Injectable Hydrogel Guides Neurons Growth with Specific Directionality
Abstract
1. Introduction
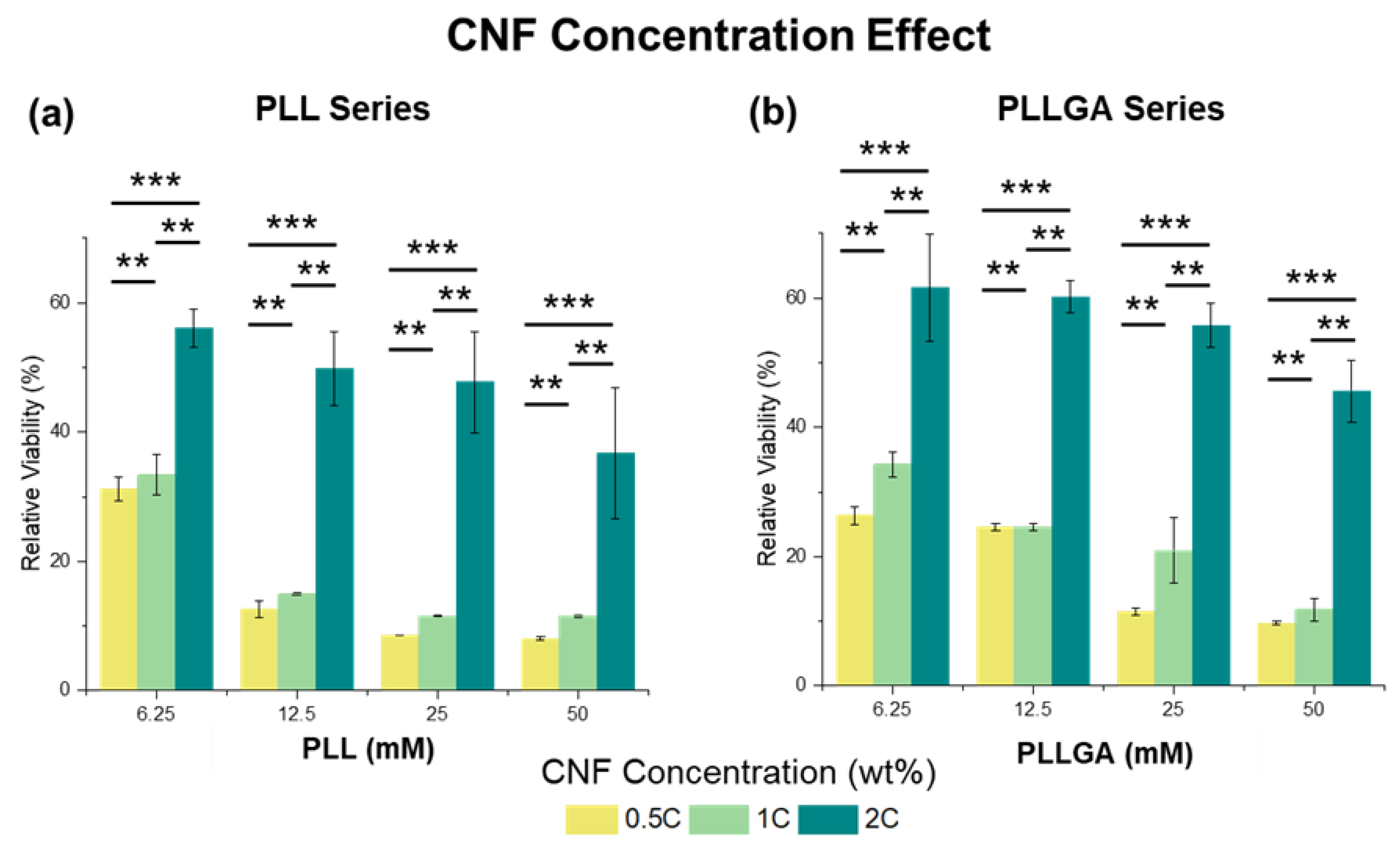
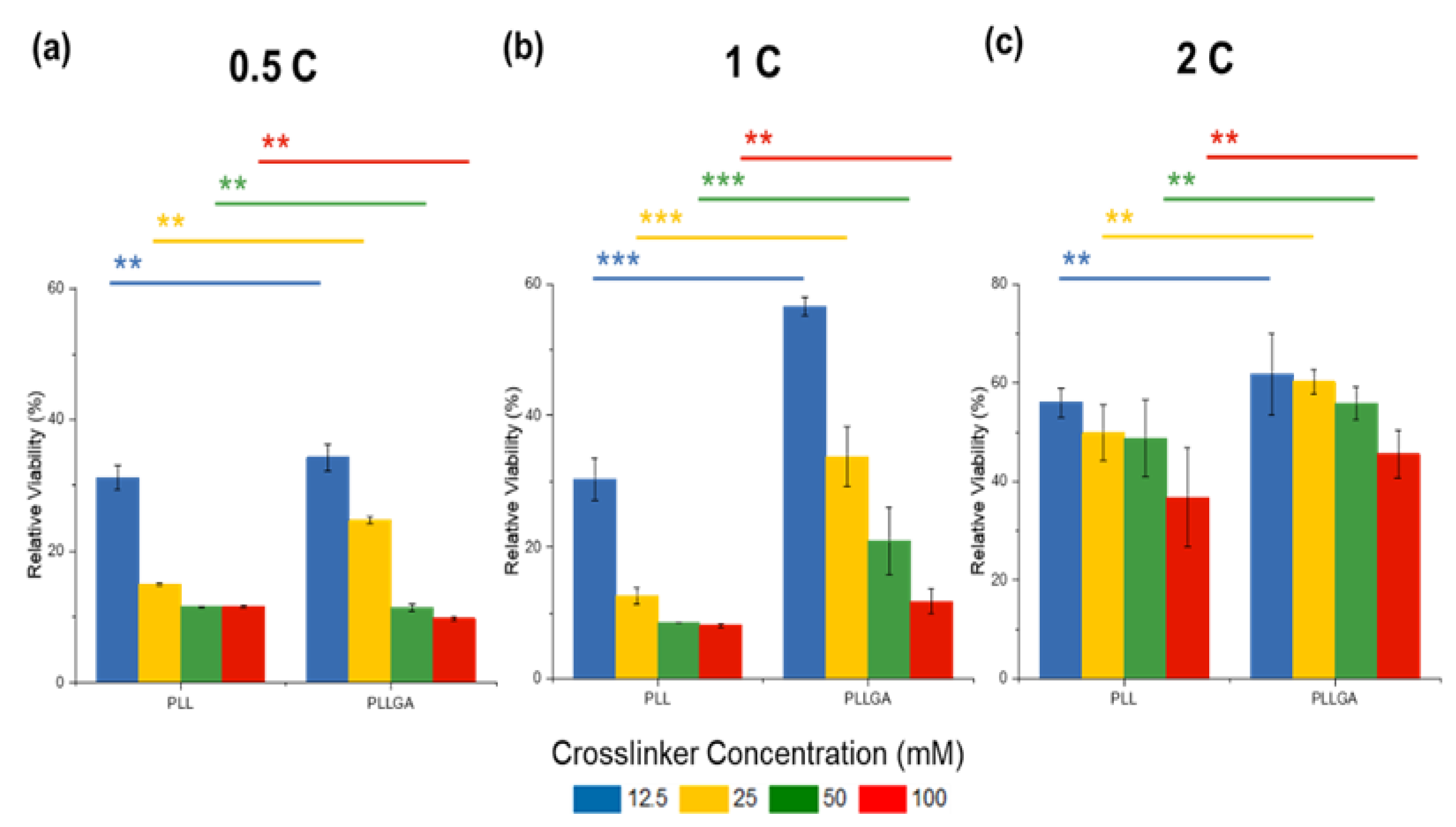
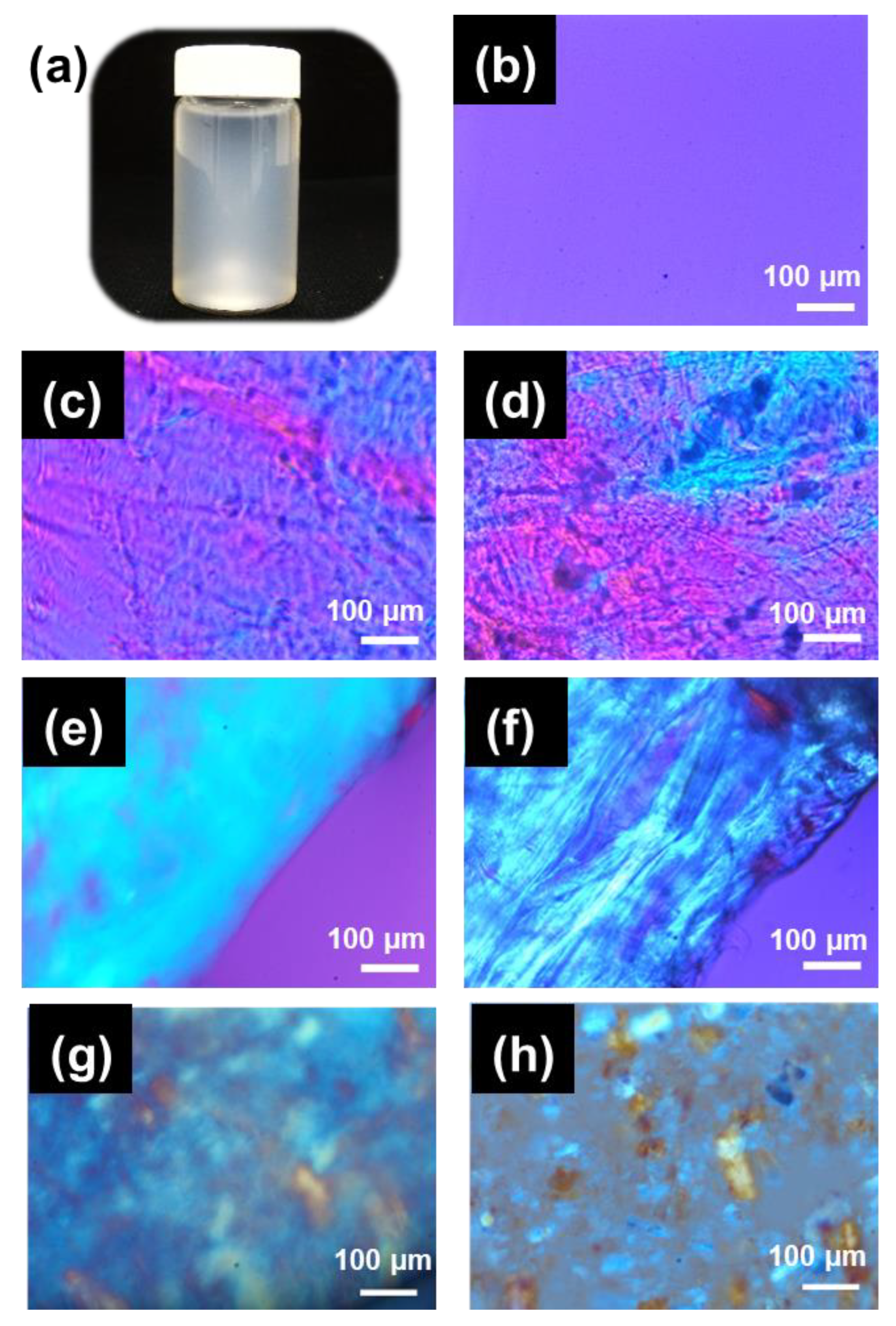
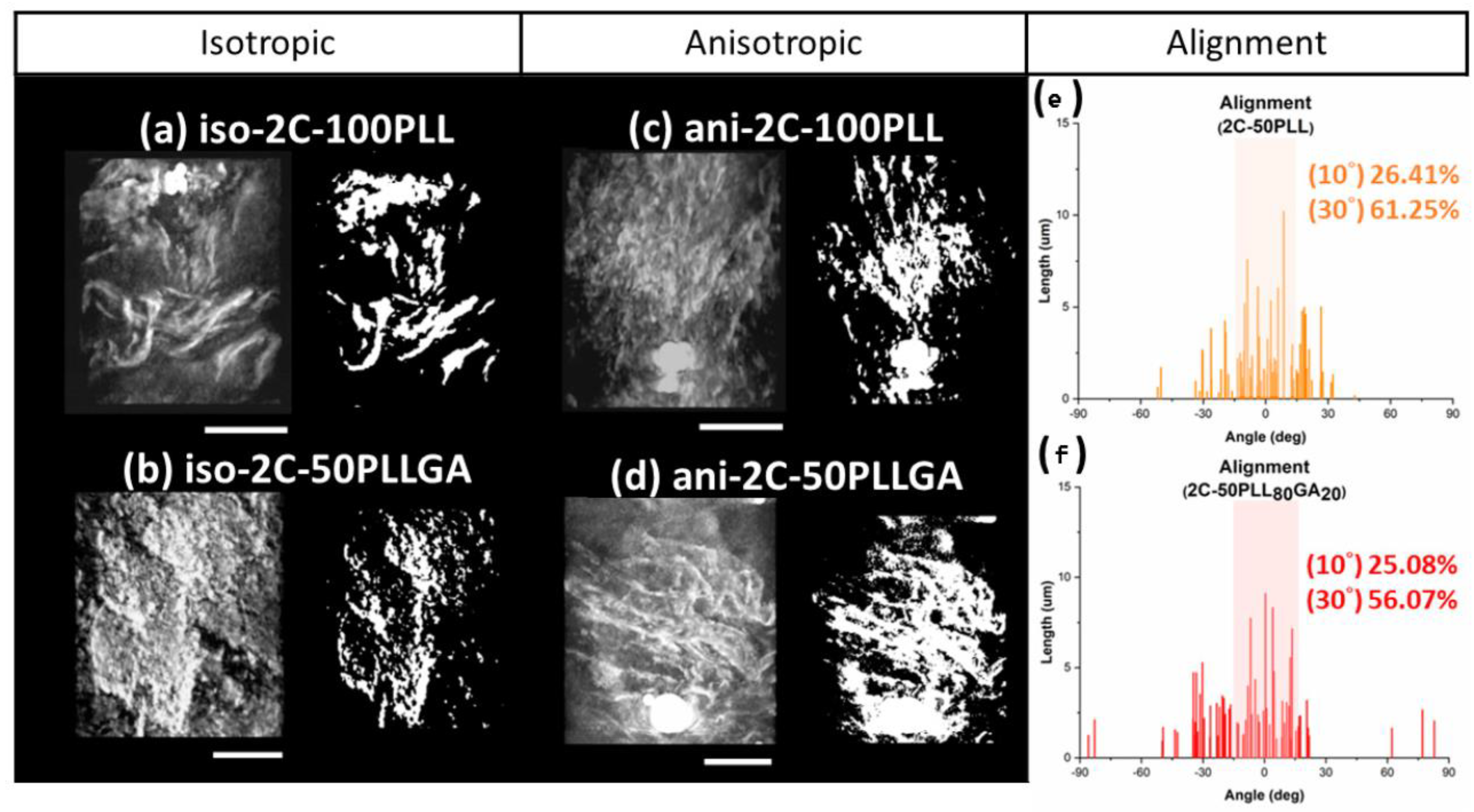
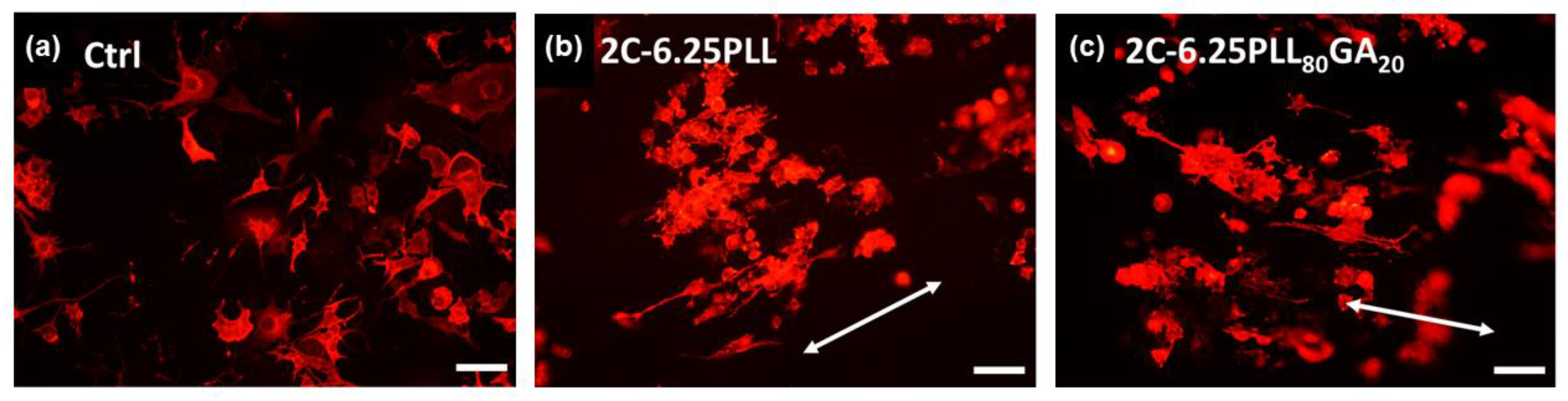
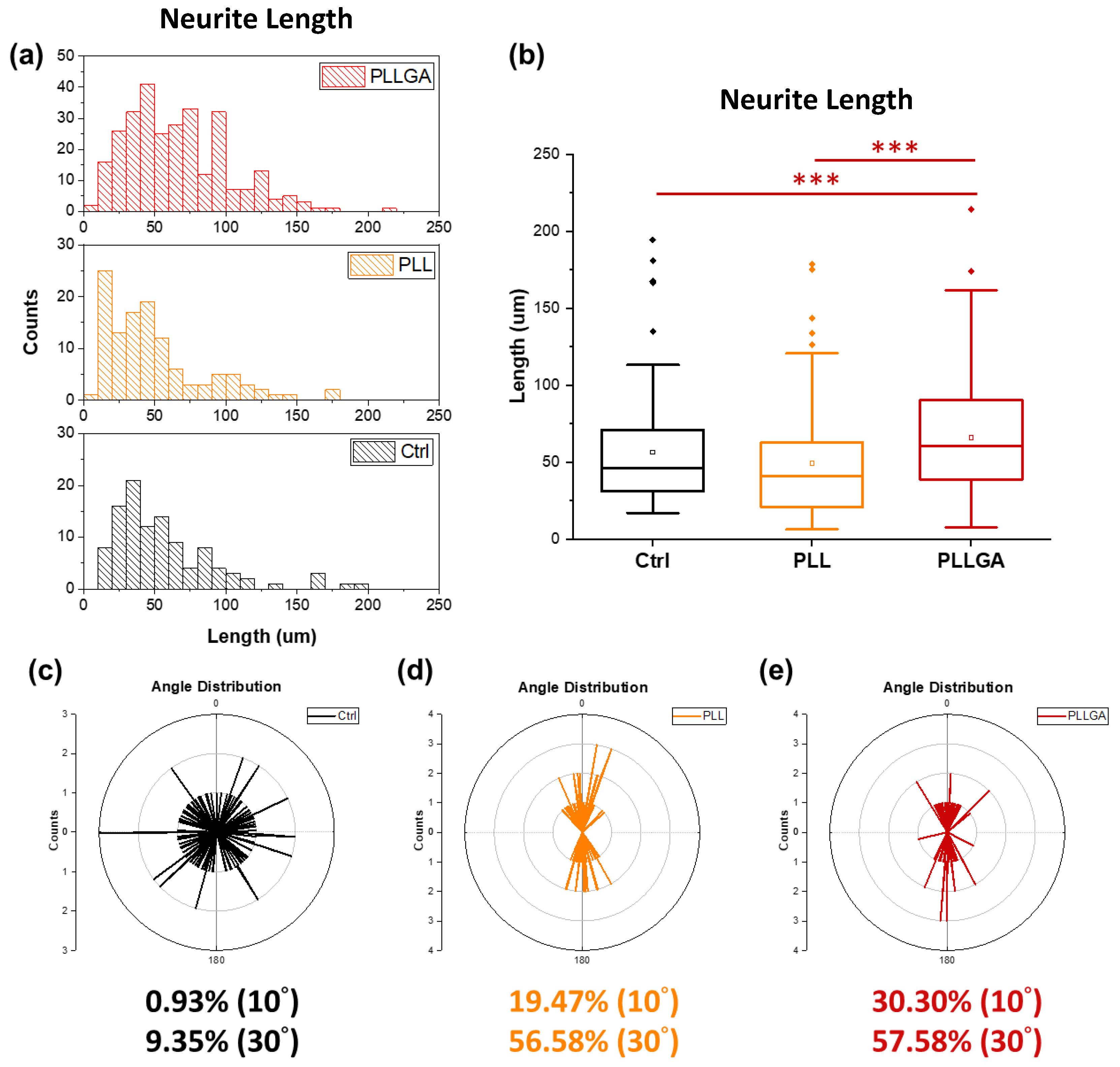
2. Results and Discussion
3. Materials and Methods
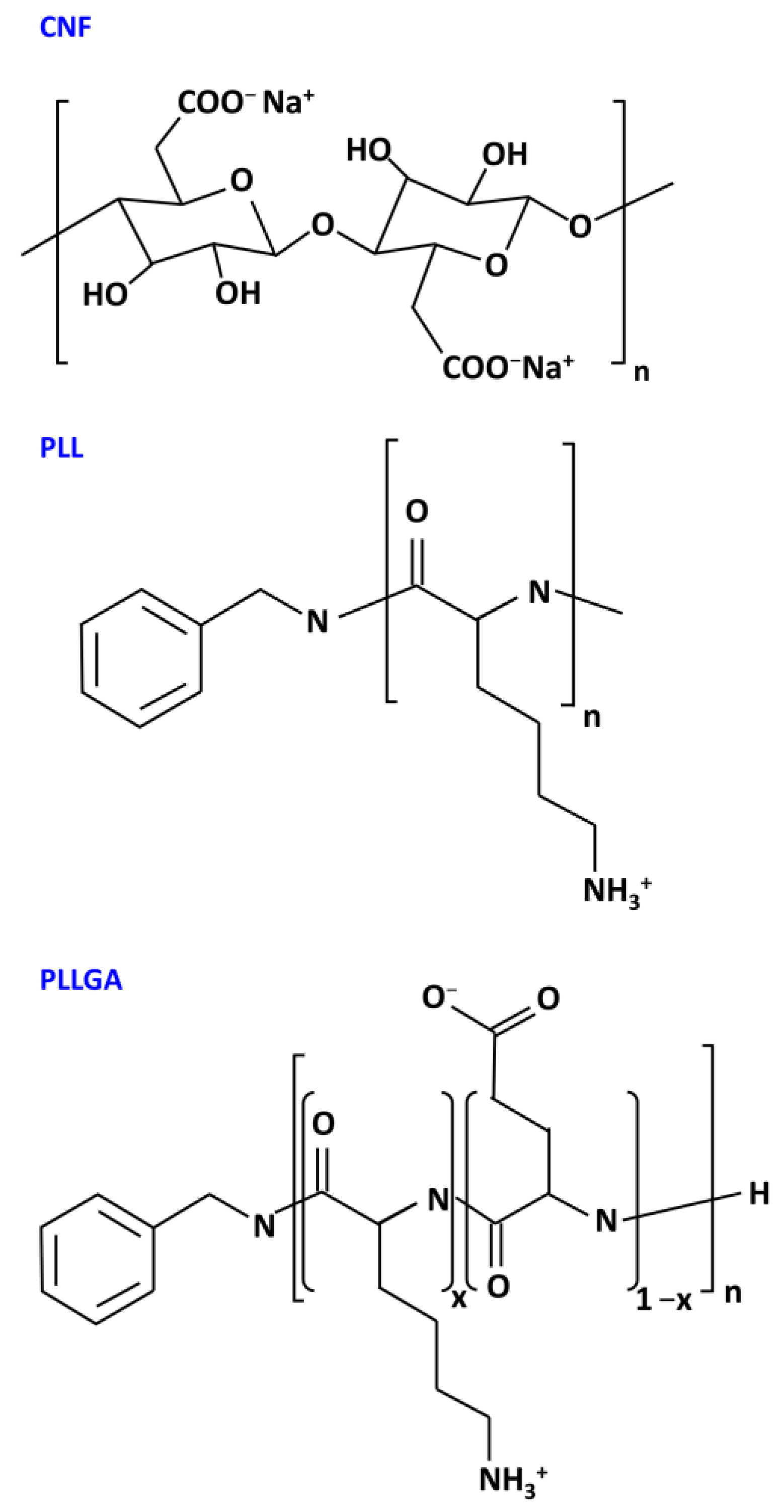
3.1. Materials
3.2. Nomenclature
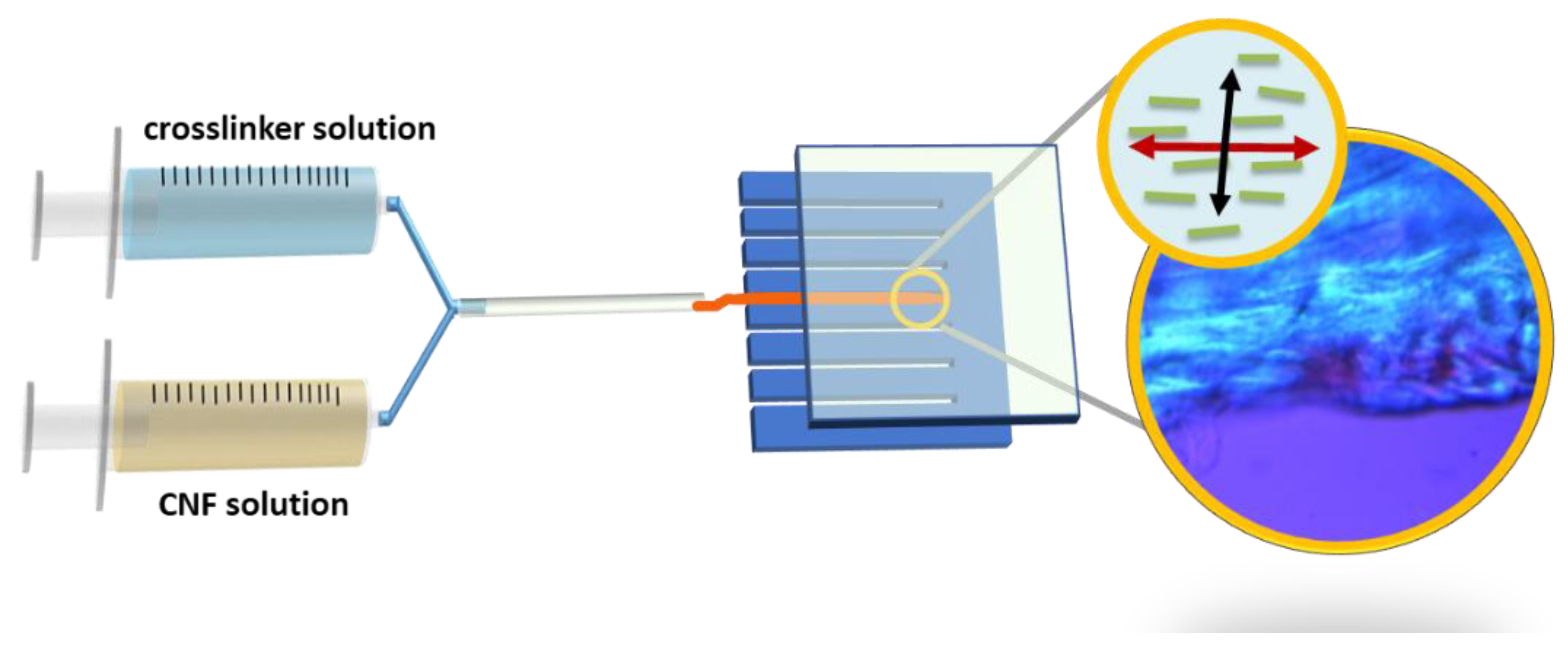
3.3. Hydrogel Preparation
3.4. Characterization of Hydrogel Morphology
3.4.1. Polarized Optical Microscopy
3.4.2. Transmission X-ray Microscope
3.5. In Vitro Studies Using Hydrogel
3.5.1. Cell Viability
- A: Absorption of tested sample
- A′: Absorption of blank
3.5.2. Calcium Imaging
3.5.3. Measurement of Length, Distribution, and the Extent of the Alignment of Neurites
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ha, A.; Kim, Y.K.; Park, Y.J.; Jeoung, J.W.; Park, K.H. Intraocular pressure change during reading or writing on smartphone. PLoS ONE 2018, 13, e0206061. [Google Scholar] [CrossRef] [PubMed]
- Qudsiya, S.M.; Khatoon, F.; Khader, A.A.; Ali, M.A.; Hazari, M.A.H.; Sultana, F.; Farheen, A. Study of intraocular pressure among individuals working on computer screens for long hours: Effect of exposure to computer screens on IOP. Ann. Med. Physiol. 2017, 1, 22–25. [Google Scholar] [CrossRef]
- Nassr, M.A.; Morris, C.L.; Netland, P.A.; Karcioglu, Z.A. Intraocular Pressure Change in Orbital Disease. Surv. Ophthalmol. 2009, 54, 519–544. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wu, A.; Zhang, L.; Wang, W.; Chen, X.; Yu, X.; Wang, K. The increasing prevalence of myopia and high myopia among high school students in Fenghua city, eastern China: A 15-year population-based survey. BMC Ophthalmol. 2018, 18, 159. [Google Scholar] [CrossRef]
- Matsumura, H.; Hirai, H. Prevalence of Myopia and Refractive Changes in Students from 3 to 17 Years of Age. Surv. Ophthalmol. 1999, 44, S109–S115. [Google Scholar] [CrossRef]
- Saw, S.-M.; Gazzard, G.; Shih-Yen, E.C.; Chua, W.-H. Myopia and associated pathological complications. Ophthalmic Physiol. Opt. 2005, 25, 381–391. [Google Scholar] [CrossRef]
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020, 12, e11686. [Google Scholar] [CrossRef]
- Kingman, S. Glaucoma is second leading cause of blindness globally. Bull. World Health Organ. 2004, 82, 887–888. [Google Scholar]
- Goel, M.; Pacciani, R.G.; Lee, R.K.; Battacharya, S.K. Aqueous Humor Dynamics: A Review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Vargas, J.L.C.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef]
- Yang, P.; Wei, L.; Tian, H.; Yu, F.; Shi, Y.; Gao, L. Gadolinium chloride protects neurons by regulating the activation of microglia in the model of optic nerve crush. Biochem. Biophys. Res. Commun. 2022, 618, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hu, C.; Tang, Z.; Yu, Q.; Liu, X.; Chen, H. Tissue-engineered Vascular Grafts: Balance of the Four Major Requirements. Colloid Interface Sci. Commun. 2018, 23, 34–44. [Google Scholar] [CrossRef]
- Wei, Y.; Baskaran, N.; Wang, H.-Y.; Su, Y.-C.; Nabilla, S.C.; Chung, R.-J. Study of polymethylmethacrylate/tricalcium silicate composite cement for orthopedic application. Biomed. J. 2022. [Google Scholar] [CrossRef] [PubMed]
- Deegan, A.J.; Hendrikson, W.J.; El Haj, A.J.; Rouwkema, J.; Yang, Y. Regulation of endothelial cell arrangements within hMSC–HUVEC co-cultured aggregates. Biomed. J. 2019, 42, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Carrier, R.L.; Papadaki, M.; Rupnick, M.; Schoen, F.J.; Bursac, N.; Langer, R.; Freed, L.E.; Vunjak-Novakovic, G. Cardiac tissue engineering: Cell seeding, cultivation parameters, and tissue construct characterization. Biotechnol. Bioeng. 1999, 64, 580–589. [Google Scholar] [CrossRef]
- Chen, Q.-Z.; Harding, S.E.; Ali, N.N.; Lyon, A.R.; Boccaccini, A.R. Biomaterials in cardiac tissue engineering: Ten years of research survey. Mater. Sci. Eng. R Rep. 2008, 59, 1–37. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Luo, S.-C.; Yu, J.-S.; Chen, T.-C.; Su, W.-F. Peptide-Based Polyelectrolyte Promotes Directional and Long Neurite Outgrowth. ACS Appl. Bio Mater. 2018, 2, 518–526. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Chang, Y.-Y.; Wu, J.-G.; Lin, C.-Y.; An, H.-L.; Luo, S.-C.; Tang, T.K.; Su, W.-F. Novel 3D Neuron Regeneration Scaffolds Based on Synthetic Polypeptide Containing Neuron Cue. Macromol. Biosci. 2017, 18, 1700251. [Google Scholar] [CrossRef]
- Steed, M.B.; Mukhatyar, V.; Valmikinathan, C.; Bellamkonda, R.V. Advances in Bioengineered Conduits for Peripheral Nerve Regeneration. Atlas Oral Maxillofac. Surg. Clin. 2011, 19, 119–130. [Google Scholar] [CrossRef]
- Petersen, O.W.; Rønnov-Jessen, L.; Howlett, A.R.; Bissell, M.J. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. USA 1992, 89, 9064. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk, B.; Sajkiewicz, P.; Kolbuk, D. Injectable hydrogels as novel materials for central nervous system regeneration. J. Neural Eng. 2018, 15, 051002. [Google Scholar] [CrossRef]
- He, L.; Xiao, Q.; Zhao, Y.; Li, J.; Reddy, S.; Shi, X.; Su, X.; Chiu, K.; Ramakrishna, S. Engineering an Injectable Electroactive Nanohybrid Hydrogel for Boosting Peripheral Nerve Growth and Myelination in Combination with Electrical Stimulation. ACS Appl. Mater. Interfaces 2020, 12, 53150–53163. [Google Scholar] [CrossRef] [PubMed]
- Chetia, M.; Debnath, S.; Chowdhury, S.; Chatterjee, S. Self-assembly and multifunctionality of peptide organogels: Oil spill recovery, dye absorption and synthesis of conducting biomaterials. RSC Adv. 2020, 10, 5220–5233. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Bai, S.; Ding, Z.; Guo, H.; Shao, Z.; Zhu, H.; Kaplan, D.L. Hydrogel Assembly with Hierarchical Alignment by Balancing Electrostatic Forces. Adv. Mater. Interfaces 2016, 3, 1500687. [Google Scholar] [CrossRef]
- Omidinia-Anarkoli, A.; Boesveld, S.; Tuvshindorj, U.; Rose, J.C.; Haraszti, T.; De Laporte, L. Aninjectable hybrid hydrogel with oriented short fibers induces unidirectional growth of functional nerve cells. Small 2017, 13, 1702207. [Google Scholar] [CrossRef]
- Wang, L.; Song, D.; Zhang, X.; Ding, Z.; Kong, X.; Lu, Q.; Kaplan, D.L. Silk–Graphene Hybrid Hydrogels with Multiple Cues to Induce Nerve Cell Behavior. ACS Biomater. Sci. Eng. 2018, 5, 613–622. [Google Scholar] [CrossRef]
- De France, K.J.; Yager, K.G.; Chan, K.J.W.; Corbett, B.; Cranston, E.D.; Hoare, T. Injectable Anisotropic Nanocomposite Hydrogels Direct in Situ Growth and Alignment of Myotubes. Nano Lett. 2017, 17, 6487–6495. [Google Scholar] [CrossRef]
- Ghaderinejad, P.; Najmoddin, N.; Bagher, Z.; Saeed, M.; Karimi, S.; Simorgh, S.; Pezeshki-Modaress, M. An injectable anisotropic alginate hydrogel containing oriented fibers for nerve tissue engineering. Chem. Eng. J. 2021, 420, 130465. [Google Scholar] [CrossRef]
- Vader, D.; Kabla, A.; Weitz, D.; Mahadevan, L. Strain-Induced Alignment in Collagen Gels. PLoS ONE 2009, 4, e5902. [Google Scholar] [CrossRef]
- Antman-Passig, M.; Levy, S.; Gartenberg, C.; Schori, H.; Shefi, O. Mechanically Oriented 3D Collagen Hydrogel for Directing Neurite Growth. Tissue Eng. Part A 2017, 23, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Im, S.K.; Oh, S.J.; Jeong, S.; Yoon, E.S.; Lee, C.J.; Choi, N.; Hur, E.M. Anisotropically organized three-dimensional culture platform for reconstruction of a hippocampal neural network. Nat. Commun. 2017, 8, 14346. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Jeong, S.; Chung, J.J.; Kim, S.H.; Choi, N.; Jung, Y. Development of an Anisotropically Organized Brain dECM Hydrogel-Based 3D Neuronal Culture Platform for Recapitulating the Brain Microenvironment in Vivo. ACS Biomater. Sci. Eng. 2019, 6, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Liu, J.; Yao, S.; Mao, H.; Peng, J.; Sun, X.; Cao, Z.; Yang, Y.; Xiao, B.; Wang, Y.; et al. Prompt peripheral nerve regeneration induced by a hierarchically aligned fibrin nanofiber hydrogel. Acta Biomater. 2017, 55, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Yu, S.; Cao, Z.; Yang, Y.; Yu, X.; Mao, H.-Q.; Wang, L.-N.; Sun, X.; Zhao, L.; Wang, X. Hierarchically aligned fibrin nanofiber hydrogel accelerated axonal regrowth and locomotor function recovery in rat spinal cord injury. Int. J. Nanomed. 2018, 13, 2883–2895. [Google Scholar] [CrossRef]
- Perez, R.A.; Kim, M.; Kim, T.-H.; Kim, J.-H.; Lee, J.H.; Park, J.-H.; Knowles, J.C.; Kim, H.-W. Utilizing Core–Shell Fibrous Collagen-Alginate Hydrogel Cell Delivery System for Bone Tissue Engineering. Tissue Eng. Part A 2014, 20, 103–114. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Mikkelsen, D.; Flanagan, B.M.; Rehm, C.; de Campo, L.; Gidley, M.J.; Gilbert, E.P. Investigation of the micro- and nano-scale architecture of cellulose hydrogels with plant cell wall polysaccharides: A combined USANS/SANS study. Polymer 2016, 105, 449–460. [Google Scholar] [CrossRef]
- Hong-Ping, G.; Bao-Shan, K. Neuroprotective effect of L-lysine monohydrochloride on acute iterative anoxia in rats with quantitative analysis of electrocorticogram. Life Sci. 1999, 65, PL19–PL25. [Google Scholar] [CrossRef]
- Cheng, J.; Tang, J.C.; Pan, M.X.; Chen, S.F.; Zhao, D.; Zhang, Y.; Liao, H.B.; Zhuang, Y.; Lei, R.X.; Wang, S.; et al. l-lysine confers neuroprotection by suppressing inflammatory response via microRNA-575/PTEN signaling after mouse intracerebral hemorrhage injury. Exp. Neurol. 2020, 327, 113214. [Google Scholar] [CrossRef]
- Kondoh, T.; Kameishi, M.; Mallick, H.N.; Ono, T.; Torii, K. Lysine and arginine reduce the effects of cerebral ischemic insults and inhibit glutamate-induced neuronal activity in rats. Front. Integr. Neurosci. 2010, 4, 18. [Google Scholar] [CrossRef]
- Yavin, E.; Yavin, Z. Attachment and culture of dissociated cells from rat embryo cerebral hemispheres on polylysine-coated surface. J. Cell Biol. 1974, 62, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Low, C.M.; Wee, K.S. New insights into the not-so-new NR3 subunits of N-methyl-D-aspartate receptor: Localization, structure, and function. Mol. Pharm. 2010, 78, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pin, J.-P.; Acher, F. The metabotropic glutamate receptors: Structure, activation mechanism and pharmacology. Curr. Drug Target CNS Neurol. Disord. 2002, 1, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Yang, S.; Luo, S.; Chen, W.; Liao, S.; Su, W. Dual-Function Fibrous Co-Polypeptide Scaffolds for Neural Tissue Engineering. Macromol. Biosci. 2022, 23, 2200286. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Tseng, Y.; Wang, C.; Lin, T.; Wu, M.; Tsao, C.; Su, W. Three-Level Hierarchical 3D Network Formation and Structure Elucidation of Wet Hydrogel of Tunable-High-Strength Nanocomposites. Macromol. Mater. Eng. 2022, 307, 2100871. [Google Scholar] [CrossRef]
- Shui, G. 5.44-Development of in Vitro Neural Models for Drug Discovery and Toxicity Screening, in Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, VT, USA, 2011; pp. 565–572. [Google Scholar]
- Koizumi, S.; Bootman, M.D.; Bobanović, L.K.; Schell, M.J.; Berridge, M.J.; Lipp, P. Characterization of Elementary Ca2+ Release Signals in NGF-Differentiated PC12 Cells and Hippocampal Neurons. Neuron 1999, 22, 125–137. [Google Scholar] [CrossRef]
- Wittig, D.; Wang, X.; Walter, C.; Gerdes, H.-H.; Funk, R.H.W.; Roehlecke, C. Multi-Level Communication of Human Retinal Pigment Epithelial Cells via Tunneling Nanotubes. PLoS ONE 2012, 7, e33195. [Google Scholar] [CrossRef]
- Hasanzadeh, E.; Seifalian, A.; Mellati, A.; Saremi, J.; Asadpour, S.; Enderami, S.E.; Nekounam, H.; Mahmoodi, N. Injectable hydrogels in central nervous system: Unique and novel platforms for promoting extracellular matrix remodeling and tissue engineering. Mater. Today Bio 2023, 20, 100614. [Google Scholar] [CrossRef]
- Khan, J.; Rudrapal, M.; Bhat, E.A.; Ali, A.; Alaidarous, M.; Alshehri, B.; Banwas, S.; Ismail, R.; Egbuna, C. Perspective Insights to Bio-Nanomaterials for the Treatment of Neurological Disorders. Front. Bioeng. Biotechnol. 2021, 9, 724158. [Google Scholar] [CrossRef]
- Grimaudo, M.; Krishnakumar, G.; Giusto, E.; Furlani, F.; Bassi, G.; Rossi, A.; Molinari, F.; Lista, F.; Montesi, M.; Panseri, S. Bioactive injectable hydrogels for on demand molecule/cell delivery and for tissue regeneration in the central nervous system. Acta Biomater. 2021, 140, 88–101. [Google Scholar] [CrossRef]
- Li, S.; Ke, Z.; Peng, X.; Fan, P.; Chao, J.; Wu, P.; Xiao, P.; Zhou, Y. Injectable and fast gelling hyaluronate hydrogels with rapid self-healing ability for spinal cord injury repair. Carbohydr. Polym. 2022, 298, 120081. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.-L.; Yang, S.-C.; Cheng, T.; Chen, M.-Y.; Wu, J.-H.; Liao, S.-L.; Chen, W.-L.; Su, W.-F. Exploration of biomimetic poly(γ-benzyl-l-glutamate) fibrous scaffolds for corneal nerve regeneration. J. Mater. Chem. B 2022, 10, 6372–6379. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011, 3, a003947. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Geng, X.; Yonkunas, M.; Su, A.; Densmore, E.; Tang, P.; Drain, P. Ligand-dependent Linkage of the ATP Site to Inhibition Gate Closure in the KATP Channel. J. Gen. Physiol. 2005, 126, 285–299. [Google Scholar] [CrossRef]
- Duman, J.G.; Chen, L.; Hille, B. Calcium Transport Mechanisms of PC12 Cells. J. Gen. Physiol. 2008, 131, 307–323. [Google Scholar] [CrossRef]
- Li, G.-Y.; Fan, B.; Zheng, Y.-C. Calcium Overload Is A Critical Step in Programmed Necrosis of ARPE-19 Cells Induced by High-Concentration H2O2. Biomed. Environ. Sci. 2010, 23, 371–377. [Google Scholar] [CrossRef]
- Strauss, O.; Abdusalamova, K.; Huber, C.; Rohrer, B.; Busch, C. Interactive Ca2+ signaling in the RPE by anaphylatoxins. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2277. [Google Scholar]
- Nakatani, K.; Chen, C.; Yau, K.-W.; Koutalos, Y. Calcium and Phototransduction. Adv. Exp. Med. Biol. 2002, 514, 1–20. [Google Scholar] [CrossRef]
- Zhang, L.; Hui, Y.-N.; Wang, Y.-S.; Ma, J.-X.; Wang, J.-B.; Ma, L.-N. Calcium overload is associated with lipofuscin formation in human retinal pigment epithelial cells fed with photoreceptor outer segments. Eye 2011, 25, 519–527. [Google Scholar] [CrossRef]
- Dunn, K.C.; Aotaki-Keen, A.E.; Putkey, F.R.; Hjelmeland, L.M. ARPE-19, A Human Retinal Pigment Epithelial Cell Line with Differentiated Properties. Exp. Eye Res. 1996, 62, 155–170. [Google Scholar] [CrossRef]
- Hazim, R.A.; Volland, S.; Yen, A.; Burgess, B.L.; Williams, D.S. Rapid differentiation of the human RPE cell line, ARPE-19, induced by nicotinamide. Exp. Eye Res. 2019, 179, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Cristaldi, M.; Giurdanella, G.; Perrotta, R.E.; Furno, D.L.; Giuffrida, R.; Rusciano, D. ARPE-19 conditioned medium promotes neural differentiation of adipose-derived mesenchymal stem cells. World J. Stem Cells 2021, 13, 1783–1796. [Google Scholar] [CrossRef]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W.; Ding, J.; et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 2014, 10, 1989–2005. [Google Scholar] [CrossRef] [PubMed]
- Reigada, D.; Lu, W.; Zhang, X.; Friedman, C.; Pendrak, K.; McGlinn, A.; Stone, R.A.; Laties, A.M.; Mitchell, C.H. Degradation of extracellular ATP by the retinal pigment epithelium. Am. J. Physiol. Physiol. 2005, 289, C617–C624. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Camargo, L.M.; Yeboah, F.; Digan, M.E.; Niu, H.; Pan, Y.; Reiling, S.; Soler-Llavina, G.; Weihofen, W.A.; Wang, H.-R.; et al. A NMDA-receptor calcium influx assay sensitive to stimulation by glutamate and glycine/D-serine. Sci. Rep. 2017, 7, 11608. [Google Scholar] [CrossRef]
- Jan, Y.-N.; Jan, L. Branching out: Mechanisms of dendritic arborization. Nat. Rev. Neurosci. 2010, 11, 316–328. [Google Scholar] [CrossRef]
- Kirch, C.; Gollo, L.L. Single-neuron dynamical effects of dendritic pruning implicated in aging and neurodegeneration: Towards a measure of neuronal reserve. Sci. Rep. 2021, 11, 1309. [Google Scholar] [CrossRef]
- López-Doménech, G.; Higgs, N.F.; Vaccaro, V.; Roš, H.; Arancibia-Cárcamo, I.L.; MacAskill, A.F.; Kittler, J.T. Loss of Dendritic Complexity Precedes Neurodegeneration in a Mouse Model with Disrupted Mitochondrial Distribution in Mature Dendrites. Cell Rep. 2016, 17, 317–327. [Google Scholar] [CrossRef]
- Guo, W.-H.; Frey, M.T.; Burnham, N.A.; Wang, Y.-L. Substrate Rigidity Regulates the Formation and Maintenance of Tissues. Biophys. J. 2006, 90, 2213–2220. [Google Scholar] [CrossRef]
- Sharma, A.; Goring, A.; Staines, K.A.; Emery, R.J.; Pitsillides, A.A.; Oreffo, R.O.; Mahajan, S.; Clarkin, C.E. Raman spectroscopy links differentiating osteoblast matrix signatures to pro-angiogenic potential. Matrix Biol. Plus 2020, 5, 100018. [Google Scholar] [CrossRef]
- Orlowska, A.; Perera, P.T.; Al Kobaisi, M.; Dias, A.; Nguyen, H.K.D.; Ghanaati, S.; Baulin, V.; Crawford, R.J.; Ivanova, E.P. The Effect of Coatings and Nerve Growth Factor on Attachment and Differentiation of Pheochromocytoma Cells. Materials 2017, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Santiago, L.Y.; Katagiri, Y.; Geller, H.M. Myosin II activity regulates neurite outgrowth and guidance in response to chondroitin sulfate proteoglycans. J. Neurochem. 2011, 120, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.; Levitz, J. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef]
- Takaichi, S.; Saito, T.; Tanaka, R.; Isogai, A. Improvement of nanodispersibility of oven-dried TEMPO-oxidized celluloses in water. Cellulose 2014, 21, 4093–4103. [Google Scholar] [CrossRef]
- Jagan, S.; Paganessi, L.A.; Frank, R.R.; Venugopal, P.; Larson, M.; Christopherson, K.W. Bone Marrow and Peripheral Blood AML Cells Are Highly Sensitive to CNDAC, the Active Form of Sapacitabine. Adv. Hematol. 2012, 2012, 727683. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.H.; Kao, L.S.; Liu, P.S.; Huang, C.C.; Yang, D.M.; Pan, C.Y. Dopamine elevates intracellular zinc concentration in cultured rat embryonic cortical neurons through the cAMP-nitric oxide signaling cascade. Mol. Cell Neurosci. 2017, 82, 35–45. [Google Scholar] [CrossRef]
- Hung, H.-H.; Huang, W.-P.; Pan, C.-Y. Dopamine- and zinc-induced autophagosome formation facilitates PC12 cell survival. Cell Biol. Toxicol. 2013, 29, 415–429. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, Y.-H.; Ma, T.-L.; Tan, D.-H.; Su, A.-J.A.; Washington, K.M.; Wang, C.-C.; Huang, Y.-C.; Wu, M.-C.; Su, W.-F. Injectable Hydrogel Guides Neurons Growth with Specific Directionality. Int. J. Mol. Sci. 2023, 24, 7952. https://doi.org/10.3390/ijms24097952
Tseng Y-H, Ma T-L, Tan D-H, Su A-JA, Washington KM, Wang C-C, Huang Y-C, Wu M-C, Su W-F. Injectable Hydrogel Guides Neurons Growth with Specific Directionality. International Journal of Molecular Sciences. 2023; 24(9):7952. https://doi.org/10.3390/ijms24097952
Chicago/Turabian StyleTseng, Yun-Hsiu, Tien-Li Ma, Dun-Heng Tan, An-Jey A. Su, Kia M. Washington, Chun-Chieh Wang, Yu-Ching Huang, Ming-Chung Wu, and Wei-Fang Su. 2023. "Injectable Hydrogel Guides Neurons Growth with Specific Directionality" International Journal of Molecular Sciences 24, no. 9: 7952. https://doi.org/10.3390/ijms24097952
APA StyleTseng, Y.-H., Ma, T.-L., Tan, D.-H., Su, A.-J. A., Washington, K. M., Wang, C.-C., Huang, Y.-C., Wu, M.-C., & Su, W.-F. (2023). Injectable Hydrogel Guides Neurons Growth with Specific Directionality. International Journal of Molecular Sciences, 24(9), 7952. https://doi.org/10.3390/ijms24097952









