An Agrobacterium-Mediated Transient Expression Method for Functional Assay of Genes Promoting Disease in Monocots
Abstract
:1. Introduction
2. Results
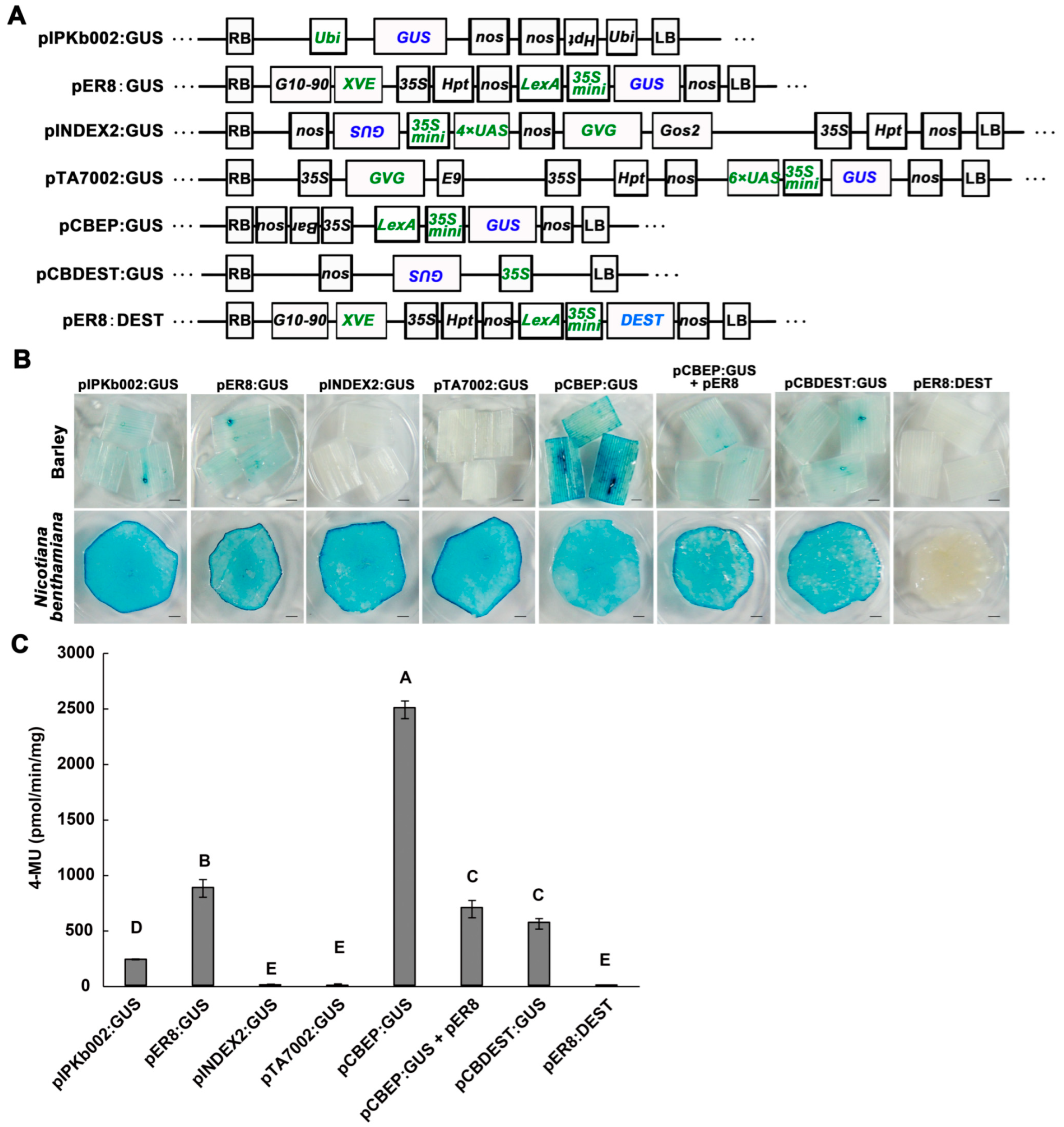
2.1. Binary Vectors Differed in Transient Expression Efficiency of the β-Glucuronidase Gene in Barley
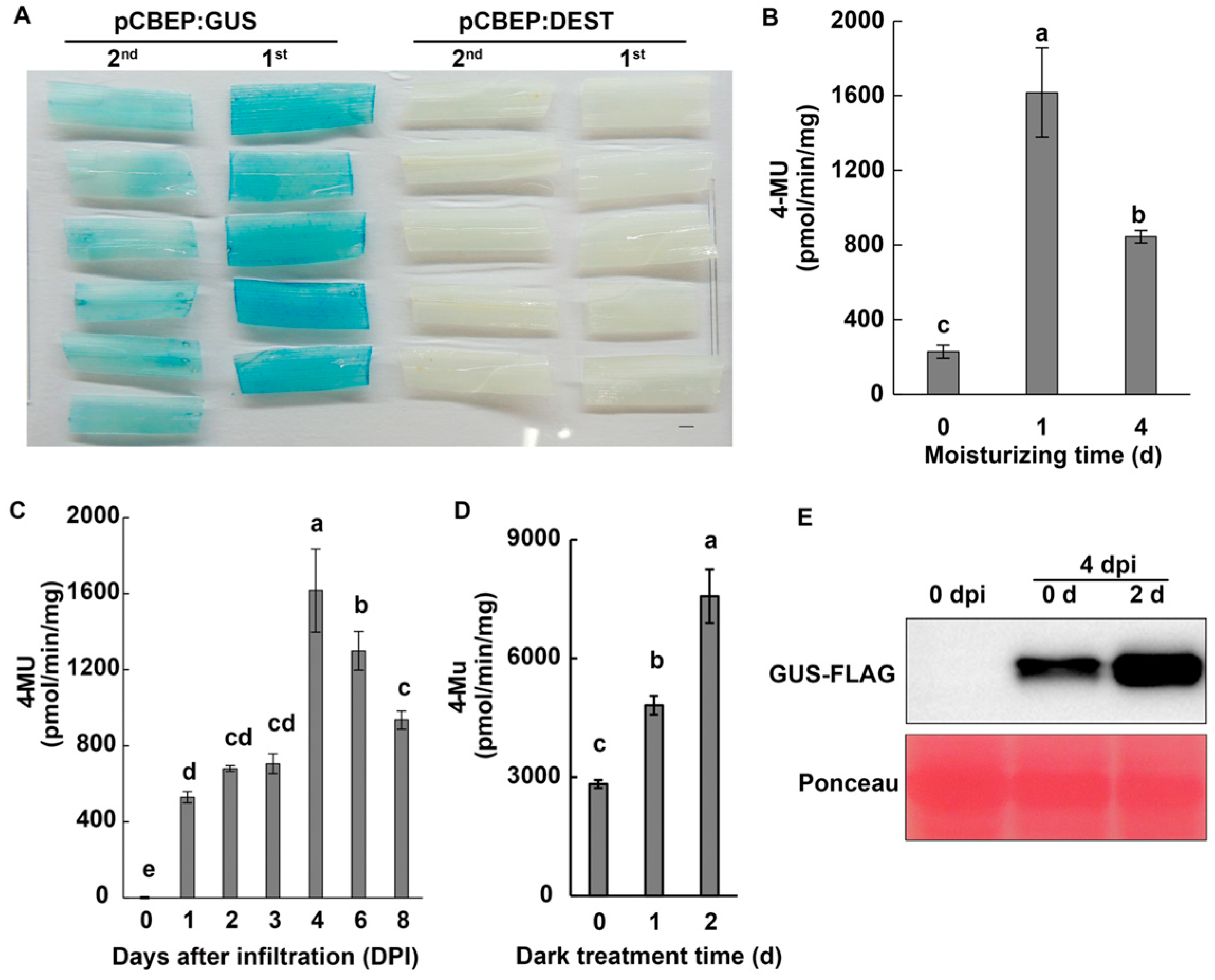
2.2. Optimization for Higher Efficiency of Transient Expression in Barley Leaves
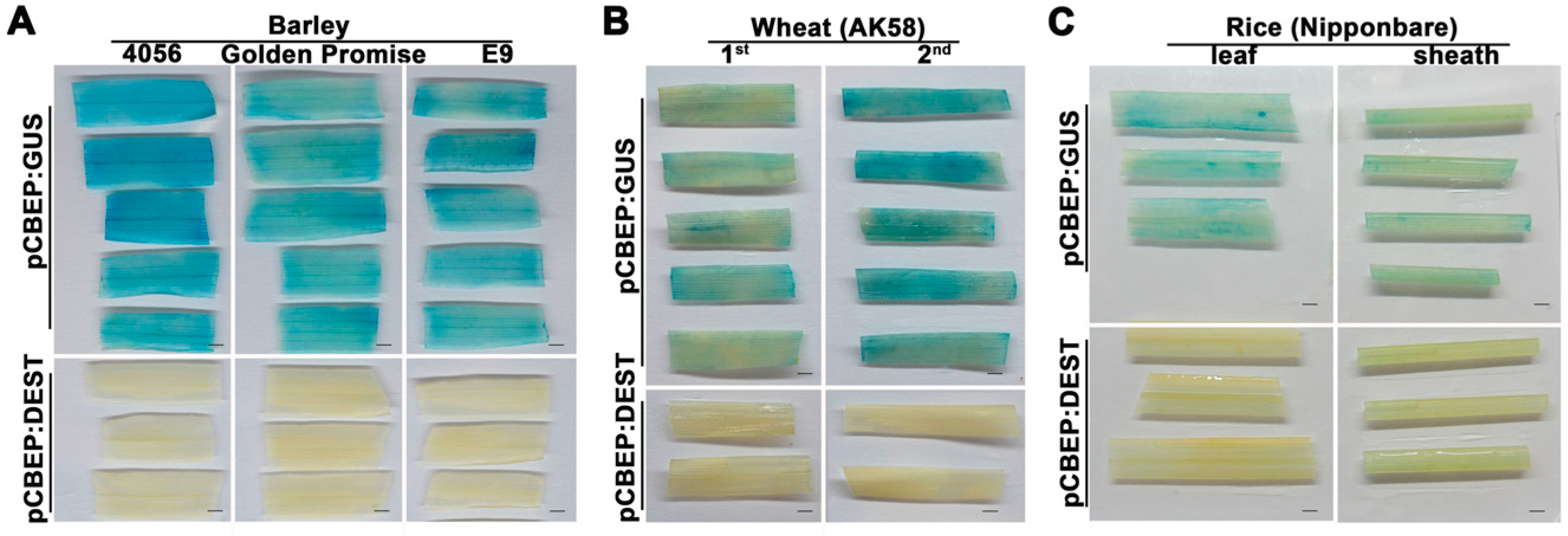
2.3. Transient Expression with pCBEP:GUS in Other Monocot Plants
2.4. AGL1 (pCBEP)-Mediated Transient Expression for Analyzing Protein-Protein Interactions in Monocots
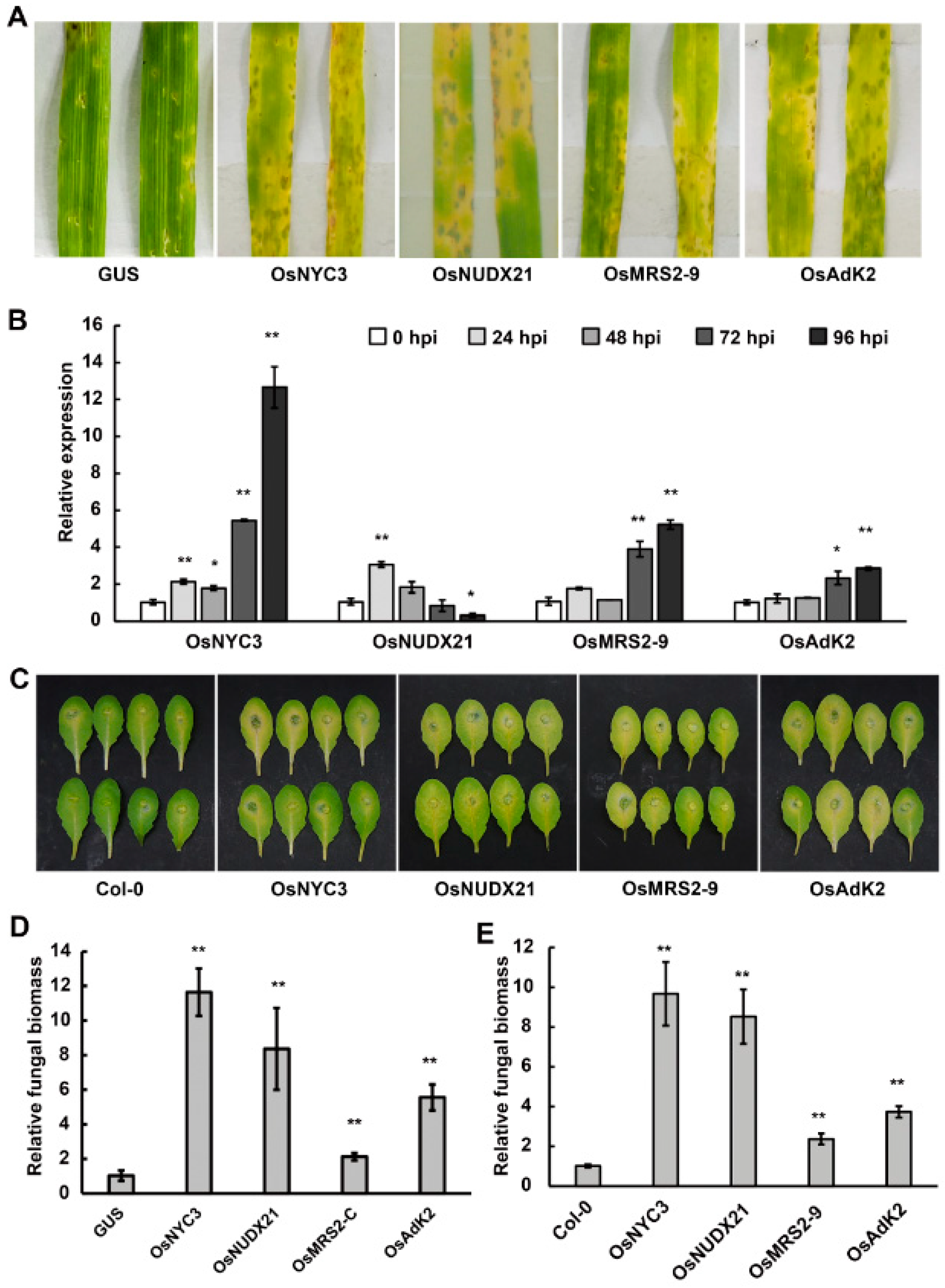
2.5. Functional Screening with AGL1 (pCBEP)-Mediated Transient Expression for Rice Genes Conferring Disease Susceptibility
2.6. Overexpression of the Chloroplast-Related Proteins Promoted Disease Susceptibility in Barley and Arabidopsis
3. Discussion
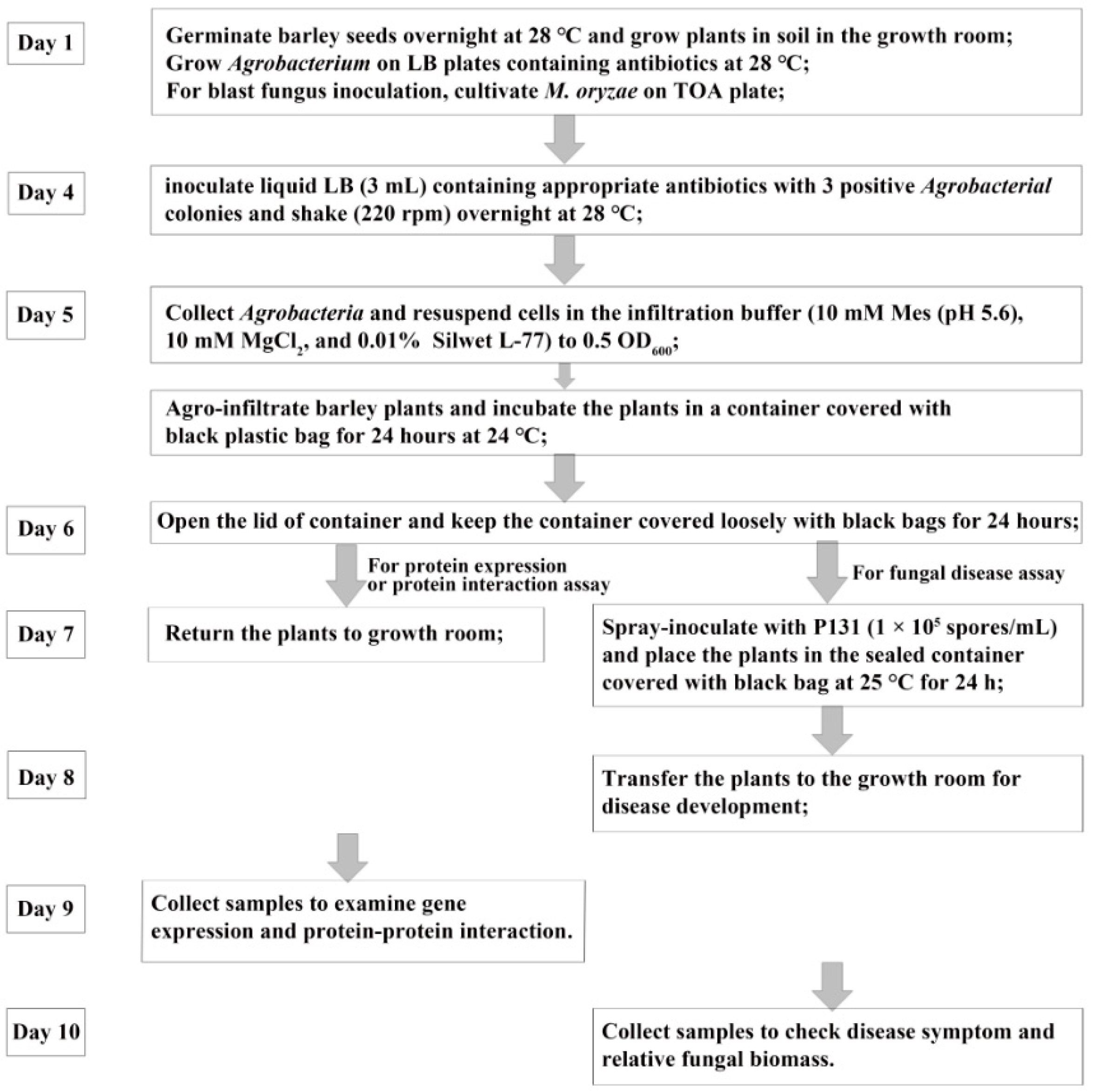
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Vector Construction and Primers
4.3. Agrobacterium Preparations
4.4. Agrobacterium-Mediated Transient Transformation
4.5. GUS Assays
4.6. Western Blot Assay of GUS Protein
4.7. Split-Luciferase Assay
4.8. Transformation of Arabidopsis Plants
4.9. Plant Inoculations and Disease Assay
4.10. Relative Fungal Biomass Assay
4.11. Gene Expression Analysis
4.12. Statistical Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canto, T. Transient expression systems in plants: Potentialities and constraints. Adv. Exp. Med. Biol. 2016, 896, 287–301. [Google Scholar] [CrossRef]
- Mardanova, E.S.; Ravin, N.V. Transient expression of recombinant proteins in plants using potato virus X based vectors. Methods Enzym. 2021, 660, 205–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Siemiatkowska, B.; Toleco, M.R.; Jing, Y.; Strotmann, V.; Zhang, J.; Stahl, Y.; Fernie, A.R. A highly efficient Agrobacterium-mediated method for transient gene expression and functional studies in multiple plant species. Plant Commun. 2020, 1, 100028. [Google Scholar] [CrossRef]
- Lu, J.; Bai, M.; Ren, H.; Liu, J.; Wang, C. An efficient transient expression system for gene function analysis in rose. Plant Methods 2017, 13, 116. [Google Scholar] [CrossRef]
- Chang, Q.-L.; Xu, H.-J.; Peng, Y.-L.; Fan, J. Subtractive hybridization-assisted screening and characterization of genes involved in the rice-Magnaporthe oryzae interaction. Phytopathol. Res. 2019, 1, 21. [Google Scholar] [CrossRef]
- Marx, V. Plants: A toolbox of cell-based assays. Nat. Methods 2016, 13, 551–554. [Google Scholar] [CrossRef]
- He, F.; Chen, S.; Ning, Y.; Wang, G.L. Rice (Oryza sativa) protoplast isolation and its application for transient expression analysis. Curr. Protoc. Plant Biol. 2016, 1, 373–383. [Google Scholar] [CrossRef]
- Leissing, F.; Reinstädler, A.; Thieron, H.; Panstruga, R. Gene gun-mediated transient gene expression for functional studies in plant immunity. Methods Mol. Biol. 2022, 2523, 63–77. [Google Scholar] [CrossRef]
- Page, M.T.; Parry, M.A.J.; Carmo-Silva, E. A high-throughput transient expression system for rice. Plant Cell Env. 2019, 42, 2057–2064. [Google Scholar] [CrossRef]
- Usami, S.; Morikawa, S.; Takebe, I.; Machida, Y. Absence in monocotyledonous plants of the diffusible plant factors inducing T-DNA circularization and vir gene expression in Agrobacterium. Mol. Gen. Genet. MGG 1987, 209, 221–226. [Google Scholar] [CrossRef]
- Sahi, S.V.; Chilton, M.D.; Chilton, W.S. Corn metabolites affect growth and virulence of Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 1990, 87, 3879–3883. [Google Scholar] [CrossRef]
- Xu, D.H.; Xu, S.B.; Li, B.; Liu, Y.; Huang, Z.S.; Gu, L.Q. Identification of rice signal factor inhibiting the growth and transfer of Agrobacterium tumefaciens. J. Integr. Plant Biol. 1999, 41, 1283–1286. [Google Scholar]
- Coy, M.R.; Abbitt, S.E.; Frank, M.J. Protoplast isolation and transfection in maize. Methods Mol. Biol. 2022, 2464, 91–104. [Google Scholar] [CrossRef]
- Wu, B.; Chen, Z.; Xu, X.; Chen, R.; Wang, S.; Xu, H.; Lin, F. Harnessing a transient gene expression system in Nicotiana benthamiana to explore plant agrochemical transporters. Plants 2021, 10, 524. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, J.; Duan, S.; Ao, Y.; Dai, J.; Liu, J.; Wang, P.; Li, Y.; Liu, B.; Feng, D.; et al. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 2011, 7, 30. [Google Scholar] [CrossRef]
- Nostadt, R.; Hilbert, M.; Nizam, S.; Rovenich, H.; Wawra, S.; Martin, J.; Küpper, H.; Mijovilovich, A.; Ursinus, A.; Langen, G.; et al. A secreted fungal histidine- and alanine-rich protein regulates metal ion homeostasis and oxidative stress. New Phytol. 2020, 227, 1174–1188. [Google Scholar] [CrossRef]
- Lee, W.S.; Hammond-Kosack, K.E.; Kanyuka, K. Barley stripe mosaic virus-mediated tools for investigating gene function in cereal plants and their pathogens: Virus-induced gene silencing, host-mediated gene silencing, and virus-mediated overexpression of heterologous protein. Plant Physiol. 2012, 160, 582–590. [Google Scholar] [CrossRef]
- Wood, A.K.M.; Walker, C.; Lee, W.S.; Urban, M.; Hammond-Kosack, K.E. Functional evaluation of a homologue of plant rapid alkalinisation factor (RALF) peptides in Fusarium graminearum. Fungal Biol. 2020, 124, 753–765. [Google Scholar] [CrossRef]
- Garcia-Gimenez, G.; Schreiber, M.; Dimitroff, G.; Little, A.; Singh, R.; Fincher, G.B.; Burton, R.A.; Waugh, R.; Tucker, M.R.; Houston, K. Identification of candidate MYB transcription factors that influence CslF6 expression in barley grain. Front. Plant Sci. 2022, 13, 883139. [Google Scholar] [CrossRef]
- Lin, Y.; Meng, F.; Fang, C.; Zhu, B.; Jiang, J. Rapid validation of transcriptional enhancers using agrobacterium-mediated transient assay. Plant Methods 2019, 15, 21. [Google Scholar] [CrossRef]
- Ouwerkerk, P.B.; de Kam, R.J.; Hoge, J.H.; Meijer, A.H. Glucocorticoid-inducible gene expression in rice. Planta 2001, 213, 370–378. [Google Scholar] [CrossRef]
- Aoyama, T.; Chua, N.H. A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 1997, 11, 605–612. [Google Scholar] [CrossRef]
- Lu, X.; Kracher, B.; Saur, I.M.; Bauer, S.; Ellwood, S.R.; Wise, R.; Yaeno, T.; Maekawa, T.; Schulze-Lefert, P. Allelic barley MLA immune receptors recognize sequence-unrelated avirulence effectors of the powdery mildew pathogen. Proc. Natl. Acad. Sci. USA 2016, 113, E6486–E6495. [Google Scholar] [CrossRef]
- Zuo, J.; Niu, Q.W.; Chua, N.H. Technical advance: An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 2000, 24, 265–273. [Google Scholar] [CrossRef]
- Fang, Y.L.; Peng, Y.L.; Fan, J. The Nep1-like protein family of Magnaporthe oryzae is dispensable for the infection of rice plants. Sci. Rep. 2017, 7, 4372. [Google Scholar] [CrossRef]
- Burman, N.; Chandran, D.; Khurana, J.P. A rapid and highly efficient method for transient gene expression in rice plants. Front. Plant Sci. 2020, 11, 584011. [Google Scholar] [CrossRef]
- Mortensen, S.; Bernal-Franco, D.; Cole, L.F.; Sathitloetsakun, S.; Cram, E.J.; Lee-Parsons, C.W.T. EASI transformation: An efficient transient expression method for analyzing gene function in Catharanthus roseus seedlings. Front. Plant Sci. 2019, 10, 755. [Google Scholar] [CrossRef]
- Meng, F.; Yang, C.; Cao, J.; Chen, H.; Pang, J.; Zhao, Q.; Wang, Z.; Qing Fu, Z.; Liu, J. A bHLH transcription activator regulates defense signaling by nucleo-cytosolic trafficking in rice. J. Integr. Plant Biol. 2020, 62, 1552–1573. [Google Scholar] [CrossRef]
- de Torres Zabala, M.; Littlejohn, G.; Jayaraman, S.; Studholme, D.; Bailey, T.; Lawson, T.; Tillich, M.; Licht, D.; Bölter, B.; Delfino, L.; et al. Chloroplasts play a central role in plant defence and are targeted by pathogen effectors. Nat. Plants 2015, 1, 15074. [Google Scholar] [CrossRef]
- Kretschmer, M.; Damoo, D.; Djamei, A.; Kronstad, J. Chloroplasts and plant immunity: Where are the fungal effectors? Pathogens 2019, 9, 19. [Google Scholar] [CrossRef]
- Straus, M.R.; Rietz, S.; Ver Loren van Themaat, E.; Bartsch, M.; Parker, J.E. Salicylic acid antagonism of EDS1-driven cell death is important for immune and oxidative stress responses in Arabidopsis. Plant J. 2010, 62, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ji, Z.; Yan, H.; Xu, J.; Zhao, X.; Zhou, Z. Effector Sntf2 interacted with chloroplast-related protein Mdycf39 promoting the colonization of Colletotrichum gloeosporioides in apple leaf. Int. J. Mol. Sci. 2022, 23, 6379. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Huai, B.; Lu, Y.; Cai, K.; Guo, J.; Zhu, X.; Kang, Z.; Guo, J. A stripe rust effector Pst18363 targets and stabilises TaNUDX23 that promotes stripe rust disease. New Phytol. 2020, 225, 880–895. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Islam, M.A.; Cai, K.; Tian, S.; Liu, Y.; Kang, Z.; Guo, J. TaClpS1, negatively regulates wheat resistance against Puccinia striiformis f. sp. tritici. BMC Plant Biol. 2020, 20, 555. [Google Scholar] [CrossRef]
- Lin, Y.; Zhao, H.; Kotlarz, M.; Jiang, J. Enhancer-mediated reporter gene expression in Arabidopsis thaliana: A forward genetic screen. Plant J. 2021, 106, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Tsuboyama, S.; Kodama, Y. Highly efficient G-AgarTrap-mediated transformation of the Marchantia polymorpha model strains Tak-1 and Tak-2. Plant Biotechnol. 2018, 35, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Yang, Y. Transient expression assay by agroinfiltration of leaves. In Arabidopsis Protocols; Salinas, J., Sanchez-Serrano, J.J., Eds.; Humana Press: Totowa, NJ, USA, 2006; pp. 225–229. [Google Scholar]
- Go, Y.S.; Kim, H.; Kim, H.J.; Suh, M.C. Arabidopsis cuticular wax biosynthesis is negatively regulated by the DEWAX gene encoding an AP2/ERF-Type transcription factor. Plant Cell 2014, 26, 1666–1680. [Google Scholar] [CrossRef]
- Oberpichler, I.; Rosen, R.; Rasouly, A.; Vugman, M.; Ron, E.Z.; Lamparter, T. Light affects motility and infectivity of Agrobacterium tumefaciens. Environ. Microbiol. 2008, 10, 2020–2029. [Google Scholar] [CrossRef]
- Roberts, M.R.; Paul, N.D. Seduced by the dark side: Integrating molecular and ecological perspectives on the influence of light on plant defence against pests and pathogens. New Phytol. 2006, 170, 677–699. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Zhang, X.; Zhang, Q.; Huang, J.; Chen, J.-Q.; Hartl, D.L.; Tian, D. Rapidly evolving R genes in diverse grass species confer resistance to rice blast disease. Proc. Natl. Acad. Sci. USA 2013, 110, 18572–18577. [Google Scholar] [CrossRef]
- Shen, Z.F.; Li, L.; Wang, J.Y.; Zhang, Y.R.; Wang, Z.H.; Liang, S.; Zhu, X.M.; Lu, J.P.; Lin, F.C.; Liu, X.H. A subunit of the COP9 signalosome, MoCsn6, is involved in fungal development, pathogenicity, and autophagy in rice blast fungus. Microbiol. Spectr. 2022, 10, e0202022. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, Z.; Chern, M.; Yin, J.; Yang, C.; Ran, L.; Cheng, M.; He, M.; Wang, K.; Wang, J.; et al. A natural allele of a transcription factor in rice confers broad-spectrum blast resistance. Cell 2017, 170, 114–126.e15. [Google Scholar] [CrossRef] [PubMed]
- Ribot, C.; Hirsch, J.; Balzergue, S.; Tharreau, D.; Nottéghem, J.-L.; Lebrun, M.-H.; Morel, J.-B. Susceptibility of rice to the blast fungus, Magnaporthe grisea. J. Plant Physiol. 2008, 165, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Aubert, Y.; Widemann, E.; Miesch, L.; Pinot, F.; Heitz, T. CYP94-mediated jasmonoyl-isoleucine hormone oxidation shapes jasmonate profiles and attenuates defence responses to Botrytis cinerea infection. J. Exp. Bot. 2015, 66, 3879–3892. [Google Scholar] [CrossRef]
- Heitz, T.; Widemann, E.; Lugan, R.; Miesch, L.; Ullmann, P.; Désaubry, L.; Holder, E.; Grausem, B.; Kandel, S.; Miesch, M.; et al. Cytochromes P450 CYP94C1 and CYP94B3 catalyze two successive oxidation steps of plant hormone Jasmonoyl-isoleucine for Catabolic Turnover. J. Biol. Chem. 2012, 287, 6296–6306. [Google Scholar] [CrossRef]
- Woo, H.R.; Kim, H.J.; Lim, P.O.; Nam, H.G. Leaf senescence: Systems and dynamics aspects. Annu. Rev. Plant Biol. 2019, 70, 347–376. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.; Wang, H.-L.; Kan, C.; Li, Z.; Yang, X.; Yin, W.; Xia, X.; Nam, H.G.; Li, Z.; et al. Verticillium dahliae secretory effector PevD1 induces leaf senescence by promoting ORE1-mediated ethylene biosynthesis. Mol. Plant 2021, 14, 1901–1917. [Google Scholar] [CrossRef]
- Morita, R.; Sato, Y.; Masuda, Y.; Nishimura, M.; Kusaba, M. Defect in non-yellow coloring 3, an α/β hydrolase-fold family protein, causes a stay-green phenotype during leaf senescence in rice. Plant J. 2009, 59, 940–952. [Google Scholar] [CrossRef]
- Schelbert, S.; Aubry, S.; Burla, B.; Agne, B.; Kessler, F.; Krupinska, K.; Hörtensteiner, S. Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. Plant Cell 2009, 21, 767–785. [Google Scholar] [CrossRef]
- Teng, K.; Yue, Y.; Zhang, H.; Li, H.; Xu, L.; Han, C.; Fan, X.; Wu, J. Functional characterization of the pheophytinase gene, ZjPPH, from Zoysia japonica in regulating chlorophyll degradation and photosynthesis. Front. Plant Sci. 2021, 12, 786570. [Google Scholar] [CrossRef]
- Breia, R.; Conde, A.; Badim, H.; Fortes, A.M.; Gerós, H.; Granell, A. Plant SWEETs: From sugar transport to plant-pathogen interaction and more unexpected physiological roles. Plant Physiol. 2021, 186, 836–852. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, M.; Croll, D.; Kronstad, J.W. Chloroplast-associated metabolic functions influence the susceptibility of maize to Ustilago maydis. Mol. Plant Pathol. 2017, 18, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Ueda, Y.; Yoshimura, K.; Shigeoka, S. Comprehensive analysis of cytosolic Nudix hydrolases in Arabidopsis thaliana. J. Biol. Chem. 2005, 280, 25277–25283. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Yoshimura, K.; Miyake, H.; Ishikawa, K.; Ito, D.; Tanabe, N.; Shigeoka, S. Molecular characterization of organelle-type Nudix hydrolases in Arabidopsis. Plant Physiol. 2008, 148, 1412–1424. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, M.; Gobbato, E.; Bednarek, P.; Debey, S.; Schultze, J.L.; Bautor, J.; Parker, J.E. Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the Nudix hydrolase NUDT7. Plant Cell 2006, 18, 1038–1051. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, X.; Li, H.; Shuai, Y.; Chen, W.; Ma, D.; Lü, Z. Uncovering the role of wheat magnesium transporter family genes in abiotic responses. Front. Plant Sci. 2023, 14, 1078299. [Google Scholar] [CrossRef]
- Yang, L.; Cao, H.; Zhang, X.; Gui, L.; Chen, Q.; Qian, G.; Xiao, J.; Li, Z. Genome-wide identification and expression analysis of tomato ADK gene family during development and stress. Int. J. Mol. Sci. 2021, 22, 7708. [Google Scholar] [CrossRef]
- Dong, Y.; Li, Y.; Zhao, M.; Jing, M.; Liu, X.; Liu, M.; Guo, X.; Zhang, X.; Chen, Y.; Liu, Y.; et al. Global genome and transcriptome analyses of Magnaporthe oryzae epidemic isolate 98-06 uncover novel effectors and pathogenicity-related genes, revealing gene gain and lose dynamics in genome evolution. PLoS Pathog. 2015, 11, e1004801. [Google Scholar] [CrossRef]
- Jantasuriyarat, C.; Gowda, M.; Haller, K.; Hatfield, J.; Lu, G.; Stahlberg, E.; Zhou, B.; Li, H.; Kim, H.; Yu, Y.; et al. Large-scale identification of expressed sequence tags involved in rice and rice blast fungus interaction. Plant Physiol. 2005, 138, 105–115. [Google Scholar] [CrossRef]
- Chong, J.; Piron, M.C.; Meyer, S.; Merdinoglu, D.; Bertsch, C.; Mestre, P. The SWEET family of sugar transporters in grapevine: VvSWEET4 is involved in the interaction with Botrytis cinerea. J. Exp. Bot. 2014, 65, 6589–6601. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Yang, C.; Tian, Z.; Li, J. AtSWEET4, a hexose facilitator, mediates sugar transport to axial sinks and affects plant development. Sci. Rep. 2016, 6, 24563. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.B.; Bao, S.W.; Fang, Y.L.; Wei, L.Y.; Zhu, W.S.; Peng, Y.L.; Fan, J. An LRR-only protein promotes NLP-triggered cell death and disease susceptibility by facilitating oligomerization of NLP in Arabidopsis. New Phytol. 2021, 232, 1808–1822. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Romero-Calvo, I.; Ocón, B.; Martínez-Moya, P.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; de Medina, F.S. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal. Biochem. 2010, 401, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, G.; Macho, A.P.; Lozano-Durán, R. Split-luciferase complementation imaging assay to study protein-protein interactions in Nicotiana benthamiana. Bio Protoc. 2021, 11, e4237. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Peng, Y.-L.; Shishiyama, J.J.B. Temporal sequence of cytological events in rice leaves infected with Pyricularia oryzae. Can. J. Bot. 1988, 66, 730–735. [Google Scholar] [CrossRef]
- Chen, M.M.; Yang, S.R.; Wang, J.; Fang, Y.L.; Peng, Y.L.; Fan, J. Fungal oxysterol-binding protein-related proteins promote pathogen virulence and activate plant immunity. J. Exp. Bot. 2022, 73, 2125–2141. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, D.; Zheng, L.; Hsiang, T.; Wei, Y.; Fu, Y.; Huang, J. Identification of virulence genes in the crucifer anthracnose fungus Colletotrichum higginsianum by insertional mutagenesis. Microb. Pathog. 2013, 64, 6–17. [Google Scholar] [CrossRef]
- Elliott, K.; Berry, J.C.; Kim, H.; Bart, R.S. A comparison of ImageJ and machine learning based image analysis methods to measure cassava bacterial blight disease severity. Plant Methods 2022, 18, 86. [Google Scholar] [CrossRef] [PubMed]
- Minas, K.; McEwan, N.R.; Newbold, C.J.; Scott, K.P. Optimization of a high-throughput CTAB-based protocol for the extraction of qPCR-grade DNA from rumen fluid, plant and bacterial pure cultures. FEMS Microbiol. Lett. 2011, 325, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Yang, Y. Quantification of Magnaporthe grisea during infection of rice plants using real-time polymerase chain reaction and northern blot/phosphoimaging analyses. Phytopathology 2002, 92, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Clone No. | Gene | Encoding Protein | Disease Severity a |
|---|---|---|---|
| 7B210 | Os06g0354700 | Pheophytinase, NYC3 | +++ |
| 27A6 | Os08g0109300 | Adenylate monophosphate kinase 2, AdK2 | ++ |
| 15A5 | Os02g0734300 | Nudix hydrolase 21, NUDX21 | ++ |
| 15C1 | Os04g0501100 | Magnesium transporter MRS2-C | ++ |
| 357 | Os12g0150200 | Cytochrome P450, CYP94C1 | ++ |
| 367 | Os03g0327100 | NAC transcription factor 39, OsNAC92 | ++ |
| 5C8 | Os04g0498200 | Cytochrome c oxidase subunit 6b-2,COX6b2 | ++ |
| 32B1 | Os03g0437200 | C2H2 type zinc finger protein 36-like, ZFP36 (Bsr-d1) | + |
| 1D8 | Os03g0145600 | CCCH type zinc finger protein 48 | + |
| 4F7 | Os05g0494200 | Cysteine proteinase inhibitor 2-like | + |
| 6B3 | Os04g0587400 | Purine permease 11 | + |
| 9C7 | Os01g0856800 | Pleckstrin homology (PH) domain protein 1 | + |
| 9C3 | Os07g0656100 | C-type lectin (CTL)-like protein | + |
| 2B21 | Os06g0181566 | 60S ribosomal protein L39 | + |
| 4A46 | Os01g0562600 | Uncharacterized protein | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Chang, Q.; Huang, L.; Wei, P.; Song, Y.; Guo, Z.; Peng, Y.-L.; Fan, J. An Agrobacterium-Mediated Transient Expression Method for Functional Assay of Genes Promoting Disease in Monocots. Int. J. Mol. Sci. 2023, 24, 7636. https://doi.org/10.3390/ijms24087636
Xu H, Chang Q, Huang L, Wei P, Song Y, Guo Z, Peng Y-L, Fan J. An Agrobacterium-Mediated Transient Expression Method for Functional Assay of Genes Promoting Disease in Monocots. International Journal of Molecular Sciences. 2023; 24(8):7636. https://doi.org/10.3390/ijms24087636
Chicago/Turabian StyleXu, Haijiao, Qingle Chang, Luli Huang, Peiyao Wei, Yulu Song, Zejian Guo, You-Liang Peng, and Jun Fan. 2023. "An Agrobacterium-Mediated Transient Expression Method for Functional Assay of Genes Promoting Disease in Monocots" International Journal of Molecular Sciences 24, no. 8: 7636. https://doi.org/10.3390/ijms24087636






