Identification, Baculoviral Expression, and Biochemical Characterization of a Novel Cholinesterase of Amblyomma americanum (Acari: Ixodidae)
Abstract
:1. Introduction
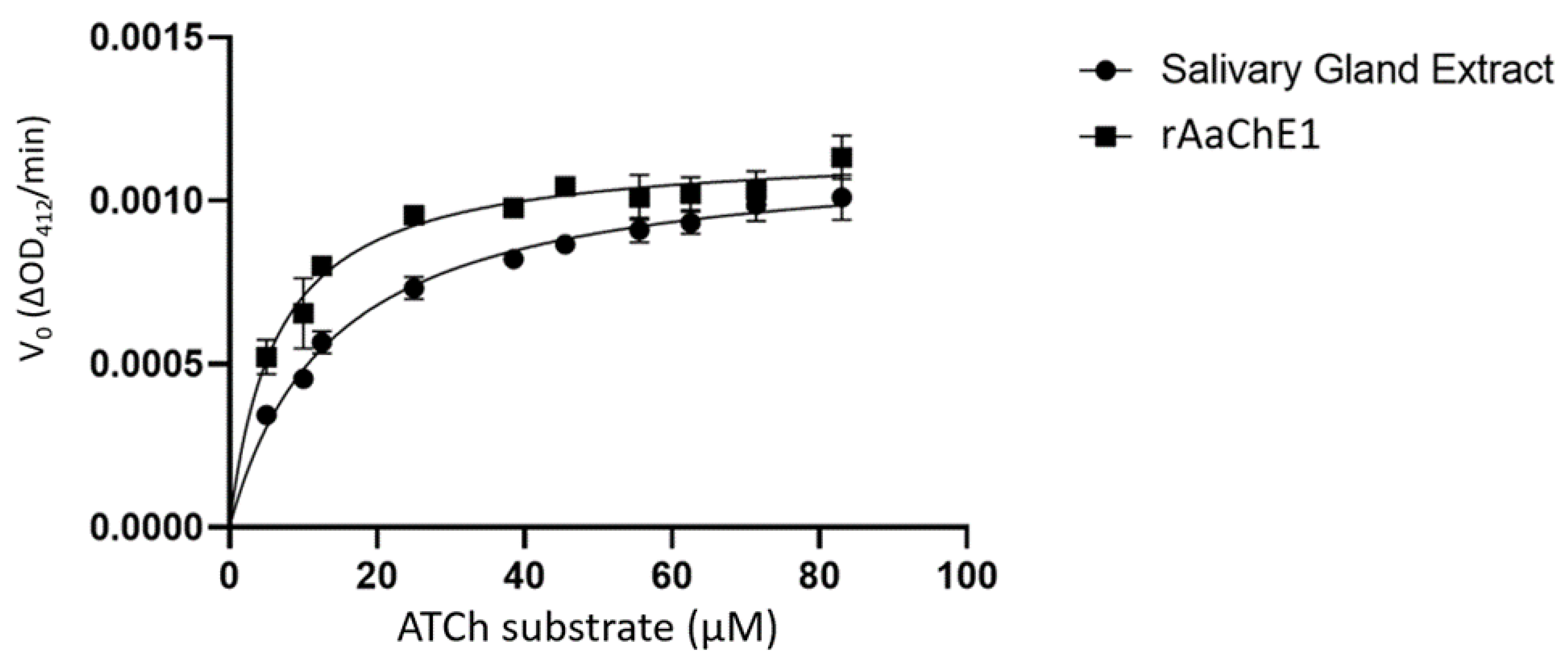
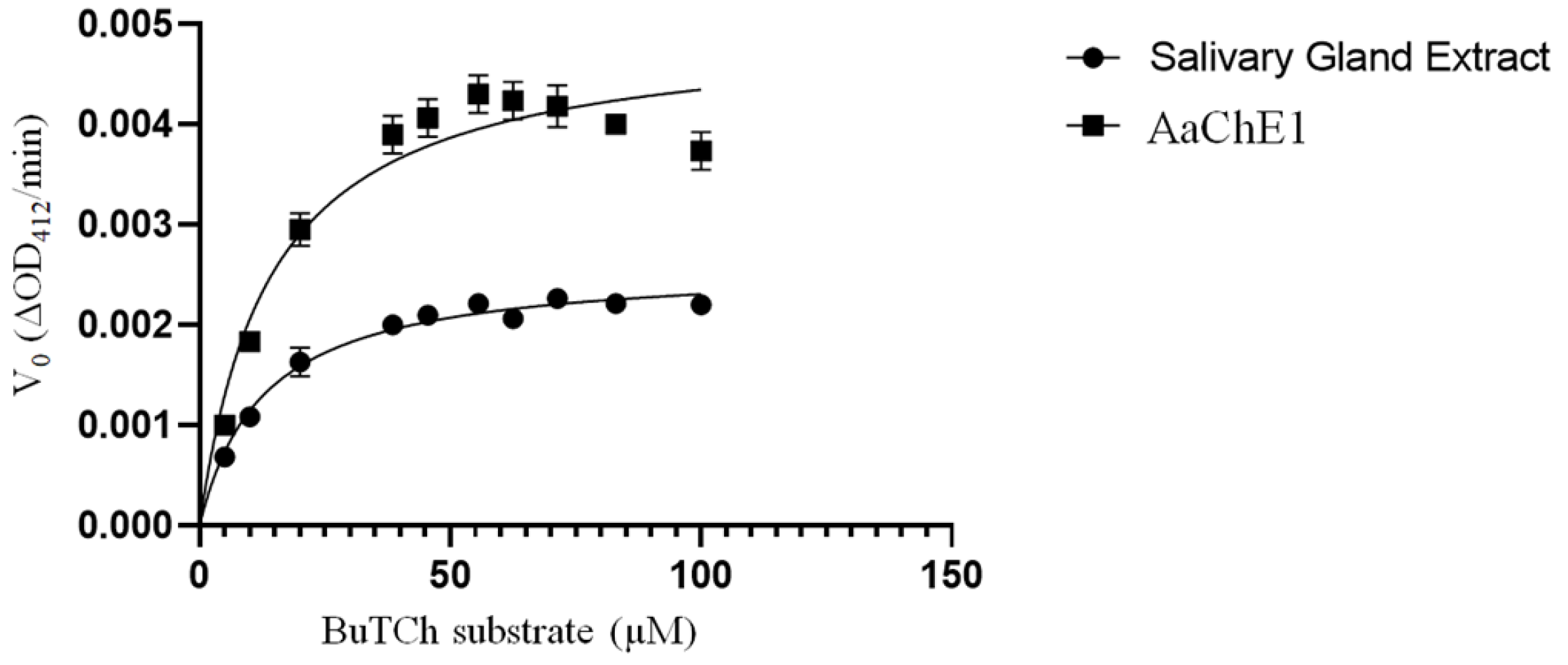
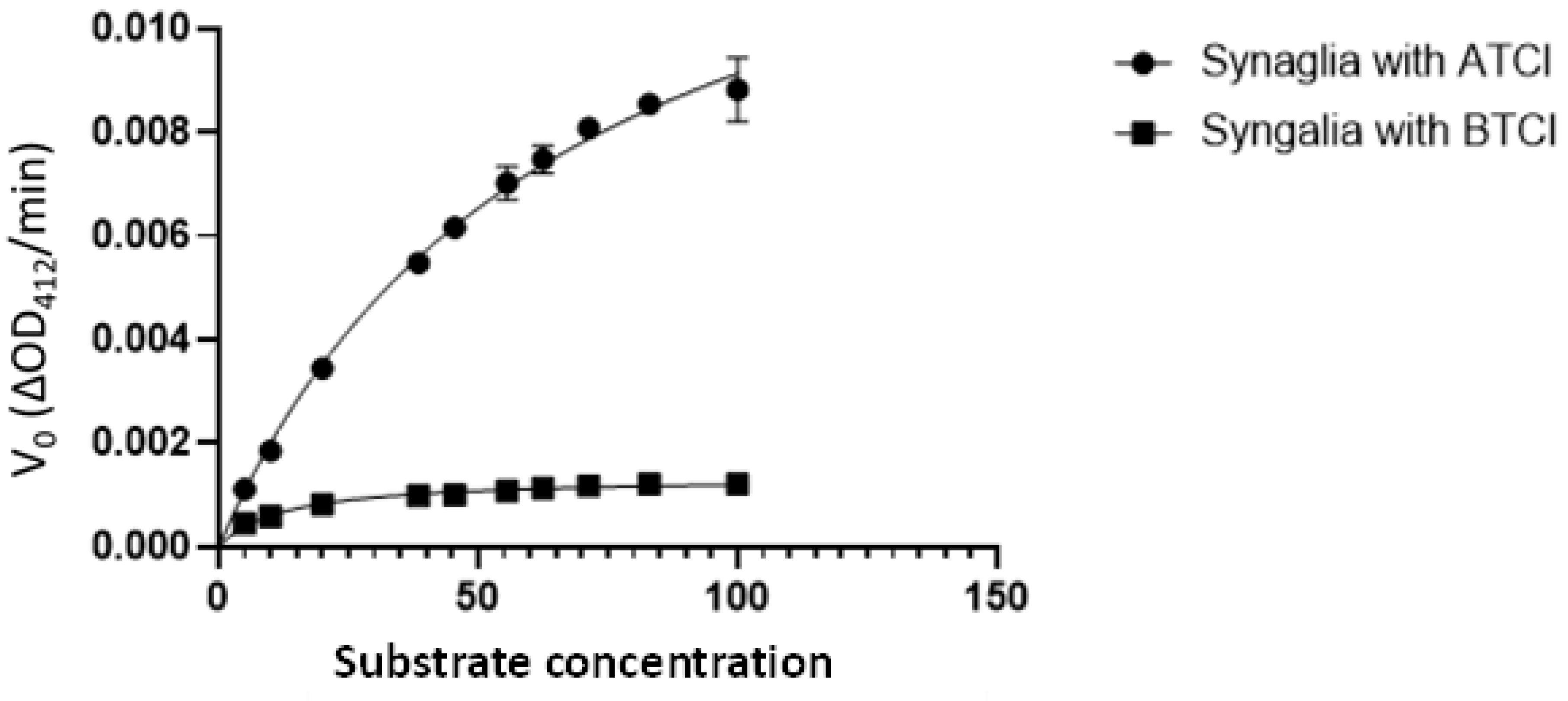
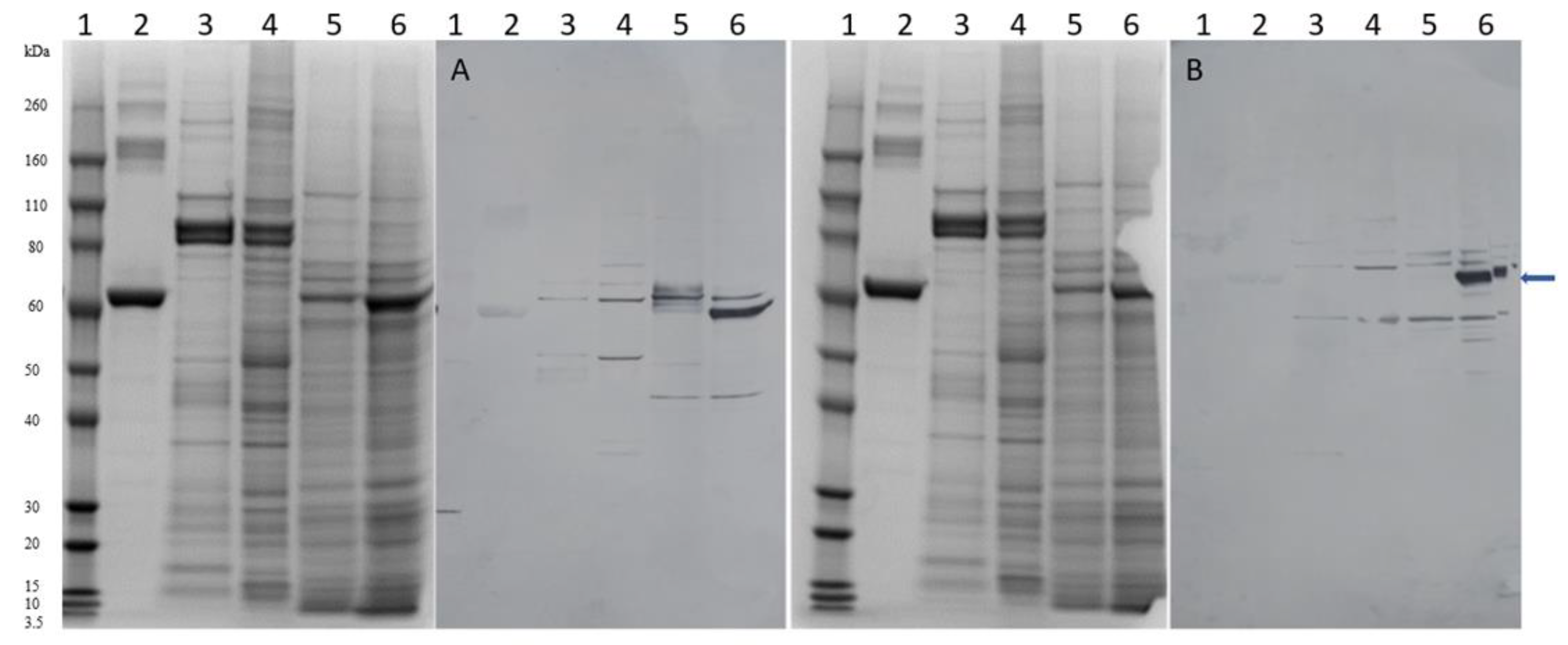
2. Results
3. Discussion
4. Materials and Methods
4.1. Ticks, Salivary Gland Extract, Synganglion Extract, and Total RNA Preparations
4.2. RT-PCR, cDNA Construction, Cloning, and Sequencing
4.3. Baculoviral Expression of Recombinant Cholinesterases
4.4. Cholinesterase Assays
4.5. AaChE-Specific Antibody and SDS-PAGE Western Blot
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Goddard, J.; Varela-Stokes, A.S. Role of the lone star tick, Amblyomma americanum (L.), in human and animal diseases. Vet. Parasitol. 2009, 160, 1–12. [Google Scholar] [CrossRef]
- Kennedy, A.C.; Marshall, E. Lone Star Ticks (Amblyomma americanum): An emerging threat in Delaware. Del. J. Public Health 2021, 7, 66–71. [Google Scholar] [CrossRef]
- Ma, D.; Lun, X.; Li, C.; Zhou, R.; Zhao, Z.; Wang, J.; Zhang, Q.; Liu, Q. Predicting the potential global distribution of Amblyomma americanum (Acari: Ixodidae) under near current and future climatic conditions, using the maximum entropy model. Biology 2021, 10, 1057. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.K.; Peterson, A.T.; Cobos, M.E.; Ganta, R.; Foley, D. Current and future distribution of the lone star tick, Amblyomma americanum (L.) (Acari: Ixodidae) in North America. PLoS ONE 2019, 14, e0209082. [Google Scholar] [CrossRef]
- Crispell, G.; Commins, S.P.; Archer-Hartman, S.A.; Choudhary, S.; Dharmarajan, G.; Azadi, P.; Karim, S. Discovery of alpha-gal-containing antigens in North American tick species believed to induce red meat allergy. Front Immunol. 2019, 10, 1056. [Google Scholar] [CrossRef] [Green Version]
- Steinke, J.W.; Thomas, A.E.; Platts-Mills, M.D.; Commins, P.S. The alpha gal story: Lessons learned from connecting the dots. J. Allergy Clin. Immunol. 2015, 135, 589–597. [Google Scholar] [CrossRef] [Green Version]
- Wolver, S.E.; Sun, D.R.; Commins, S.P.; Schwartz, L.B. A peculiar cause of anaphylaxis: No more steak? The journey to discovery of a newly recognized allergy to galactose-alpha-1,3-galactose found in mammalian meat. J. Gen. Intern. Med. 2012, 28, 322–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.R.; Karim, S. Tick saliva and the alpha-gal syndrome: Finding a needle in a haystack. Front. Cell. Infect. Microbiol. 2021, 11, 680264. [Google Scholar] [CrossRef]
- Temeyer, K.B.; Tuckow, A.P. Tick salivary cholinesterase: A probable immunomodulator of host-parasite interactions. J. Med. Entomol. 2016, 53, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Temeyer, K.B. Molecular biology of tick acetylcholinesterases. Front. Biosci. (Landmark Ed) 2018, 23, 1320–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temeyer, K.B.; Schlechte, K.G.; Olafson, P.U.; Drolet, B.S.; Tidwell, J.P.; Osbrink, W.L.A.; Showler, A.T.; Gross, A.D.; Pérez de León, A.A. Association of salivary cholinesterase with arthropod vectors of disease. J. Med. Entomol. 2020, 57, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.; De Jaco, A.; Comoletti, D.; Miller, M.; Camp, S. Cholinesterase confabs and cousins: Approaching forty years. Chem. Biol. Interact. 2013, 203, 10–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wessler, I.; Kirkpatrick, C.J. Cholinergic signaling controls immune functions and promotes homeostasis. Int. Immunopharmacol. 2020, 83, 106345. [Google Scholar] [CrossRef] [PubMed]
- Temeyer, K.B.; Pruett, J.H.; Olafson, P.U. Baculovirus expression, biochemical characterization and organophosphate sensitivity of rBmAChE1, rBmAChE2, and rBmAChE3 of Rhipicephalus (Boophilus) microplus. Vet. Parasitol. 2010, 172, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Temeyer, K.B.; Olafson, P.U.; Brake, D.K.; Tuckow, A.P.; Li, A.Y.; Pérez de León, A.A. Acetylcholinesterase of Rhipicephalus (Boophilus) microplus and Phlebotomus papatasi: Gene identification, expression, and biochemical properties of recombinant proteins. Pest. Biochem. Physiol. 2013, 106, 118–123. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bo, Y.; Han, I.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLR: Functional classification of prteins via subfamily domain architechtures. Nuclis Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teufel, F.; Armenteros, J.J.A.; Johansen, A.R.; Gislason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; Heijne, G.V.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Tirloni, L.; Reck, J.; Terra, R.M.S.; Martins, J.R.; Mulenga, A.; Sherman, N.E.; Fox, J.W.; Yates, J.R.; Termignoni, C.; Pinto, A.F.M.; et al. Proteomic analysis of cattle tick Rhipicephalus (Boophilus) microplus saliva: A comparison between partially and fully engorged females. PLoS ONE 2014, 9, e94831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Expression and function of the cholinergic system in immune cells. Front. Immunol. 2017, 8, 1085. [Google Scholar] [CrossRef] [Green Version]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Physiological functions of the cholinergic system in immune cells. J. Pharmacol. Sci. 2017, 134, 1–21. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujii, T.; Moriwaki, Y.; Misawa, H. Critical roles of acetylcholine and the muscarinic and nicotinic acetylcholine receptors in the regulation of immune function. Life Sci. 2012, 91, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Fujii, T.; Moriwaki, Y.; Misawa, H.; Horiguchi, K. Reconciling neuronally and nonneuronally derived acetylcholine in the regulation of immune function. Ann. N. Y. Acad. Sci. 2012, 1261, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, J.; Lips, K.S. The non-neuronal cholinergic system in health and disease. Pharmacology 2013, 92, 286–302. [Google Scholar] [CrossRef] [PubMed]
- Nizri, E.; Brenner, T. Modulation of inflammatory pathways by the immune cholinergic system. Amino Acids 2013, 45, 73–85. [Google Scholar] [CrossRef]
- Zdanowski, R.; Krzyzowska, M.; Ujazdowska, D.; Lewicka, A.; Lewicki, S. Role of alpha-7 nicotinic receptor in the immune system and intracellular signaling pathways. Cent. Eur. J. Immunol. 2015, 40, 373–379. [Google Scholar] [CrossRef]
- Adamo, S.A. Parasites: Evolution’s neurobiologists. J. Exp. Biol. 2013, 216, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Šimo, L.; Kazimirova, M.; Richardson, J.; Bonnet, S.I. The essential role of tick salivary glands and saliva in tick feeding and pathogen transmission. Front. Cell. Infect. Microbiol. 2017, 7, 281. [Google Scholar] [CrossRef]
- Giordani, G.; Cattabriga, G.; Becchimanzi, A.; Lelio, I.D.; Leva, G.D.; Gigliotti, S.; Pennacchio, F.; Gargiulo, G.; Cavaliere, V. Role of neuronal and non-neuronal acetylcholine signaling in Drosophila humoral immunity. Insect. Biochem. Mol. Biol. 2023, 153, 103899. [Google Scholar] [CrossRef]
- Shaw, D.K.; Tate, A.T.; Schneider, D.S.; Levashina, E.A.; Kagan, J.C.; Pal, U.; Fikrig, E.; Pedra, J.H.F. Vector immunity and evolutionary ecology: The harmonious dissonance. Trends Immunol. 2018, 39, 862–873. [Google Scholar] [CrossRef]
- Kotál, J.; Langhansová, H.; Lieskovská, J.; Andersenc, J.F.; Francischetti, I.M.B.; Chavakis, T.; Kopecký, J.; Pedra, J.H.F.; Kotsyfakis, M.; Chmelař, J. Modulation of host immunity by tick saliva. J. Proteom. 2015, 128, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nutall, P.A. Tick saliva and its role in pathogen transmission. Wien. Klin. Wochenschr. 2023, 135, 165–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demarta-Gatsi, C.; Méchri, S. Vector saliva controlled inflammatory response of the host may represent the Achilles heel during pathogen transmission. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20200155. [Google Scholar] [CrossRef]
- Renthal, R.; Manghnani, L.; Bernal, S.; Qu, Y.; Griffith, W.P.; Lohmeyer, K.; Guerrero, F.D.; Borges, L.M.F.; Pérez de León, A. The chemosensory appendage proteome of Amblyomma americanum (Acari: Ixodidae) reveals putative odorant-binding and other chemoreception-related proteins. Insect Sci. 2017, 24, 730–742. [Google Scholar] [CrossRef]
- Pruett, J.H.; Pound, J.M. Biochemical diagnosis of organophosphate-insensitivity with neural acetylcholinesterase extracted by sonication from the adult tick synganglion. Vet. Parasitol. 2006, 135, 355–363. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goujon, M.; McWilliam, H.; Li, W.; Valentin, F.; Squizzato, S.; Paern, J.; Lopez, R. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 2010, 38, W695–W699. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Soding, J.; et al. Fast scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Temeyer, K.B.; Schlechte, K.G.; McDonough, W.P. Baculoviral expression of presumptive OP-resistance mutations in BmAChE1 of Rhipicephalus (Boophilus) microplus and biochemical resistance to OP inhibition. J. Med. Entomol. 2019, 56, 1318–1323. [Google Scholar] [CrossRef]
- Pruett, J.H.; Temeyer, K.B.; Fisher, W.F.; Beetham, P.K.; Kunz, S.E. Evaluation of natural Psoroptes ovis (Acarina: Psoroptidae) soluble proteins as candidate vaccine immunogens. J. Med. Entomol. 1998, 35, 861–871. [Google Scholar] [CrossRef] [PubMed]
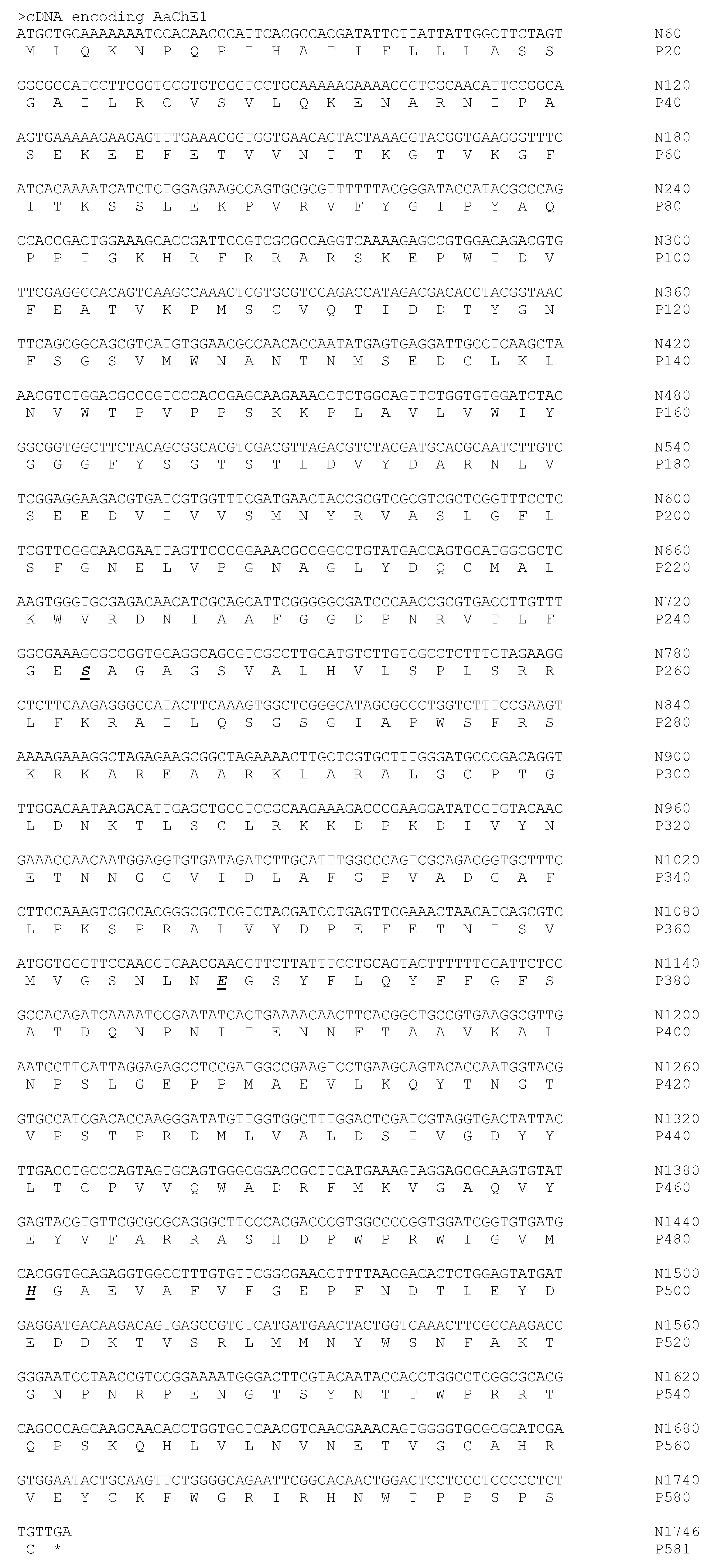
| Name | Sequence (5′-3′) | Purpose |
|---|---|---|
| BmAChE1-725U19 | TGGAAATGCCGGACTATAC | RT-PCR A. americanum larval RNA |
| BmAChE1-1646L17 | CGGTCTTGGCGAAGTTG | RT-PCR A. americanum larval RNA |
| AaChE573U18 | CGGCGGTGGCTTCTACAG | 3′-RACE A. americanum larval RNA |
| AaChE186L18 | CTTGAGCGCCATGCACTG | 5′-RACE A. americanum larval RNA |
| AaChE-Fwd | AGAATTGCCCTTATGCTGCAAAAAATCCACACAAG | Clone into TOPO XL-2 plasmid |
| AaChE-Rvs | CGAATTGCCCTTTCAACAAGAGGGGGAGGGAG | Clone into TOPO XL-2 plasmid |
| pBB-BmAChE1-AaFwd | TGAAAGGGCAATTCGAAGC | InFusion clone into baculoviral expression plasmid |
| pBB-BmAChE1-AaRvs | CATAAGGGCAATTCTAGACC | InFusion clone into baculoviral expression plasmid |
| Inhibitor 1 | (IC50) 2 | |
|---|---|---|
| rAaChE1 (ATCh Substrate) | rAaChE1 (BuTCh Substrate) | |
| Eserine | 2.28 × 10−10 (2.37–3.26 × 10−10) | 9.06 × 10−10 (5.46–14.96 × 10−10) |
| Iso-OMPA | 3.48 × 10−05 (2.76–4.43 × 10−5) | 2.77 × 10−5 (2.42–3.17 × 10−5) |
| Ethopropazine | 6.82 × 10−5 (N.D.) | 2.27 × 10−04 (N.D.) |
| BW284c51 | 2.96 × 10−7 (2.50–3.50 × 10−7) | 1.46 × 10−7 (1.29–1.64 × 10−7) |
| Coroxon | 2.35 × 10−11 (1.93–2.84 × 10−11) | 9.07 × 10−12 (0.91–2.26 × 10−11) |
| Malaoxon | 2.77 × 10−8 (2.45–3.14 × 10−8) | 3.59 × 10−08 (1.40–1.93 × 10−8) |
| Paraoxon | 1.31 × 10−9 (1.13–1.52 × 10−9) | 1.08 × 10−9 (0.92–1.27 × 10−9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temeyer, K.B.; Schlechte, K.G.; Gross, A.D.; Lohmeyer, K.H. Identification, Baculoviral Expression, and Biochemical Characterization of a Novel Cholinesterase of Amblyomma americanum (Acari: Ixodidae). Int. J. Mol. Sci. 2023, 24, 7681. https://doi.org/10.3390/ijms24097681
Temeyer KB, Schlechte KG, Gross AD, Lohmeyer KH. Identification, Baculoviral Expression, and Biochemical Characterization of a Novel Cholinesterase of Amblyomma americanum (Acari: Ixodidae). International Journal of Molecular Sciences. 2023; 24(9):7681. https://doi.org/10.3390/ijms24097681
Chicago/Turabian StyleTemeyer, Kevin B., Kristie G. Schlechte, Aaron D. Gross, and Kimberly H. Lohmeyer. 2023. "Identification, Baculoviral Expression, and Biochemical Characterization of a Novel Cholinesterase of Amblyomma americanum (Acari: Ixodidae)" International Journal of Molecular Sciences 24, no. 9: 7681. https://doi.org/10.3390/ijms24097681





