High-Loaded Copper-Containing Sol–Gel Catalysts for Furfural Hydroconversion
Abstract
:1. Introduction
2. Results and Discussion
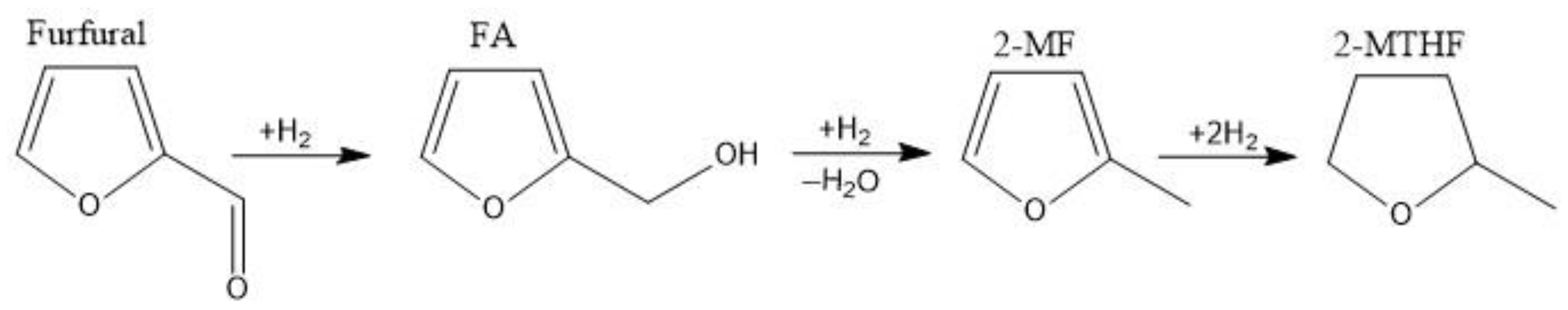
2.1. Furfural Hydroconversion in the Presence of Monometallic Copper Catalysts
2.2. Furfural Hydroconversion in the Presence of Copper-Containing Catalysts Modified with Iron and Aluminum
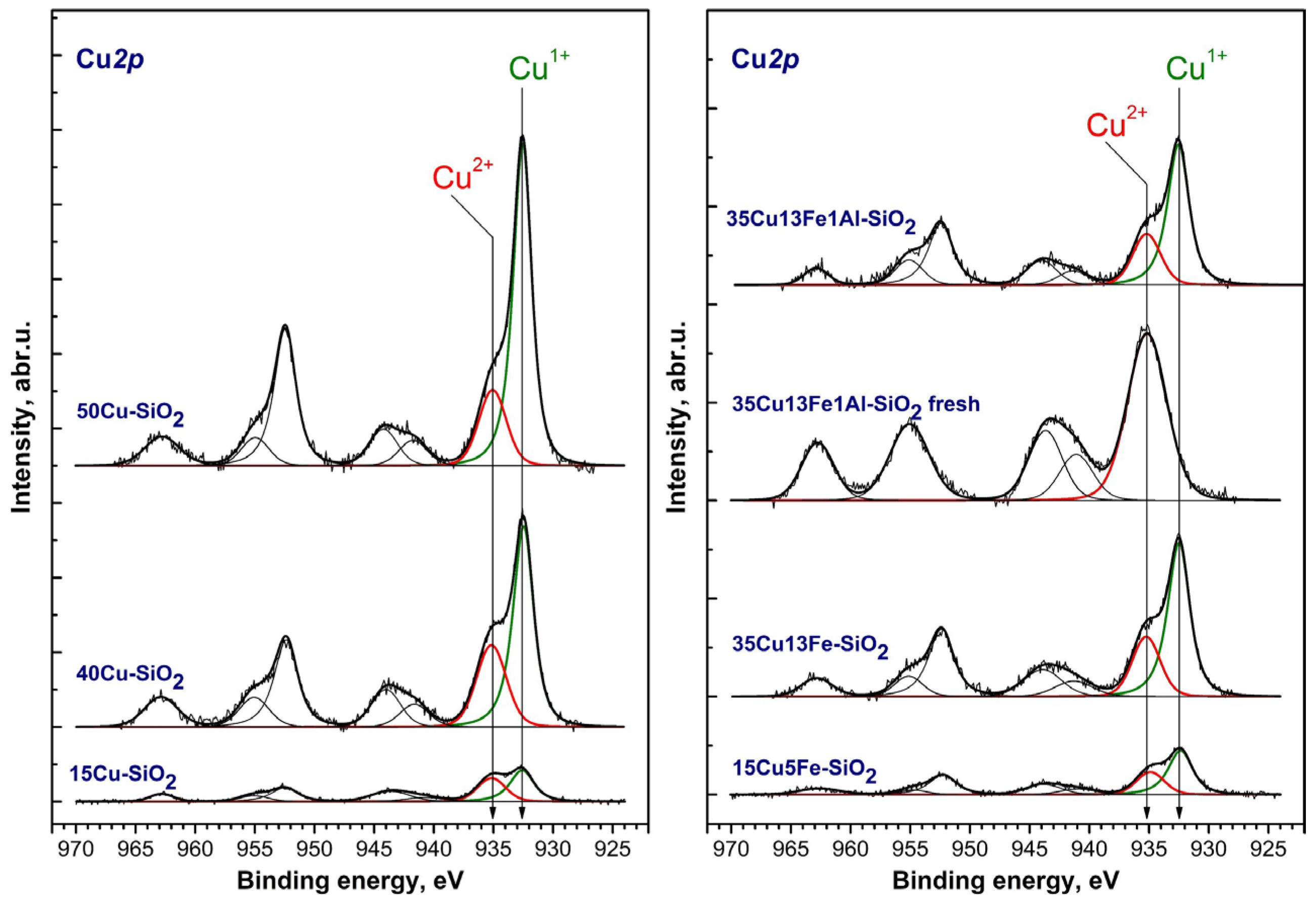
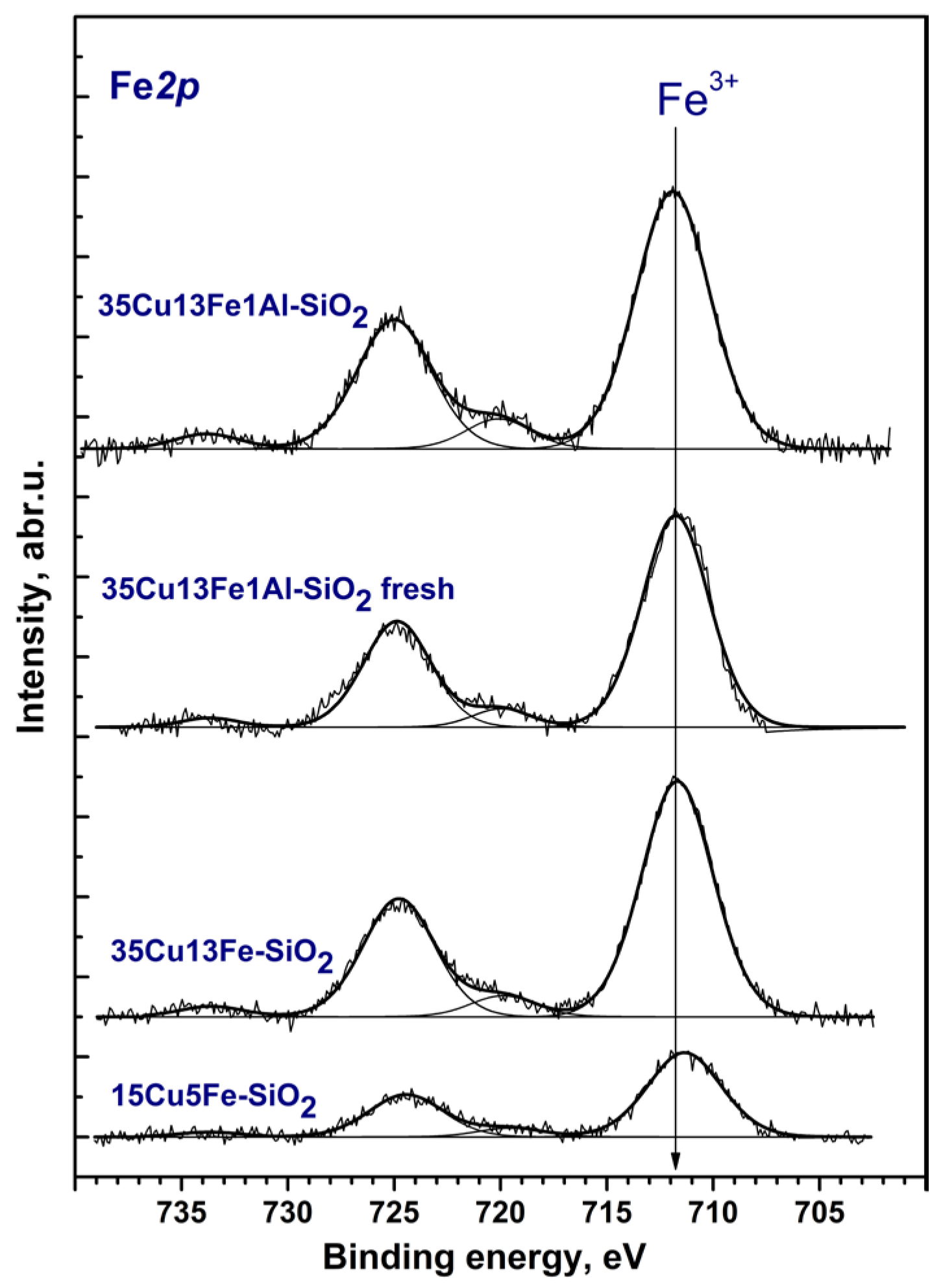
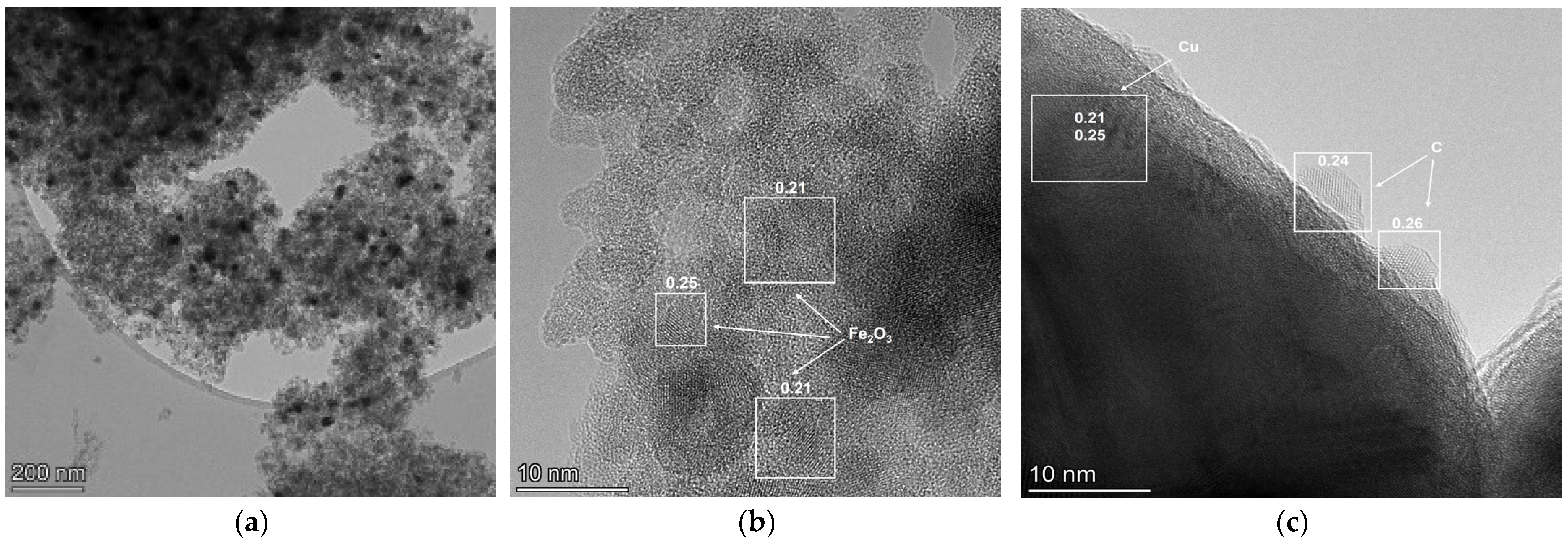
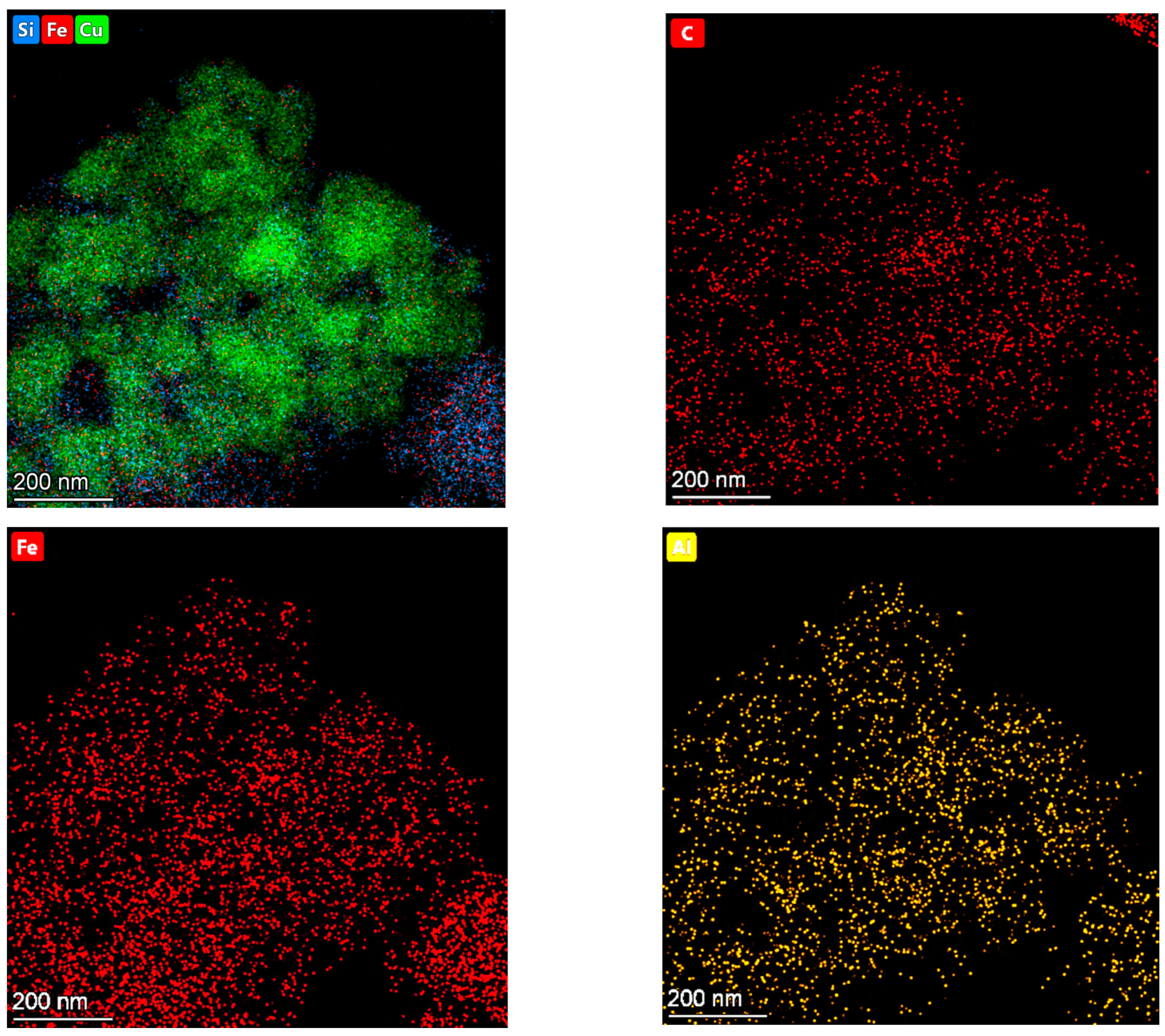
2.3. Catalyst Characterization
3. Materials and Methods
3.1. Chemicals
3.2. Catalyst Preparation
3.3. Catalyst Activity Tests
3.4. Product Analysis
3.5. Elemental Composition of Catalysts
3.6. Texture Characteristics
3.7. CO Chemosorption
3.8. High-Resolution Transmission Electron Microscopy
3.9. X-ray Diffraction
3.10. X-ray Photoelectron Spectroscopy (XPS)
3.11. Determination of Carbon Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; US Department of Energy: Washington, DC, USA, 2004. [Google Scholar]
- Cai, C.M.; Zhang, T.; Kumar, R.; Wyman, C.E. Integrated Furfural Production as a Renewable Fuel and Chemical Platform from Lignocellulosic Biomass. J. Chem. Technol. Biotechnol. 2014, 89, 2–10. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; Granados, M.L. Furfural: A Renewable and Versatile Platform Molecule for the Synthesis of Chemicals and Fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Khemthong, P.; Yimsukanan, C.; Narkkun, T.; Srifa, A.; Witoon, T.; Pongchaiphol, S.; Kiatphuengporn, S.; Faungnawakij, K. Advances in Catalytic Production of Value-Added Biochemicals and Biofuels via Furfural Platform Derived Lignocellulosic Biomass. Biomass Bioenergy 2021, 148, 106033. [Google Scholar] [CrossRef]
- Jaswal, A.; Singh, P.P.; Mondal, T. Furfural—A Versatile, Biomass-Derived Platform Chemical for the Production of Renewable Chemicals. Green Chem. 2022, 24, 510–551. [Google Scholar] [CrossRef]
- Xian, M. Sustainable Production of Bulk Chemicals: Integration of Bio-,Chemo- Resources and Processes (Springerbriefs in Molecular Science), 1st ed.; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 94-017-7473-0. [Google Scholar]
- Chheda, J.N.; Dumesic, J.A. An Overview of Dehydration, Aldol-Condensation and Hydrogenation Processes for Production of Liquid Alkanes from Biomass-Derived Carbohydrates. Catal. Today 2007, 123, 59–70. [Google Scholar] [CrossRef]
- Huber, G.W.; Chheda, J.N.; Barrett, C.J.; Dumesic, J.A. Production of Liquid Alkanes by Aqueous-Phase Processing of Biomass-Derived Carbohydrates. Science 2005, 308, 1446–1450. [Google Scholar] [CrossRef]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-Phase Catalytic Processing of Biomass-Derived Oxygenated Hydrocarbons to Fuels and Chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Chen, A. Efficient Hydrogenation of Biomass-Derived Furfural and Levulinic Acid on the Facilely Synthesized Noble-Metal-Free Cu–Cr Catalyst. Energy 2013, 58, 357–363. [Google Scholar] [CrossRef]
- Sulmonetti, T.P.; Hu, B.; Ifkovits, Z.; Lee, S.; Agrawal, P.K.; Jones, C.W. Vapor Phase Hydrogenolysis of Furanics Utilizing Reduced Cobalt Mixed Metal Oxide Catalysts. ChemCatChem 2017, 9, 1815–1823. [Google Scholar] [CrossRef]
- Date, N.S.; Hengne, A.M.; Huang, K.-W.; Chikate, R.C.; Rode, C.V. Single Pot Selective Hydrogenation of Furfural to 2-Methylfuran over Carbon Supported Iridium Catalysts. Green Chem. 2018, 20, 2027–2037. [Google Scholar] [CrossRef]
- Varila, T.; Mäkelä, E.; Kupila, R.; Romar, H.; Hu, T.; Karinen, R.; Puurunen, R.L.; Lassi, U. Conversion of Furfural to 2-Methylfuran over CuNi Catalysts Supported on Biobased Carbon Foams. Catal. Today 2021, 367, 16–27. [Google Scholar] [CrossRef]
- Corma, A.; de la Torre, O.; Renz, M.; Villandier, N. Production of High-Quality Diesel from Biomass Waste Products. Angew. Chem. Int. Ed. 2011, 50, 2375–2378. [Google Scholar] [CrossRef]
- Corma, A.; de la Torre, O.; Renz, M. Production of High Quality Diesel from Cellulose and Hemicellulose by the Sylvan Process: Catalysts and Process Variables. Energy Environ. Sci. 2012, 5, 6328–6344. [Google Scholar] [CrossRef]
- Zeitsch, K.J. The Chemistry and Technology of Furfural and Its Many By-Products; Elsevier: Amsterdam, The Netherlands, 2000; ISBN 978-0-08-052899-1. [Google Scholar]
- Taylor, M.J.; Durndell, L.J.; Isaacs, M.A.; Parlett, C.M.A.; Wilson, K.; Lee, A.F.; Kyriakou, G. Highly Selective Hydrogenation of Furfural over Supported Pt Nanoparticles under Mild Conditions. Appl. Catal. B Environ. 2016, 180, 580–585. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, Properties and Catalytic Hydrogenation of Furfural to Fuel Additives and Value-Added Chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Rodríguez-Padrón, D.; Len, C. Recent Advances in Catalytic Hydrogenation of Furfural. Catalysts 2019, 9, 796. [Google Scholar] [CrossRef]
- Schneider, M.H.; Phillips, J.G. Furfuryl Alcohol and Lignin Adhesive Composition. U.S. Patent 6,747,076, 8 June 2004. [Google Scholar]
- Barr, J.B.; Wallon, S.B. The Chemistry of Furfuryl Alcohol Resins. J. Appl. Polym. Sci. 1971, 15, 1079–1090. [Google Scholar] [CrossRef]
- An, Z.; Li, J. Recent Advances in the Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol over Heterogeneous Catalysts. Green Chem. 2022, 24, 1780–1808. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Mahajani, V.V. Kinetics of Liquid-Phase Hydrogenation of Furfuraldehyde to Furfuryl Alcohol over a Pt/C Catalyst. Ind. Eng. Chem. Res. 2003, 42, 3881–3885. [Google Scholar] [CrossRef]
- Sitthisa, S.; Sooknoi, T.; Ma, Y.; Balbuena, P.B.; Resasco, D.E. Kinetics and Mechanism of Hydrogenation of Furfural on Cu/SiO2 Catalysts. J. Catal. 2011, 277, 1. [Google Scholar] [CrossRef]
- Vasil’ev, S.N.; Gamova, I.A.; de Vekki, A.V. Novy Spravochnik Himika i Tehnologa. Syr’e i Produkty Promyshlennosti Organicheskih i Neorganicheskih Veshchestv; NPO “Professional”: Sankt-Peterburg, Russia, 2005; p. 1142. [Google Scholar]
- Adkins, H.; Connor, R. The Catalytic Hydrogenation of Organic Compounds over Copper Chromite. J. Am. Chem. Soc. 1931, 53, 1091–1095. [Google Scholar] [CrossRef]
- Wojcik, B.H. Catalytic Hydrogenation of Furan Compounds. Ind. Eng. Chem. 1948, 40, 210–216. [Google Scholar] [CrossRef]
- Zhang, H.; Lei, Y.; Kropf, A.J.; Zhang, G.; Elam, J.W.; Miller, J.T.; Sollberger, F.; Ribeiro, F.; Akatay, M.C.; Stach, E.A.; et al. Enhancing the Stability of Copper Chromite Catalysts for the Selective Hydrogenation of Furfural Using ALD Overcoating. J. Catal. 2014, 317, 284–292. [Google Scholar] [CrossRef]
- Liu, D.; Zemlyanov, D.; Wu, T.; Lobo-Lapidus, R.J.; Dumesic, J.A.; Miller, J.T.; Marshall, C.L. Deactivation Mechanistic Studies of Copper Chromite Catalyst for Selective Hydrogenation of 2-Furfuraldehyde. J. Catal. 2013, 299, 336–345. [Google Scholar] [CrossRef]
- Nguyen-Huy, C.; Kim, J.S.; Yoon, S.; Yang, E.; Kwak, J.H.; Lee, M.S.; An, K. Supported Pd Nanoparticle Catalysts with High Activities and Selectivities in Liquid-Phase Furfural Hydrogenation. Fuel 2018, 226, 607–617. [Google Scholar] [CrossRef]
- Mäkelä, E.; Lahti, R.; Jaatinen, S.; Romar, H.; Hu, T.; Puurunen, R.L.; Lassi, U.; Karinen, R. Study of Ni, Pt, and Ru Catalysts on Wood-Based Activated Carbon Supports and Their Activity in Furfural Conversion to 2-Methylfuran. ChemCatChem 2018, 10, 3269–3283. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Zhang, C.; Arai, M.; Zhou, L.; Zhao, F. Selective Hydrogenation of Furfural to Furfuryl Alcohol over Pd/TiH2 Catalyst. Mol. Catal. 2021, 508, 111599. [Google Scholar] [CrossRef]
- Sitthisa, S.; Resasco, D.E. Hydrodeoxygenation of Furfural Over Supported Metal Catalysts: A Comparative Study of Cu, Pd and Ni. Catal. Lett. 2011, 141, 784. [Google Scholar] [CrossRef]
- Villaverde, M.M.; Bertero, N.M.; Garetto, T.F.; Marchi, A.J. Selective Liquid-Phase Hydrogenation of Furfural to Furfuryl Alcohol over Cu-Based Catalysts. Catal. Today 2013, 213, 87–92. [Google Scholar] [CrossRef]
- Vetere, V.; Merlo, A.B.; Ruggera, J.F.; Casella, M.L. Transition Metal-Based Bimetallic Catalysts for the Chemoselective Hydrogenation of Furfuraldehyde. J. Braz. Chem. Soc. 2010, 21, 914–920. [Google Scholar] [CrossRef]
- Yan, K.; Chen, A. Selective Hydrogenation of Furfural and Levulinic Acid to Biofuels on the Ecofriendly Cu–Fe Catalyst. Fuel 2014, 115, 101–108. [Google Scholar] [CrossRef]
- Rao, T.U.; Suchada, S.; Choi, C.; Machida, H.; Huo, Z.; Norinaga, K. Selective Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol in 2-Butanol over an Equimolar Ni-Cu-Al Catalyst Prepared by the Co-Precipitation Method. Energy Convers. Manag. 2022, 265, 115736. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, D. Aqueous Phase Catalytic Hydrogenation of Furfural to Furfuryl Alcohol over In-Situ Synthesized Cu–Zn/SiO2 Catalysts. Mater. Chem. Phys. 2021, 260, 124152. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, G.; Zhu, Q.; Kong, X. CuZn@N-doped Graphene Layer for Upgrading of Furfural to Furfuryl Alcohol. Mol. Catal. 2022, 517, 112066. [Google Scholar] [CrossRef]
- Smirnov, A.A.; Shilov, I.N.; Alekseeva, M.V.; Selishcheva, S.A.; Yakovlev, V.A. Study of the Composition Effect of Molybdenum-Modified Nickel–Copper Catalysts on Their Activity and Selectivity in the Hydrogenation of Furfural to Different Valuable Chemicals. Catal. Ind. 2018, 10, 228–236. [Google Scholar] [CrossRef]
- Vargas-Hernández, D.; Rubio-Caballero, J.M.; Santamaría-González, J.; Moreno-Tost, R.; Mérida-Robles, J.M.; Pérez-Cruz, M.A.; Jiménez-López, A.; Hernández-Huesca, R.; Maireles-Torres, P. Furfuryl Alcohol from Furfural Hydrogenation over Copper Supported on SBA-15 Silica Catalysts. J. Mol. Catal. A Chem. 2014, 383–384, 106–113. [Google Scholar] [CrossRef]
- Rajabi, F.; Arancon, R.A.D.; Luque, R. Oxidative Esterification of Alcohols and Aldehydes Using Supported Iron Oxide Nanoparticle Catalysts. Catal. Commun. 2015, 59, 101–103. [Google Scholar] [CrossRef]
- Luo, Z.; Li, R.; Zhu, T.; Liu, C.-F.; Feng, H.; Nartey, K.A.; Liu, Q.; Xu, X. Iron-Catalyzed Oxidative Decabonylation/Radical Cyclization of Aliphatic Aldehydes with Biphenyl Isocyanides: A New Pathway For the Synthesis of 6-Alkylphenanthridines. Asian J. Org. Chem. 2021, 10, 926–930. [Google Scholar] [CrossRef]
- Wang, W.D.; Wang, F.; Chang, Y.; Dong, Z. Biomass Chitosan-Derived Nitrogen-Doped Carbon Modified with Iron Oxide for the Catalytic Ammoxidation of Aromatic Aldehydes to Aromatic Nitriles. Mol. Catal. 2021, 499, 111293. [Google Scholar] [CrossRef]
- Yeletsky, P.M.; Zaikina, O.O.; Sosnin, G.A.; Kukushkin, R.G.; Yakovlev, V.A. Heavy Oil Cracking in the Presence of Steam and Nanodispersed Catalysts Based on Different Metals. Fuel Process. Technol. 2020, 199, 106239. [Google Scholar] [CrossRef]
- Ma, M.; Hou, P.; Zhang, P.; Cao, J.; Liu, H.; Yue, H.; Tian, G.; Feng, S. Magnetic Fe3O4 Nanoparticles as Easily Separable Catalysts for Efficient Catalytic Transfer Hydrogenation of Biomass-Derived Furfural to Furfuryl Alcohol. Appl. Catal. A Gen. 2020, 602, 117709. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Ko, B.-T.; Huang, B.-H.; Lin, C.-C. Reduction of Aldehydes and Ketones Catalyzed by a Novel Aluminum Alkoxide: Mechanistic Studies of Meerwein−Ponndorf−Verley Reaction. Organometallics 2002, 21, 2066–2069. [Google Scholar] [CrossRef]
- Ermakova, M.A.; Ermakov, D.Y. High-Loaded Nickel–Silica Catalysts for Hydrogenation, Prepared by Sol–Gel: Route: Structure and Catalytic Behavior. Appl. Catal. A Gen. 2003, 245, 277–288. [Google Scholar] [CrossRef]
- Ermakov, D.Y.; Bykova, M.V.; Selishcheva, S.A.; Khromova, S.A.; Yakovlev, V.A. Method of Preparing Hydrotreatment Catalyst. Patent RU2496580, 27 October 2013. [Google Scholar]
- Asedegbega Nieto, E.; Ruiz, A.; Rodriguez-Ramos, I. Study of CO Chemisorption on Graphite-Supported Ru–Cu and Ni–Cu Bimetallic Catalysts. Thermochim. Acta 2005, 434, 113–118. [Google Scholar] [CrossRef]
- Parris, G.E.; Klier, K. The Specific Copper Surface Areas in CuZnO Methanol Synthesis Catalysts by Oxygen and Carbon Monoxide Chemisorption: Evidence for Irreversible CO Chemisorption Induced by the Interaction of the Catalyst Components. J. Catal. 1986, 97, 374–384. [Google Scholar] [CrossRef]
- Phillips, J.M.; Leibsle, F.M.; Holder, A.J.; Keith, T. A Comparative Study of Chemisorption by Density Functional Theory, Ab Initio, and Semiempirical Methods: Carbon Monoxide, Formate, and Acetate on Cu(110). Surf. Sci. 2003, 545, 1–7. [Google Scholar] [CrossRef]
- Smirnov, A.A.; Khromova, S.A.; Bulavchenko, O.A.; Kaichev, V.V.; Saraev, A.A.; Reshetnikov, S.I.; Bykova, M.V.; Trusov, L.I.; Yakovlev, V.A. Effect of the Ni/Cu Ratio on the Composition and Catalytic Properties of Nickel-Copper Alloy in Anisole Hydrodeoxygenation. Kinet Catal. 2014, 55, 69–78. [Google Scholar] [CrossRef]
- Gutowski, M.; Jaffe, J.E.; Liu, C.-L.; Stoker, M.; Hegde, R.I.; Rai, R.S.; Tobin, P.J. Thermodynamic Stability of High-K Dielectric Metal Oxides ZrO2 and HfO2 in Contact with Si and SiO2. Appl. Phys. Lett. 2002, 80, 1897–1899. [Google Scholar] [CrossRef]
- Khassin, A.A.; Yurieva, T.M.; Demeshkina, M.P.; Kustova, G.N.; Itenberg, I.S.; Kaichev, V.V.; Plyasova, L.M.; Anufrienko, V.F.; Molina, I.Y.; Larina, T.V.; et al. Characterization of the Nickel-Amesite-Chlorite-Vermiculite System. Phys. Chem. Chem. Phys. 2003, 5, 4025–4031. [Google Scholar] [CrossRef]
- Kim, S.; Park, Y.M.; Choi, S.-H.; Kim, K.J.; Choi, D.H. Temperature-Dependent Carrier Recombination Processes in Nanocrystalline Si/SiO2 Multilayers Studied by Continuous-Wave and Time-Resolved Photoluminescence. J. Phys. D Appl. Phys. 2007, 40, 1339. [Google Scholar] [CrossRef]
- Batista, J.; Mandrino, D.; Jenko, M.; Martin, V. XPS and TPR Examinations of γ-Alumina-Supported Pd-Cu Catalysts. Appl. Catal. A Gen. 2001, 206, 113–124. [Google Scholar] [CrossRef]
- Wöllner, A.; Lange, F.; Schmelz, H.; Knözinger, H. Characterization of Mixed Copper-Manganese Oxides Supported on Titania Catalysts for Selective Oxidation of Ammonia. Appl. Catal. A Gen. 1993, 94, 181–203. [Google Scholar] [CrossRef]
- Moretti, G. Auger Parameter and Wagner Plot in the Characterization of Chemical States: Initial and Final State Effects. J. Electron Spectrosc. Relat. Phenom. 1995, 76, 365–370. [Google Scholar] [CrossRef]
- Descostes, M.; Mercier, F.; Thromat, N.; Beaucaire, C.; Gautier-Soyer, M. Use of XPS in the Determination of Chemical Environment and Oxidation State of Iron and Sulfur Samples: Constitution of a Data Basis in Binding Energies for Fe and S Reference Compounds and Applications to the Evidence of Surface Species of an Oxidized Pyrite in a Carbonate Medium. Appl. Surf. Sci. 2000, 165, 288–302. [Google Scholar] [CrossRef]
- Tan, B.J.; Klabunde, K.J.; Sherwood, P.M. X-ray Photoelectron Spectroscopy Studies of Solvated Metal Atom Dispersed Catalysts. Monometallic Iron and Bimetallic Iron-Cobalt Particles on Alumina. Chem. Mater. 1990, 2, 186–191. [Google Scholar] [CrossRef]
- Selishchev, D.; Svintsitskiy, D.; Kovtunova, L.; Gerasimov, E.; Gladky, A.; Kozlov, D. Surface Modification of TiO2 with Pd Nanoparticles for Enhanced Photocatalytic Oxidation of Benzene Micropollutants. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125959. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater Subshell Photoionization Cross-Sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Copyright© 2005 Casa Software Ltd. Available online: http://www.casaxps.com/ (accessed on 28 February 2023).
- Kovalevskiy, N.; Svintsitskiy, D.; Cherepanova, S.; Yakushkin, S.; Martyanov, O.; Selishcheva, S.; Gribov, E.; Kozlov, D.; Selishchev, D. Visible-Light-Active N-Doped TiO2 Photocatalysts: Synthesis from TiOSO4, Characterization, and Enhancement of Stability Via Surface Modification. Nanomaterials 2022, 12, 4146. [Google Scholar] [CrossRef] [PubMed]
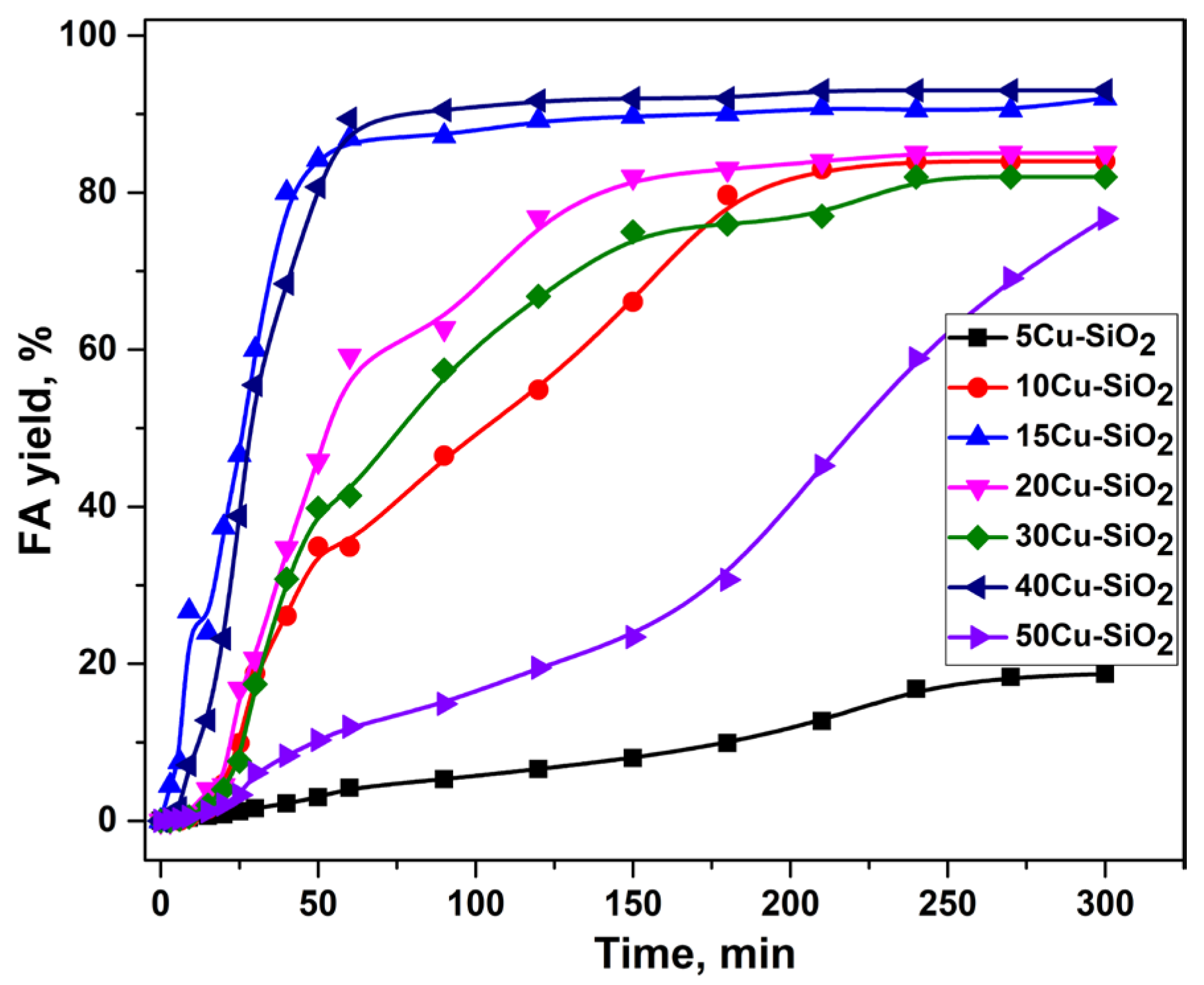

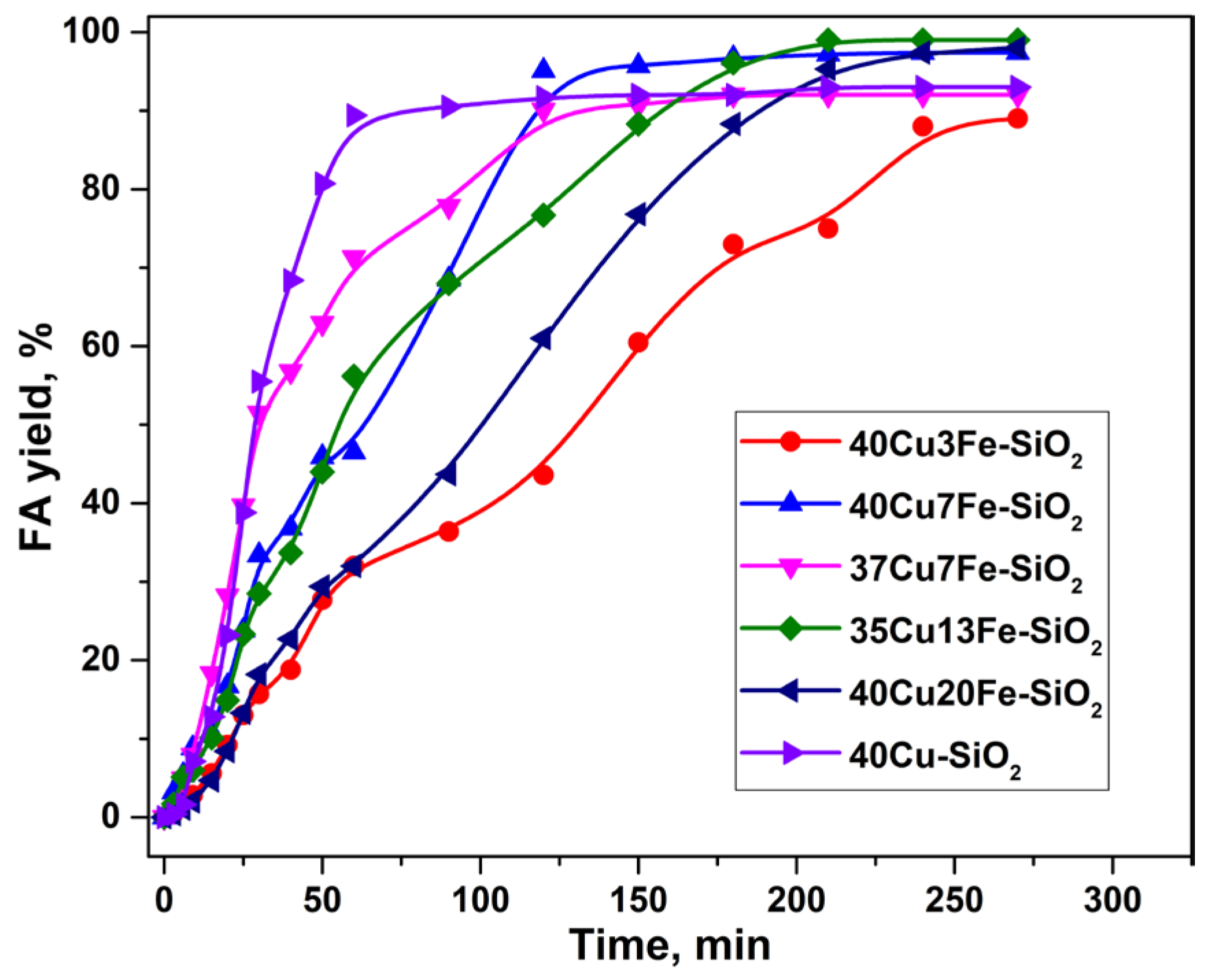
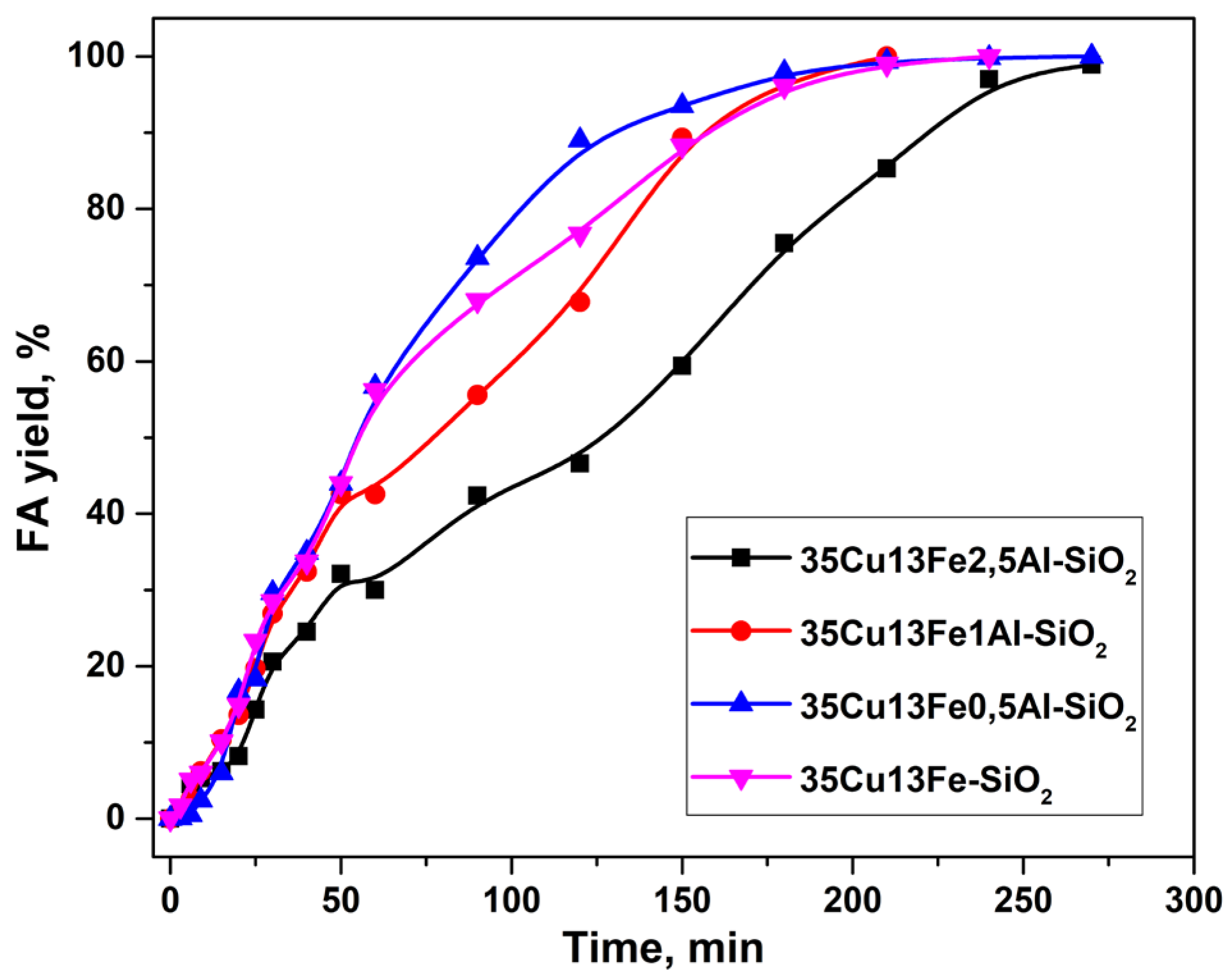
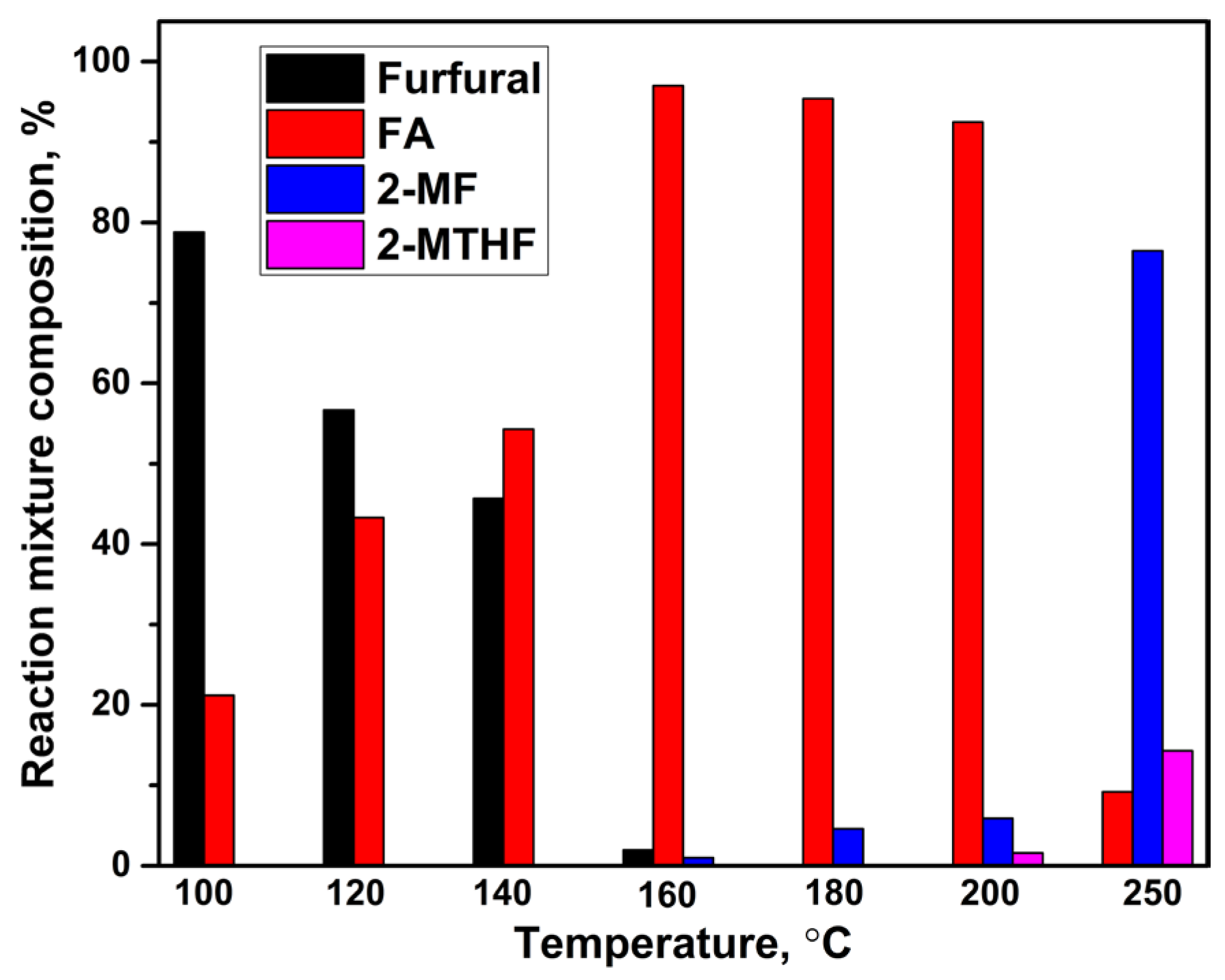

| Catalyst | CO Uptake, µmol/gcat. | Yield of FA, % |
|---|---|---|
| HomSG series a | ||
| 5Cu-SiO2 | 26 | 19 |
| 10Cu-SiO2 | 65 | 84 |
| 15Cu-SiO2 | 89 | 92 |
| 20Cu-SiO2 | 80 | 85 |
| 30Cu-SiO2 | 65 | 82 |
| HetSG series b | ||
| 40Cu-SiO2 | 86 | 93 |
| 50Cu-SiO2 | 45 | 77 |
| Catalyst a,b | SSA c, m2/g | V∑, cm3/g | Phase Composition/CSR, Å d |
|---|---|---|---|
| HomSG series e | |||
| 5Cu-SiO2 | 156 | 0.71 | SiO2 (amorph. f)/- g |
| 10Cu-SiO2 | 210 | 0.73 | SiO2 (amorph.)/- CuO/40 |
| 15Cu-SiO2 | 290 | 0.78 | SiO2 (amorph.)/- CuO/40 |
| 20Cu-SiO2 | 320 | 0.78 | SiO2 (amorph.)/- CuO/50 |
| 30Cu-SiO2 | 329 | 0.82 | SiO2 (amorph.)/- CuO/90 |
| 10Cu10Fe-SiO2 (0.9) h | 215 | 1.05 | SiO2 (amorph.)/- |
| 15Cu5Fe-SiO2 (2.4) | 257 | 1.03 | SiO2 (amorph.)/- |
| 15Cu3Fe-SiO2 (4.4) | 262 | 1.00 | SiO2 (amorph.)/- |
| 15Cu2Fe-SiO2 (5.5) | 270 | 0.99 | SiO2 (amorph.)/- |
| HetSG series i | |||
| 40Cu-SiO2 | 83 | 0.45 | SiO2 (amorph.)/- CuO/130 |
| 50Cu-SiO2 | 40 | 0.20 | CuO/170 |
| 40Cu20Fe-SiO2 (1.8) | 360 | 0.72 | SiO2 (amorph.)/- CuO (trace) |
| 35Cu13Fe-SiO2 (2.4) | 348 | 0.73 | SiO2 (amorph.)/- CuO (trace)/- |
| 37Cu7Fe-SiO2 (4.4) | 330 | 0.72 | SiO2 (amorph.)/- CuO (trace)/- |
| 40Cu7Fe-SiO2 (5.5) | 323 | 0.72 | SiO2 (amorph.)/- CuO (trace)/- |
| 40Cu3Fe-SiO2 (11.6) | 310 | 0.72 | SiO2 (amorph.)/- CuO (trace)/- |
| 35Cu13Fe2.5Al-SiO2 (1) j | 235 | 0.75 | CuO/160 |
| 35Cu13Fe1Al-SiO2 (2) | 240 | 0.78 | CuO/170 |
| 35Cu13Fe0.5Al-SiO2 (4.6) | 260 | 0.82 | CuO/320 |
| Catalyst after Reaction | Phase Composition, % | CSR, Å |
|---|---|---|
| 40Cu-SiO2 | 30% Cu2O 70% Cu | 30 140 |
| 35Cu13Fe-SiO2 | 50% Cu2O 50% Cu | 50 140 |
| 35Cu13Fe1Al-SiO2 | 25% Cu2O 75% Cu | 55 240 |
| Catalyst | [Cu]/[Si] | [Fe]/[Si] | [O]/[Si] | [C]/[Si] | %, Cu2+ | %, Cu1+ | %, Cu0 |
|---|---|---|---|---|---|---|---|
| 15Cu-SiO2 | 0.082 | 0.000 | 2.27 | 0.51 | 55 | 45 | 0 |
| 40Cu-SiO2 | 0.378 | 0.000 | 2.33 | 1.75 | 47 | 53 | 0 |
| 50Cu-SiO2 | 0.519 | 0.000 | 2.40 | 2.32 | 36 | 64 | 0 |
| 15Cu5Fe-SiO2 | 0.074 | 0.025 | 2.32 | 0.77 | 47 | 53 | 0 |
| 35Cu13Fe-SiO2 | 0.224 | 0.068 | 2.44 | 0.87 | 43 | 57 | 0 |
| 35Cu13Fe1Al-SiO2 (fresh) | 0.336 | 0.053 | 2.27 | 0 | 100 | 0 | 0 |
| 35Cu13Fe1Al-SiO2 | 0.195 | 0.073 | 2.42 | 0.85 | 42 | 58 | 0 |
| Catalyst | Carbon Content, wt.% |
|---|---|
| 5Cu-SiO2 | 7.6 ± 0.5 |
| 10Cu-SiO2 | 4.5 ± 0.4 |
| 15Cu-SiO2 | 2.80 ± 0.05 |
| 20Cu-SiO2 | 5.68 ± 0.08 |
| 30Cu-SiO2 | 3.16 ± 0.02 |
| 40Cu-SiO2 | 3.0 ± 0.1 |
| 50Cu-SiO2 | 6.3 ± 0.6 |
| 10Cu10Fe-SiO2 | 8.8 ± 0.3 |
| 15Cu5Fe-SiO2 (2.4) | 5.8 ± 0.1 |
| 15Cu3Fe-SiO2 (4.4) | 6.82 ± 0.10 |
| 15Cu2Fe-SiO2 (5.5) | 6.2 ± 0.8 |
| 35Cu13Fe-SiO2 | 4.0 ± 0.2 |
| 40Cu20Fe-SiO2 | 4.33 ± 0.03 |
| 37Cu7Fe-SiO2 | 4.72 ± 0.03 |
| 40Cu7Fe-SiO2 | 3.9 ± 0.1 |
| 40Cu3Fe-SiO2 | 4.8 ± 0.5 |
| 35Cu13Fe0,5Al-SiO2 | 4.2 ± 0.6 |
| 35Cu13Fe1Al-SiO2 | 3.5 ± 0.2 |
| 35Cu13Fe2.5Al-SiO2 | 4.3 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selishcheva, S.; Sumina, A.; Gerasimov, E.; Selishchev, D.; Yakovlev, V. High-Loaded Copper-Containing Sol–Gel Catalysts for Furfural Hydroconversion. Int. J. Mol. Sci. 2023, 24, 7547. https://doi.org/10.3390/ijms24087547
Selishcheva S, Sumina A, Gerasimov E, Selishchev D, Yakovlev V. High-Loaded Copper-Containing Sol–Gel Catalysts for Furfural Hydroconversion. International Journal of Molecular Sciences. 2023; 24(8):7547. https://doi.org/10.3390/ijms24087547
Chicago/Turabian StyleSelishcheva, Svetlana, Anastasiya Sumina, Evgeny Gerasimov, Dmitry Selishchev, and Vadim Yakovlev. 2023. "High-Loaded Copper-Containing Sol–Gel Catalysts for Furfural Hydroconversion" International Journal of Molecular Sciences 24, no. 8: 7547. https://doi.org/10.3390/ijms24087547







