Photochemically Aided Arteriovenous Fistula Creation to Accelerate Fistula Maturation
Abstract
1. Introduction
2. Results
2.1. Sheep AVF Maturation at 1 Week and 4 Weeks Following Surgery
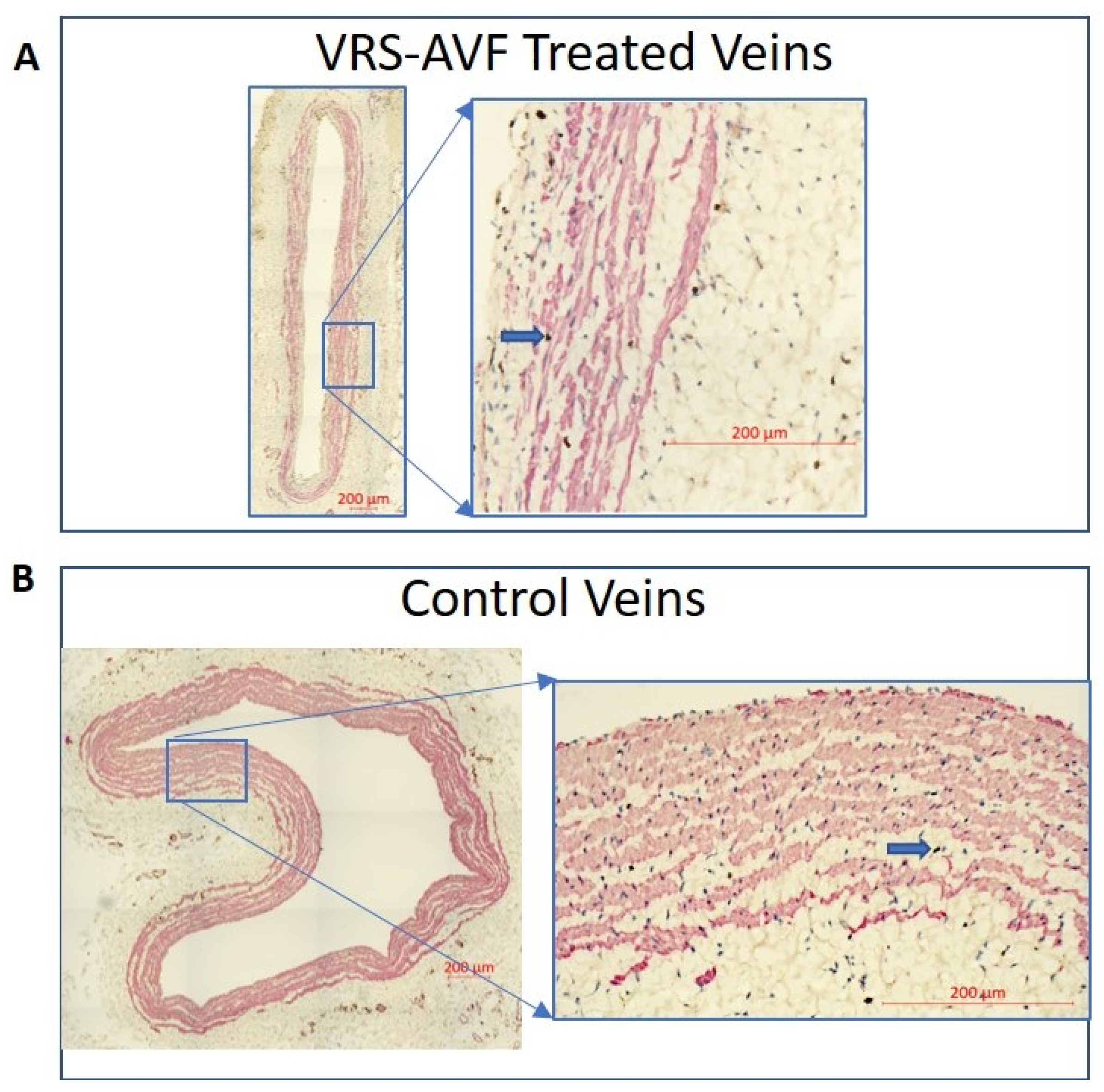
2.2. Evidence of Facilitated Outward Remodeling Following VRS-AVF Treatment (Ki67 and SMC Staining)
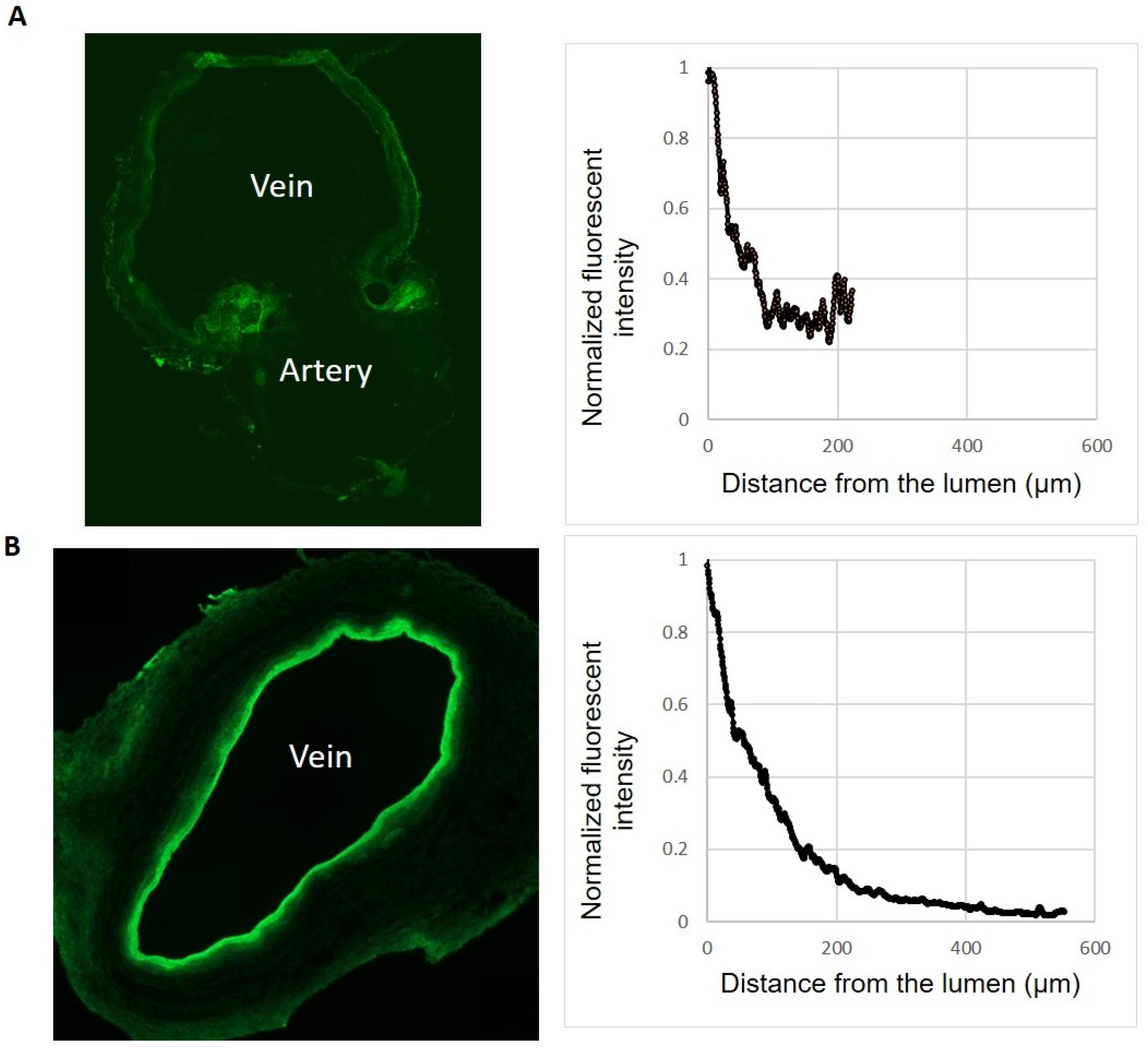
2.3. Sheep vs. Human Vein Wall Size and Delivery of the 10-8-10 Dimer
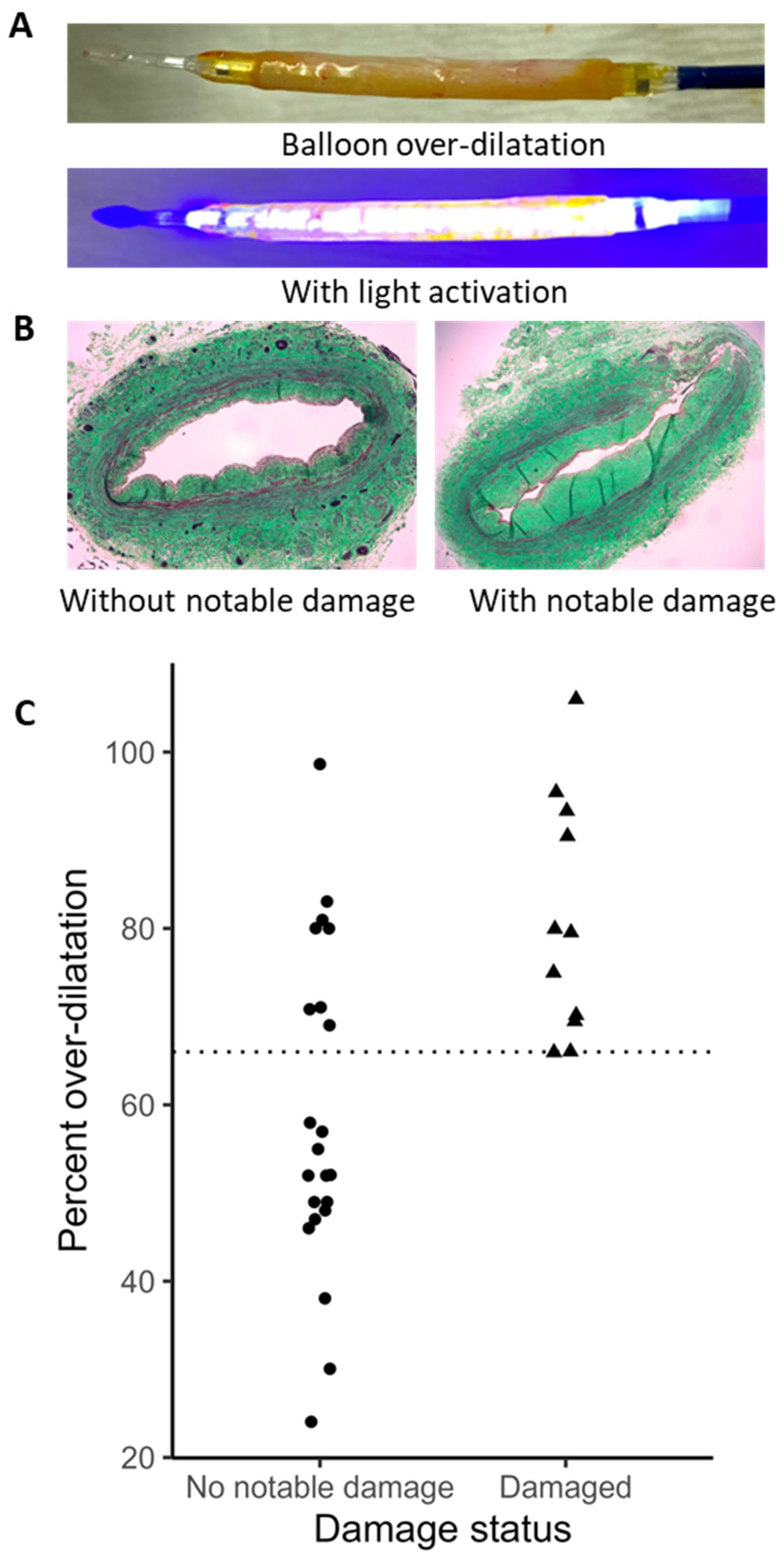
2.4. Human Vein Distension and Biomechanics
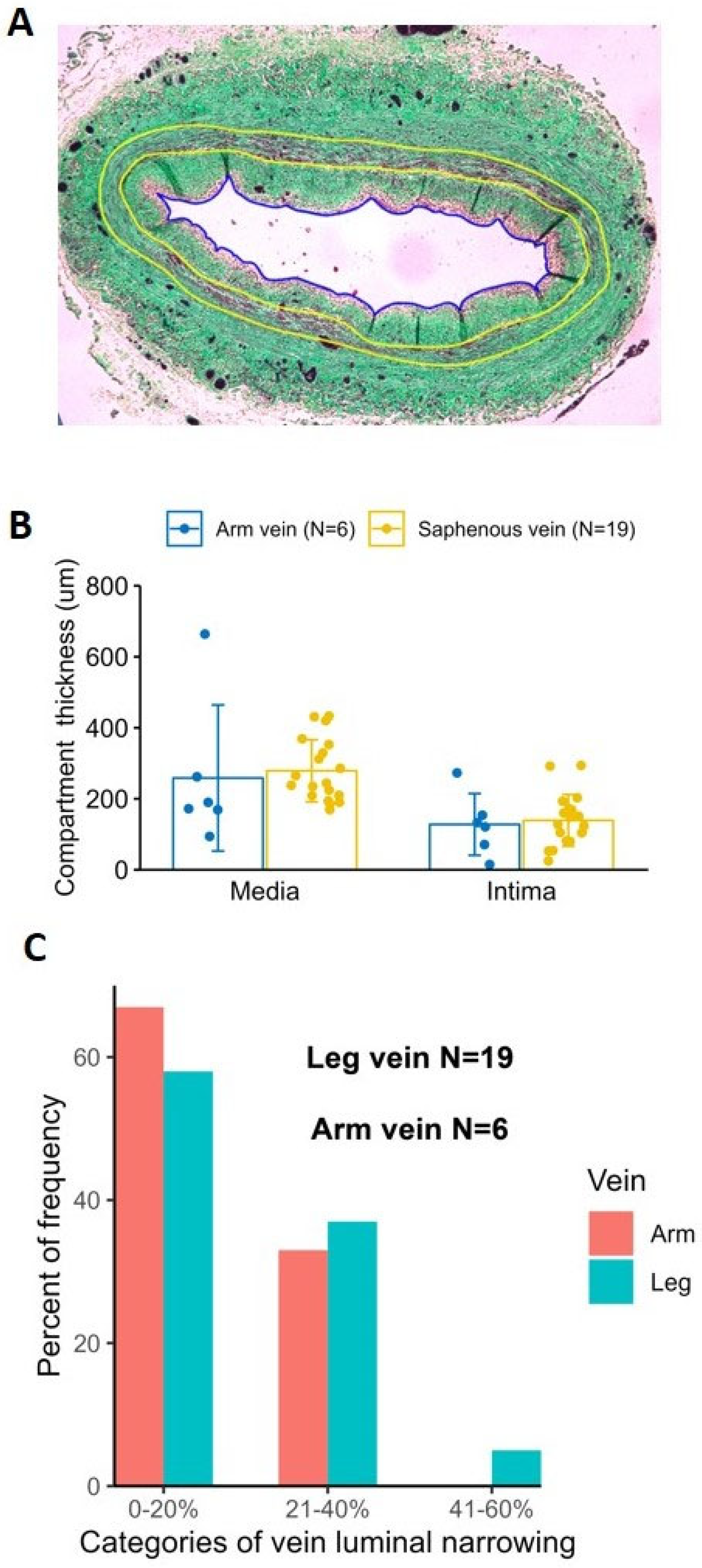
2.5. Histological Comparison of Human Arm and Leg Veins
3. Discussion
4. Materials and Methods
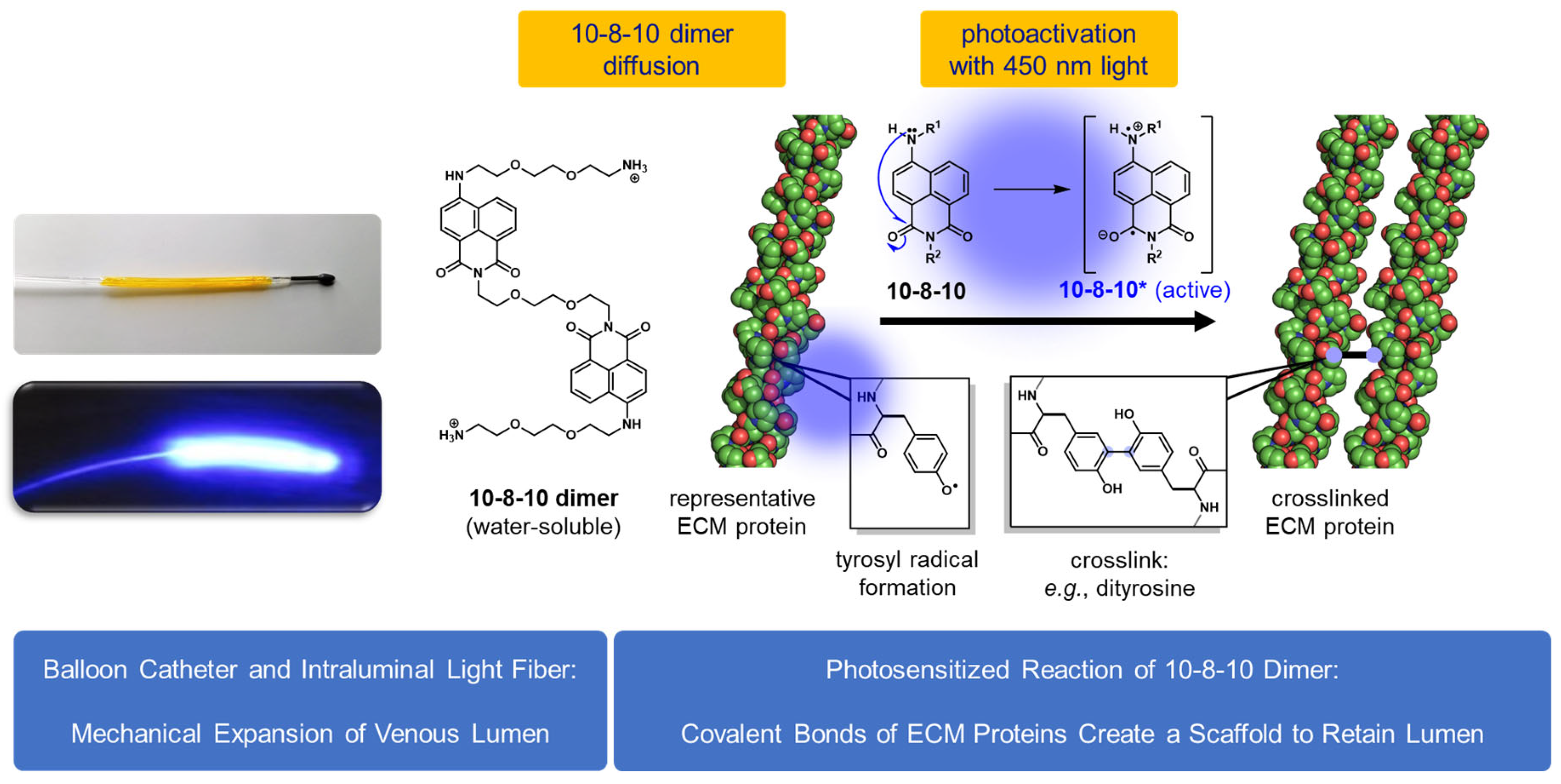
4.1. VRS-AVF Treatment
4.2. Sheep Model of AVF Using Superficial Veins of the Forelimbs
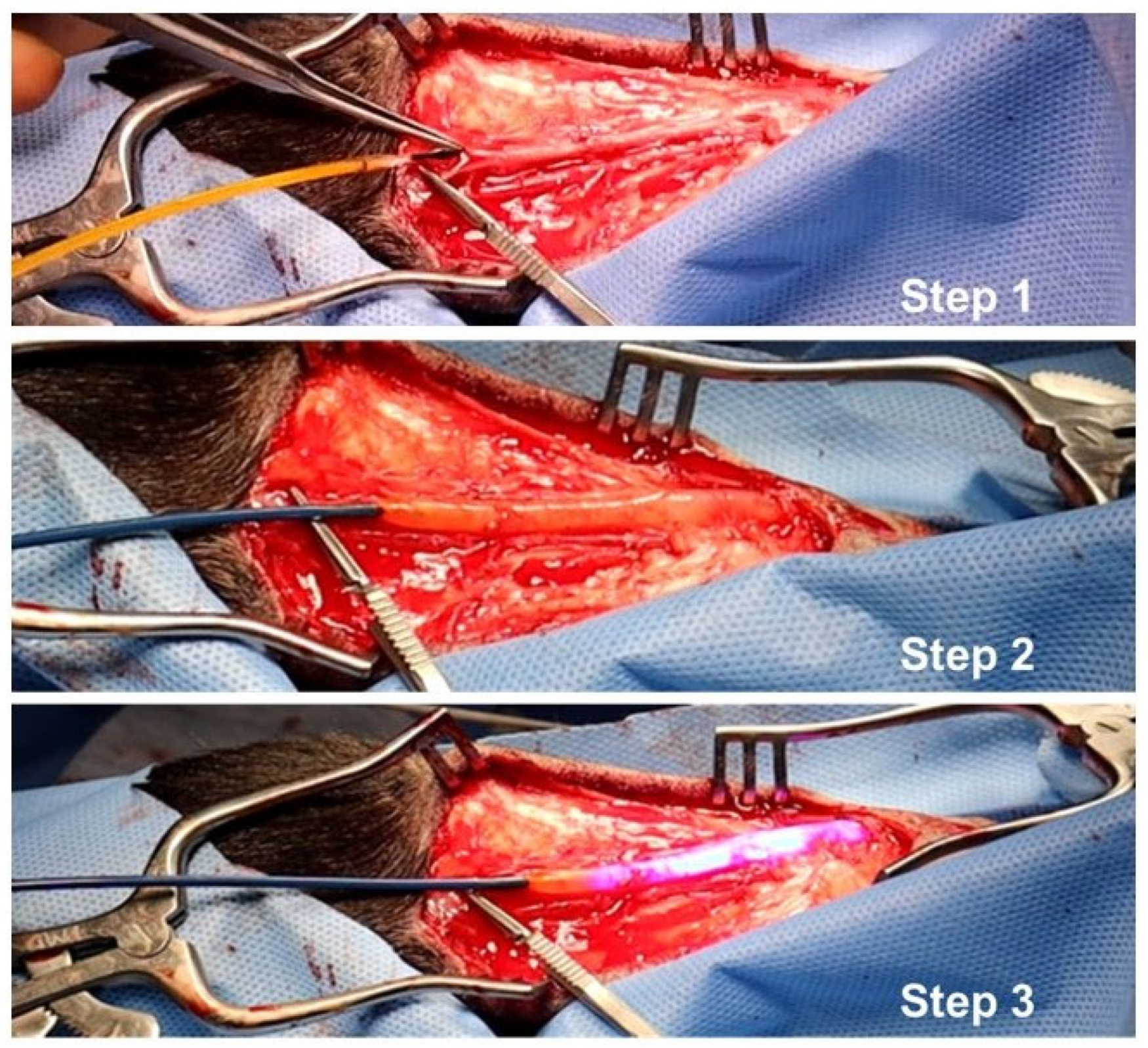
4.3. VRS-AVF Procedural Steps during Fistula Creation Surgery
4.4. Morphometric Analysis and Immunohistochemistry Staining
4.5. Human Vein Studies
4.6. Fluorescent Images of Treated Veins to Confirm Drug Delivery
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huber, T.S.; Berceli, S.A.; Scali, S.T.; Neal, D.; Anderson, E.M.; Allon, M.; Cheung, A.K.; Dember, L.M.; Himmelfarb, J.; Roy-Chaudhury, P.; et al. Arteriovenous Fistula Maturation, Functional Patency, and Intervention Rates. JAMA Surg. 2021, 156, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Allon, M.; Imrey, P.B.; Cheung, A.K.; Radeva, M.; Alpers, C.E.; Beck, G.J.; Dember, L.M.; Farber, A.; Greene, T.; Himmelfarb, J.; et al. Relationships Between Clinical Processes and Arteriovenous Fistula Cannulation and Maturation: A Multicenter Prospective Cohort Study. Am. J. Kidney Dis. 2018, 71, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Rothuizen, T.C.; Wong, C.; Quax, P.H.A.; van Zonneveld, A.J.; Rabelink, T.J.; Rotmans, J.I. Arteriovenous access failure: More than just intimal hyperplasia? Nephrol. Dial. Transplant. 2013, 28, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Allon, M.; Greene, T.; Dember, L.M.; Vita, J.A.; Cheung, A.K.; Hamburg, N.M.; Imrey, P.B.; Kaufman, J.S.; Robbin, M.L.; Shiu, Y.T.; et al. Association between preoperative vascular function and postoperative arteriovenous fistula development. J. Am. Soc. Nephrol. 2016, 27, 3788–3795. [Google Scholar] [CrossRef]
- Remuzzi, A.; Bozzetto, M. Biological and physical factors involved in the maturation of arteriovenous fistula for hemodialysis. Cardiovasc. Eng. Technol. 2017, 3, 273–279. [Google Scholar] [CrossRef]
- Shih, Y.C.; Chen, P.Y.; Ko, T.M.; Huang, P.H.; Ma, H.; Tarng, D.C. MMP-9 Deletion Attenuates Arteriovenous Fistula Neointima through Reduced Perioperative Vascular Inflammation. Int. J. Mol. Sci. 2021, 22, 5448. [Google Scholar] [CrossRef]
- Matsubara, Y.; Kiwan, G.; Fereydooni, A.; Langford, J.; Dardik, A. Distinct subsets of T cells and macrophages impact venous remodeling during arteriovenous fistula maturation. JVS Vasc. Sci. 2020, 1, 207–218. [Google Scholar] [CrossRef]
- Linn, Y.L.; Choke, E.T.C.; Yap, C.J.Q.; Tan, R.Y.; Patel, A.; Tang, T.Y. Utility of sirolimus coated balloons in the peripheral vasculature—A review of the current literature. CVIR Endovasc. 2022, 5, 29. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef]
- Wagenseil, J.E.; Mecham, R.P. Vascular extracellular matrix and arterial mechanics. Physiol. Rev. 2009, 89, 957–989. [Google Scholar] [CrossRef]
- Del Monte-Nieto, G.; Fischer, J.W.; Gorski, D.J.; Harvey, R.P.; Kovacic, J.C. Basic Biology of Extracellular Matrix in the Cardiovascular System, Part 1/4: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 2169–2188. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, Y.; Yanagisawa, H. The molecular mechanism of mechanotransduction in vascular homeostasis and disease. Clin. Sci. 2020, 134, 2399–2418. [Google Scholar] [CrossRef]
- Goldschmidt, M.E.; McLeod, K.J.; Taylor, W.R. Integrin-mediated mechanotransduction in vascular smooth muscle cells: Frequency and force response characteristics. Circ. Res. 2001, 88, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Kauser, K.; Warner, K.S.; Anderson, B.; Keyes, E.D.; Hayes, R.; Kawamoto, E.; Perkins, D.; Scott, R.; Isaacson, J.; Haberer, B.; et al. Creating a Natural Vascular Scaffold by Photochemical Treatment of the Extracellular Matrix for Vascular Applications. Int. J. Mol. Sci. 2022, 23, 683. [Google Scholar] [CrossRef]
- Keyes, E.D.; Kauser, K.; Warner, K.S.; Roberts, A.G. Photosensitized Oxidative Dimerization at Tyrosine by a Water-Soluble 4-Amino-1,8-naphthalimide. ChemBioChem 2021, 22, 2703–2710. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.A.; Roll, M.; Joseph, J.; Seenisamy, J.; Barrett, J.; Kauser, K.; Warner, K.S. Solvent-Dependent Photophysics and Reactivity of Monomeric and Dimeric 4-Amino-1,8-Naphthalimides. J. Phys. Chem. A 2021, 125, 2294–2307. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.R.; Yamamoto, K.; Protack, C.D.; Tsuneki, M.; Kuwahara, G.; Assi, R.; Brownson, K.E.; Bai, H.; Madri, J.A.; Dardik, A. Temporal regulation of venous extracellular matrix components during arteriovenous fistula maturation. J. Vasc. Access. 2015, 16, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Chen, E.Y.; Wong, D.J.; Yamamoto, K.; Protack, C.D.; Williams, W.T.; Assi, R.; Hall, M.R.; Sadaghianloo, N.; Dardik, A. Vein graft adaptation and fistula maturation in the arterial environment. J. Surg. Res. 2014, 188, 162–173. [Google Scholar] [CrossRef]
- Hu, H.; Patel, S.; Hanisch, J.J.; Santana, J.M.; Hashimoto, T.; Bai, H.; Kudze, T.; Foster, T.R.; Guo, J.; Yatsula, B.; et al. Future research directions to improve fistula maturation and reduce access failure. Semin. Vasc. Surg. 2016, 29, 153–171. [Google Scholar] [CrossRef]
- Wong, C.Y.; Rothuizen, T.C.; de Vries, M.R.; Rabelink, T.J.; Hamming, J.F.; van Zonneveld, A.J.; Quax, P.H.A.; Rotmans, J.I. Elastin is a Key Regulator of Outward Remodeling in Arteriovenous Fistulas. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 480–486. [Google Scholar] [CrossRef]
- Weigand, A.; Boos, A.M.; Ringwald, J.; Mieth, M.; Kneser, U.; Arkudas, A.; Bleiziffer, O.; Klumpp, D.; Horch, R.E.; Beier, J.P. New aspects on efficient anticoagulation and antiplatelet strategies in sheep. BMC Vet. Res. 2013, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Geread, R.S.; Morreale, P.; Dony, R.D.; Brouwer, E.; Wood, G.A.; Androutsos, D.; Khademi, A. IHC Color Histograms for Unsupervised Ki67 Proliferation Index Calculation. Front. Bioeng. Biotechnol. 2019, 7, 226. [Google Scholar] [CrossRef]
- Ando, J.; Yamamoto, K. Hemodynamic forces, endothelial mechanotransduction, and vascular diseases. Magn. Reson. Med. Sci. 2021, 21, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Nugent, H.M.; Groothuis, A.; Seifert, P.; Guerraro, J.L.; Nedelman, M.; Mohanakumar, T.; Edelman, E.R. Perivascular endothelial implants inhibit intimal hyperplasia in a model of arteriovenous fistulae: A safety and efficacy study in the pig. J. Vasc. Res. 2002, 39, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.S.; Nugent, H.M.; Gaccione, P.; Guleria, I.; Roy-Chaudhury, P.; Lawson, J.H. Multicenter phase I/II trial of the safety of allogeneic endothelial cell implants after the creation of arteriovenous access for hemodialysis use: The V-HEALTH study. J. Vasc. Surg. 2009, 50, 1359–1368.e1. [Google Scholar] [CrossRef]
- De Marco Garcia, L.P.; Davila-Santini, L.R.; Feng, Q.; Calderin, J.; Krishnasastry, K.V.; Panetta, T.F. Primary balloon angioplasty plus balloon angioplasty maturation to upgrade small-caliber veins (<3 mm) for arteriovenous fistulas. J. Vasc. Surg. 2010, 52, 139–144. [Google Scholar] [CrossRef]
- Veroux, P.; Giaquinta, A.; Tallarita, T.; Sinagra, N.; Virgilio, C.; Zerbo, D.; Gloviczki, P.; Veroux, M. Primary balloon angioplasty of small (≤2 mm) cephalic veins improves primary patency of arteriovenous fistulae and decreases reintervention rates. J. Vasc. Surg. 2013, 57, 131–136. [Google Scholar] [CrossRef]
- Roy-Chaudhury, P.; Lee, T.; Woodle, B.; Wadehra, D.; Campos-Naciff, B.; Munda, R. Balloon-assisted maturation (BAM) of the arteriovenous fistula: The good, the bad, and the ugly. Semin. Nephrol. 2012, 32, 558–563. [Google Scholar] [CrossRef]
- Hye, R.J.; Peden, E.K.; O’Connor, T.P.; Browne, B.J.; Dixon, B.S.; Schanzer, A.S.; Jensik, S.C.; Dember, L.M.; Jaff, M.R.; Burke, S.K. Human type I pancreatic elastase treatment of arteriovenous fistulas in patients with chronic kidney disease. J. Vasc. Surg. 2014, 60, 454–461.e1. [Google Scholar] [CrossRef]
- Peden, E.K.; Leeser, D.B.; Dixon, B.S.; El-Khatib, M.T.; Roy-Chaudhury, P.; Lawson, J.H.; Menard, M.T.; Dember, L.M.; Glickman, M.H.; Gustafson, P.N.; et al. A multi-center, dose-escalation study of human type I pancreatic elastase (PRT-201) administered after arteriovenous fistula creation. J. Vasc. Access 2013, 14, 143–151. [Google Scholar] [CrossRef]
- Bleyer, A.J.; Scavo, V.A.; Wilson, S.E.; Browne, B.J.; Ferris, B.L.; Ozaki, C.K.; Lee, T.; Peden, E.K.; Dixon, B.S.; Mishler, R.; et al. A randomized trial of vonapanitase (PATENCY-1) to promote radiocephalic fistula patency and use for hemodialysis. J. Vasc. Surg. 2019, 69, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Peden, E.K.; Lucas, J.F.; Browne, B.J.; Settle, S.M.; Scavo, V.A.; Bleyer, A.J.; Ozaki, C.K.; Teruya, T.H.; Wilson, S.E.; Mishler, R.E.; et al. PATENCY-2 trial of vonapanitase to promote radiocephalic fistula use for hemodialysis and secondary patency. J. Vasc. Access 2022, 23, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.R.; Applewhite, B.; Martinez, L.; Laurito, T.; Tabbara, M.; Rojas, M.G.; Wei, Y.; Selman, G.; Knysheva, M.; Velazquez, O.C.; et al. Inhibition of Lysyl Oxidase with beta-aminopropionitrile Improves Venous Adaptation after Arteriovenous Fistula Creation. Kidney360 2021, 2, 270–278. [Google Scholar] [CrossRef]
- Shiu, Y.T.; He, Y.; Tey, J.C.S.; Knysheva, M.; Anderson, B.; Kauser, K. Natural vascular scaffolding treatment promotes outward remodeling during arteriovenous fistula development in rats. Front. Bioeng. Biotechnol. 2021, 9, 622617. [Google Scholar] [CrossRef] [PubMed]
- Kokozidou, M.; Katsargyris, A.; Verhoeven, E.L.G.; Schulze-Tanzil, G. Vascular access animal models used in research. Ann. Anat. 2019, 225, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Florescu, M.C.; Foster, K.W.; Sacks, A.R.; Lof, J.; Stolze, E.A.; Fry, G.M.; Bumgardner, D.P.; Tysinger, T.; Kuchta, M.J.; Runge, H.J.; et al. Sheep Model of Hemodialysis Arteriovenous Fistula Using Superficial Veins. Semin. Dial. 2015, 28, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Tabbara, M.; Duque, J.C.; Martinez, L.; Escobar, L.A.; Wu, W.; Pan, Y.; Fernandez, N.; Velazquez, O.C.; Jaimes, E.A.; Salman, L.H.; et al. Pre-existing and Postoperative Intimal Hyperplasia and Arteriovenous Fistula Outcomes. Am. J. Kidney Dis. 2016, 68, 455–464. [Google Scholar] [CrossRef]
- Munger, K.A.; Downey, T.M.; Haberer, B.; Pohlson, K.; Marshall, L.L.; Utecht, R.E. A novel photochemical cross-linking technology to improve luminal gain, vessel compliance, and buckling post-angioplasty in porcine arteries. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 375–384. [Google Scholar] [CrossRef]
- Alpers, C.E.; Imrey, P.B.; Hudkins, K.L.; Wietecha, T.A.; Radeva, M.; Allon, M.; Cheung, A.K.; Dember, L.M.; Roy-Chaudhury, P.; Shiu, Y.-T.; et al. Histopathology of veins obtained at hemodialysis arteriovenous fistula creation surgery. J. Am. Soc. Nephrol. 2017, 28, 3076–3088. [Google Scholar] [CrossRef]


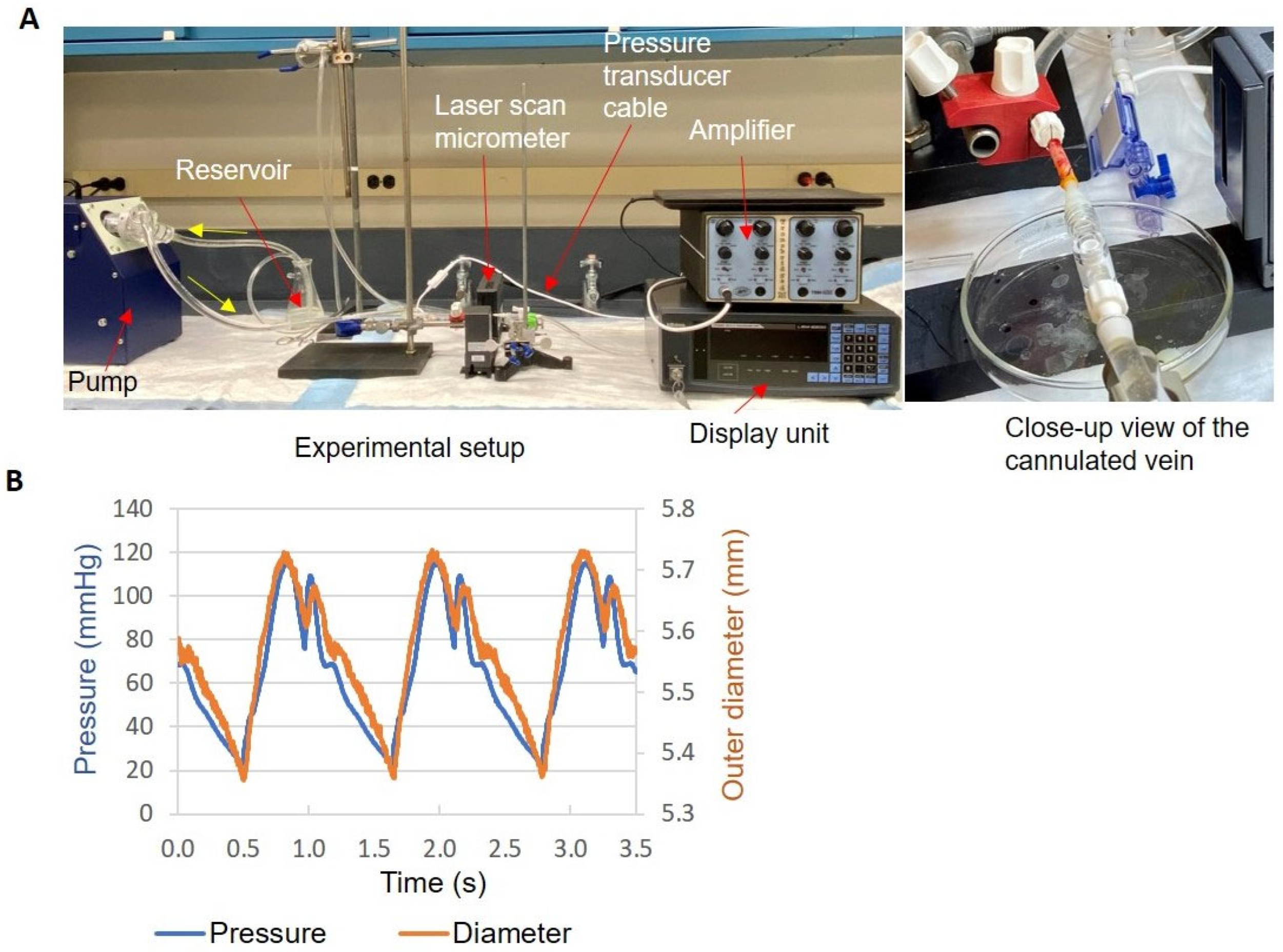
| Light Activation | Pr Min (mmHg) | Pr Max (mmHg) | Pulse Pr (mmHg) | Mean D (mm) | D Change (%) | Distensibility (10−3/mmHg) |
|---|---|---|---|---|---|---|
| Yes | 20 ± 5 | 110 ± 6 | 91 ± 2 | 5.33 ± 0.55 | 3.9 ± 1.9 | 0.87 ± 0.41 |
| No | 18 ± 1 | 108 ± 2 | 90 ± 1 | 5.39 ± 0.58 | 5.1 ± 1.7 | 1.17 ± 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Anderson, B.; Hu, Q.; Hayes, R.; Huff, K.; Isaacson, J.; Warner, K.S.; Hauser, H.; Greenberg, M.; Chandra, V.; et al. Photochemically Aided Arteriovenous Fistula Creation to Accelerate Fistula Maturation. Int. J. Mol. Sci. 2023, 24, 7571. https://doi.org/10.3390/ijms24087571
He Y, Anderson B, Hu Q, Hayes R, Huff K, Isaacson J, Warner KS, Hauser H, Greenberg M, Chandra V, et al. Photochemically Aided Arteriovenous Fistula Creation to Accelerate Fistula Maturation. International Journal of Molecular Sciences. 2023; 24(8):7571. https://doi.org/10.3390/ijms24087571
Chicago/Turabian StyleHe, Yong, Blake Anderson, Qiongyao Hu, RB Hayes, Kenji Huff, Jim Isaacson, Kevin S. Warner, Hank Hauser, Myles Greenberg, Venita Chandra, and et al. 2023. "Photochemically Aided Arteriovenous Fistula Creation to Accelerate Fistula Maturation" International Journal of Molecular Sciences 24, no. 8: 7571. https://doi.org/10.3390/ijms24087571
APA StyleHe, Y., Anderson, B., Hu, Q., Hayes, R., Huff, K., Isaacson, J., Warner, K. S., Hauser, H., Greenberg, M., Chandra, V., Kauser, K., & Berceli, S. A. (2023). Photochemically Aided Arteriovenous Fistula Creation to Accelerate Fistula Maturation. International Journal of Molecular Sciences, 24(8), 7571. https://doi.org/10.3390/ijms24087571









