The Impact of Light Wavelength and Darkness on Metabolite Profiling of Korean Ginseng: Evaluating Its Anti-Cancer Potential against MCF-7 and BV-2 Cell Lines
Abstract
1. Introduction
2. Results
2.1. Analysis of Ginseng Metabolome by GC-TOF-MS
2.2. Ginsenosides Composition under Different Treatments
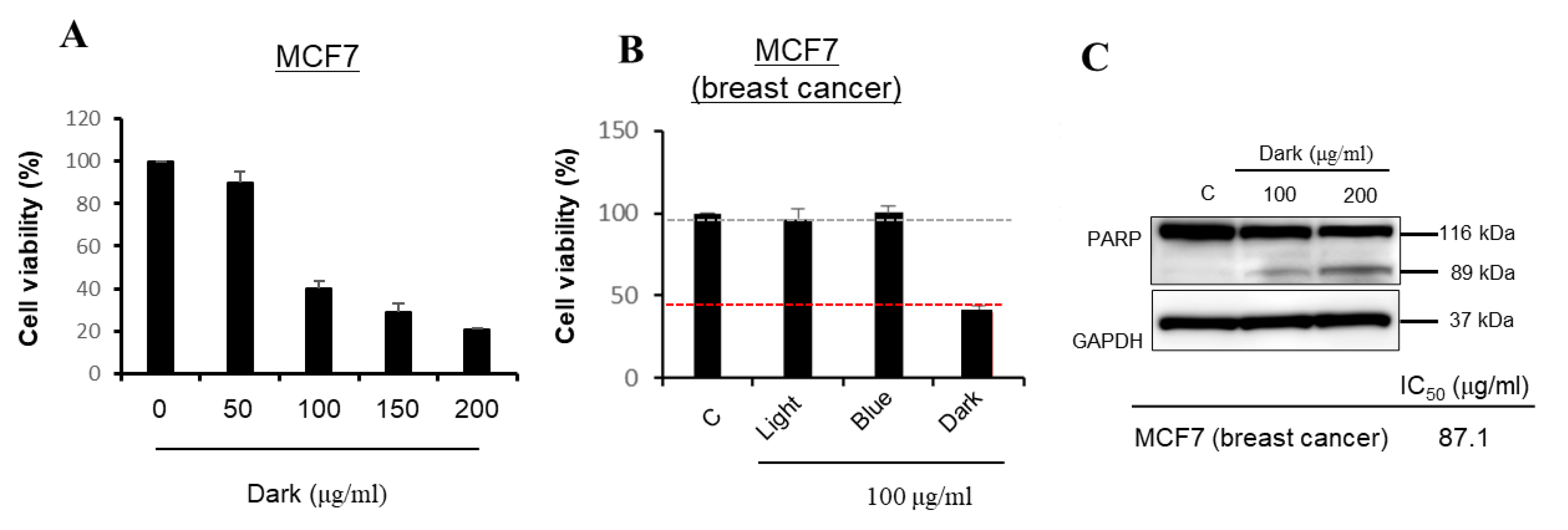
2.3. Dark-Treated Ginseng Extract Reduces the Viability of MCF-7 Cancer Cells
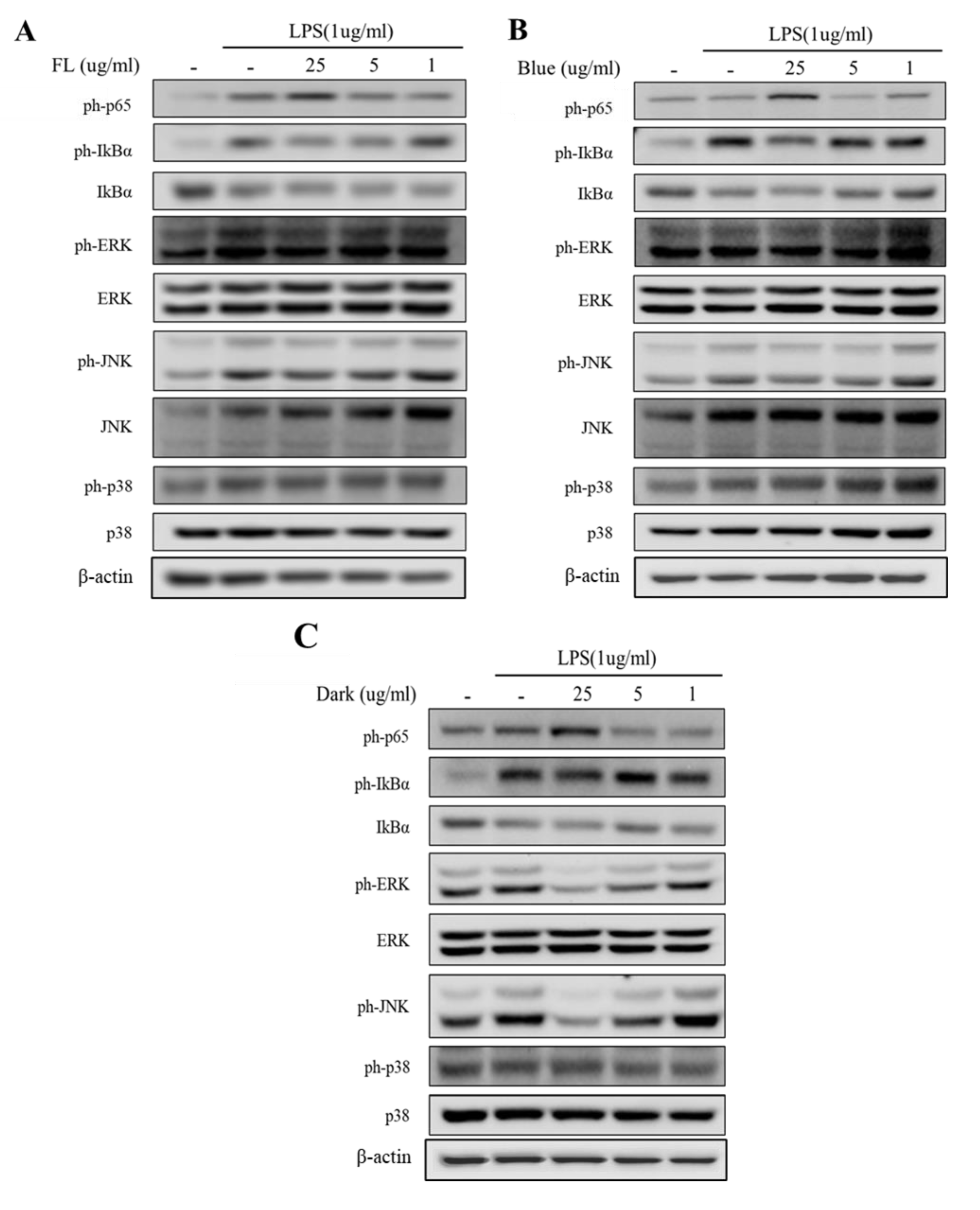
2.4. Dark-Treated Ginseng Extract Inhibits the Pathway of NF-κB Signaling
2.5. Activation of MAPK Signaling Was Inhibited by Dark Treated Ginseng Extract
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Light Treatments and Ginsenosides Extraction
4.3. Ginsenosides Analysis by HPLC—Evaporative Light Scattering Detection (ELSD)
4.4. Sample Preparation for Primary Metabolites Profiling
4.5. Profiling of Primary Metabolites
4.6. Cell Cultures
4.7. Cell Viability Assay
4.8. Preparation of Nuclear Extract, Whole-Cell Lysates and Immunoblot Analysis
4.9. MTT Assay
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ratan, Z.A.; Haidere, M.F.; Hong, Y.H.; Park, S.H.; Lee, J.-O.; Lee, J.; Cho, J.Y. Pharmacological potential of ginseng and its major component ginsenosides. J. Ginseng Res. 2020, 45, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.W.; Luo, K.; Quan, Y.; Cui, S.; Shin, Y.J.; Ko, E.J.; Chung, B.H.; Yang, C.W. The safety, immunological benefits, and efficacy of ginseng in organ transplantation. J. Ginseng Res. 2020, 44, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Unlu, A.; Nayir, E.; Kirca, O.; Ay, H.; Ozdogan, M. Ginseng and cancer. J. BUON 2016, 21, 1383–1387. [Google Scholar] [PubMed]
- Shi, Z.-Y.; Zeng, J.-Z.; Wong, A.S.T. Chemical structures and pharmacological profiles of ginseng saponins. Molecules 2019, 24, 2443. [Google Scholar] [CrossRef]
- Sadeghian, M.; Rahmani, S.; Zendehdel, M.; Hosseini, S.A.; Zare Javid, A. Ginseng and cancer-related fatigue: A systematic review of clinical trials. Nutr. Cancer 2021, 73, 1270–1281. [Google Scholar] [CrossRef]
- Farh, M.E.-A.; Kim, Y.-J.; Sukweenadhi, J.; Singh, P.; Yang, D.-C. Aluminium resistant, plant growth promoting bacteria induce overexpression of Aluminium stress related genes in Arabidopsis thaliana and increase the ginseng tolerance against Aluminium stress. Microbiol. Res. 2017, 200, 45–52. [Google Scholar] [CrossRef]
- Lee, S.; Rhee, D.-K. Effects of ginseng on stress-related depression, anxiety, and the hypothalamic–pituitary–adrenal axis. J. Ginseng Res. 2017, 41, 589–594. [Google Scholar] [CrossRef]
- Bae, M.; Jang, S.; Lim, J.W.; Kang, J.; Bak, E.J.; Cha, J.-H.; Kim, H. Protective effect of Korean Red Ginseng extract against Helicobacter pylori-induced gastric inflammation in Mongolian gerbils. J. Ginseng Res. 2014, 38, 8–15. [Google Scholar] [CrossRef]
- Im, D.-S. Pro-resolving effect of ginsenosides as an anti-inflammatory mechanism of Panax ginseng. Biomolecules 2020, 10, 444. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Park, C.W.; Lee, S.J.; Park, H.-R.; Kim, S.H.; Son, S.-U.; Park, J.; Shin, K.-S. Anti-cancer effects of Panax ginseng berry polysaccharides via activation of immune-related cells. Front. Pharmacol. 2019, 10, 1411. [Google Scholar] [CrossRef]
- Park, K.-C.; Jin, H.; Zheng, R.; Kim, S.; Lee, S.-E.; Kim, B.-H.; Yim, S.-V. Cognition enhancing effect of panax ginseng in Korean volunteers with mild cognitive impairment: A randomized, double-blind, placebo-controlled clinical trial. Transl. Clin. Pharmacol. 2019, 27, 92–97. [Google Scholar] [CrossRef]
- Hyun, S.H.; Bhilare, K.D.; In, G.; Park, C.-K.; Kim, J.-H. Effects of Panax ginseng and ginsenosides on oxidative stress and cardiovascular diseases: Pharmacological and therapeutic roles. J. Ginseng Res. 2022, 46, 33–38. [Google Scholar] [CrossRef]
- You, L.; Cha, S.; Kim, M.-Y.; Cho, J.Y. Ginsenosides are active ingredients in Panax ginseng with immunomodulatory properties from cellular to organismal levels. J. Ginseng Res. 2022, 46, 711–721. [Google Scholar] [CrossRef]
- Hwang, C.R.; Lee, S.H.; Jang, G.Y.; Hwang, I.G.; Kim, H.Y.; Woo, K.S.; Lee, J.; Jeong, H.S. Changes in ginsenoside compositions and antioxidant activities of hydroponic-cultured ginseng roots and leaves with heating temperature. J. Ginseng Res. 2014, 38, 180–186. [Google Scholar] [CrossRef]
- Alrifai, O.; Hao, X.; Marcone, M.F.; Tsao, R. Current review of the modulatory effects of LED lights on photosynthesis of secondary metabolites and future perspectives of microgreen vegetables. J. Agric. Food Chem. 2019, 67, 6075–6090. [Google Scholar] [CrossRef]
- Al Murad, M.; Razi, K.; Jeong, B.R.; Samy, P.M.A.; Muneer, S. Light emitting diodes (LEDs) as agricultural lighting: Impact and its potential on improving physiology, flowering, and secondary metabolites of crops. Sustainability 2021, 13, 1985. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C. Unraveling the role of red: Blue LED lights on resource use efficiency and nutritional properties of indoor grown sweet basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Abd_Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Selenium modulates dynamics of antioxidative defence expression, photosynthetic attributes and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. plants. Environ. Exp. Bot. 2019, 161, 180–192. [Google Scholar] [CrossRef]
- Maroga, G.M.; Soundy, P.; Sivakumar, D. Different postharvest responses of fresh-cut sweet peppers related to quality and antioxidant and phenylalanine ammonia lyase activities during exposure to light-emitting diode treatments. Foods 2019, 8, 359. [Google Scholar] [CrossRef]
- Lian, T.T.; Cha, S.-Y.; Moe, M.M.; Kim, Y.J.; Bang, K.S. Effects of different colored LEDs on the enhancement of biologically active ingredients in callus cultures of Gynura procumbens (Lour.) Merr. Molecules 2019, 24, 4336. [Google Scholar] [CrossRef]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Dhakal, R.; Baek, K.-H. Short period irradiation of single blue wavelength light extends the storage period of mature green tomatoes. Postharvest Biol. Technol. 2014, 90, 73–77. [Google Scholar] [CrossRef]
- Taulavuori, E.; Taulavuori, K.; Holopainen, J.K.; Julkunen-Tiitto, R.; Acar, C.; Dincer, I. Targeted use of LEDs in improvement of production efficiency through phytochemical enrichment. J. Sci. Food Agric. 2017, 97, 5059–5064. [Google Scholar] [CrossRef] [PubMed]
- Amrane, M.; Oukid, S.; Gagaoua, I.; Ensari, T. Breast cancer classification using machine learning. In Proceedings of the 2018 Electric Electronics, Computer Science, Biomedical Engineerings’ Meeting (EBBT), Istanbul, Turkey, 18–19 April 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–4. [Google Scholar]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA A Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R. Global, regional, national burden of breast cancer in 185 countries: Evidence from GLOBOCAN 2018. Breast Cancer Res. Treat. 2021, 187, 557–567. [Google Scholar] [CrossRef]
- Camorani, S.; Granata, I.; Collina, F.; Leonetti, F.; Cantile, M.; Botti, G.; Fedele, M.; Guarracino, M.R.; Cerchia, L. Novel aptamers selected on living cells for specific recognition of triple-negative breast cancer. iScience 2020, 23, 100979. [Google Scholar] [CrossRef]
- Dutta, S.; Mahalanobish, S.; Saha, S.; Ghosh, S.; Sil, P.C. Natural products: An upcoming therapeutic approach to cancer. Food Chem. Toxicol. 2019, 128, 240–255. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, J.; Zhou, C.; Li, Y.; Duan, W.; Zhang, B.; Wang, M.; Fang, J. 20 (S)-Ginsenoside Rh2 displays efficacy against T-cell acute lymphoblastic leukemia through the PI3K/Akt/mTOR signal pathway. J. Ginseng Res. 2020, 44, 725–737. [Google Scholar] [CrossRef]
- Lee, K.-W.; Jung, S.Y.; Choi, S.-M.; Yang, E.J. Effects of ginsenoside Re on LPS-induced inflammatory mediators in BV2 microglial cells. BMC Complement. Altern. Med. 2012, 12, 196. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Park, J.-S.; Jung, J.-S.; Kim, D.-H.; Kim, H.-S. Anti-inflammatory effect of ginsenoside Rg5 in lipopolysaccharide-stimulated BV2 microglial cells. Int. J. Mol. Sci. 2013, 14, 9820–9833. [Google Scholar] [CrossRef]
- Kim, M.; Choi, S.Y.; Kim, K.-T.; Rhee, Y.K.; Hur, J. Ginsenoside Rg18 suppresses lipopolysaccharide-induced neuroinflammation in BV2 microglia and amyloid-β-induced oxidative stress in SH-SY5Y neurons via nuclear factor erythroid 2-related factor 2/heme oxygenase-1 induction. J. Funct. Foods 2017, 31, 71–78. [Google Scholar] [CrossRef]
- Lu, D.; Zhu, L.-H.; Shu, X.-M.; Zhang, C.-J.; Zhao, J.-Y.; Qi, R.-B.; Wang, H.-D.; Lu, D.-X. Ginsenoside Rg1 relieves tert-Butyl hydroperoxide-induced cell impairment in mouse microglial BV2 cells. J. Asian Nat. Prod. Res. 2015, 17, 930–945. [Google Scholar] [CrossRef]
- Jang, S.-w.; Sadiq, N.B.; Hamayun, M.; Jung, J.; Lee, T.; Yang, J.-S.; Lee, B.; Kim, H.-Y. Silicon foliage spraying improves growth characteristics, morphological traits, and root quality of Panax ginseng CA Mey. Ind. Crops Prod. 2020, 156, 112848. [Google Scholar] [CrossRef]
- CHU, L.L.; Hanhong, B. Bacterial endophytes from ginseng and their biotechnological application. J. Ginseng Res. 2021, 46, 1–10. [Google Scholar] [CrossRef]
- Hong, H.; Baatar, D.; Hwang, S.G. Anticancer activities of ginsenosides, the main active components of ginseng. Evid. Based Complement. Altern. Med. 2021, 2021, 8858006. [Google Scholar] [CrossRef]
- Choi, P.; Park, J.Y.; Kim, T.; Park, S.-H.; Kim, H.-k.; Kang, K.S.; Ham, J. Improved anticancer effect of ginseng extract by microwave-assisted processing through the generation of ginsenosides Rg3, Rg5 and Rk1. J. Funct. Foods 2015, 14, 613–622. [Google Scholar] [CrossRef]
- Park, S.-E.; Seo, S.-H.; Lee, K.I.; Na, C.-S.; Son, H.-S. Metabolite profiling of fermented ginseng extracts by gas chromatography mass spectrometry. J. Ginseng Res. 2018, 42, 57–67. [Google Scholar] [CrossRef]
- Cui, S.; Wu, J.; Wang, J.; Wang, X. Discrimination of American ginseng and Asian ginseng using electronic nose and gas chromatography–mass spectrometry coupled with chemometrics. J. Ginseng Res. 2017, 41, 85–95. [Google Scholar] [CrossRef]
- Liu, Z.; Wen, X.; Wang, C.-Z.; Li, W.; Huang, W.-H.; Xia, J.; Ruan, C.-C.; Yuan, C.-S. Remarkable impact of amino acids on ginsenoside transformation from fresh ginseng to red ginseng. J. Ginseng Res. 2020, 44, 424–434. [Google Scholar] [CrossRef]
- Kim, H.W.; Han, S.H.; Lee, S.W.; Choi, H.S.; Suh, H.J.; Hong, K.B. Enzymatic hydrolysis increases ginsenoside content in Korean red ginseng (Panax ginseng CA Meyer) and its biotransformation under hydrostatic pressure. J. Sci. Food Agric. 2019, 99, 6806–6813. [Google Scholar] [CrossRef]
- Choi, M.-K.; Jin, S.; Jeon, J.-H.; Kang, W.Y.; Seong, S.J.; Yoon, Y.-R.; Han, Y.-H.; Song, I.-S. Tolerability and pharmacokinetics of ginsenosides Rb1, Rb2, Rc, Rd, and compound K after single or multiple administration of red ginseng extract in human beings. J. Ginseng Res. 2020, 44, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Yi, E.S.; Kang, C.H.; Liu, Y.; Lee, Y.-G.; Choi, H.S.; Jang, H.B.; Huo, Y.; Baek, N.-I.; Yang, D.C. Whitening and inhibiting NF-κB-mediated inflammation properties of the biotransformed green ginseng berry of new cultivar K1, ginsenoside Rg2 enriched, on B16 and LPS-stimulated RAW 264.7 cells. J. Ginseng Res. 2021, 45, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Reierstad, S.; Lu, M.; Lin, Z.; Ishikawa, H.; Bulun, S.E. Regulation of breast cancer-associated aromatase promoters. Cancer Lett. 2009, 273, 15–27. [Google Scholar] [CrossRef]
- Ahmad, G.; Mir, S.A.; Anand, L.K.; Pottoo, F.H.; Dhiman, N.; Malik, F.; Ali, A. Myricanol-9-acetate, a novel naturally occurring derivative of myricanol, induces ROS-dependent mitochondrial-mediated Apoptosis in MCF-7 cancer cells. Curr. Top. Med. Chem. 2021, 21, 1418–1427. [Google Scholar] [CrossRef]
- Lee, G.H.; Jin, S.W.; Kim, S.J.; Pham, T.H.; Choi, J.H.; Jeong, H.G. Tetrabromobisphenol A induces MMP-9 expression via NADPH oxidase and the activation of ROS, MAPK, and Akt pathways in human breast cancer MCF-7 cells. Toxicol. Res. 2019, 35, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Boulares, A.H.; Yakovlev, A.G.; Ivanova, V.; Stoica, B.A.; Wang, G.; Iyer, S.; Smulson, M. Role of poly (ADP-ribose) polymerase (PARP) cleavage in apoptosis: Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J. Biol. Chem. 1999, 274, 22932–22940. [Google Scholar] [CrossRef]
- Mullen, P. PARP cleavage as a means of assessing apoptosis. In Cancer Cell Culture; Springer: Berlin/Heidelberg, Germany, 2004; pp. 171–181. [Google Scholar]
- Leisching, G.; Loos, B.; Botha, M.; Engelbrecht, A.-M. Bcl-2 confers survival in cisplatin treated cervical cancer cells: Circumventing cisplatin dose-dependent toxicity and resistance. J. Transl. Med. 2015, 13, 328. [Google Scholar] [CrossRef]
- Vo, P.H.T.; Nguyen, T.D.T.; Tran, H.T.; Nguyen, Y.N.; Doan, M.T.; Nguyen, P.H.; Lien, G.T.K.; To, D.C.; Tran, M.H. Cytotoxic components from the leaves of Erythrophleum fordii induce human acute leukemia cell apoptosis through caspase 3 activation and PARP cleavage. Bioorg. Med. Chem. Lett. 2021, 31, 127673. [Google Scholar] [CrossRef]
- Sayed, A.E.-D.H.; Hamed, H.S. Induction of apoptosis and DNA damage by 4-nonylphenol in African catfish (Clarias gariepinus) and the antioxidant role of Cydonia oblonga. Ecotoxicol. Environ. Saf. 2017, 139, 97–101. [Google Scholar] [CrossRef]
- Ajji, P.K.; Binder, M.J.; Walder, K.; Puri, M. Balsamin induces apoptosis in breast cancer cells via DNA fragmentation and cell cycle arrest. Mol. Cell. Biochem. 2017, 432, 189–198. [Google Scholar] [CrossRef]
- Villanueva, P.J.; Martinez, A.; Baca, S.T.; DeJesus, R.E.; Larragoity, M.; Contreras, L.; Gutierrez, D.A.; Varela-Ramirez, A.; Aguilera, R.J. Pyronaridine exerts potent cytotoxicity on human breast and hematological cancer cells through induction of apoptosis. PLoS ONE 2018, 13, e0206467. [Google Scholar]
- Wang, H.-M.; Yang, H.-L.; Thiyagarajan, V.; Huang, T.-H.; Huang, P.-J.; Chen, S.-C.; Liu, J.-Y.; Hsu, L.-S.; Chang, H.-W.; Hseu, Y.-C. Coenzyme Q0 enhances ultraviolet B–induced apoptosis in human estrogen receptor–positive breast (MCF-7) cancer cells. Integr. Cancer Ther. 2017, 16, 385–396. [Google Scholar] [CrossRef]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res. Bull. 2012, 87, 10–20. [Google Scholar] [CrossRef]
- Vinoth Kumar, R.; Oh, T.W.; Park, Y.-K. Anti-inflammatory effects of ginsenoside-Rh2 inhibits LPS-induced activation of microglia and overproduction of inflammatory mediators via modulation of TGF-β1/Smad pathway. Neurochem. Res. 2016, 41, 951–957. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Park, J.-S.; Lee, E.-J.; Lee, S.-Y.; Kim, D.-H.; Kang, J.L.; Kim, H.-S. Anti-inflammatory mechanism of ginseng saponin metabolite Rh3 in lipopolysaccharide-stimulated microglia: Critical role of 5′-adenosine monophosphate-activated protein kinase signaling pathway. J. Agric. Food Chem. 2015, 63, 3472–3480. [Google Scholar] [CrossRef]
- Yu, R.; Li, Q.; Feng, Z.; Cai, L.; Xu, Q. m6A reader YTHDF2 regulates LPS-induced inflammatory response. Int. J. Mol. Sci. 2019, 20, 1323. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Liu, Q.-P.; An, P.; Jia, M.; Luan, X.; Tang, J.-Y.; Zhang, H. Ginsenoside Rd: A promising natural neuroprotective agent. Phytomedicine 2022, 95, 153883. [Google Scholar] [CrossRef]
- Kim, G.-S.; Lee, S.-E.; Noh, H.-J.; Kwon, H.; Lee, S.-W.; Kim, S.-Y.; Kim, Y.-B. Effects of natural bioactive products on the growth and ginsenoside contents of Panax ginseng cultured in an aeroponic system. J. Ginseng Res. 2012, 36, 430. [Google Scholar] [CrossRef]
- Wei, Y.; Hou, B.; Fang, H.; Sun, X.; Ma, F. Salting-out extraction of ginsenosides from the enzymatic hydrolysates of Panax quinquefolium based on ethanol/sodium carbonate system. J. Ginseng Res. 2020, 44, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, P.; Liu, W.; Jiang, Y.; Wang, W.; Bao, L.; Jin, Y.; Li, X. Determination of common ginsenosides in Kang’ai injection by aqueous two-phase extraction with deep eutectic solvents and HPLC-UV/DAD. Microchem. J. 2018, 137, 302–308. [Google Scholar] [CrossRef]
- Xu, L.; Xu, J.; Shi, G.; Xiao, S.; Dai, R.; Wu, S.; Sun, B.; Zhang, X.; Zhao, Y. Optimization of flash extraction, separation of ginsenosides, identification by HPLC-FT-ICR-MS and determination of rare ginsenosides in mountain cultivated ginseng. RSC Adv. 2020, 10, 44050–44057. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Song, K.-W.; Hong, S.-P. Simultaneous quantification of six nonpolar ginsenosides in white ginseng by reverse-phase high-performance liquid chromatography coupled with integrated pulsed amperometric detection. J. Ginseng Res. 2020, 44, 563–569. [Google Scholar] [CrossRef]
- Kim, T.J.; Choi, J.; Kim, K.W.; Ahn, S.K.; Ha, S.H.; Choi, Y.; Park, N.I.; Kim, J.K. Metabolite profiling of peppers of various colors reveals relationships between tocopherol, carotenoid, and phytosterol content. J. Food Sci. 2017, 82, 2885–2893. [Google Scholar] [CrossRef]
- Ghosson, H.; Schwarzenberg, A.; Jamois, F.; Yvin, J.-C. Simultaneous untargeted and targeted metabolomics profiling of underivatized primary metabolites in sulfur-deficient barley by ultra-high performance liquid chromatography-quadrupole/time-of-flight mass spectrometry. Plant Methods 2018, 14, 62. [Google Scholar] [CrossRef]
- Al-Rubaye, A.F.; Hameed, I.H.; Kadhim, M.J. A review: Uses of gas chromatography-mass spectrometry (GC-MS) technique for analysis of bioactive natural compounds of some plants. Int. J. Toxicol. Pharmacol. Res. 2017, 9, 81–85. [Google Scholar] [CrossRef]
- Lim, W.-C.; Kim, H.; Kim, Y.-J.; Jeon, B.-N.; Kang, H.-B.; Ko, H. Catechol inhibits epidermal growth factor-induced epithelial-to-mesenchymal transition and stem cell-like properties in hepatocellular carcinoma cells. Sci. Rep. 2020, 10, 7620. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the MTT assay. Cold Spring Harb. Protoc. 2018, 2018, prot095505. [Google Scholar] [CrossRef]
- Litovchick, L. Preparing whole-cell lysates for immunoblotting. Cold Spring Harb. Protoc. 2018, 2018, prot098400. [Google Scholar] [CrossRef]
- Gessi, S.; Borea, P.A.; Bencivenni, S.; Fazzi, D.; Varani, K.; Merighi, S. The activation of μ-opioid receptor potentiates LPS-induced NF-kB promoting an inflammatory phenotype in microglia. FEBS Lett. 2016, 590, 2813–2826. [Google Scholar] [CrossRef]
- Santa-Cecília, F.V.; Socias, B.; Ouidja, M.O.; Sepulveda-Diaz, J.E.; Acuna, L.; Silva, R.L.; Michel, P.P.; Del-Bel, E.; Cunha, T.M.; Raisman-Vozari, R. Doxycycline suppresses microglial activation by inhibiting the p38 MAPK and NF-kB signaling pathways. Neurotox. Res. 2016, 29, 447–459. [Google Scholar] [CrossRef]
- Karakaş, D.; Ari, F.; Ulukaya, E. The MTT viability assay yields strikingly false-positive viabilities although the cells are killed by some plant extracts. Turk. J. Biol. 2017, 41, 919–925. [Google Scholar] [CrossRef]
- Sousa, N.A.; Oliveira, G.A.; de Oliveira, A.P.; Lopes, A.L.F.; Iles, B.; Nogueira, K.M.; Araújo, T.S.; Souza, L.K.; Araújo, A.R.; Ramos-Jesus, J. Novel ocellatin peptides mitigate LPS-induced ROS formation and NF-kB activation in microglia and hippocampal neurons. Sci. Rep. 2020, 10, 2696. [Google Scholar] [CrossRef]
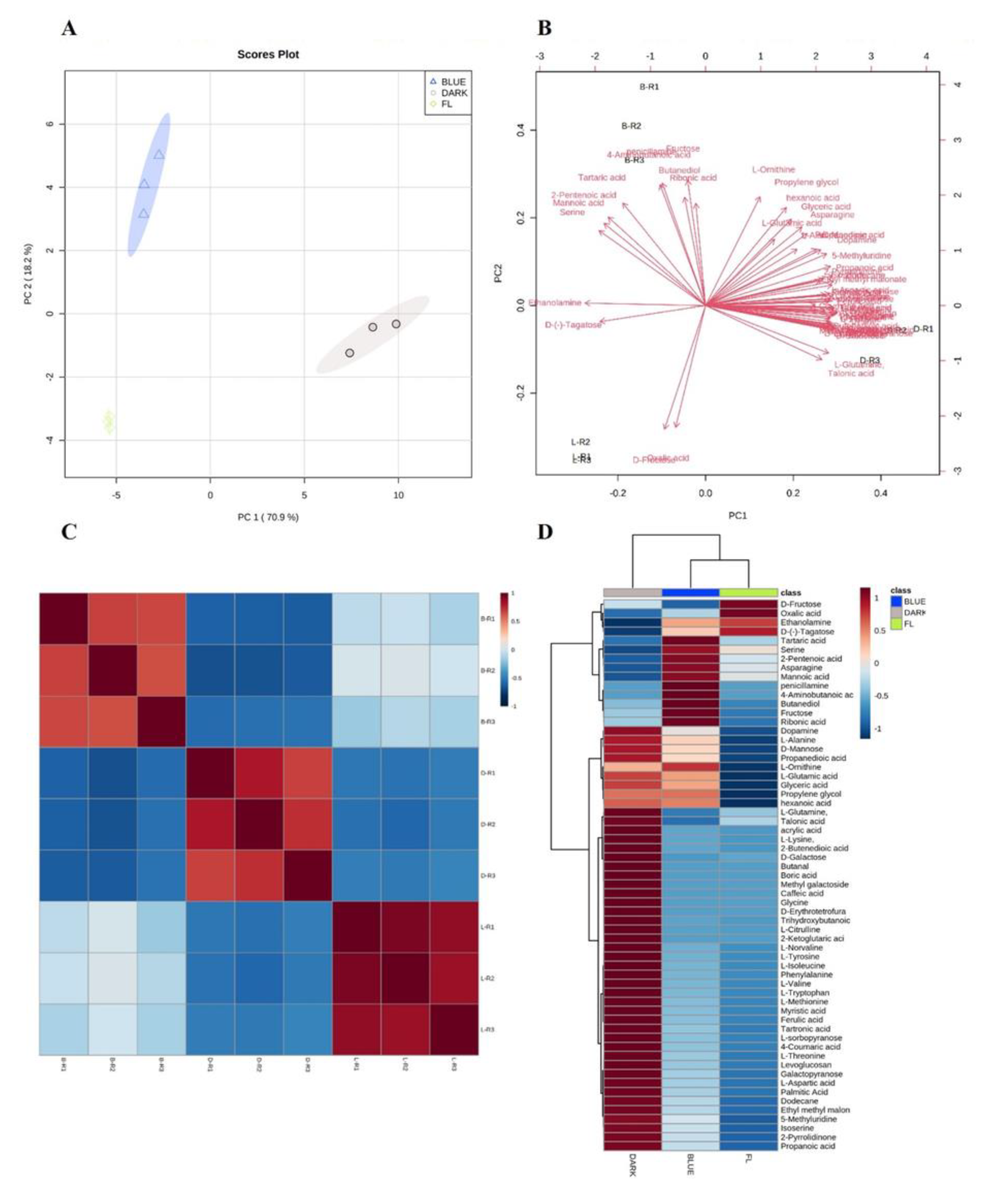
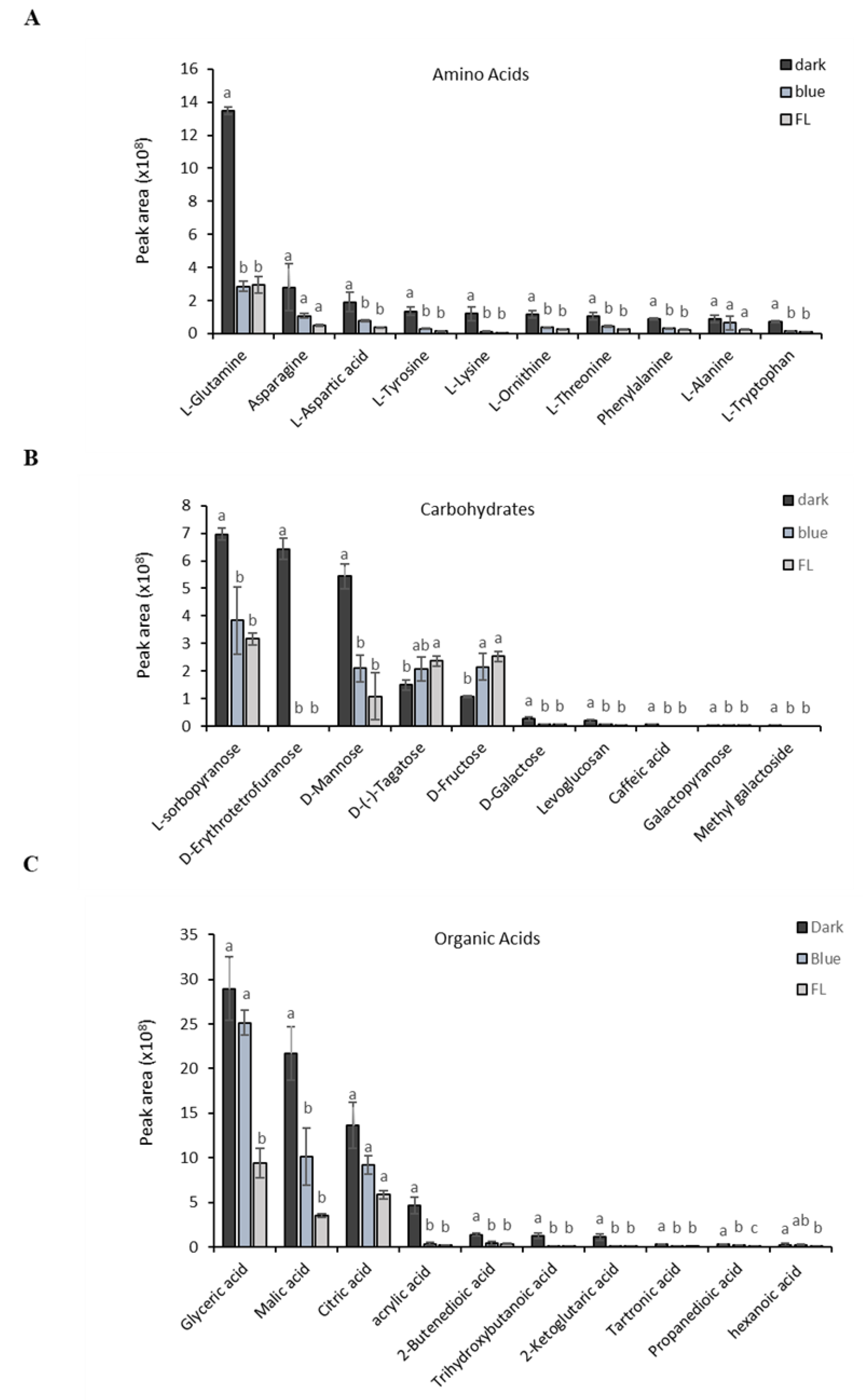

| NO | RT(SEC) | Identified Compound | Formula | Treatment | Peak Area | p-Value |
|---|---|---|---|---|---|---|
| Organic Acids | ||||||
| 1 | 274.6 | Boric acid | H3BO3 | FL | ND | |
| Dark | 55,158 ± 4979 | 0.003 | ||||
| Blue | ND | |||||
| 2 | 326.8 | Ethyl methyl malonate | C6H10O4 | FL | ND | |
| Dark | 2,648,561 ± 77,905 | 0.000 | ||||
| Blue | 907,763 ± 450,128 | |||||
| 3 | 414.5 | Propanedioic acid | C3H4O4 | FL | 11,837,242 ± 963,277 | |
| Dark | 26,345,064 ± 807,597 | 0.000 | ||||
| Blue | 20,531,543 ± 1,296,059 | 0.01 | ||||
| 4 | 471 | 4-Aminobutanoic acid | C4H9NO2 | FL | ND | |
| Dark | ND | |||||
| Blue | 35,057,390 ± 5,863,536 | 0.001 | ||||
| 5 | 490 | Glyceric acid | C3H6O4 | FL | 941,334,205 ± 162,244,741 | |
| Dark | 2,894,792,786 ± 755,578,224 | |||||
| Blue | 2,514,331,459 ± 142,931,824 | 0.01 | ||||
| 6 | 495.1 | 2-Butenedioic acid | C6H8O4 | FL | 3,388,837 ± 3,149,704 | |
| Dark | 138,224,506 ± 14,064,424 | 0.008 | ||||
| Blue | 38,551,250 ± 20,640,438 | |||||
| 7 | 603 | Trihydroxybutanoic acid | C4H8O5 | FL | 6,946,451 ± 1,197,940 | |
| Dark | 102,284,588.33 ± 3,523,581 | 0.000 | ||||
| Blue | 9,506,910 ± 901,734.6 | |||||
| 8 | 628 | Oxalic acid | C2H2O4 | FL | 15,000,978 ± 2,279,165 | |
| Dark | ND | |||||
| Blue | 5,048,996 ± 997,737 | 0.03 | ||||
| 9 | 666.3 | Hexanoic acid | C6H12O2 | FL | 6,361,231 ± 2,906,165 | |
| Dark | 25,147,663 ± 3,163,963 | 0.03 | ||||
| Blue | 23,949,708 ± 4,094,744 | 0.003 | ||||
| 10 | 703.3 | Ribonic acid | C5H9O6 | FL | 10,727,733 ± 6,149,856 | |
| Dark | 13,826,780 ± 866,318 | |||||
| Blue | 23,317,685 ± 7,507,867 | 0.01 | ||||
| 11 | 745.7 | 2-Ketoglutaric acid | C5H6O5 | FL | 5,572,062 ± 1,486,034 | |
| Dark | 110,162,880 ± 33,201,327 | 0.04 | ||||
| Blue | 6,303,697 ± 4,026,419 | |||||
| 12 | 775.5 | Acrylic acid | C3H4O2 | FL | 17,604,061 ± 1,032,006 | |
| Dark | 463,504,027 ± 92,918,712 | 0.01 | ||||
| Blue | 3,5014,339 ± 14,354,648 | |||||
| 13 | 780.4 | 4-Coumaric acid | C9H8O3 | FL | ND | |
| Dark | 8,441,648 ± 4,174,601 | |||||
| Blue | 1,794,541 ± 258,199.4 | 0.008 | ||||
| 14 | 785.4 | Tartronic acid | C3H4O5 | FL | 2,751,360 ± 304,388 | |
| Dark | 27,093,984 ± 4,230,208 | 0.001 | ||||
| Blue | 7,538,675 ± 1,832,029 | 0.05 | ||||
| 15 | 826 | Tartaric acid | C4H6O6 | FL | 3,5980,414 ± 2,407,682 | |
| Dark | ND | |||||
| Blue | 109,798,116 ± 3,291,842 | 0.000 | ||||
| 16 | 854.5 | Ferulic acid | C10H10O4 | FL | ND | |
| Dark | 19,429,576 ± 7,087,994 | 0.05 | ||||
| Blue | 3,751,615 ± 607,332 | 0.01 | ||||
| 17 | 871 | 2-Pentenoic acid | C5H8O2 | FL | 2,427,381 ± 154,542 | |
| Dark | ND | |||||
| Blue | 5,276,122 ± 243,489 | 0.005 | ||||
| 18 | 936.2 | Propanoic acid | C₃H₆O₂ | FL | ND | |
| Dark | 4,142,119 ± 68,430 | 0.000 | ||||
| Blue | 1,682,445 ± 172,712 | 0.004 | ||||
| 19 | 1484 | Mannoic acid | C6H12O7 | FL | 103,339,880 ± 7,836,722 | |
| Dark | ND | |||||
| Blue | 204,832,544 ± 15,541,722 | 0.01 | ||||
| Amino Acids | ||||||
| 20 | 341.2 | L-Valine | C5H11NO2 | FL | 648,024 ± 38,340 | |
| Dark | 21,271,780 ± 5,902,237 | 0.03 | ||||
| Blue | 3,254,685 ± 1,830,534 | |||||
| 21 | 350.5 | L-Alanine | C3H7NO2 | FL | 22,921,160 ± 2,208,680 | |
| Dark | 98,293,721 ± 15,568,039 | 0.01 | ||||
| Blue | 64,064,382 ± 40,670,416 | |||||
| 22 | 506.2 | Serine | C3H7NO3 | FL | 38,823,176 ± 3,976,486 | |
| Dark | ND | |||||
| Blue | 66,865,059 ± 8,216,446 | 0.01 | ||||
| 23 | 512.4 | Isoserine | C3H7NO3 | FL | 1,655,877 ± 625,229 | |
| Dark | 5,951,890 ± 19,049 | 0.01 | ||||
| Blue | 3,295,161 ± 1,544,319 | |||||
| 24 | 521.4 | L-Threonine | C4H9NO3 | FL | 26,596,544 ± 3,132,799 | |
| Dark | 106,533,538 ± 20,954,160 | 0.03 | ||||
| Blue | 4,396,874 ± 792,504 | 0.02 | ||||
| 25 | 562.2 | L-Citrulline | C6H13N3O3 | FL | ND | |
| Dark | 4,730,208 ± 335,083 | 0.002 | ||||
| Blue | 68,736 ± 53,704 | |||||
| 26 | 589.9 | L-Aspartic acid | C4H7NO4 | FL | 36,974,682 ± 3,990,717 | |
| Dark | 189,220,043 ± 58,130,937 | 0.01 | ||||
| Blue | 76,458,966 ± 3,877,746 | 0.006 | ||||
| 27 | 591.1 | L-Methionine | C4H7NO4 | FL | 3,918,664 ± 755,737 | |
| Dark | 25,062,068 ± 579,209 | 0.000 | ||||
| Blue | 6,590,229 ± 635,920 | |||||
| 28 | 634.6 | L-Ornithine | C5H12N2O2 | FL | 25,776,495 ± 2,926,910 | |
| Dark | 114,425,820 ± 21,676,407 | 0.02 | ||||
| Blue | 36,284,134 ± 3,763,759 | |||||
| 29 | 636.9 | L-Glutamic acid | C5H9NO4 | FL | ND | |
| Dark | 30,258,688 ± 4,385,870 | 0.008 | ||||
| Blue | 22,155,999 ± 26,019,046 | |||||
| 30 | 589.9 | L-Aspartic acid | C4H7NO4 | FL | 36,974,682 ± 3,990,717 | |
| Dark | 155,886,710 ± 18,716,099 | 0.01 | ||||
| Blue | 76,458,966 ± 3,877,746 | 0.006 | ||||
| 31 | 644.7 | Phenylalanine | C9H11NO2 | FL | 22,839,808 ± 2,262,238 | |
| Dark | 89,290,303 ± 6,606,822 | 0.005 | ||||
| Blue | 30,596,843 ± 2,963,083 | 0.04 | ||||
| 32 | 676.7 | L-Lysine | C6H14N2O2 | FL | 6,874,649 ± 415,258 | |
| Dark | 120,874,869 ± 42,373,390 | 0.05 | ||||
| Blue | 12,535,775 ± 3,810,434 | |||||
| 33 | 707.9 | L-Glutamine | C5H10N2O3 | FL | 294,061,961 ± 52,227,860 | |
| Dark | 1,347,690,969 ± 20,051,935 | 0.000 | ||||
| Blue | 285,096,879 ± 30,214,602 | |||||
| 34 | 662.9 | Asparagine | C4H8N2O3 | FL | 51,785,180 ± 5,177,064 | |
| Dark | 150,042,799 ± 25,531,561 | 0.02 | ||||
| Blue | 105,437,258 ± 14,148,732 | 0.03 | ||||
| 35 | 781.7 | L-Tyrosine | C9H11NO3 | FL | 16,119,127 ± 1,204,190 | |
| Dark | 135,002,280 ± 26,479,454 | 0.02 | ||||
| Blue | 28,514,941 ± 3,558,700 | 0.02 | ||||
| 36 | 932.3 | L-Tryptophan | C11H12N2O2 | FL | 8,223,776 ± 1,547,891 | |
| Dark | 70,186,274 ± 6,300,278 | 0.003 | ||||
| Blue | 16,435,122 ± 782,277 | 0.02 | ||||
| Carbohydrates | ||||||
| 37 | 725.7 | D-Mannose | C6H12O6 | FL | 107,738,818 ± 84,582,603 | |
| Dark | 543,957,084 ± 46,193,939 | 0.02 | ||||
| Blue | 209,192,752 ± 48,618,900 | |||||
| 38 | 733.3 | L-sorbopyranose | C6H12O6 | FL | 316,158,562 ± 20,999,619 | |
| Dark | 697,213,182 ± 20,642,808 | 0.001 | ||||
| Blue | 383,562,829 ± 121,668,384 | |||||
| 39 | 755.2 | D-Fructose | C6H12O6 | FL | 253,630,327 ± 18,247,145 | |
| Dark | 107,158,254 ± 3,685,079 | 0.009 | ||||
| Blue | 214,588,479 ± 48,192,251 | |||||
| 40 | 759.7 | D-(-)-Tagatose | C6H12O6 | FL | 235,944,910 ± 19,541,216 | |
| Dark | 149,426,526 ± 7,315,272 | 0.03 | ||||
| Blue | 205,866,117 ± 43,378,085 | |||||
| 41 | 809.3 | D-Erythrofuranose | C4H8O4 | FL | ND | |
| Dark | 643,197,453 ± 38,582,722 | 0.001 | ||||
| Blue | ND | |||||
| 42 | 851 | D-Galactose | C6H12O6 | FL | 6,796,773 ± 416,588 | |
| Dark | 25,968,973 ± 686,2756 | 0.04 | ||||
| Blue | 6,104,887 ± 607,559 | |||||
| 43 | 876.4 | Caffeic acid | C9H8O4 | FL | ND | |
| Dark | 6,097,960 ± 405,390 | 0.001 | ||||
| Blue | ND | |||||
| 44 | 908.9 | Methyl galactoside | C7H14O6 | FL | ND | |
| Dark | 1,748,082 ± 248,700 | 0.008 | ||||
| Blue | ND | |||||
| 45 | 1020.5 | Levoglucosan | C6H10O5 | FL | 3,822,565 ± 81,039 | |
| Dark | 18,672,292 ± 536,7540 | 0.05 | ||||
| Blue | 7,096,685 ± 264,684 | 0.001 | ||||
| 46 | 1160.6 | Galactopyranose | C6H12O6 | FL | 1,243,798 ± 314,085 | |
| Dark | 3,954,698 ± 428,917 | 0.01 | ||||
| Blue | 1,937,122 ± 233,853 | 0.02 | ||||
| Others | ||||||
| d47 | 410 | Dodecane | C12H26 | FL | ND | |
| Dark | 2,089,678 ± 289,395 | 0.007 | ||||
| Blue | 750,133 ± 109,095 | 0.08 | ||||
| 48 | 453.8 | Ethanolamine | C2H7NO | FL | 27,440,780 ± 624,097 | |
| Dark | 1,463,234 ± 97,721 | 0.000 | ||||
| Blue | 22,216,551 ± 8,680,885 | |||||
| 49 | 533 | Dopamine | C8H11NO2 | FL | ND | |
| Dark | 1,463,234 ± 97,721 | 0.000 | ||||
| Blue | 2,322,461 ± 76,435 | 0.000 | ||||
| 50 | 608 | Penicillamine | C5H11NO2S | FL | ND | |
| Dark | ND | |||||
| Blue | 2,036,085 ± 189,334 | 0.003 | ||||
| 51 | 823.4 | Palmitic Acid | C16H32O2 | FL | 12,165,495 ± 236,559 | |
| Dark | 30,359,205 ± 4,795,821 | 0.02 | ||||
| Blue | 16,927,244 ± 2,657,439 | |||||
| 52 | 927 | Myristic acid | C14H28O2 | FL | 7,061,525 ± 491,473 | |
| Dark | 12,418,666 ± 1,559,438 | 0.02 | ||||
| Blue | 7,921,258 ± 489,889 | |||||
| 53 | 1467.7 | 5-Methyluridine | C10H14N2O6 | FL | ND | |
| Dark | 8,401,912 ± 758,232 | 0.003 | ||||
| Blue | 3,838,136 ± 443,175 | 0.005 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadiq, N.B.; Kwon, H.; Park, N.I.; Hamayun, M.; Jung, J.-H.; Yang, S.-H.; Jang, S.-W.; Kabadayı, S.N.; Kim, H.-Y.; Kim, Y.-J. The Impact of Light Wavelength and Darkness on Metabolite Profiling of Korean Ginseng: Evaluating Its Anti-Cancer Potential against MCF-7 and BV-2 Cell Lines. Int. J. Mol. Sci. 2023, 24, 7768. https://doi.org/10.3390/ijms24097768
Sadiq NB, Kwon H, Park NI, Hamayun M, Jung J-H, Yang S-H, Jang S-W, Kabadayı SN, Kim H-Y, Kim Y-J. The Impact of Light Wavelength and Darkness on Metabolite Profiling of Korean Ginseng: Evaluating Its Anti-Cancer Potential against MCF-7 and BV-2 Cell Lines. International Journal of Molecular Sciences. 2023; 24(9):7768. https://doi.org/10.3390/ijms24097768
Chicago/Turabian StyleSadiq, Nooruddin Bin, Hyukjoon Kwon, Nam Il Park, Muhammad Hamayun, Je-Hyeong Jung, Seung-Hoon Yang, Soo-Won Jang, Seda Nur Kabadayı, Ho-Youn Kim, and Young-Joo Kim. 2023. "The Impact of Light Wavelength and Darkness on Metabolite Profiling of Korean Ginseng: Evaluating Its Anti-Cancer Potential against MCF-7 and BV-2 Cell Lines" International Journal of Molecular Sciences 24, no. 9: 7768. https://doi.org/10.3390/ijms24097768
APA StyleSadiq, N. B., Kwon, H., Park, N. I., Hamayun, M., Jung, J.-H., Yang, S.-H., Jang, S.-W., Kabadayı, S. N., Kim, H.-Y., & Kim, Y.-J. (2023). The Impact of Light Wavelength and Darkness on Metabolite Profiling of Korean Ginseng: Evaluating Its Anti-Cancer Potential against MCF-7 and BV-2 Cell Lines. International Journal of Molecular Sciences, 24(9), 7768. https://doi.org/10.3390/ijms24097768








