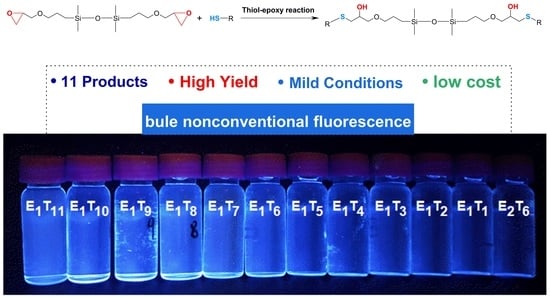
Facile Synthesis of Sulfur-Containing Functionalized Disiloxanes with Nonconventional Fluorescence by Thiol–Epoxy Click Reaction
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Reaction Condition
2.2. Synthesis and Characterization of Sulfur-Containing Functionalized Disiloxanes
2.3. Fluorescent Properties
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. General Procedure for the Synthesis of Sulfur-Containing Functionalized Disiloxanes E1T1 to E1T11
3.4. Synthesis of bis(2-(ethyl-3-acetate-thiopropoxy)-2-ol-ethyl)-ether (E2T6)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yilgör, E.; Yilgör, I. Silicone containing copolymers: Synthesis, properties and applications. Prog. Polym. Sci. 2014, 39, 1165–1195. [Google Scholar] [CrossRef]
- Rücker, C.; Kümmerer, K. Environmental Chemistry of Organosiloxanes. Chem. Rev. 2015, 115, 466–524. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yao, Y.; Li, X.; Zhang, Z. Fabrication of advanced polydimethylsiloxane-based functional materials: Bulk modifications and surface functionalizations. Chem. Eng. J. 2021, 408, 127262. [Google Scholar] [CrossRef]
- Cazacu, M.; Dascalu, M.; Stiubianu, G.-T.; Bele, A.; Tugui, C.; Racles, C. From passive to emerging smart silicones. Rev. Chem. Eng. 2022. [Google Scholar] [CrossRef]
- Qi, D.; Zhang, K.; Tian, G.; Jiang, B.; Huang, Y. Stretchable Electronics Based on PDMS Substrates. Adv. Mater. 2021, 33, 2003155. [Google Scholar] [CrossRef]
- Wang, D.; Klein, J.; Mejía, E. Catalytic Systems for the Cross-Linking of Organosilicon Polymers. Chem. Asian J. 2017, 12, 1180–1197. [Google Scholar] [CrossRef]
- Brook, M.A. Silicon in Organic, Organometallic, and Polymer Chemistry; Wiley: New York, NY, USA, 2000; Volume 123. [Google Scholar]
- Pawlenko, S. Organosilicon Chemistry; De Gruyter: Berlin, Germany, 1986. [Google Scholar]
- Chojnowski, J.; Marciniec, B. Progress in Organosilicon Chemistry; Gordon and Breach: Philadelphia, PA, USA, 1995. [Google Scholar]
- Wang, D.; Cao, J.; Hang, D.; Li, W.; Feng, S. Novel Organosilicon Synthetic Methodologies. Prog. Chem. 2019, 31, 110–120. [Google Scholar]
- Jones, R.; Ando, W.; Chojnowski, J. The Science and Technology of Their Synthesis and Applications. Silicon-Containing Polymers; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- LaRonde, F.J.; Brook, M.A.; Hu, G. Amino acid and peptide chemistry on silicones. Silicon Chem. 2002, 1, 215–222. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, M.; Gao, Y.; Zhou, Q.; Zhang, Y.; Yu, J.; Xu, W.; Yan, J.; Liu, H.; Lei, Z.; et al. Stepwise on-demand functionalization of multihydrosilanes enabled by a hydrogen-atom-transfer photocatalyst based on eosin Y. Nat. Chem. 2023. [Google Scholar] [CrossRef]
- Fuchise, K.; Sato, K.; Igarashi, M. Precise Synthesis of Linear Polysiloxanes End-Functionalized with Alkynylsilyl Groups by Organocatalytic Ring-Opening Polymerization of Cyclotrisiloxanes. Macromolecules 2021, 54, 5765–5773. [Google Scholar] [CrossRef]
- Siripanich, P.; Bureerug, T.; Chanmungkalakul, S.; Sukwattanasinitt, M.; Ervithayasuporn, V. Mono and Dumbbell Silsesquioxane Cages as Dual-Response Fluorescent Chemosensors for Fluoride and Polycyclic Aromatic Hydrocarbons. Organometallics 2022, 41, 201–210. [Google Scholar] [CrossRef]
- Yi, B.; Wang, S.; Hou, C.; Huang, X.; Cui, J.; Yao, X. Dynamic siloxane materials: From molecular engineering to emerging applications. Chem. Eng. J. 2021, 405, 127023. [Google Scholar] [CrossRef]
- Golas, P.L.; Matyjaszewski, K. Marrying click chemistry with polymerization: Expanding the scope of polymeric materials. Chem. Soc. Rev. 2010, 39, 1338–1354. [Google Scholar] [CrossRef]
- Qin, A.; Lam, J.W.Y.; Tang, B.Z. Click polymerization. Chem. Soc. Rev. 2010, 39, 2522–2544. [Google Scholar] [CrossRef] [PubMed]
- Franc, G.; Kakkar, A.K. “Click” methodologies: Efficient, simple and greener routes to design dendrimers. Chem. Soc. Rev. 2010, 39, 1536–1544. [Google Scholar] [CrossRef]
- Rambarran, T.; Bertrand, A.; Gonzaga, F.; Boisson, F.; Bernard, J.; Fleury, E.; Ganachaud, F.; Brook, M.A. Sweet supramolecular elastomers from α,ω-(β-cyclodextrin terminated) PDMS. Chem. Commun. 2016, 52, 6681–6684. [Google Scholar] [CrossRef]
- Tucker-Schwartz, A.K.; Farrell, R.A.; Garrell, R.L. Thiol–ene Click Reaction as a General Route to Functional Trialkoxysilanes for Surface Coating Applications. J. Am. Chem. Soc. 2011, 133, 11026–11029. [Google Scholar] [CrossRef]
- Zuo, Y.; Cao, J.; Feng, S. Sunlight-Induced Cross-Linked Luminescent Films Based on Polysiloxanes and d-Limonene via Thiol–ene “Click” Chemistry. Adv. Funct. Mater. 2015, 25, 2754–2762. [Google Scholar] [CrossRef]
- Genest, A.; Binauld, S.; Pouget, E.; Ganachaud, F.; Fleury, E.; Portinha, D. Going beyond the barriers of aza-Michael reactions: Controlling the selectivity of acrylates towards primary amino-PDMS. Polym. Chem. 2017, 8, 624–630. [Google Scholar] [CrossRef]
- Lu, H.; Feng, L.; Li, S.; Zhang, J.; Lu, H.; Feng, S. Unexpected Strong Blue Photoluminescence Produced from the Aggregation of Unconventional Chromophores in Novel Siloxane–Poly(amidoamine) Dendrimers. Macromolecules 2015, 48, 476–482. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, Y.; Gou, Z.; Lin, W. Facile construction of imidazole functionalized polysiloxanes by thiol-ene “Click” reaction for the consecutive detection of Fe3+ and amino acids. Sensors Actuat. Chem. 2019, 291, 235–242. [Google Scholar] [CrossRef]
- Bezlepkina, K.A.; Ardabevskaia, S.N.; Klokova, K.S.; Ryzhkov, A.I.; Migulin, D.A.; Drozdov, F.V.; Cherkaev, G.V.; Muzafarov, A.M.; Milenin, S.A. Environment Friendly Process toward Functional Polyorganosiloxanes with Different Chemical Structures through CuAAC Reaction. ACS Appl. Polym. Mater. 2022, 4, 6770–6783. [Google Scholar] [CrossRef]
- Milenin, S.A.; Drozdov, F.V.; Bezlepkina, K.A.; Majorov, V.Y.; Muzafarov, A.M. Acid-Catalyzed Rearrangement of Azidopropyl-Siloxane Monomers for the Synthesis of Azidopropyl-Polydimethylsiloxane and Their Carboxylic Acid Derivatives. Macromolecules 2021, 54, 2921–2935. [Google Scholar] [CrossRef]
- Zuo, Y.; Gou, Z.; Zhang, C.; Feng, S. Polysiloxane-Based Autonomic Self-Healing Elastomers Obtained through Dynamic Boronic Ester Bonds Prepared by Thiol–Ene “Click” Chemistry. Macromol. Rapid Commun. 2016, 37, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Huang, T.; Li, S.; Li, K.; Li, C.; Liu, Y.; Wang, Y.; Yu, C.; Zhou, Y. Emulsion-Assisted Polymerization-Induced Hierarchical Self-Assembly of Giant Sea Urchin-like Aggregates on a Large Scale. Angew. Chem. 2018, 130, 8175–8179. [Google Scholar] [CrossRef]
- Stuparu, M.C.; Khan, A. Thiol-epoxy “click” chemistry: Application in preparation and postpolymerization modification of polymers. J. Polym. Sci. Polym. Chem. 2016, 54, 3057–3070. [Google Scholar] [CrossRef]
- Hoang, M.V.; Chung, H.-J.; Elias, A.L. Irreversible bonding of polyimide and polydimethylsiloxane (PDMS) based on a thiol-epoxy click reaction. J. Microme. Microeng. 2016, 26, 105019. [Google Scholar] [CrossRef]
- Shevchenko, V.; Bliznyuk, V.; Gumenna, M.; Klimenko, N.; Stryutsky, A.; Wang, J.; Binek, C.; Chernyakova, M.; Belikov, K. Coordination Polymers Based on Amphiphilic Oligomeric Silsesquioxanes and Transition Metal Ions (Co2+, Ni2+): Structure and Stimuli-Responsive Properties. Macromol. Mater. Eng. 2021, 306, 2100085. [Google Scholar] [CrossRef]
- Shibasaki, S.; Sasaki, Y.; Nakabayashi, K.; Mori, H. Synthesis and metal complexation of dual-functionalized silsesquioxane nanoparticles by sequential thiol–epoxy click and esterification reactions. React. Funct. Polym. 2016, 107, 11–19. [Google Scholar] [CrossRef]
- Lin, H.; Chen, L.; Ou, J.; Liu, Z.; Wang, H.; Dong, J.; Zou, H. Preparation of well-controlled three-dimensional skeletal hybrid monoliths via thiol–epoxy click polymerization for highly efficient separation of small molecules in capillary liquid chromatography. J. Chromatog. A 2015, 1416, 74–82. [Google Scholar] [CrossRef]
- Lungu, A.; Ghitman, J.; Cernencu, A.I.; Serafim, A.; Florea, N.M.; Vasile, E.; Iovu, H. POSS-containing hybrid nanomaterials based on thiol-epoxy click reaction. Polymer 2018, 145, 324–333. [Google Scholar] [CrossRef]
- Bekin Acar, S.; Ozcelik, M.; Uyar, T.; Tasdelen, M.A. Polyhedral oligomeric silsesquioxane-based hybrid networks obtained via thiol-epoxy click chemistry. Iran. Polym. J. 2017, 26, 405–411. [Google Scholar] [CrossRef]
- Lin, G.; Yin, J.; Lin, Z.; Zhu, Y.; Li, W.; Li, H.; Liu, Z.; Xiang, H.; Liu, X. Facile thiol-epoxy click chemistry for transparent and aging-resistant silicone/epoxy composite as LED encapsulant. Prog. Org. Coat. 2021, 156, 106269. [Google Scholar] [CrossRef]
- Murphy, Z.; Kent, M.; Freeman, C.; Landge, S.; Koricho, E. Halloysite nanotubes functionalized with epoxy and thiol organosilane groups to improve fracture toughness in nanocomposites. SN Appl. Sci. 2020, 2, 213. [Google Scholar] [CrossRef]
- Bera, S.; Rout, T.K.; Udayabhanu, G.; Narayan, R. Water-based & eco-friendly epoxy-silane hybrid coating for enhanced corrosion protection & adhesion on galvanized steel. Prog. Org. Coat. 2016, 101, 24–44. [Google Scholar]
- De, S.; Khan, A. Efficient synthesis of multifunctional polymersviathiol–epoxy “click” chemistry. Chem. Commun. 2012, 48, 3130–3132. [Google Scholar] [CrossRef]
- Gadwal, I.; Stuparu, M.C.; Khan, A. Homopolymer bifunctionalization through sequential thiol–epoxy and esterification reactions: An optimization, quantification, and structural elucidation study. Polym. Chem. 2015, 6, 1393–1404. [Google Scholar] [CrossRef]
- From, M.; Larsson, P.T.; Andreasson, B.; Medronho, B.; Svanedal, I.; Edlund, H.; Norgren, M. Tuning the properties of regenerated cellulose: Effects of polarity and water solubility of the coagulation medium. Carbohyd. Polym. 2020, 236, 116068. [Google Scholar] [CrossRef]
- Azizi, N.; Khajeh-Amiri, A.; Ghafuri, H.; Bolourtchian, M. LiOH-Catalyzed simple ring opening of epoxides under solvent-free conditions. Phosphorus Sulfur. 2010, 185, 1550–1557. [Google Scholar] [CrossRef]
- Zuo, Y.; Gou, Z.; Quan, W.; Lin, W. Silicon-assisted unconventional fluorescence from organosilicon materials. Coord. Chem. Rev. 2021, 438, 213887. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, C.; Chen, W.; Li, J.; Li, M.; Liu, X.; Mao, L.; Yuan, J.; Tao, L.; Wei, Y. A polymerizable Aggregation Induced Emission (AIE)-active dye with remarkable pH fluorescence switching based on benzothiazole and its application in biological imaging. Dye. Pigment. 2021, 196, 109793. [Google Scholar] [CrossRef]
- Razi, S.S.; Gupta, R.C.; Ali, R.; Dwivedi, S.K.; Srivastava, P.; Misra, A. A new D–π–A type intramolecular charge transfer Dyad System to detect F−: Anion induced CO2 sensing. Sensors Actuat. Chem. 2016, 236, 520–528. [Google Scholar] [CrossRef]
- Cao, J.; Zuo, Y.; Lu, H.; Yang, Y.; Feng, S. An unconventional chromophore in water-soluble polysiloxanes synthesized via thiol-ene reaction for metal ion detection. J. Photo. Photo. A Chem. 2018, 350, 152–163. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, J.; Feng, S. Controllable photophysical properties and self-assembly of siloxane-poly(amidoamine) dendrimers. Phys. Chem. Chem. Phys. 2015, 17, 26783–26789. [Google Scholar] [CrossRef]
- Fu, P.-Y.; Li, B.-N.; Zhang, Q.-S.; Mo, J.-T.; Wang, S.-C.; Pan, M.; Su, C.-Y. Thermally Activated Fluorescence vs Long Persistent Luminescence in ESIPT-Attributed Coordination Polymer. J. Am. Chem. Soc. 2022, 144, 2726–2734. [Google Scholar] [CrossRef]
- Lu, H.; Hu, Z.; Feng, S. Nonconventional Luminescence Enhanced by Silicone-Induced Aggregation. Chem. Asian J. 2017, 12, 1213–1217. [Google Scholar] [CrossRef]









| Entry a | Catalyst | Cat. Loading (mol%) | Time (h) | Temp (°C) | Conversion (%) c |
|---|---|---|---|---|---|
| 1 | LiOH | 5 | 6 | 25 | 63.1 |
| 2 | LiOH | 5 | 12 | 25 | 99.5 |
| 3 | LiOH | 3 | 12 | 25 | 80.3 |
| 4 | LiOH | 1 | 12 | 25 | 39.8 |
| 5 | TBAF b | 8 | 12 | 25 | 81.3 |
| 6 | TBAF b | 8 | 12 | 50 | 99.5 |
| 7 | TBAF b | 5 | 12 | 50 | 99.4 |
| 8 | TBAF b | 3 | 12 | 50 | 98.6 |
| 9 | TBAF b | 1 | 12 | 50 | 92.3 |
| 10 | TBAF b | 0.8 | 12 | 50 | 80.0 |
| 11 | TBAF b | 3 | 6 | 50 | 96.7 |
| 12 | TBAF b | 3 | 3 | 50 | 91.8 |
| Entry a | Substrates | Products | Time (h) | Conversion (%) b | Yields (%) c | ||
|---|---|---|---|---|---|---|---|
| 1 | T1 |  | E1T1 |  | 6 | 96.0 | 96.0 |
| 2 | T2 |  | E1T2 |  | 6 | 99.9 | 95.5 |
| 3 | T3 |  | E1T3 |  | 6 | 99.9 | 95.4 |
| 4 | T4 |  | E1T4 |  | 6 | 99.9 | 94.3 |
| 5 | T5 |  | E1T5 |  | 6 | 99.0 | 90.3 |
| 6 | T6 |  | E1T6 |  | 6 | 98.0 | 95.0 |
| 7 | T7 |  | E1T7 |  | 6 | 99.9 | 94.7 |
| 8 | T8 |  | E1T8 |  | 6 | 99.9 | 92.3 |
| 9 | T9 |  | E1T9 |  | 6 | 99.9 | 96.5 |
| 10 | T10 |  | E1T10 |  | 6 | 99.9 | 93.8 |
| 11 | T11 |  | E1T11 |  | 6 | 98.0 | 89.3 |
| 12 | T2 | E1T2 | 3 | 99.9 | 95.5 | ||
| 13 | T3 | E1T3 | 5 | 99.9 | 95.3 | ||
| 14 | T4 | E1T4 | 5 | 99.9 | 93.8 | ||
| 15 | T8 | E1T8 | 5 | 99.9 | 92.1 | ||
| 16 | T9 | E1T9 | 3 | 99.9 | 96.4 | ||
| Entry | Ex/nm | E2T6 | E1T6 | ||
|---|---|---|---|---|---|
| Em/nm | Intensity | Em/nm | Intensity | ||
| 1 | 310 | 345 | 299.1 | 349 | 8069 |
| 2 | 320 | 357 | 245 | 363 | 5824 |
| 3 | 330 | 370 | 212.3 | 366 | 3534 |
| 4 | 340 | 381 | 184.8 | 386 | 2730 |
| 5 | 350 | 392 | 147.9 | 410 | 1959 |
| 6 | 360 | 404 | 139.7 | 409 | 2310 |
| 7 | 370 | 417 | 131.8 | 409 | 1951 |
| 8 | 380 | 430 | 102 | 412 | 1700 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Feng, S.; Wang, D. Facile Synthesis of Sulfur-Containing Functionalized Disiloxanes with Nonconventional Fluorescence by Thiol–Epoxy Click Reaction. Int. J. Mol. Sci. 2023, 24, 7785. https://doi.org/10.3390/ijms24097785
Tang J, Feng S, Wang D. Facile Synthesis of Sulfur-Containing Functionalized Disiloxanes with Nonconventional Fluorescence by Thiol–Epoxy Click Reaction. International Journal of Molecular Sciences. 2023; 24(9):7785. https://doi.org/10.3390/ijms24097785
Chicago/Turabian StyleTang, Jing, Shengyu Feng, and Dengxu Wang. 2023. "Facile Synthesis of Sulfur-Containing Functionalized Disiloxanes with Nonconventional Fluorescence by Thiol–Epoxy Click Reaction" International Journal of Molecular Sciences 24, no. 9: 7785. https://doi.org/10.3390/ijms24097785
APA StyleTang, J., Feng, S., & Wang, D. (2023). Facile Synthesis of Sulfur-Containing Functionalized Disiloxanes with Nonconventional Fluorescence by Thiol–Epoxy Click Reaction. International Journal of Molecular Sciences, 24(9), 7785. https://doi.org/10.3390/ijms24097785