. 2005, 79, 140–149. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, L.; Zhang, J.; Xiao, L.; Wu, Q.; Chen, D.; Li, J.K. Purification and characterization of White Spot Syndrome Virus (WSSV) produced in an alternate host: Crayfish, Cambarus clarkia. Virus Res. 2001, 76, 115–125. [Google Scholar] [CrossRef]
- Fraser, M.J. Ultrastructural observations of virion maturation in Autographa californica nuclear polyhedrosis virus infected Spodoptera frugiperda cell cultures. J. Ultrastruct. Mol. Struct. Res. 1986, 95, 189–195. [Google Scholar] [CrossRef]
- Chelikani, V.; Ranjan, T.; Kondabagil, K. Revisiting the genome packaging in viruses with lessons from the “Giants”. Virology 2014, 466, 15–26. [Google Scholar] [CrossRef]
- Liu, Y.T.; Jih, J.; Dai, X.; Bi, G.Q.; Zhou, Z.H. CryoEM structures of herpes simplex virus type 1 portal vertex and packaged genome. Nature 2019, 570, 257–261. [Google Scholar] [CrossRef]
- Zhao, S.; He, G.; Yang, Y.; Liang, C. Nucleocapsid Assembly of Baculoviruses. Viruses 2019, 11, 595. [Google Scholar] [CrossRef]
- Dedeo, C.L.; Cingolani, G.; Teschke, C.M. Portal protein: The orchestrator of capsid assembly for the dsDNA tailed bacteriophages and herpesviruses. Annu. Rev. Virol. 2019, 6, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, M.; Shan, H.; Li, K.; Wang, P.; Guo, H.; Zhao, Y.; Wang, R.; Tao, Y.; Yang, L.; et al. Ring-stacked capsids of white spot syndrome virus and structural transitions with genome ejection. Sci. Adv. 2023, 9, eadd2796. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Liu, W.; Lee, C.C.; Chou, T.L.; Lee, Y.T.; Wu, T.S.; Huang, J.Y.; Huang, W.T.; Lee, T.L.; Kou, G.H.; et al. A 3D model of the membrane protein complex formed by the white spot syndrome virus structural proteins. PLoS ONE 2010, 5, e10718. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Bosch, B.J.; Vlak, J.M.; Van Oers, M.M.; Rottier, P.J.; Ven Lent, J.W.M. Budded baculovirus particle structure revisited. J. Invertebr. Pathol. 2016, 134, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wege, C.; Koch, C. From stars to stripes: RNA-directed shaping of plant viral protein templates-structural synthetic virology for smart biohybrid nanostructures. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 12, e1591. [Google Scholar] [CrossRef]
- Lata, R.; Conway, J.F.; Cheng, N.; Duda, R.L.; Hendrix, R.W.; Wikoff, W.R.; Johnson, J.E.; Tsuruta, H.; Steven, A.C. Maturation dynamics of a viral capsid: Visualization of transitional intermediate states. Cell 2000, 100, 253–263. [Google Scholar] [CrossRef]
- Escobedo-Bonilla, C.M.; Alday-Sanz, V.; Wille, M.; Sorgeloos, P.; Pensaert, M.B.; Nauwynck, H.J. A review on the morphology, molecular characterization, morphogenesis and pathogenesis of white spot syndrome virus. J. Fish Dis. 2008, 31, 1–18. [Google Scholar] [CrossRef]
- Sánchez-Paz, A. White spot syndrome virus: An overview on an emergent concern. Vet. Res. 2010, 41, 43. [Google Scholar] [CrossRef]
- Verbruggen, B.; Bickley, L.K.; van Aerle, R.; Bateman, K.S.; Stentiford, G.D.; Santos, E.M.; Tyler, C.R. Molecular mechanisms of white spot syndrome virus infection and perspectives on treatments. Viruses 2016, 8, 23. [Google Scholar] [CrossRef]
- Huang, Z.J.; Kang, S.T.; Leu, J.H.; Chen, L.L. Endocytic pathway is indicated for white spot syndrome virus (WSSV) entry in shrimp. Fish Shellfish Immunol. 2013, 35, 707–715. [Google Scholar] [CrossRef]
- Huang, J.; Li, F.; Wu, J.; Yang, F. White spot syndrome virus enters crayfish hematopoietic tissue cells via clathrin-mediated endocytosis. Virology 2015, 486, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Shen, K.; Chen, Z.; Fan, W.; Xie, X.; Meng, C.; Chang, X.; Zheng, L.; Jeswin, J.; Li, C.; et al. White spot syndrome virus entry is dependent on multiple endocytic routes and strongly facilitated by Cq-GABARAP in a CME-dependent manner. Sci. Rep. 2016, 6, 28694. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Desmarets, L.M.B.; De Gryse, G.M.A.; Theuns, S.; Van Tuan, V.; Van Thuong, K.; Bossier, P.; Nauwynck, H.J. Virus replication cycle of white spot syndrome virus in secondary cell cultures from the lymphoid organ of Litopenaeus vannamei. J. Gen. Virol. 2015, 96, 2844–2854. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Liu, L.K.; Li, D.L.; Gao, R.L.; Fan, W.W.; Wang, K.J.; Wang, H.C.; Liu, H.P. White spot syndrome virus benefits from endosomal trafficking, substantially facilitated by a valosin-containing protein, to escape autophagic elimination and propagate in crustacean Cherax quadricarinatus. J. Virol. 2020, 94, e01570-20. [Google Scholar] [CrossRef]
- Wang, H.C.; Lin, A.T.; Yii, D.M.; Chang, Y.S.; Kou, G.H.; Lo, C.F. DNA microarrays of the white spot syndrome virus genome: Genes expressed in the gills of infected shrimp. Mar. Biotechnol. 2004, 6, S106–S111. [Google Scholar]
- Chen, I.T.; Aoki, T.; Huang, Y.T.; Hirono, I.; Chen, T.C.; Huang, J.Y.; Chang, G.D.; Lo, C.F.; Wang, H.C. White spot syndrome virus induces metabolic changes resembling the Warburg effect in shrimp hemocytes in the early stage of infection. J. Virol. 2011, 85, 12919–12928. [Google Scholar] [CrossRef]
- Wang, Y.G.; Hassan, M.D.; Shariff, M.; Zamri, S.M.; Chen, X. Histopathology and cytopathology of white spot syndrome virus (WSSV) in cultured Penaeus monodon from peninsular Malaysia with emphasis on pathogenesis and the mechanism of white spot formation. Dis. Aquat. Organ. 1999, 39, 1–11. [Google Scholar] [CrossRef]
- Witteveldt, J.; Vermeesch, A.M.; Langenhof, M.; de Lang, A.; Vlak, J.M.; van Hulten, M.C. Nucleocapsid protein VP15 is the basic DNA binding protein of white spot syndrome virus of shrimp. Arch. Virol. 2005, 150, 1121–1133. [Google Scholar] [CrossRef]








| Type of WSSV Nucleocapsid | Narrow Type | Wide Type |
|---|---|---|
| Electron microscope | Talos Arctica | Talos Arctica |
| Detector | Falcon Ⅲ | Falcon Ⅲ |
| Magnification | 73,000× | 73,000× |
| Voltage(kV) | 200 | 200 |
| Electron exposure (e−/Å2) | 48 | 48 |
| Exposure (s) | 2.5 | 2.5 |
| Frames (no.) | 50 | 50 |
| Defocus range (um) | −0.5~−2.5 | −0.5~−2.5 |
| Pixel size (Å) | 2.76 | 2.76 |
| Symmetry imposed | C14 | C14 |
| Initial particles | 55,723 | 55,723 |
| Final particles | 15,279 | 15,243 |
| Map resolution (Å) | 5.52 | 5.52 |
| FSC threshold | 0.143 | 0.143 |
| Software | Relion and cisTEM | Relion and cisTEM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-J.; Tang, S.-L.; Chang, Y.-C.; Wang, H.-C.; Ng, T.H.; Garmann, R.F.; Chen, Y.-W.; Huang, J.-Y.; Kumar, R.; Chang, S.-H.; et al. Multiple Nucleocapsid Structural Forms of Shrimp White Spot Syndrome Virus Suggests a Novel Viral Morphogenetic Pathway. Int. J. Mol. Sci. 2023, 24, 7525. https://doi.org/10.3390/ijms24087525
Huang H-J, Tang S-L, Chang Y-C, Wang H-C, Ng TH, Garmann RF, Chen Y-W, Huang J-Y, Kumar R, Chang S-H, et al. Multiple Nucleocapsid Structural Forms of Shrimp White Spot Syndrome Virus Suggests a Novel Viral Morphogenetic Pathway. International Journal of Molecular Sciences. 2023; 24(8):7525. https://doi.org/10.3390/ijms24087525
Chicago/Turabian StyleHuang, Hui-Ju, Sen-Lin Tang, Yuan-Chih Chang, Hao-Ching Wang, Tze Hann Ng, Rees F. Garmann, Yu-Wen Chen, Jiun-Yan Huang, Ramya Kumar, Sheng-Hsiung Chang, and et al. 2023. "Multiple Nucleocapsid Structural Forms of Shrimp White Spot Syndrome Virus Suggests a Novel Viral Morphogenetic Pathway" International Journal of Molecular Sciences 24, no. 8: 7525. https://doi.org/10.3390/ijms24087525
APA StyleHuang, H.-J., Tang, S.-L., Chang, Y.-C., Wang, H.-C., Ng, T. H., Garmann, R. F., Chen, Y.-W., Huang, J.-Y., Kumar, R., Chang, S.-H., Wu, S.-R., Chao, C.-Y., Matoba, K., Kenji, I., Gelbart, W. M., Ko, T.-P., Wang, H.-J., Lo, C.-F., Chen, L.-L., & Wang, H.-C. (2023). Multiple Nucleocapsid Structural Forms of Shrimp White Spot Syndrome Virus Suggests a Novel Viral Morphogenetic Pathway. International Journal of Molecular Sciences, 24(8), 7525. https://doi.org/10.3390/ijms24087525








